Abstract
The non-human primate is an important translational species for understanding the normal function and disease processes of the human brain. Unbiased stereology, the method accepted as state-of-the-art for quantification of biological objects in tissue sections2, generates reliable structural data for biological features in the mammalian brain3. The key components of the approach are unbiased (systematic-random) sampling of anatomically defined structures (reference spaces), combined with quantification of cell numbers and size, fiber and capillary lengths, surface areas, regional volumes and spatial distributions of biological objects within the reference space4. Among the advantages of these stereological approaches over previous methods is the avoidance of all known sources of systematic (non-random) error arising from faulty assumptions and non-verifiable models. This study documents a biological application of computerized stereology to estimate the total neuronal population in the frontal cortex of the vervet monkey brain (Chlorocebus aethiops sabeus), with assistance from two commercially available stereology programs, BioQuant Life Sciences and Stereologer (Figure 1). In addition to contrast and comparison of results from both the BioQuant and Stereologer systems, this study provides a detailed protocol for the Stereologer system.
Protocol
Part 1: Pre-processing of tissue should be done according to Burke et al. (2009)5.
Briefly, tissue should be well perfused with paraformaldehyde, glutaraldehyde, or formalin. This can be achieved through standard transcardial perfusion typically used to harvest other organs. In the present study the subject was deeply sedated with ketamine hydrochloride (10 mg/kg, i.m.), euthanized with an overdose of sodium pentobarbital (25 mg/kg, i.v.) and perfused transcardially with 0.1 M PBS until completely exsanguinated. This is followed by a 4% paraformaldehyde solution in PBS for 5 min (~1 liter). Tissue should be well perfused with paraformaldeyhde, gluteraldehyde, or formalin. The brain should be stereotaxically blocked, removed from the skull, weighed, volume determined, cryoprotected, and frozen5.
Part 2: Systematic Sampling of tissue, according to Burke et al. (2009)6.
In this example we focused on the frontal lobe of the vervet brain. The reference space was defined to include cortical tissue from the central sulcus to the frontal pole of the left hemisphere. For our purposes the series were set at 1/10 sections throughout the cortex, sectioned at 50µm. One complete series was stained with cresyl violet (series #1). Series 2 was split so that half of the sections were stained with gold chloride for myelin revelation and the other half stained for acetylcholine esterase (for delineation of certain subcortical areas). All other series were banked in antigen preserve as part of our long-term research plans.
Part 3: Stereology (for more details, see Mouton 2002)4
The total estimation of cell numbers (N) is calculated based on the following equation: N = ssf-1 x asf-1 x tsf-1 x ΣQ- Where ssf is the section sampling fraction, asf is the area sampling fraction, tsf if the thickness sampling fraction (where the measured thickness of the tissue is divided by the dissector height), and ∑Q- is the total number of objects of interest counted within the dissector. The next sections of this protocol show how to determine the ssf, asf, tsf and ∑Q.
- Section Sampling Fraction (non-computer based)
- The entire reference space must be well defined from anterior to posterior and dorsal to ventral. In this example we were interested in the frontal lobe and have defined it as the region from the tip of the frontal pole (anterior) to the central sulcus (posterior) and the lateral sulcus (ventral) and excluded the insula.
- For the frontal lobe of this particular subject a total of 760 sections were systematically collected with 76 stained for cresyl violet. Since the target was 10 sections throughout the reference space 1/6 cresyl violet stained sections were sampled, leading to a Section Sample Fraction of about 1/60. We randomly started with one of the first 6 cresyl violet stained sections and systematically sampled 1/6 thereafter. A total of 13 sections were sampled for the selected subject (Figure 2).
- Area Sample Fraction (computer-based)
- A pilot study will indicate the optimal grid size, the Area Sampling Fraction, to sample the reference space with about 100-300 disectors. Within the frontal lobe, a grid size of 2500 µm2 yielded an average of 254 disectors for this subject (Figure 2). The size of the disector should yield between about 0-5 counted objects, in this case, neurons.
