Abstract
Black individuals have lower 25-hydroxyvitamin D [25(OH)D] levels and experience a disproportionate burden of ESRD compared with white individuals. Animal studies suggest that vitamin D has renoprotective effects. We evaluated the contribution of low 25(OH)D levels on incidence of ESRD using data from the Third National Health and Nutrition Examination Survey–linked Medicare claims files (n = 13,328). We included baseline (1988 through 1994) measurements of 25(OH)D and assessed the incidence of ESRD through July 31, 2001. Overall, 34% of non-Hispanic black individuals had 25(OH)D levels <15 ng/ml compared with 5% of non-Hispanic white individuals (P < 0.001). During a median of 9.1 yr, 65 participants developed ESRD. After adjustment for demographic, socioeconomic, and clinical and laboratory factors (including diabetes, hypertension, estimated GFR, and albuminuria), participants with 25(OH)D levels <15 ng/ml had a 2.6-fold greater incidence of ESRD than those with levels ≥15 ng/ml (incidence rate ratio 2.64; 95% confidence interval [CI] 1.00 to 7.05; P = 0.05). After adjustment for clinical covariates but not 25(OH)D levels, non-Hispanic black individuals had a 2.83-fold (95% CI 1.03 to 7.77) higher risk for developing ESRD compared with non-Hispanic white individuals. Additional adjustment for 25(OH)D levels reduced the risk by 58% (incidence rate ratio 1.77; 95% CI 0.38 to 8.21). In summary, low 25(OH)D levels associate with development of ESRD even after adjustment for multiple risk factors. Low 25(OH)D levels may account for a substantial proportion of the increased risk for ESRD experienced by black individuals.
Non-Hispanic black and Hispanic individuals have a higher risk for ESRD compared with non-Hispanic white individuals. Even after adjustment for demographic, socioeconomic, and lifestyle factors, non-Hispanic black participants in the Second National Health and Nutrition Examination Survey (NHANES) had a relative risk for progressing to ESRD of 1.95 compared with non-Hispanic white individuals.1
Low vitamin D levels, measured as 25-hydroxyvitamin D [25(OH)D], the main circulating form, have been associated with incident hypertension,2 insulin resistance,3 peripheral arterial disease,4 cardiovascular disease,5,6 and mortality.7 Vitamin D may also have effects on the progression of kidney disease. Activated vitamin D, 1,25-dihydroxyvitamin D [1,25(OH)2D], suppresses renin biosynthesis in mice, and vitamin D deficiency stimulates renin production.8 In different animal models, 1,25(OH)2D or its analogs reduced proteinuria levels; preserved glomerular podocyte structure9; decreased levels of TGF-β1, an inducer of renal fibrosis; and inhibited mesangial cell proliferation, a marker of renal injury.10 Low 25(OH)D levels were associated with albuminuria in a cross-sectional analysis of NHANES III.11 Reports of human studies of vitamin D and progression of kidney disease are rare. Three reports, each with limited sample sizes, suggested no harm from and a potential benefit of active vitamin D use in slowing the progression of kidney disease.12–14 On the basis of its suppression of the renin-angiotensin system and on animal models showing beneficial effects on albuminuria, mesangial cell proliferation and inflammation, and extracellular matrix formation, low vitamin D levels are a candidate novel risk factor for the progression of renal disease.
Non-Hispanic black and Hispanic individuals have lower levels of 25(OH)D,15 potentially because of darker skin pigmentation, leading to decreased synthesis of vitamin D in the skin.16–18 If low 25(OH)D levels are a risk factor for progression of kidney disease, then this may explain some of the excess risk for ESRD in non-Hispanic black and Hispanic individuals. We tested the hypothesis that low 25(OH)D levels are associated with progression of kidney disease using data from the NHANES III follow-up study, a cohort study based on a nationally representative sample of US adults in which 25(OH)D levels were measured in 1988 through 1994 and participants were followed for up to 12 yr through linkage with Medicare files and the National Death Index.
Results
Participant Characteristics
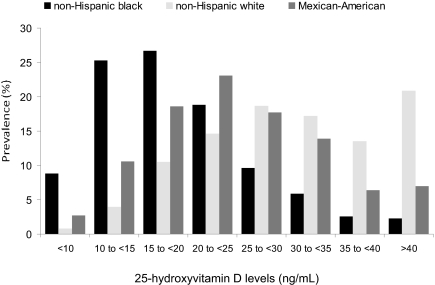
Participants with 25(OH)D deficiency (<15 ng/ml) were more likely to be female, non-Hispanic black, Mexican-American, or other race; have diabetes and hypertension; and be former smokers (Table 1, Figure 1). Participants with 25(OH)D deficiency were also more likely to be of low socioeconomic status (SES); to be examined during the winter months; have higher body mass index (BMI), HDL cholesterol, phosphate, and C-reactive protein (CRP) levels; and to have albuminuria and lower serum albumin and hemoglobin. Participants who exercised more and those who took vitamin D supplements were less likely to be 25(OH)D deficient.
Table 1.
