Abstract
Background
Sensory neuropathy (paresthesias), tinnitus, hearing impairment, and Raynaud phenomena are side effects of cisplatin-based chemotherapy used to treat testicular cancer patients. We assessed the long-term occurrence of these side effects among testicular cancer survivors according to the treatment they received.
Methods
A total of 1814 men who were treated for unilateral testicular cancer in Norway during 1980–1994 were invited to participate in a national multicenter follow-up survey conducted during 1998–2002. The men were allocated to six groups according to the treatment they had received. Self-reported symptoms were assessed by a mailed questionnaire that included the Scale for Chemotherapy-Induced Neurotoxicity. A total of 1409 participants who responded to the questionnaire and/or underwent audiometry were assessable in this study. Respondents to the questionnaire (n = 1402) scored the relevant symptoms according to how troubled they were by each (not at all, a little, quite a bit, or very much). Hearing impairment was objectively assessed by audiometry at 4000 Hz in 755 men (seven of whom did not respond to the questionnaire). Group comparisons of symptom assessments were performed with χ2 or Kruskal–Wallis tests. Associations between relevant factors and self-reported symptoms or hearing impairment measured by audiometry were assessed using proportional odds ordinal logistic regression models and linear regression models, respectively. All statistical tests were two-sided.
Results
The median follow-up for the 1409 assessable men was 10.7 years (range = 4–21 years). All chemotherapy groups had statistically significantly higher odds for increasing severity of all assessed symptoms and inferior audiometric results compared with men who did not receive chemotherapy. Among chemotherapy-treated men, 39% (95% confidence interval [CI] = 35% to 43%) reported Raynaud-like phenomena (defined as white or cold hands or fingers [or feet or toes] on cold exposure), 29% (95% CI = 25% to 33%) reported paresthesias in the hands or feet, 21% (95% CI = 18% to 25%) reported hearing impairment, and 22% (95% CI = 19% to 26%) reported tinnitus as major symptoms troubling them quite a bit or very much. Hearing impairment (odds ratio [OR] = 5.3, 95% CI = 3.0 to 9.2) and tinnitus (OR = 7.1, 95% CI = 4.1 to 12.4) were particularly common in the dose-intensive chemotherapy group compared with the no chemotherapy group. Men who were treated with radiotherapy had higher odds of self-reported paresthesias in feet compared with those not treated with radiotherapy (OR = 1.5, 95% CI = 1.01 to 2.1, P = .04).
Conclusion
Long-term survivors of testicular cancer who were treated with cisplatin-based chemotherapy were more often troubled by dose-dependent neurological side effects and Raynaud-like phenomena compared with those who were not treated with chemotherapy.
CONTEXT AND CAVEATS
Prior knowledge
The side effects of cisplatin-based chemotherapy used to treat testicular cancer patients include sensory neuropathy, tinnitus, hearing impairment, and Raynaud phenomena.
Study design
A cross-sectional study that assessed the long-term occurrence of cisplatin-associated side effects among testicular cancer survivors according to the treatment they received. Men who received chemotherapy were allocated to three groups based on cisplatin administration (100 mg/m2 cisplatin over 5 consecutive days in first two groups): up to four cycles, five or more cycles, or dose-intensive chemotherapy (100 mg/m2 cisplatin over 2 consecutive days or a total dose exceeding 100 mg/m2). Self-reported symptoms were assessed by a mailed questionnaire, and hearing impairment was objectively assessed by audiometry at 4000 Hz.
Contribution
At 4–21 years after the initiation of treatment for testicular cancer, compared with men not treated with chemotherapy, those who had received any chemotherapy had higher risk for increasing severity of all assessed symptoms and, except for those who received five or more cycles, statistically significantly worse audiometric results.
Implications
These findings support the current approach of using risk-specific recommendations in the treatment of testicular cancer to minimize side effects in good- and intermediate-risk patients and suggest that cisplatin should be administered over the course of 5 days rather than 2 days.
Limitations
The findings are largely based on self-reported symptoms, and the reported toxic effects were, with the exception of hearing threshold measurements, not objectively confirmed. The screening instrument was brief and did not address each symptom in detail. There was no information about the existence of symptoms before diagnoses or whether they changed over time.
From the Editors
Testicular germ cell cancer is the most common cancer among 20- to 40-year-old men, and the cure rate for this cancer currently exceeds 95% (1,2). Long-term side effects of treatment may therefore have an impact on the daily lives of testicular cancer survivors for several decades.
Sensory neuropathy, tinnitus, and hearing impairment are well-known neurotoxic side effects of cisplatin (3,4), which, for three decades, has been the cornerstone for chemotherapeutic treatment of testicular cancer patients. Raynaud phenomenon—well-demarcated discoloration of the fingers and toes upon exposure to cold—is another common side effect following chemotherapy for testicular cancer (3,5,6), and both vascular and neurological mechanisms may be involved in the abnormal vasoregulation that causes vasospasms of the digital arterioles leading to Raynaud phenomenon (7). Bleomycin, which is commonly a part of cisplatin-based combination regimens that are used to treat testicular cancer, is considered to be an important causal factor for the development of Raynaud phenomenon (5,8), and cisplatin may also be associated with this phenomenon (9).
In some testicular cancer patients, these side effects of chemotherapy may decrease in severity and even disappear during the first few years after treatment (3,10–13). In other patients, these side effects are long lasting and can influence the patient's quality of life, daily activities, and ability to work (3,4,14,15). Proper counseling regarding the risk of long-term treatment-related side effects is an essential part of the care of cancer patients and survivors. High-quality long-term follow-up data are needed to guide physicians in their recommendations about choice of optimal treatment for patients and for informing patients about lifestyle changes that may reduce the occurrence of some side effects.