- Thickness Sample Fraction (computer-based)
- At each disector location, the height of the tissue is measured, and a fraction of this height is sampled; this is known as the Thickness Sampling Fraction. To determine the measured thickness, focus through the z-plane until the first cell comes into focus, then back up slightly to the top of the section, i.e., until the last object appears just out of focus. To determine the bottom of the tissue, focus through the z-plane until the cells are barely out of focus.
- Focusing through the z-plane, cells should be stained at every depth; if not, this may indicate an incomplete penetration of the stain or non-uniform dehydration of the tissue, in which case the sections should be re-stained. This precaution is especially important for immunostained tissue that requires penetration of immunoprobes through relatively thick tissue sections. For this reason, immunostained tissue should be lightly counterstained with a basophilic stain (e.g., cresyl violet, hematoxylin) in order to accurately determine top and bottom of the section and to confirm penetration of the antibody. In this study the sections were sliced at a microtome setting of 50µm. After all tissue processing was complete, the measured tissue thickness averaged 17.9µm (Figure 2), with a mean shrinkage of about 65%. Note that these results represent typical shrinkage for tissue processed for routine histological preparation7.
- The default disector height for the typical study is 10µm. The difference between the disector height and the measured section thickness is the guard height, the volume of tissue where no biological features are counted. The use of a guard height avoids tissue damage at the sectioning surfaces (e.g., lost caps).
- The total number of objects counted, ΣQ-
- To avoid bias from recognition errors, it is imperative that a standard definition is followed for the particular biological feature of interest. In this case, we were interested in counting neurons. Therefore, a neuron was defined as having a visible centrally located nucleolus and a clearly defined cytoplasm, whereas glial cells generally lacked visible nucleoli and cytoplasm. For this subject 457 neurons were counted.
Part 4: Stereologer -- A Computerized Stereology System (Stereology Resource Center, Chester, MD)
The Stereologer System prompts the user to fill out the necessary information in a step-by-step fashion. In the “Study Information” section the parameters of the study are established. Since a reference space must be defined for the study, the volume parameter should be selected (for the reference space the Cavalieri estimator should be selected). Object volume may also be selected here to estimate the number-weighted volume for the population of objects of interest. For our example, only the volume of the reference space was selected. For each object, select the number and define the feature of interest, in this case Neurons.
Case Initialization: In this section the sampling information is established. Enter the slab sampling interval (if separate slabs of tissue were sliced exhaustively and each section is placed in sequential order then enter 1 here). Enter the total number of sections taken through the reference space (in this case 760). Then enter the section sampling interval (in this case 59). The system then calculates the number of sections to be sampled (in this case 13).
Probe Parameters: This section defines the grid and disector size. For the volume, use a low magnification 2.5x-10x to define the grid spacing under the edit menu. Object magnification should be performed at 100x (N.A. 1.3 or 1.4). Frame area is the size of the disector, in this case we used 50% Screen. The frame height is the thickness of the disector; set at 10µm here. The frame spacing is the size of the grid. In our pilot study we found that 2500µm yields between 150-200 frames spaced in a systematic-uniform manner through a relatively large reference space. For smaller areas, a smaller grid size should be used. A pilot study will identify the optical probe parameters for each particular study.
Once the study parameters have been established, sampling through the tissue may proceed. The program will prompt you to sample the first section. Step 1: The program will prompt the user, under low magnification, to trace the reference space on the section. The system will then place a grid over the section based on the probe parameters and the user will then verify that points fall within the reference space. If a point is not within the reference space simply click on the point and will not be calculated into the volume. Step 2: The system will place a new grid over the reference space, based on the frame spacing parameters. Verify that the intersections fall within the reference space. Step 3: The system will then prompt the user to switch to the higher magnification objective and move the stage to the first dissector. Step 4: At this point the system prompts the user to define the top and bottom of the section then the system sets the sampling space in the z-axis. Step 5: The user will then click on each object that falls within the disector through the z-plane. Objects that touch the red lines or the bottom of the disector may not be counted. Once each object is counted, click next. The stage will move to the next disector and steps 4 and 5 will be repeated. Once all of the disectors for the section have been counted, the system will prompt the user to insert the next sequential section to be probed. Steps 1-5 will be repeated until all of the sequential sections have been sampled. The system will then provide the calculations for asf, ssf, tsf, and ∑Q. Based on these parameters, the system will generate an estimated N, reference volume and CE’s for both the estimated number and volume. It will also give recommendations to become more efficient or to reduce the CE (Figure 2).