Baseline characteristics of 13,328 NHANES III participants by serum 25(OH)D level
| Characteristic | 25(OH)D Levels (ng/ml) |
|||
|---|---|---|---|---|
| Total(n = 13,328) | ≥15(n = 11,238) | <15(n = 2090) | P | |
| Age (yr; mean [SE]) | 44.3 (0.5) | 44.3 (0.5) | 44.8 (0.6) | 0.42 |
| Female gender (% [SE]) | 52.1 (0.5) | 50.4 (0.5) | 69.5 (1.5) | <0.001 |
| Race/ethnicity (% [SE]) | ||||
| non-Hispanic white | 77.8 (1.2) | 81.2 (1.1) | 42.3 (2.7) | <0.001 |
| non-Hispanic black | 10.0 (0.6) | 7.3 (0.5) | 38.8 (2.2) | <0.001 |
| Mexican American | 4.8 (0.4) | 4.5 (0.4) | 7.2 (0.8) | <0.001 |
| other | 7.4 (0.8) | 7.0 (0.9) | 11.6 (1.8) | 0.004 |
| Diabetes (% [SE]) | 6.6 (0.3) | 6.3 (0.3) | 9.0 (1.0) | 0.003 |
| Hypertension (% [SE]) | 23.7 (0.8) | 23.4 (0.9) | 26.9 (1.5) | 0.02 |
| Smoking (% [SE]) | ||||
| former | 26.2 (0.6) | 26.7 (0.7) | 20.9 (1.5) | 0.001 |
| current | 28.3 (0.8) | 27.9 (0.9) | 32.3 (2.3) | 0.06 |
| History of CVD (% [SE]) | 7.6 (0.4) | 7.6 (0.5) | 7.6 (0.9) | 0.99 |
| Low SES (% [SE]) | 32.8 (1.2) | 32.0 (1.3) | 41.0 (1.8) | <0.001 |
| Season (% [SE]) | ||||
| Winter (January through March) | 16.4 (2.9) | 15.1 (2.6) | 30.3 (5.6) | <0.001 |
| Spring (April through June) | 25.9 (4.1) | 26.1 (4.2) | 23.8 (4.2) | 0.39 |
| Summer (July through September) | 33.3 (4.9) | 34.7 (5.1) | 18.8 (3.7) | <0.001 |
| Fall (October through December) | 24.3 (4.2) | 24.1 (4.3) | 27.0 (4.4) | 0.36 |
| Use of vitamin D supplementation (% [SE]) | 28.9 (0.7) | 30.3 (0.7) | 13.9 (1.5) | <0.001 |
| Physical activity level (% [SE]) | ||||
| moderate | 54.3 (0.9) | 55.0 (1.0) | 46.9 (1.7) | <0.001 |
| high | 21.4 (0.9) | 22.5 (0.9) | 10.8 (1.1) | <0.001 |
| BMI (kg/m2; mean [SE]) | 26.5 (0.1) | 26.4 (0.1) | 28.2 (0.3) | <0.001 |
| Albumin excretion ≥30 mg/g of creatinine (% [SE]) | 8.2 (0.4) | 7.9 (0.4) | 12.1 (1.2) | <0.001 |
| Use of cholesterol lowering medication (% [SE]) | 2.9 (0.2) | 2.8 (0.2) | 3.9 (1.0) | 0.14 |
| HDL cholesterol (mg/dl; mean [SE]) | 50.8 (0.3) | 50.6 (0.4) | 52.3 (0.6) | 0.002 |
| Total cholesterol (mg/dl; mean [SE]) | 203.7 (0.8) | 203.8 (0.9) | 202.2 (1.7) | 0.38 |
| Serum phosphate (mg/dl; mean [SE]) | 3.45 (0.01) | 3.44 (0.01) | 3.49 (0.02) | 0.005 |
| Serum hemoglobin (g/dl; mean [SE]) | 14.00 (0.03) | 14.20 (0.03) | 13.50 (0.05) | <0.001 |
| Serum albumin (g/dl; mean [SE]) | 4.18 (0.02) | 4.20 (0.02) | 4.05 (0.02) | <0.001 |
| C-reactive protein >0.021 (mg/L; % [SE]) | 28.1 (1.2) | 27.3 (1.3) | 37.2 (2.0) | <0.001 |
| eGFR <60 ml/min per 1.73 m2 (% [SE]) | 4 (0.3) | 4.0 (0.3) | 4.5 (0.7) | 0.48 |
CVD, cardiovascular disease.
Figure 1.
Distribution of 25(OH)D levels by race/ethnicity in the NHANES III is shown.
Associations between 25(OH)D Deficiency and Incident ESRD
During a median of 9.1 yr (interquartile range 7.6 to 10.7), 65 NHANES III participants developed ESRD. Of those who developed ESRD, the 2728 form revealed that approximately 54% developed ESRD secondary to diabetes, 32% secondary to hypertension, and 6% secondary to glomerulonephritis; the causes for the remaining 8% of cases were either not reported or were causes other than those listed. In unadjusted analysis, the incidence rate ratio (IRR) of ESRD associated with 25(OH)D deficiency was 4.26 (95% confidence interval [CI] 2.02 to 8.98). After age, gender, race/ethnicity, and season adjustment, the IRR was 2.88 (95% CI 1.10 to 7.55), and after further multivariable adjustment, the IRR was 2.64 (95% CI 1.00 to 7.05; Figure 2).
Figure 2.
IRRs for ESRD in those with 25(OH)D levels <15 ng/ml compared with those with levels ≥15 ng/ml after various levels of propensity score adjustment are shown. Adjusted 1 includes age, gender, race/ethnicity, and season. Adjusted 2 includes the variables in adjusted 1 and hypertension, diabetes, BMI, HDL cholesterol, eGFR <60 ml/min per 1.73 m2, serum albumin, log urinary ACR, log CRP, level of physical activity, vitamin D supplement use, and low SES.