Typically, the prevalence of side effects detected using objective criteria is greater than that reported by the patients (10,11). There are several reasons for this difference. Patients may adapt to their symptoms over time and may therefore underreport their complaints (16), and some toxic effects may not affect a patient's daily life (eg, reduced hearing at frequencies >4000 Hz) (10,11). Although subjective and objective assessments of toxicity are important for the clinical investigator who wants to understand the etiology of side effects, the level of long-term self-reported toxicity probably best reflects the effect of treatment on a cancer survivor's well-being during daily life.
The aim of this study was to assess the occurrence of self-reported paresthesias (sensory neuropathies), Raynaud-like phenomena (white or cold hands or fingers [toes or feet] on exposure to cold), tinnitus, and impaired hearing (also measured objectively via audiometry at 4000 Hz) according to detailed treatment classifications in a large cohort of unselected testicular cancer survivors 4–21 years after treatment. In particular, we assessed the occurrence of these side effects following standard cisplatin-based chemotherapy courses (20 mg/m2 for five consecutive days) separately from those who received more dose-intensive regimens. In a secondary analysis, we tested the hypothesis that these long-term side effects may be associated with tobacco smoking.
Patients and Methods
Population and Study Design
From January 4, 1998, through April 11, 2002, a national follow-up survey was performed to assess physical and psychological morbidity in long-term survivors of testicular cancer. All men who were treated for unilateral germ cell testicular cancer in Norway from January 1, 1980, through December 31, 1994, and were alive as of 1 month before invitation were identified through the Cancer Registry of Norway and the regional university hospitals. Testicular cancer survivors aged 18–75 years were invited to participate. Following our receipt of written informed consent to participate, the study population received a mailed questionnaire, which included questions that assessed the relevant symptoms (Table 1), and an appointment for a clinical examination. In three of the five collaborating oncology centers, the clinical examination included pure tone audiometry. Men were excluded from this study if they had undergone bilateral orchiectomy for any reason, had been diagnosed with extragonadal germ cell cancer or other malignancies except skin cancer, or had mental retardation. Further details of the study design are described elsewhere (17).
Table 1.
Questions used to assess the relevant symptoms*
| Symptom assessed | Question asked |
| Raynaud-like phenomena, hands | Are you troubled by white/cold hands/fingers when it is cold? |
| Raynaud-like phenomena, feet | Are you troubled by white/cold feet/toes when it is cold? |
| Paresthesias, hands | Are you troubled by pain, tingling, or numbness in your hands/fingers? |
| Paresthesias, feet | Are you troubled by pain, tingling, or numbness in your feet/toes? |
| Tinnitus | Are you troubled by ringing in your ears? |
| Hearing impairment | Are you troubled by impaired hearing? |
From the Scale for Chemotherapy-Induced Neurotoxicity. The possible answers were 1) not at all, 2) a little, 3) quite a bit, and 4) very much.
Of the 1814 men who were invited, we included 1462 (80.6%) as participants (10 men were untraceable, 340 did not respond, one was deceased, and one withdrew from the study at a later stage). Most of the participants (n = 1271) answered the questionnaire and underwent the clinical examination, 25 participants only underwent the clinical examination, and 166 participants only answered the questionnaire. The study sample in this study on Raynaud-like phenomena and neurological side effects consisted of 1409 participants (78% of the men originally invited): four men were excluded because they had undergone cranial irradiation, 25 were excluded because their testicular cancers had been treated with a carboplatin-based chemotherapy regimen without a cisplatin-based regimen, one was excluded because of treatment with dactinomycin only, and 23 were excluded because data on all symptoms that were assessed and audiometry were missing.
Primary data on all invited men regarding tumor histology (seminoma or nonseminoma), tumor stage according to the Royal Marsden Hospital staging system (18), type of treatment, and history of relapse were retrieved from the patient's medical records. We retrieved additional details about the chemotherapy treatment from the medical records of all men who participated in this study. This study was approved by the Committee for Medical Research Ethics of the Southern Health Region of Norway.
Standards of Treatment, 1980–1994
All men included in this study were treated either within the Swedish–Norwegian testicular cancer project (SWENOTECA collaboration) or according to the European Organization for Research and Treatment of Cancer and Medical Research Council protocols, as previously described (17). All men in the study sample who were treated with chemotherapy received cisplatin (n = 528). The initial regimen was cisplatin in combination with vinblastine and bleomycin (CVB; n = 202), bleomycin and etoposide (BEP; n = 287), or etoposide (n = 9). Thirty men received other combinations as the initial regimen. Cisplatin was usually administered at dose of 100 mg/m2 over the course of 5 days per cycle. However, for 57 men who were treated according to specific protocols, cisplatin was given at higher dose intensities in at least one of the administered cycles, either at a dose that exceeded 100 mg/m2 per cycle or by administering the cisplatin dose over the course of 2 days (19–22). Eight patients received carboplatin-based chemotherapy regimens (median cumulative dose = 2975 mg, range = 1590–3575 mg) in addition to cisplatin-based regimens. Bleomycin was given as part of the chemotherapy treatment in 511 of the 528 chemotherapy-treated patients and was administered as a bolus dose, mostly within a limited cumulative dose range (median dose = 300 000 IU, range = 30 000–540 000 IU; 77% received a cumulative dose between 240 000 and 360 000 IU). Vincristine was administered in 40 of the cases at cumulative doses of 2–27 mg (median dose = 7 mg). A rigorous hydration regimen was standard for all regimens.
Seminomas.
Most patients with early-stage seminomas (ie, stage I or IIA) were treated with infradiaphragmatic radiotherapy. The L-field or dogleg technique (23,24) was typically used, but in 42 cases, the fields were limited to the para-aortic area (23). Only eight seminoma patients received no additional treatment after orchiectomy. The standard target radiation dose for these stages was gradually reduced from 36–40 Gy in the early 1980s to 25.2–27 Gy in the mid-1990s. Patients with advanced-stage seminomas (ie, stage IIB or higher) were treated with cisplatin-based chemotherapy, and in some patients, this was followed by radiation or resection of enlarged lymph nodes or by retroperitoneal lymph node dissection.