Part 5: Representative Results:
Unbiased stereology provides efficient and reliable estimates of cell populations within a reference space. There are a number of computer-based stereological systems available, all of which rely on systematic sampling and a defined reference space. We have used the BioQuant Life Sciences system to estimate the total neuronal population of the cerebral cortex of 2-year-old vervets to be over 828 millions (Figure 3) with an average CE of 0.042. We have subsequently used the Stereologer system to estimate that the frontal lobe accounts for about half the number of cortical neurons8.
 Figure 1 Computer-based Stereology Systems. The basic set-up of the stereology systems is the same, a microscope with a motorized stage (x-y-z), a stage controller, video camera, computer, and software. The system that is ultimately chosen should be based on efficiency, needs, and cost analysis.
Figure 1 Computer-based Stereology Systems. The basic set-up of the stereology systems is the same, a microscope with a motorized stage (x-y-z), a stage controller, video camera, computer, and software. The system that is ultimately chosen should be based on efficiency, needs, and cost analysis.
| STUDY INFORMATION | |
| Study Name | Frontal Cortex |
| Principal Investigator | MB |
| Species | AG |
| Reference Space Frontal | Cortex |
| Notes | |
| CASE INFORMATION | |
| Data Collector | MB |
| Date | Wednesday, April 30, 2008 |
| Group | Control |
| Subject | O26 |
| Notes | |
| SAMPLING CHARACTERISTICS | |
| Slab Sampling Interval | 1 |
| Total Number of Sections | 760 |
| Section Sampling Interval | 59 |
| Starting Section | 2 |
| FRACTIONS | |
| ASF | 0.0002 |
| SlabSF | 1.0000 |
| SSF | 0.0169 |
| TSF | 0.5587 |
| RESULTS SUMMARY | |||||
| Parameter | Probe | Name | Result | CE | SD |
| Thickness | --- | --- | 17.8996 µ | --- | --- |
| Number | Disector | neuron | 243833769.1473 | 0.0474 | N/A |
| Volume | Cavalieri Point Grid | volume | 1719769397954.1284 µ^3 | 0.0078 | N/A |
| RECOMMENDATIONS | |
| Probe | Object Number (neuron) |
| CE | 0.0474 |
| Recommendations | CE is acceptable. |
| Number of Disectors Viewed is Too High, Increase Disector Spacing. | |
| Probe | Region Volume (volume) |
| CE | 0.0078 |
| Recommendations | CE is acceptable. |
| SECTION RESULTS | ||
| Section | neuron NumObjects neuron NumCorners | volume RegPoints |
| 1. 40 | 48 | 34 |
| 2. 26 | 52 | 44 |
| 3. 29 | 56 | 48 |
| 4. 39 | 100 | 83 |
| 5. 49 | 112 | 83 |
| 6. 53 | 156 | 120 |
| 7. 65 | 128 | 106 |
| 8. 45 | 108 | 100 |
| 9. 52 | 100 | 77 |
| 10. 26 | 64 | 60 |
| 11. 21 | 44 | 44 |
| 12. 11 | 36 | 32 |
| 13. 1 | 12 | 6 |
Figure 2 Results from the Fontal Lobe Obtained with the Stereologer System. For the frontal lobe, the longest part of the stereological process was in the systematic sampling and sectioning. Topography, volume and neuronal estimate were completed in about a day for each subject. The left hemisphere was sampled and the number is doubled in the text to approximate an estimate for both hemispheres.