Subgroup Analysis
The association between 25(OH)D deficiency and incident ESRD was similar in subgroups defined by baseline estimated GFR (eGFR) (Table 2). There were interactions in the association between 25(OH)D levels <15 ng/ml and ESRD by age (P = 0.03 for interaction) and gender (P = 0.07 for interaction). The association was more pronounced in participants who were younger than 65 yr and in women. The association was also stronger in non-Hispanic white and Mexican-American individuals than in non-Hispanic black individuals (P = 0.06 for interaction for non-Hispanic white versus black). Incidence rates (95% CI) by race/ethnicity were 198 (72 to 301) per 1,000,000 person-years for non-Hispanic white individuals, 767 (498 to 1006) per 1,000,000 person-years for non-Hispanic black individuals, and 387 (200 to 526) per 1,000,000 person-years for Mexican-American individuals.
Table 2.
Associations between 25(OH)D levels and incident ESRD in 13,328 participants of NHANES III overall and in patient subgroups
| Parameter | 25(OH)D Levels (ng/ml; IRR [95% CI]) |
P (Interaction) | |
|---|---|---|---|
| ≥15(n = 11,238) | <15(n = 2090) | ||
| IRR | |||
| overall | 1.00 (reference) | 2.64 (1.00 to 7.05) | |
| age | 0.03 | ||
| <65 (10,259/27/15) | 1.00 (reference) | 3.20 (1.02 to 10.04) | |
| ≥65 (3069/17/6) | 1.00 (reference) | 1.32 (0.26 to 6.53) | |
| gender | 0.07 | ||
| male (6275/30/8) | 1.00 (reference) | 0.64 (0.24 to 1.71) | |
| female (7053/14/13) | 1.00 (reference) | 5.67 (1.57 to 20.57) | |
| race | |||
| non-Hispanic white (5696/8/3) | 1.00 (reference) | 3.71 (1.06 to 12.95) | |
| non-Hispanic black (3.595/21/9) | 1.00 (reference) | 1.17 (0.43 to 3.17) | 0.06 |
| Mexican American (3,510/13/9) | 1.00 (reference) | 5.26 (2.20 to 12.55) | 0.99 |
| other (527/2/0) | 1.00 (reference) | a | |
| baseline eGFR | 0.25 | ||
| ≥60 ml/min per 1.73 m2 (12,587/17/8) | 1.00 (reference) | 5.24 (0.84 to 32.77) | |
| <60 ml/min per 1.73 m2 (741/27/13) | 1.00 (reference) | 1.49 (0.58 to 3.90) | |
| IRRs (per 1,000,000 person-years) | |||
| race | |||
| non-Hispanic white | 153 (48 to 238) | 1139 (0 to 2121) | |
| non-Hispanic black | 698 (439 to 904) | 929 (145 to 1491) | |
| Mexican American | 207 (101 to 283) | 1574 (387 to 2416) | |
Numbers after subgroups represent total N/events in ≥15 ng/ml/events in <15 ng/ml. Results are adjusted for age, gender, race/ethnicity, season, hypertension, diabetes, BMI, HDL cholesterol, baseline eGFR < or >60 ml/min per 1.73 m2, serum albumin, log urinary ACR, log CRP, physical activity level, vitamin D supplementation, and low SES except in specific subgroup models, the subgroup categorical variable is not included in the model.
aNumbers too small to yield valid estimates.
25(OH)D Deficiency and Race and the Development of ESRD
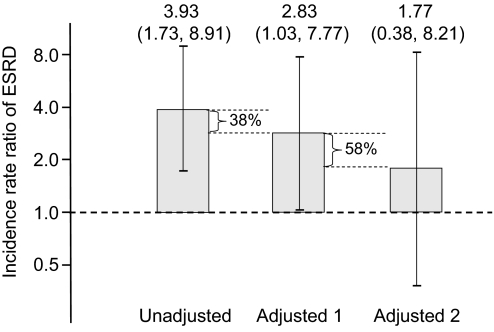
Non-Hispanic black individuals had an unadjusted IRR of 3.93 (95% CI 1.73 to 8.91) of developing ESRD compared with non-Hispanic white individuals (Figure 3). After adjustment for potential confounders including age, gender, season, hypertension, diabetes, BMI, HDL cholesterol, eGFR <60 ml/min per 1.73 m,2 serum albumin, log urinary albumin-creatinine ratio (ACR), log CRP, physical activity level, and low SES in a propensity score, the IRR of ESRD was 2.83 (95% CI 1.03 to 7.77). The IRR after further adjustment for 25(OH)D <15 ng/ml was 1.77 (95% CI 0.38 to 8.21), suggesting 25(OH)D deficiency explained 58% of the increased risk for ESRD experienced by non-Hispanic black individuals.
Figure 3.
IRRs of ESRD in non-Hispanic black compared with non-Hispanic white individuals after various levels of propensity score adjustment are shown. Adjusted 1 includes age, season, hypertension, diabetes, BMI, HDL cholesterol, eGFR <60 ml/min per 1.73 m2, serum albumin, log urinary ACR, log CRP, level of physical activity, and low SES. Adjusted 2 includes variables in adjusted 1 and 25(OH)D <15 ng/ml. The values 38% and 58% represent the reduction in the IRR associated with adjustment for variables in adjusted model 1 and adjusted model 2, respectively.