Nonseminomas.
Patients with early-stage nonseminomas (stage I or IIA) were, until approximately 1990, treated with a primary modified bilateral or ipsilateral template retroperitoneal lymph node dissection; the nerve-sparing technique was introduced in 1989. Retroperitoneal lymph node dissection was followed by adjuvant chemotherapy if lymph node metastases were present. After 1990, patients with stage I nonseminomas were either subjected to surveillance following orchiectomy or treated with one to three cycles of adjuvant chemotherapy. Patients with advanced-stage nonseminomas (before 1990: stages IIB–IV; 1990 or later: stages IIA–IV) generally received at least three cycles of cisplatin-based chemotherapy, often combined with a formal retroperitoneal lymph node dissection or more limited resection of retroperitoneal lymph nodes.
Treatment Groups.
All participating testicular cancer survivors were allocated to one of six mutually exclusive groups according to the treatment they received following orchiectomy at the initial diagnosis and at relapse: 1) surveillance; 2) retroperitoneal surgery only; 3) radiotherapy only; 4) chemotherapy with up to four cycles of standard schedule cisplatin-based regimens (20 mg/m2 per day for five consecutive days), with or without retroperitoneal surgery or radiotherapy; 5) chemotherapy with five or more cycles of standard schedule cisplatin-based regimens, with or without retroperitoneal surgery or radiotherapy; or 6) chemotherapy in which at least one of the cisplatin-based cycles was administered more than 20 mg/m2 per day, either because of same dose (100 mg/m2 per cycle) given over fewer days or higher dose per cycle, irrespective of total number of cisplatin-based cycles with or without retroperitoneal surgery or radiotherapy (dose-intensive chemotherapy).
For participating testicular cancer survivors who underwent ototoxicity evaluation, the three first treatment groups (surveillance, retroperitoneal surgery, and radiotherapy) were combined into one treatment group (no chemotherapy) because these treatments are unlikely to have an impact on hearing.
Measurements
The 219-item mailed questionnaire included the six-item Scale for Chemotherapy-Induced Neurotoxicity (SCIN) (25,26), which we used to assess the relevant side effects (Table 1). The participants were asked to score how troubled they were—not at all, a little, quite a bit, or very much—by the relevant symptoms. As in the EORTC QLQ C-30, the responses to the questions and their scaling in the SCIN were intended to reflect both the severity and the frequency of symptoms as experienced by the patient and, thus, assessed the individual's subjective perception of the symptoms. Based on its psychometric properties and its brevity, the SCIN has been recommended (25) for use as a brief screening instrument for chemotherapy-induced neurotoxicity. The SCIN has been used in other studies (12,19,21,26). For most data analyses, we used the ordinal scales (four categories). When the data were dichotomized as minor vs major symptoms, the latter were defined as those with responses of “quite a bit” or “very much.”
The questionnaire also assessed tobacco smoking habits, and the survivors were asked whether they had ever smoked daily, currently smoked daily, or smoked only occasionally. They were also asked how much they smoked or had smoked per day, how old they were when they started, and for how many years they smoked daily. On the basis of their answers, they were grouped as never daily smokers, former daily smokers, or current daily smokers. Cigarette smoking as well as tobacco consumption by pipe (n = 4) or cigar (n = 10) were included.
We considered white or cold fingers or hands (or toes or feet) as Raynaud-like phenomena because the questions about these symptoms did not specifically address the classical well-demarcated discoloration or the pain and numbness that characterize Raynaud phenomenon (7).
Pure tone audiometry was performed as part of the outpatient examination in three of the five participating medical centers, one of which performed audiometry exclusively in chemotherapy-treated patients. Although a range of frequencies may have been tested, only the air conduction threshold at 4000 Hz was entered into the study database per the study protocol. The ability to detect this frequency, which represents the upper limit of the language communication range, is often compromised by exposure to cisplatin, which preferentially affects the higher frequencies (27,28). The 4000-Hz hearing thresholds for both ears were averaged for each testicular cancer survivor. Published normative data from a large unscreened Norwegian male reference population, including the median and the quartiles of hearing threshold levels at 4000 Hz averaged for both ears for seven age groups (20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and 80–89 years), were used to create four groups of audiometric results in our study population (29). Each testicular cancer survivor was allocated to one of the four groups corresponding to the respective quartile (quartiles 1–4) of the reference population according to age and averaged 4000-Hz hearing threshold. The group corresponding to quartile 4 had the most severe hearing impairment.
Statistical Analysis
Group comparisons of continuous variables were performed by using the Student t test (for comparing age at diagnoses and at follow-up in the study sample vs in nonassessable men) or the Kruskal–Wallis test (all comparisons of continuous data within the study sample). Group comparisons were performed with χ2 tests for nominal variables or with Kruskal–Wallis tests for ordinal variables (the symptom assessments in Figures 1 and 2). The McNemar test was used to analyze paired data. Proportional odds ordinal logistic regression models were used to assess the associations between relevant factors and self-reported symptoms. All factors of interest were included in the models. For Raynaud-like phenomena and paresthesias, the treatment-related side effects were assessed by separate variables for receipt of retroperitoneal surgery (yes vs no [referent]), radiotherapy (yes vs no [referent]), and chemotherapy (no chemotherapy [referent], one to four cycles, five or more cycles, or dose intensive). The analyses for chemotherapy were repeated by using one to four cycles and five or more cycles as the referent group. The assumption of proportional odds was checked as recommended by Harrell (30), mainly by graphical checks of plots of partial and score residuals. We concluded that the assumption of proportionality was valid. Associations between relevant factors and audiometric results were analyzed by linear regression models in which the outcome variable corresponds to hearing impairment, in decibels (dB), at 4000 Hz (increasing values correspond to more severe hearing loss).
Figure 1.