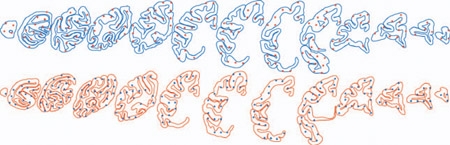 Figure 3 Cortex Neuronal Population Estimate Obtained with BioQuant. The BioQuant system requires an exact topography prior to cell counting. Here an average of 13 sections were sampled. Cortical topography and subsequent volume estimation took between 15-20 hours for each subject. Topography was performed under a 2.5x objective and counting was done using a 100x oil immersion objective (N.A. 1.3). Every 100th section was chosen throughout the left hemisphere with an average of 180 disectors sampled. Once the topography was completed neuronal counting took about a day. The left hemisphere was sampled and the number is doubled in the text to approximate an estimate for both hemispheres.
Figure 3 Cortex Neuronal Population Estimate Obtained with BioQuant. The BioQuant system requires an exact topography prior to cell counting. Here an average of 13 sections were sampled. Cortical topography and subsequent volume estimation took between 15-20 hours for each subject. Topography was performed under a 2.5x objective and counting was done using a 100x oil immersion objective (N.A. 1.3). Every 100th section was chosen throughout the left hemisphere with an average of 180 disectors sampled. Once the topography was completed neuronal counting took about a day. The left hemisphere was sampled and the number is doubled in the text to approximate an estimate for both hemispheres.
Discussion
Unbiased stereology in a primate brain is similar to that of other biological specimens. However, given the size of the primate brain, a careful processing plan is recommended since a single hemisphere of the vervet monkey yields more than 1200 sections when sliced at 50µm. First we recommend stereotaxically blocking the brain into 1cm blocks, which provides a standard plane of section between blocks and subjects, minimizes partial sections between blocks, and provides manageable sized blocks5. A crucial step for stereology is systematic sampling such that 6-10 sections are obtained through the reference area. For the primate brain, this leaves a significant amount of material unprocessed, so in order to maximize the potential data obtained from each brain we suggest banking the tissue in antigen preserve for long-term research plans6. When tissue is banked, a systematic immunodetection approach for identifying target proteins in the monkey brain can be prepared for stereology9. Larger areas such as the cortex or the lobes may require 10-14 sections, so prior to sectioning a plan should be made to have at least 10 sections through the smallest reference space of potential interest. A pilot study should also be performed on a control subject to set the parameters for the stereological study. Furthermore when presenting or interpreting published stereological data the asf, tsf, ssf, and ∑Q should be reported along with the appropriate CE values.
Acknowledgments
The authors would like to thank Ikiel Ptito for his continued technical support. NSERC grant to MP.
References
- West M, Slomianka L, Gundersen H. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Aat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Saper CB. Any way you cut it: a new journal policy for the use of unbiased counting methods. J Comp Neurol. 1996;364:5–5. doi: 10.1002/(SICI)1096-9861(19960101)364:1<5::AID-CNE1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and Practices of Unbiased Stereology. Baltimore: The Johns Hopkins University Press; 2002. [Google Scholar]
- Burke MW, Zangenehpour S, Boire D, Ptito M. Dissecting the non-human primate brain in stereotaxic space. J Vis Exp. 2009;29 doi: 10.3791/1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MW, Zangenehpour S, Boire D, Ptito M. Brain banking: Making the most of your research. J Vis Exp. 2009;29 doi: 10.3791/1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaye KF, Wang P, O'Neil J, Huafu S, Tizabi Y, Thompson N, Ottinger MA, Ingram DK, Mouton PR. Neuropathological Quantification Of Dtg APP/PS1: Neuroimaging, Stereology, And Biochemistry. AGE. 2009;29:87–96. doi: 10.1007/s11357-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MW, Palmour RM, Ervin FR, Ptito M. Neuronal reduction in frontal cortex of primates after prenatal alcohol exposure. Neuroreport. 2009;20:13–17. doi: 10.1097/WNR.0b013e32831b449c. [DOI] [PubMed] [Google Scholar]
- Zangenehpour S, Burke MW, Chaudhuri A, Ptito M. Batch immunostaining for large-scale protein detection in the whole monkey brain. J Vis Exp. 2009;29 doi: 10.3791/1286. [DOI] [PMC free article] [PubMed] [Google Scholar]