Associations between 25(OH)D Deficiency and Incident ESRD or Death with Nephropathy
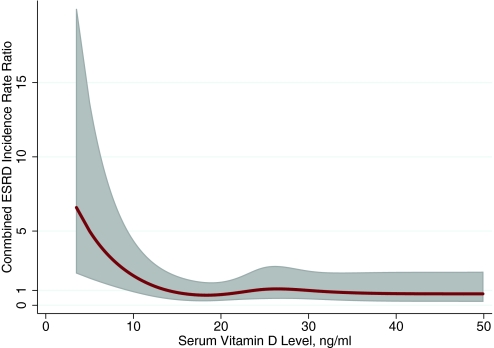
A total of 170 participants reached the combined end point of initiating ESRD or dying with nephropathy. The unadjusted IRR for the combined end point of initiating ESRD or dying with nephropathy among those with 25(OH)D <15 ng/ml was 2.67 (95% CI 1.65 to 4.31). Adjustment for age, gender, race/ethnicity, and season decreased the IRR to 2.05 (95% CI 1.10 to 3.81), and multivariable adjustment further decreased the IRR to 1.89 (95% CI 1.00 to 3.57). The continuous association between 25(OH)D levels and the combined outcome is shown in Figure 4.
Figure 4.
Spline shows the continuous association between 25(OH)D levels and the development of dialysis or death with underlying kidney disease among 13,328 participants of NHANES III. Line represents IRR; shaded area represents 95% CI.
Sensitivity Analyses
To confirm that our findings were robust, we conducted analyses adjusting for both serum hemoglobin and phosphate levels. The multivariable adjusted IRR for dialysis was 2.47 (95% CI 0.98 to 6.27; P = 0.056). For the combined end point of dialysis and death with underlying nephropathy, the IRR was 1.87 (95% CI 1.00 to 3.50; P = 0.050). After adjustment for total caloric and protein intake as markers of nutritional adequacy, the IRR for dialysis was 2.56 (95% CI 0.98 to 6.69; P = 0.055) and the IRR for the combined end point was 1.86 (95% CI 1.00 to 3.45; P = 0.049). Excluding the “other races” category, the multivariable adjusted IRR for dialysis was 3.20 (95% CI 1.13 to 9.08), and the IRR for the combined end point was 2.06 (95% CI 1.08 to 3.96). An additional model with no variables in the model with variance inflation factors (VIF) >10 revealed an IRR for dialysis of 2.72 (95% CI 1.08 to 6.84).
Discussion
In this US nationally representative adult population, 25(OH)D levels <15 ng/ml were associated with incident ESRD and the combined end point of incident ESRD and death as a result of nephropathy. In subgroup analyses, 25(OH)D levels <15 ng/ml seemed to be more strongly associated with the outcomes in particular subgroups (age <65, female, non-Hispanic white and Mexican-American individuals). We present these results as potentially hypothesis generating but urge caution in their interpretation because of the small sample sizes on which they are based. Interestingly, in previous analyses, the associations between low 25(OH)D levels and a variety of outcomes (e.g., mortality and peripheral vascular disease) have not been significant in the non-Hispanic black subgroup.7,19,20 Explanations include that cut points do not differentiate adequately in black individuals because of the different underlying distribution in the population and different levels of other related hormones, such as parathyroid hormone (PTH) or fibroblast growth factor 23, which may influence these associations, or that low 25(OH)D are not as detrimental in non-Hispanic black individuals. This last possibility seems unlikely when examining evidence from studies of bone health. Although skeletal resistance to PTH and vitamin D has been shown among non-Hispanic black individuals,21,22 vitamin D supplements decrease PTH levels and markers of bone turnover in black individuals, and most researchers suggested that black individuals require 25(OH)D levels similar to white individuals for bone health.16,23 This area warrants further investigation.
Most studies show that non-Hispanic black individuals have a similar prevalence of stages 1 through 4 chronic kidney disease (CKD) as white individuals but have a higher incidence of ESRD.1,24,25 This suggests that either non-Hispanic black individuals progress faster through the stages of CKD than do non-Hispanic white individuals26,27 or that white individuals die of competing causes before they reach ESRD.28,29 Consistent with the former possibility, black race was associated with a more rapid decline in kidney function in the Modification of Diet in Renal Disease (MDRD) study.30 Black individuals also had a faster decline in their renal function in the Multiple Risk Factor Intervention Trial (MRFIT),31 suggesting that non-Hispanic black individuals do progress faster through the stages of CKD. Previous studies investigating the higher incidence of ESRD in non-Hispanic black individuals showed that the excess risk cannot be completely explained by demographic, socioeconomic, or lifestyle factors.1,32 Other possible explanations for the racial disparity in ESRD are the low nephron mass hypothesis33,34 or genetic factors such as the recent discovery that polymorphisms of the gene encoding nonmuscle myosin heavy chain type II isoform A (MYH9) are associated with nondiabetic kidney disease.35 Although most previous studies of racial disparities in ESRD have focused on non-Hispanic black and non-Hispanic white individuals, it is important to note that Hispanic individuals also have a higher risk for ESRD and have lower vitamin D levels than white individuals.