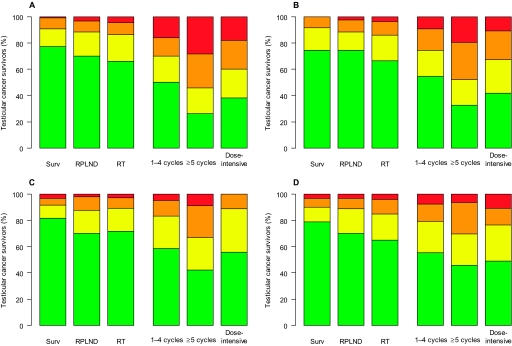
Raynaud-like phenomena and paresthesias according to self-assessed severity and treatment. Self-reported Raynaud-like phenomena in the hands (A) and feet (B) and self-reported paresthesias in the hands (C) and feet (D) are grouped according to self-assessed symptom severity for each treatment group (P < .001 for the comparison between treatment groups for each symptom [two-sided Kruskal–Wallis tests]). Symptom severity coding: red = troubled very much, orange = troubled quite a bit, yellow = troubled a little, green = not troubled at all. Surv = surveillance; RPLND = retroperitoneal lymph node dissection; RT = radiotherapy.
Figure 2.
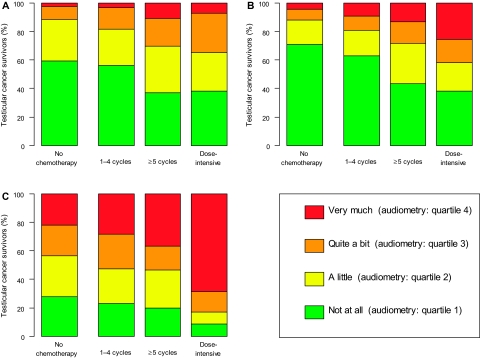
Hearing impairment and tinnitus according to severity and treatment. Self-reported hearing impairment (A) and tinnitus (B) and assessed hearing impairment (C) grouped according to severity for each treatment group. The severity assessments in (A) and (B) are those reported by the participants; for the severity assessment for objective hearing loss measured at 4000 Hz for each testicular cancer survivor, the hearing impairment was classified according quartiles of the 4000-Hz hearing thresholds (averaged for both ears) that were measured in a large unscreened reference population of Norwegian men. (P < .001 the comparison between treatment groups for each symptom [two-sided Kruskal–Wallis tests]).
To compare the side effects of vinblastine with those of etoposide as well as the impact of the cumulative bleomycin dose, we repeated the regression models for the group of men who were treated with chemotherapy by adding covariates for these cytostatic agents (vinblastine vs etoposide-containing regimens and total cumulative bleomycin dose).
Spearman correlation analysis was used to assess the association between subjective and objective measures of hearing impairment and tinnitus. Men with missing data were excluded from the relevant analyses. The treatment data were complete. All statistical tests were two-sided, and a P value less than .05 was considered statistically significant.
Results
Study Population
Of the 1814 men who were invited to participate, 1409 were assessable. Of the assessable survivors, 1402 responded to at least one of the six relevant symptom questions (the SCIN) and 1378 responded to all six questions. Pure tone audiometry was conducted in 755 men, seven of whom had only pure tone audiometry but had not responded to the relevant questions in the SCIN. The median time from orchiectomy to self-reported symptom assessment was 10.7 years and to audiometry, 11.6 years (range for both = 4–21 years). The clinical characteristics according to treatment group are shown in Table 2. Men who were included in the study sample did not statistically significantly differ from the 405 nonassessable men with respect to age at diagnosis and follow-up or by the percentage that received surveillance, retroperitoneal surgery, radiotherapy, or chemotherapy after orchiectomy (data not shown). Among the 1409 assessable survivors, 38 reported that they had diabetes. None of the survivors who reported diabetes were in the five or more cycles or the dose-intensive chemotherapy groups; the frequencies of the survivors who reported diabetes in the remaining groups were 2.7% (surveillance), 1.3% (retroperitoneal surgery), 3.5% (radiotherapy), and 3.3% (one to four cycles) (P = .4). Data regarding cardiovascular morbidity and cardiovascular risk factors, including metabolic syndrome, were published previously (31–33).
Table 2.
Characteristics of the 1409 assessable testicular cancer survivors according to treatment group*
| Characteristic | Surveillance (n = 119) | RPLND (n = 153) | Radiotherapy (n = 609) | Chemotherapy |
Total (n = 1409) | P§ | ||
| 1–4 cycles† (n = 425) | ≥5 cycles† (n = 46) | Dose intensive†‡ (n = 57) | ||||||
| Age, median (range), y | ||||||||
| At treatment | 30 (17–64) | 28 (16–58) | 35 (18–64) | 29 (15–64) | 27 (16–62) | 24 (15–57) | 32 (15–64) | <.001║ |
| At survey | 39 (24–73) | 42 (28–75) | 47 (25–75) | 42 (23–73) | 36 (23–72) | 35 (24–75) | 43 (23–75) | <.001║ |
| Histology, No. (%) | <.001 | |||||||
| Seminoma | 8 (7) | 3 (2) | 607 (99) | 79 (19) | 7 (15) | 4 (7) | 708 (50) | |
| Nonseminoma | 111 (93) | 150 (98) | 2 (1) | 346 (81) | 39 (85) | 53 (93) | 701 (50) | |
| Initial tumor stage¶, No. (%) | <.001 | |||||||
| I | 119 (100) | 147 (96) | 578 (95) | 143 (34) | 5 (11) | 9 (16) | 1001 (71) | |
| IM | — | — | — | 6 (1) | 0 | 1 (2) | 7 (1) | |
| II | — | 6 (4) | 31 (5) | 202 (48) | 18 (39) | 11 (19) | 268 (19) | |
| III | — | — | — | 18 (4) | 6 (13) | 6 (10) | 30 (2) | |
| IV | — | — | — | 56 (13) | 17 (37) | 30 (53) | 103 (7) | |
| Additional treatment and details of chemotherapy among chemotherapy-treated men, No. (%) | ||||||||
| RPLND | — | — | — | 277 (65) | 40 (87) | 40 (70) | — | .01 |
| Radiotherapy | — | — | — | 41 (10) | 5 (11) | 4 (7) | — | .7 |
| Total No. of chemotherapy cycles, median (range) | — | — | — | 4 (1–9) | 6 (5–23) | 4 (2–14) | — | |
| Total No. of cisplatin-based cycles, median (range) | — | — | — | 4 (1–4) | 6 (5–12) | 4 (2–14) | — | <.001║ |
| Total No. of cisplatin-based dose-intensive cycles, median (range) (percentage of total No. of cisplatin-based cycles, median [range]) | — | — | — | 0 | 0 | 3 (1–6) (67 [14–100]) | — | |
| Median cumulative chemotherapy dose (range) | ||||||||
| Cisplatin, mg | — | — | — | 750 (185–1000) | 1200 (800–2120) | 1120 (370–3095) | — | <.001║ |
| Bleomycin, IU | — | — | — | 300 000 (0–390 000) | 300 000 (0–435 000) | 290 000 (90 000–540 000) | — | .001║ |
| Etoposide, mg | — | — | — | 2195 (0–8550) | 4810 (0–10 580) | 3030 (0–9720) | — | <.001║ |
| Vinblastine, mg | — | — | — | 0 (0–108) | 0 (0–90) | 0 (0–93) | — | <.001║ |
| Smoking status#, No. (%) | .001 | |||||||
| Never daily | 42 (39) | 49 (33) | 178 (31) | 150 (38) | 15 (34) | 28 (55) | 462 (35) | |
| Former daily | 41 (38) | 50 (34) | 178 (31) | 96 (24) | 8 (18) | 5 (10) | 378 (29) | |
| Current daily | 25 (23) | 49 (33) | 213 (38) | 153 (38) | 21 (48) | 18 (35) | 479 (36) | |
| Educational level, No. (%)** | .37 | |||||||
| Primary, middle, or high school | 68 (58) | 100 (65) | 359 (60) | 261 (63) | 32 (70) | 38 (69) | 858 (62) | |
| College or university | 49 (42) | 53 (35) | 244 (40) | 154 (37) | 14 (30) | 17 (31) | 531 (38) | |
| Marital status, No. (%)†† | .02 | |||||||
| Unmarried or living alone | 24 (20) | 23 (15) | 134 (22) | 85 (20) | 16 (35) | 18 (33) | 300 (21) | |
| Married or cohabitating | 95 (80) | 130 (85) | 472 (78) | 334 (80) | 30 (65) | 37 (67) | 1098 (79) | |
— = not applicable; RPLND = retroperitoneal lymph node dissection; RT = radiotherapy.
Some men treated with chemotherapy also received radiotherapy or RPLND.
In the dose-intensive group, 29 patients received one to four cycles and 28 patients received five or more cycles.
Two-sided χ2 test except where indicated.
Two-sided Kruskal–Wallis test.
Royal Marsden Hospital staging system (18) stage IM indicates elevated tumor markers without radiological evidence of metastases.
Data missing for 90 patients (surveillance: 11 patients; RPLND: five patients; radiotherapy: 40 patients; one to four cycles: 26 patients; five or more cycles: two patients; dose intensive: six patients).
Data missing for 20 patients (surveillance: two patients; radiotherapy: six patients; one to four cycles: 10 patients; dose intensive: two patients).
Data missing for 11 patients (radiotherapy: three patients; one to four cycles: six patients; dose intensive: two patients).
Symptom Scores and Assessment of Hearing
The severity of the reported symptoms varied statistically significantly among the treatment groups for all side effects assessed (P < .001 for all) (Figures 1 and 2). Among chemotherapy-treated testicular cancer survivors, the most frequently reported major symptoms (ie, those the participants were troubled by “quite a bit” or “very much”) were Raynaud-like phenomena in either the hands or feet (39% [95% CI = 35% to 43%]); Raynaud-like phenomena in these patients were more common in the hands than in the feet (33% vs 28%, difference = 5%, 95% CI = −1% to 10%, P = .006). However, 12% (95% CI = 7% to 19%) of survivors who were treated by orchiectomy alone (surveillance group) reported Raynaud-like phenomena in either the hands or the feet as major symptoms. Raynaud-like phenomena in the hands or feet were reported as major symptoms statistically significantly more frequently by those who received five or more cycles of chemotherapy than by those who received one to four cycles of chemotherapy (61% vs 35%, difference = 26%, 95% CI = 11% to 40%, P = .001). These symptoms were also reported more frequently by the dose-intensive chemotherapy group than by the group that received one to four cycles of chemotherapy, but the difference was not statistically significant (49% vs 35%, difference = 14%, 95% CI = 1% to 28%, P = .052).
Paresthesias in the hands or feet were reported as major symptoms by 29% (95% CI = 25% to 33%) of all men who were treated with chemotherapy and were experienced more often in the feet than in the hands (22% vs 18%, difference = 4%, 95% CI = −1% to 9%, P = .04). More men in radiotherapy group and in the one to four chemotherapy cycles group reported paresthesias in the feet as major symptoms compared with men in the surveillance group (radiotherapy vs surveillance: 15% vs 10%, difference = 5%, 95% CI = −2% to 10%, P = .16; one to four cycles vs surveillance: 21% vs 10%, difference = 11%, 95% CI = 3% to 17%, P = .01). Paresthesias in either the hands or the feet were reported as major symptoms by statistically significantly more men who received five or more cycles of chemotherapy than by men who received one to four cycles of chemotherapy (46% vs 28%, difference = 18%, 95% CI = 4% to 33%, P = .016), whereas the frequency in the dose-intensive group was similar to that of the one to four cycles (27% vs 28%, difference = 0.5%, 95% CI = −11.6% to 13.2%, P = 1.0).