Active vitamin D, in various forms, decreases albuminuria in multiple animal models of kidney disease, including Heymann nephritis,36 murine MRL/L lupus nephritis,37 mercuric chloride–induced nephrotic syndrome,38 and subtotally nephrectomized rats.39 In the anti–Thy 1.1 model of glomerulonephritis, rats treated with 1,25(OH)2D3 had less albuminuria40 and showed preserved slit diaphragm protein morphology.9 22-Oxacalcitriol, a vitamin D analog, reduced urine albumin excretion and prevented mesangial cell proliferation and extracellular matrix expansion in two different rat models of glomerulosclerosis.10,41 Mesangial cell proliferation and extracellular matrix expansion are two of the pathologic processes that lead to progressive glomerulosclerosis. Rats treated with the same vitamin D analog had lower levels of TGF-β1 protein in the tubules and glomeruli compared with nontreated disease rats.10,39 A microarray analysis revealed that vitamin D–related genes are upregulated in the db/db mouse, and evidence suggested that perhaps this upregulation is responsible for the lack of progressive kidney disease in the mouse.42 Interestingly, vitamin D deficiency can cause a myopathy, and, recently, mutations in myosin IIA, the MYH9 gene product, were found to be associated with ESRD.35 It is unknown whether vitamin D may affect myosin IIA. The mechanism of the effect of vitamin D on proteinuria may be through downregulation of TGF-β, a cytokine-regulating cellular proliferation and extracellular matrix deposition,43 preservation of slit diaphragm integrity, anti-inflammatory effects, inhibition of the renin-angiotensin system, other effects not yet described, or, most likely, a combination of these.
Previous small studies suggested a role for active vitamin D therapy and/or low vitamin D levels in the progression of kidney disease.12–14,44 Vitamin D is a negative regulator of the renin-angiotensin system in mice.45 Vitamin D receptor knockout mice and those missing the 1α-hydroxylase enzyme develop hypertension and cardiac hypertrophy and high renin levels.45 Pharmacologic inhibitors of the renin-angiotensin system are one of the only proven therapies to delay progression of kidney disease.46 Two small studies suggested that vitamin D supplementation, one with ultraviolet B radiation (n = 20) and another with 1200 mg of calcium and 800 IU of vitamin D (n = 145), reduce systolic BP.47,48 The animal studies previously mentioned suggested that vitamin D plays a role in the progression but potentially not initiation of a wide variety of kidney diseases. Vitamin D may influence the progression of renal disease either through its effects on the renin-angiotensin system or through its effects on proteinuria as described already. If the association we observed between 25(OH)D deficiency and progression of kidney disease is seen in other observational studies and treatment with vitamin D is shown to retard the progression of kidney disease in randomized clinical trials, then this would have far-reaching public health implications for a readily available, safe medication for kidney disease.
Our study has several potential limitations. Like all observational studies, causality cannot be directly inferred. There is the possibility that unmeasured factors confound the association between 25(OH)D deficiency and incident ESRD, which we did not account for in our analysis. In addition, given the relatively low incidence of ESRD in the general population, a limited number of ESRD cases occurred during follow-up. This limits the interpretation of subgroup analyses. Also, 25(OH)D levels were measured only once at baseline and thus may not reflect lifetime vitamin D status; however, our study has several strengths, including the large number of nationally representative participants, standardized interview and examination data collection, and clinically significant outcomes including death and ESRD.
In summary, after multivariable adjustment, participants with 25(OH)D levels <15 ng/ml were 2.6 times more likely to progress to ESRD compared with participants with higher 25(OH)D levels. The increased prevalence of 25(OH)D <15 ng/ml among non-Hispanic black individuals seems to explain a substantial proportion of their excess risk for ESRD. These results need to be tested in future observational studies, and, if confirmed, then randomized, controlled trials evaluating the renoprotective effects of vitamin D supplementation may be warranted.
Concise Methods
Study Participants
NHANES III includes a sample of noninstitutionalized US civilians, enrolled through a multistage sampling design, and was conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. Details regarding the sampling design, informed consent, examination procedures, and statistical methods of NHANES III are outlined elsewhere.49 We restricted our analyses to adults who were ≥18 yr of age, who had a physical examination and laboratory testing at baseline (October 1988 through October 1994), and for whom vital status information was known at follow-up and linkage to Medicare and Medicaid files was available. Non-Hispanic black individuals, Mexican-American individuals, and the elderly were oversampled in NHANES III to allow more precise estimates in these groups.50 To ensure comparable conditions at each NHANES III survey site, northern states were surveyed during the summer and southern states were surveyed during the winter. Overall, 18,521 adults had 25(OH)D measurements performed. The main analyses were restricted to participants with an eGFR >15 ml/min per 1.73 m2 at baseline (n = 18,496). Participants were excluded when they did not have complete data on other laboratory and examination measurements (n = 3379), poverty status (n = 1477), prevalent ESRD (n = 6), or linkage data (n = 304). The remaining 13,328 participants represent approximately 175 million US adults. NHANES III was approved by the institutional review board of the National Center for Health Statistics, and each participant signed an informed consent. The institutional review board at the Albert Einstein College of Medicine determined this analysis to be exempt.