Among all men who received chemotherapy, 21% (95% CI = 18% to 25%) reported hearing impairment and 22% (95% CI = 19% to 26%) reported tinnitus as major symptoms. Tinnitus was reported as a major symptom by 12% of men who received no chemotherapy vs by 19% of those treated with one to four cycles of chemotherapy (difference = 7%, 95% CI = 3% to 11%, P = .001). Furthermore, the proportion of men who reported tinnitus as a major symptom in the dose-intensive chemotherapy group was more than twice that in the group that received one to four chemotherapy cycles (42% vs 19%, difference = 23%, 95% CI = 10% to 36%, P < .001) and was reported by 28% of those who received five or more cycles (not statistically significantly different from one to four cycles, P = .17).
Self-reported hearing impairment as a major symptom was reported by 12% of men who received no chemotherapy and by 18% of those who received one to four chemotherapy cycles (difference = 6.7%, 95% CI = 2.6% to 11.2%, P = .001). A greater proportion of men who were treated with dose-intensive chemotherapy than with one to four chemotherapy cycles reported hearing impairment as a major symptom (35% vs 18%, difference = 16.2%, 95% CI = 2.0% to 30.3%, P = .007).
We next examined the hearing thresholds at 4000 Hz, grouped according to age-specific quartiles for an unselected Norwegian male reference population, for the testicular cancer survivors by treatment group (Figure 2, C). Of the men who received no chemotherapy, 22% (95% CI = 18% to 26%) belonged to the group with most severe hearing loss, which corresponded to the fourth quartile of hearing thresholds in the reference population. Among chemotherapy-treated men, the proportion belonging to the group with most severe hearing loss increased with increasing intensity of treatment (one to four cycles: 28% [95% CI = 23% to 34%]; five or more cycles: 37% [95% CI = 20% to 56%]; dose intensive: 69% [95% CI = 52% to 82%]; dose intensive vs one to four cycles, P < .001; five or more vs one to four cycles, P = .4).
Among the 287 men who received bleomycin, etoposide, and cisplatin (BEP) as the initial chemotherapy regimen, 41 had received three complete cycles and 153 had received four cycles of BEP with cisplatin administered at 20 mg/m2 per day and no additional chemotherapy. There were no statistically significant differences between men who received three BEP cycles and those who received four BEP cycles with respect to the percentage who reported any major symptoms (Raynaud-like phenomena in either hands or feet: 42% vs 31%, P = .26; paresthesias in either hands or feet: 24% vs 26%, P = 1.0; tinnitus: 12% vs 26%, P = .09; and hearing impairment: 10% vs 17%, P = .34). A total of 17% of men had hearing thresholds at 4000 Hz corresponding to the most severe group of hearing loss following three cycles of BEP compared with 31% following four cycles of BEP (P = .2).
Association Between Self-reported and Measured Hearing Impairment and Tinnitus
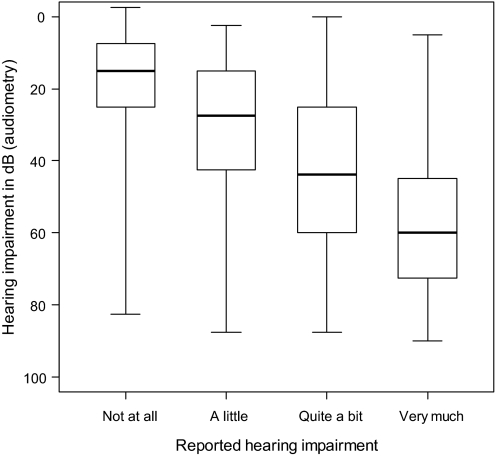
There was a moderate association between subjective and objective (hearing threshold at 4000 Hz) measures of hearing impairment (Spearman correlation coefficient = .46, P < .001). The box plot in Figure 3 illustrates the relationship between these two measures. The Spearman correlation coefficient for the association between self-reported hearing impairment and tinnitus was .49 (P < .001).
Figure 3.
Relationship between reported and assessed hearing impairment. Box plot of impaired hearing in decibels (dB) measured by pure tone audiometry at 4000 Hz in relation to reported hearing impairment for 746 testicular cancer survivors for whom both assessments were available. The dark line within the box is the median value, the lower and upper boundaries of each box represent the 25th and 75th percentiles, respectively, and the whiskers represent the minimum and maximum values.
Prediction of Side Effects
We used proportional ordinal logistic regression models to assess associations between the reported symptoms and cancer treatment and other factors of interest. Compared with no chemotherapy, all three chemotherapy groups had statistically significantly higher odds for increasing severity of all assessed self-reported symptoms (Tables 3 and 4) and, in a linear regression model, statistically significantly inferior audiometry results (except for five or more chemotherapy cycles, P = .06) (Table 5). There were no statistically significant associations between the severity of any of the self-reported or objectively measured symptoms and the length of follow-up. High education level was associated with statistically significantly lower odds for all symptoms compared with low education level, whereas marital status was statistically significantly associated only with Raynaud-like phenomena.
Table 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) for increasing severity of self-reported Raynaud-like phenomena and paresthesias in hands and feet from proportional odds ordinal logistic regression models*
| Variable | Raynaud-like phenomena |
Paresthesias |
||||||
| Hands |
Feet |
Hands |
Feet |
|||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age at follow-up† | 1.2 (1.1 to 1.4) | .002 | 1.3 (1.1 to 1.4) | <.001 | 1.2 (1.1 to 1.4) | .001 | 1.4 (1.2 to 1.6) | <.001 |
| Length of follow-up‡ | 1.01 (0.98 to 1.04) | .68 | 1.0 (0.97 to 1.03) | .90 | 1.01 (0.98 to 1.04) | .47 | 1.01 (0.98 to 1.04) | .73 |
| Chemotherapy | <.001 | <.001 | <.001 | <.001 | ||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||||
| 1–4 cycles | 2.9 (2.2 to 3.9) | <.001 | 2.4 (1.8 to 3.3) | <.001 | 2.0 (1.5 to 2.7) | <.001 | 2.2 (1.7 to 3.0) | <.001 |
| ≥5 cycles | 8.0 (4.4 to 14.7) | <.001 | 6.3 (3.4 to 11.8) | <.001 | 3.9 (2.1 to 7.3) | <.001 | 3.1 (1.7 to 5.7) | <.001 |
| Dose intensive | 5.7 (3.2 to 9.9) | <.001 | 4.7 (2.6 to 8.3) | <.001 | 2.3 (1.3 to 4.1) | .007 | 3.8 (2.1 to 6.9) | <.001 |
| Radiotherapy | .10 | .10 | .22 | .04 | ||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||||
| Yes | 1.3 (0.95 to 1.9) | 1.4 (0.95 to 1.9) | 1.3 (0.9 to 1.8) | 1.5 (1.01 to 2.1) | ||||
| RPLND | .20 | .52 | .15 | .27 | ||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||||
| Yes | 1.3 (0.9 to 1.8) | 1.1 (0.8 to 1.6) | 1.3 (.9 to 1.9) | 1.2 (0.9 to 1.7) | ||||
| Cigarette smoking | <.001 | <.001 | <.001 | <.001 | ||||
| Never daily | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||||
| Former daily | 1.1 (0.8 to 1.5) | .57 | 0.9 (0.7 to 1.3) | .71 | 1.1 (0.8 to 1.6) | .44 | 1.8 (1.4 to 2.5) | <.001 |
| Current daily | 1.7 (1.3 to 2.2) | <.001 | 1.5 (1.2 to 2.0) | .002 | 1.9 (1.4 to 2.5) | <.001 | 2.2 (1.6 to 2.8) | <.001 |
| Educational level | .03 | .01 | <.001 | .004 | ||||
| Primary, middle, or high school | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||||
| College or university | 0.8 (0.6 to 0.98) | 0.7 (0.6 to 0.9) | 0.6 (0.4 to 0.7) | 0.7 (0.6 to 0.9) | ||||
| Marital status | .04 | .003 | .81 | .31 | ||||
| Unmarried or living alone | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||||
| Married or cohabitating | 0.8 (0.6 to 0.98) | 0.7 (0.5 to 0.9) | 1.0 (.7 to 1.3) | 0.9 (0.7 to 1.4) | ||||
RPLND = retroperitoneal lymph node dissection.
Per 10-year increase.
Per 1-year increase.
Table 4.
Odds ratios (ORs) and 95% confidence intervals (CIs) for increasing severity of self-reported hearing impairment and tinnitus from proportional odds ordinal logistic regression models
| Variable | Hearing impairment |
Tinnitus |
||
| OR (95% CI) | P | OR (95% CI) | P | |
| Age at follow-up* | 1.7 (1.5 to 1.9) | <.001 | 1.3 (1.2 to 1.5) | <.001 |
| Length of follow-up† | 1.0 (.96 to 1.01) | .35 | 1.0 (.95 to 1.01) | .13 |
| Chemotherapy | <.001 | <.001 | ||
| No | 1.00 (referent) | 1.00 (referent) | ||
| 1–4 cycles | 1.5 (1.2 to 2.0) | <.001 | 1.8 (1.4 to 2.4) | <.001 |
| ≥5 cycles | 3.8 (2.1 to 6.8) | <.001 | 3.4 (1.9 to 5.9) | <.001 |
| Dose intensive | 5.3 (3.0 to 9.2) | <.001 | 7.1 (4.1 to 12.4) | <.001 |
| Cigarette smoking | .053 | .21 | ||
| Never daily | 1.00 (referent) | 1.00 (referent) | ||
| Former daily | 1.2 (.09 to 1.6) | .17 | 1.2 (0.9 to 1.7) | .16 |
| Current daily | 1.4 (1.1 to 1.8) | .02 | 1.3 (1.0 to 1.7) | .10 |
| Educational level | <.001 | <.001 | ||
| Primary, middle, or high school | 1.00 (referent) | 1.00 (referent) | ||
| College or university | 0.5 (0.4 to 0.7) | 0.6 (0.5 to 0.8) | ||
| Marital status | .66 | .62 | ||
| Unmarried or living alone | 1.00 (referent) | 1.00 (referent) | ||
| Married or cohabitating | 1.1 (.8 to 1.4) | 0.9 (0.7 to 1.2) | ||
Per 10-year increase.
Per 1-year increase.
Table 5.
Factors associated with hearing impairment at 4000 Hz in a linear regression model*
| Factor | Regression coefficient† (95% CI) | P |
| Age at follow-up† | 10.5 (9.2 to 11.9) | <.001 |
| Length of follow-up | .08 (−0.2 to .4) | .64 |
| Chemotherapy | <.001 | |
| No | 0 (referent) | |
| ≤4 cycles | 3.2 (0.4 to 6.1) | .02 |
| ≥5 cycles | 6.3 (−0.3 to 13.0) | .06 |
| Dose intensive | 20.3 (14.1 to 26.6) | <.001 |
| Cigarette smoking | .058 | |
| Never daily | 0 (referent) | |
| Former daily | 1.5 (−1.8 to 4.8) | .38 |
| Current daily | 3.7 (0.6 to 6.8) | .02 |
| Educational level | .001 | |
| Primary, middle, or high school | 0 (referent) | |
| College or university | −4.6 (−7.4 to −2.0) | |
| Marital status | .092 | |
| Unmarried or living alone | 0 (referent) | |
| Married or cohabitating | −2.7 (−5.9 to 0.4) |
CI = confidence interval.
In decibels. Increasing values correspond to more severe hearing loss.
Per 10-year increase.