Study Variables
Interview questions; physical examination; and laboratory values, including serum 25(OH)D, CRP, glucose, albumin, creatinine, phosphate, hemoglobin, and lipids and urinary albumin and creatinine levels, were assessed at baseline and processed per standard protocol.49 Serum 25(OH)D was measured using the Diasorin RIA kit (Diasorin, Stillwater, MN) which measures both 25(OH)D2 and 25(OH)D3 on frozen serum (<−20°C) between February 1994 and December 1995 (total coefficient of variation from quality control samples 13 to 19%). The RIA kit was calibrated using HPLC-purified 25(OH)D every 6 mo. 25(OH)D deficiency was defined as levels <15 ng/ml.6,51 Race/ethnicity was self-identified. Participants' height and weight were measured, and BMI was calculated as weight in kilograms divided by height in meters squared. BP was measured six times following a standard protocol, and hypertension was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, and/or use of antihypertensive medications. A participant was considered to have diabetes when he or she reported ever being told by a doctor that he or she had diabetes or “sugar diabetes” at a time other than during pregnancy, was taking insulin or a “diabetes pill” at the time of the questionnaire, or had a fasting blood glucose ≥126 mg/dl or a nonfasting blood glucose ≥200 mg/dl. Smoking was classified as never, current, or former. Low SES was defined as ≤200% of the poverty index. The use of cholesterol medication was based on the response to the question, “To lower your blood cholesterol, are you now following this advice to take prescribed medicine?” Total caloric and protein intakes were obtained from a 24-h dietary recall.
Serum creatinine levels were calibrated to the Cleveland Clinic laboratory by subtracting 0.23 mg/dl from NHANES III values, and eGFR was calculated on the basis of serum creatinine using the four-variable Modification of Diet in Renal Disease (MDRD) Study formula.52,53 Urinary ACR was based on a spot urine sample. Both ACR and CRP were log-transformed to achieve approximate normality for statistical analyses.
Physical activity level was based on the participants' metabolic expenditures in the past month and categorized as low physical activity intensity (≤3.5 metabolic equivalents [METS]), moderate intensity (3.6 to 14.9 METS), or high intensity (≥15 METS).54 No information was available about whether the physical activities were performed indoors or outdoors. Vitamin D supplementation use was coded as positive for patients who reported taking a supplement or multivitamin containing vitamin D.
ESRD Incidence
For the primary outcome, ESRD incidence was defined as initiating long-term renal replacement therapy. ESRD cases were identified through linkage of the NHANES III database with Medicare files. Probabilistic matching was used with Medicare records available through July 1, 2001. For this analysis, we considered anyone who had a record in the ESRD Patient Master File and Death Notification (Form 2746) with their initial date of dialysis occurring after their NHANES III study visit to have incident ESRD. Causes of ESRD were obtained from the ESRD Entitlement/Registration Form (Form 2728).
The occurrence of ESRD incidence or death with an underlying or contributing cause of kidney disease was analyzed as a secondary outcome. Mortality outcomes were collected for NHANES III participants through December 31, 2000, using probabilistic matching, with up to 12 identifying data elements, to National Death Index records. A selected sample of death certificates was reviewed manually to validate the process. The underlying cause of death was coded according to the ninth revision of the International Statistical Classification of Diseases, Injuries and Causes of Death (ICD-9) for deaths occurring between 1988 and 1998 and according to comparable 10th revision (ICD-10) codes for deaths occurring in 1999 and 2000.55,56 Death caused by nephropathy was coded as being present when the underlying or contributing cause of death was any one of the following ICD-9 codes: 250.4 (diabetes mellitus with nephropathy), 274.1 (gouty nephropathy), 275.4 (nephrocalcinosis), 403 (hypertensive renal disease), 404 (hypertensive heart and renal disease), 580 to 589 (nephritis, nephrotic syndrome, nephrosis), or 593.9 (renal disease not otherwise specified).1
Statistical Analyses
For accounting for the oversampling of subgroups and participant nonresponse, all analyses incorporated weights provided in the NHANES III database using the survey commands in Stata 10.0 (Stata Corp., College Station, TX). Participant characteristics were calculated for those with 25(OH)D levels above or below 15 ng/ml and compared across these levels using logistic (categorical variables) or linear (continuous variables) regression.
Poisson regression models were used to examine the associations between low 25(OH)D levels (<15 ng/ml) and incident ESRD and the combined outcome of incident ESRD and death with underlying nephropathy, separately. Follow-up time for each individual was calculated as the number of days between the baseline visit and the incidence of ESRD, death, or December 31, 2000, whichever occurred first. Incidence rates were obtained by dividing the number of survey-weighted events by the number of survey-weighted time at risk. Because of the small number of events, adjustment was made using propensity score analysis. The propensity score was obtained by performing a logistic regression with 25(OH)D levels <15 ng/ml as the outcome. Because serum 25(OH)D levels vary by season,57 all multivariable analyses were adjusted for season of examination in the propensity score. Additional variables in the propensity score for 25(OH)D <15 ng/ml included age, gender, race/ethnicity, hypertension, diabetes, BMI, HDL cholesterol, eGFR <60 ml/min per 1.73 m2, serum albumin, log urinary ACR, log CRP, physical activity level, vitamin D supplementation, and low SES. Inclusion of variables in the final regression model was based on the variable of interest changing the IRR for 25(OH)D levels <15 ng/ml by >5% in a bivariable model. Although age and eGFR <60 ml/min per 1.73 m2 did not change the IRR by 5%, they were included in the model because of their strong associations with the incidence of dialysis. No pairs of covariates included in the models had correlations ≥0.7. After testing VIFs, our final model had five variables with VIF >10. We performed another analysis without BMI and albumin and taking age out of the propensity score and modeling it separately to have all VIFs <10. Other sensitivity analyses included adding different covariates to the model and excluding the “other” race category. Interactions were tested by adding a product term for 25(OH)D levels <15 ng/ml and each of the covariates age, gender, race/ethnicity, and baseline eGFR <60 ml/min per 1.73 m2 one at a time into models with the main effects for these terms and the other variables from the multivariable model in a propensity score. To model potentially nonlinear associations between 25(OH)D and the outcome and to test our <15 ng/ml cut point, we created a restricted cubic spline using the fully adjusted analysis with the combined outcomes of dialysis and death with underlying kidney disease.
To evaluate the percentage of the excess ESRD risk experienced by non-Hispanic black individuals and explained by differences in 25(OH)D levels, we used two nested Poisson models: (1) The full propensity score model for being non-Hispanic black, including age, gender, season, hypertension, diabetes, BMI, HDL cholesterol, eGFR <60 ml/min per 1.73 m2, serum albumin, log ACR, log CRP, physical activity level, and low SES, and (2) the full propensity score model with the additional adjustment for 25(OH)D <15 ng/ml. We then calculated the excess risk explained by 25(OH)D levels using the formula % Excess risk = IRR1 − IRR2/IRR1 − 1, where IRR1 is the multivariable-adjusted IRR of incident ESRD for non-Hispanic black compared with non-Hispanic white individuals without adjustment for 25(OH)D, and IRR2 is the IRR in from a model including adjustment for 25(OH)D.1 For the comparison between non-Hispanic white and non-Hispanic black individuals in these two models, other race/ethnicity groups were excluded from the analyses. For all analyses, P < 0.05 for two-tailed tests was considered statistically significant.
Disclosures
T.H.H. has consulted for Bristol Myers Squibb, Eli Lilly, and Wyeth.
Supplementary Material
Acknowledgments
M.L.M. was supported by grant K23-DK078774, T.H.H. was supported by grants R21 DK 077326 and RO1 DK080123, and N.R.P. was supported by grants RO1 DK080123 and K24DK02643 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. M.L.M. is also supported by an American Heart Association Heritage Affiliate Clinically Applied Research Award.
This work was presented as an abstract at the National Institutes of Health summit: The Science of Eliminating Health Disparities; December 16 through 18, 2008; National Harbor, Maryland; and at the annual meeting of American Society of Nephrology; October 28 through November 1, 2009; San Diego, California.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL: Excess risk of chronic kidney disease among African-American versus white subjects in the united states: A population-based study of potential explanatory factors. J Am Soc Nephrol 13: 2363–2370, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC: Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 49: 1063–1069, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B: The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 30: 980–986, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, Raggi P: Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: Results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol 28: 1179–1185, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS: Vitamin D deficiency and risk of cardiovascular disease. Circulation 117: 503–511, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannucci E, Liu Y, Hollis BW, Rimm EB: 25-Hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch Intern Med 168: 1174–1180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melamed ML, Michos ED, Post W, Astor B: 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 168: 1629–1637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J: Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol 89–90: 387–392, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Migliori M, Giovannini L, Panichi V, Filippi C, Taccola D, Origlia N, Mannari C, Camussi G: Treatment with 1,25-dihydroxyvitamin D3 preserves glomerular slit diaphragm-associated protein expression in experimental glomerulonephritis. Int J Immunopathol Pharmacol 18: 779–790, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Makibayashi K, Tatematsu M, Hirata M, Fukushima N, Kusano K, Ohashi S, Abe H, Kuze K, Fukatsu A, Kita T, Doi T: A vitamin D analog ameliorates glomerular injury on rat glomerulonephritis. Am J Pathol 158: 1733–1741, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS: 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 50: 69–77, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hamdy NA, Kanis JA, Beneton MN, Brown CB, Juttmann JR, Jordans JG, Josse S, Meyrier A, Lins RL, Fairey IT: Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ 310: 358–363, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan JC, Kodroff MB, Landwehr DM: Effects of 1,25-dihydroxyvitamin-D3 on renal function, mineral balance, and growth in children with severe chronic renal failure. Pediatrics 68: 559–571, 1981 [PubMed] [Google Scholar]
- 14.Coen G, Mazzaferro S, Manni M, Fondi G, Perruzza I, Pasquali M, Taggi F: No acceleration and possibly slower progression of renal failure during calcitriol treatment in predialysis chronic renal failure. Nephrol Dial Transplant 9: 1520, 1994 [PubMed] [Google Scholar]
- 15.Zadshir A, Tareen N, Pan D, Norris K, Martins D: The prevalence of hypovitaminosis D among us adults: Data from the NHANES III. Ethn Dis 15: S5-97–S5-101, 2005 [PubMed] [Google Scholar]
- 16.Dawson-Hughes B: Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. Am J Clin Nutr 80: 1763S–1766S, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Loomis WF: Skin-pigment regulation of vitamin-D biosynthesis in man. Science 157: 501–506, 1967 [DOI] [PubMed] [Google Scholar]
- 18.Clemens TL, Adams JS, Henderson SL, Holick MF: Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1: 74–76, 1982 [DOI] [PubMed] [Google Scholar]
- 19.Reis JP, Michos ED, von Muhlen D, Miller ER, 3rd: Differences in vitamin D status as a possible contributor to the racial disparity in peripheral arterial disease. Am J Clin Nutr 88: 1469–1477, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Scragg R, Sowers M, Bell C: Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 27: 2813–2818, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Cosman F, Morgan DC, Nieves JW, Shen V, Luckey MM, Dempster DW, Lindsay R, Parisien M: Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res 12: 958–966, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Dawson-Hughes B, Harris SS, Finneran S, Rasmussen HM: Calcium absorption responses to calcitriol in black and white premenopausal women. J Clin Endocrinol Metab 80: 3068–3072, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Harris SS: Vitamin D and African Americans. J Nutr 136: 1126–1129, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Powe NR, Melamed ML: Racial disparities in the optimal delivery of chronic kidney disease care. Med Clin North Am 89: 475–488, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the united states. J Am Soc Nephrol 14: 2902–2907, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ: The excess incidence of diabetic end-stage renal disease among blacks: A population-based study of potential explanatory factors. JAMA 268: 3079–3084, 1992 [PubMed] [Google Scholar]
- 27.Trivedi HS, Pang MM: Discrepancy in the epidemiology of nondiabetic chronic renal insufficiency and end-stage renal disease in black and white Americans: The Third National Health and Nutrition Examination Survey and United States Renal Data System. Am J Nephrol 23: 448–457, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Newsome BB MW, Coffey CS, Allison JJ, Kiefe CI, Warnock DG: Survival advantage of black patients with kidney disease after acute myocardial infarction. Clin J Am Soc Nephrol 1: 993–999, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Smith GL, Shlipak MG, Havranek EP, Masoudi FA, McClellan WM, Foody JM, Rathore SS, Krumholz HM: Race and renal impairment in heart failure: Mortality in blacks versus whites. Circulation 111: 1270–1277, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, Rogers NL, Teschan PE: Predictors of the progression of renal disease in the Modification of Diet in Renal Disease study. Kidney Int 51: 1908–1919, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD: Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial: Racial and treatment effects. The MRFIT research group. JAMA 268: 3085–3091, 1992 [PubMed] [Google Scholar]
- 32.Krop JS, Coresh J, Chambless LE, Shahar E, Watson RL, Szklo M, Brancati FL: A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: The Atherosclerosis Risk in Communities study. Arch Intern Med 159: 1777–1783, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Hoy WE, Rees M, Kile E, Mathews JD, Wang Z: A new dimension to the Barker hypothesis: Low birthweight and susceptibility to renal disease. Kidney Int 56: 1072–1077, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Brenner BM, Chertow GM: Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23: 171–175, 1994 [PubMed] [Google Scholar]
- 35.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS: Myh9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 40: 1185–1192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Branisteanu DD, Leenaerts P, van Damme B, Bouillon R: Partial prevention of active Heymann nephritis by 1 alpha, 25 dihydroxyvitamin D3. Clin Exp Immunol 94: 412–417, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemire JM, Ince A, Takashima M: 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/L mice. Autoimmunity 12: 143–148, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Lillevang ST, Rosenkvist J, Andersen CB, Larsen S, Kemp E, Kristensen T: Single and combined effects of the vitamin D analogue KH1060 and cyclosporin A on mercuric-chloride-induced autoimmune disease in the BN rat. Clin Exp Immunol 88: 301–306, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E: Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int 53: 1696–1705, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Panichi V, Migliori M, Taccola D, Filippi C, De Nisco L, Giovannini L, Palla R, Tetta C, Camussi G: Effects of 1,25(OH)2D3 in experimental mesangial proliferative nephritis in rats. Kidney Int 60: 87–95, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Hirata M, Makibayashi K, Katsumata K, Kusano K, Watanabe T, Fukushima N, Doi T: 22-Oxacalcitriol prevents progressive glomerulosclerosis without adversely affecting calcium and phosphorus metabolism in subtotally nephrectomized rats. Nephrol Dial Transplant 17: 2132–2137, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Zhou J, Minto AW, Hack BK, Alexander JJ, Haas M, Li YC, Heilig CW, Quigg RJ: Altered vitamin D metabolism in type II diabetic mouse glomeruli may provide protection from diabetic nephropathy. Kidney Int 70: 882–891, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Aschenbrenner JK, Sollinger HW, Becker BN, Hullett DA: 1,25-(OH(2))D(3) alters the transforming growth factor beta signaling pathway in renal tissue. J Surg Res 100: 171–175, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F, Zoccali C: Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int 75: 88–95, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP: 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C: Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab 86: 1633–1637, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM: Ultraviolet B and blood pressure. Lancet 352: 709–710, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94: Series 1—Programs and collection procedures. Vital Health Stat 1 1–407, 1994 [PubMed] [Google Scholar]
- 50.Ezzati TM, Massey JT, Waksberg J, Chu A, Maurer KR: Sample design: Third National Health and Nutrition Examination Survey. Vital Health Stat 2 1–35, 1992 [PubMed] [Google Scholar]
- 51.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K: Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 167: 1159–1165, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS: Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 39: 920–929, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G: National kidney foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Zhu S, St-Onge MP, Heshka S, Heymsfield SB: Lifestyle behaviors associated with lower risk of having the metabolic syndrome. Metabolism 53: 1503–1511, 2004 [DOI] [PubMed] [Google Scholar]
- 55.International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), Washington, DC, World Health Organization, 1991 [Google Scholar]
- 56.International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Washington, DC, World Health Organization, 1992 [Google Scholar]
- 57.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR: Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30: 771–777, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.