Raynaud-Like Phenomena and Paresthesias.
The odds ratios (ORs) for Raynaud-like phenomena and paresthesias among men who received one to four cycles of chemotherapy compared with those who received no chemotherapy were 2.0–2.9 (Table 3). For the men who received five or more cycles of chemotherapy, the odds ratios for paresthesias in feet and hands were 3.1 (95% CI = 1.7 to 5.7) and 3.9 (95% CI = 2.1 to 7.3), respectively, and for Raynaud-like phenomena, 6.3 (95% CI = 3.4 to 11.8) and 8.0 (95% CI = 4.4 to 14.7), respectively (Table 3), all of which, with the exception of the odds ratio for paresthesias in feet, implied statistically significantly more severe symptoms for these men compared with men who received one to four cycles of chemotherapy (paresthesias in hands: P = .03; Raynaud-like phenomena in feet: P = .001; Raynaud-like phenomena in hands: P = .001). The odds regarding Raynaud-like phenomena were also statistically significantly higher for the dose-intensive chemotherapy group than for the one to four chemotherapy cycles group (P = .002 [hands], P = .02 [feet]).
Testicular cancer survivors who were treated with radiotherapy had statistically significantly higher odds of self-reported paresthesias in feet than those who were not treated with radiotherapy (OR = 1.5, 95% CI = 1.01 to 2.1, P = .04) (Table 3). The severity of Raynaud-like phenomena and paresthesias in hands and feet increased with increasing age (ORs of 1.2–1.4 per decade). Compared with never daily smoking, current daily smoking was statistically significantly associated with Raynaud-like phenomena and paresthesias (ORs of 1.5–2.2) (Table 3). Paresthesias in feet were also statistically significantly associated with former daily smoking (OR = 1.8, 95% CI = 1.4 to 2.5).
Hearing Impairment and Tinnitus.
Men who received one to four cycles of cisplatin-based chemotherapy were statistically significantly more inclined to report increased severity of hearing impairment (OR = 1.5, 95% CI = 1.2 to 2.0) and tinnitus (OR = 1.8, 95% CI = 1.4 to 2.4) than those who did not receive chemotherapy (Table 4). The predicted hearing threshold was 3.2 dB higher (95% CI = 0.4 to 6.1 dB, P = .02) in the one to four cycles group compared with the no chemotherapy group (Table 5). Men who received five or more cycles of chemotherapy also had more severe hearing impairment (OR = 3.8, 95% CI =2.1 to 6.8, P < .001) and tinnitus (OR = 3.4, 95% CI = 1.9 to 5.9, P < .001) compared with men who received no chemotherapy, with a predicted hearing threshold of 6.3 dB (95% CI = 0.3 to 12.9 dB), and compared with men who received one to four cycles of chemotherapy (P = .004 and P = .04, respectively). Ototoxicity was most pronounced among men who had received dose-intensive chemotherapy compared with those who received no chemotherapy (reported hearing impairment: OR = 5.3, 95% CI = 3.0 to 9.2; tinnitus: OR = 7.1, 95% CI = 4.1 to 12.4). The elevated hearing threshold among men who had received dose-intensive chemotherapy vs those who received no chemotherapy—20.3 dB (95% CI = 14.1 to 26.6 dB)—was comparable to that for a 20-year increase in age (Table 5). All ototoxicity measures were statistically significantly more severe in the dose-intensive chemotherapy group than in the one to four chemotherapy cycles group (P < .001). Statistically significantly higher hearing thresholds (P = .002) and more severe tinnitus (P = .048) were also found among men who received dose-intensive chemotherapy compared with men who received five or more chemotherapy cycles.
Compared with never daily smokers, daily smokers had statistically significant more severe reported and measured impaired hearing (P = .02 for both). However, the overall P values for the associations between smoking and self-reported as well as measured impaired hearing was not statistically significant (P = .053 and P = .058, respectively) (Tables 4 and 5).
Influence of Type of Cytostatic Drugs.
There were no statistically significant differences in the self-reported severity of any of the assessed symptoms or audiometric results between men treated with chemotherapy regimens that contained vinblastine and those treated with chemotherapy regimens that contained etoposide (data not shown). The total cumulative bleomycin dose was not statistically significantly associated with self-reported Raynaud-like phenomena or paresthesias (data not shown).
Discussion
Our results show that at 4–21 years after the initiation of treatment for testicular cancer, compared with men not treated with chemotherapy, those who had received any chemotherapy had statistically significantly higher odds for increasing severity of all assessed symptoms and, except for those who received five or more cycles, worse audiometric results. Compared with men who had received one to four cycles of chemotherapy, those who had received five or more cycles or dose-intensive chemotherapy were statistically significantly more troubled by most of the assessed symptoms. Hearing impairment and tinnitus were substantial in the dose-intensive group, whereas Raynaud-like phenomena and paresthesias were most common among men treated with five or more chemotherapy cycles. Radiotherapy was associated with paresthesias in feet. Finally, compared with never daily smoking, current daily smoking was statistically significant associated with Raynaud-like phenomena, paresthesias, and self-reported and measured hearing impairment.
A strength of this study is the large sample size of unselected testicular cancer survivors for whom we had detailed treatment information, which allowed us to compare the impact of various treatments as well as the dose intensity of cisplatin within a single cohort. Comparison between treatment groups within one cohort is particularly suitable for survivorship research, given that comparisons between studies may be difficult because of different methods of assessment and scoring systems, time to follow-up, and differences regarding treatment (3). In addition, the high participation rate increases the validity of our findings, and the long-term follow-up is, to our knowledge, quite unique.
The main limitation of this study is that it is largely based on self-reported symptoms, and the reported toxic effects were, with the exception of hearing threshold measurements, not objectively confirmed. However, the level of self-reported symptoms probably best reflects the impact of the toxic effects on a cancer survivor's well-being during daily life. Moreover, previous studies have shown that the prevalence of these toxic effects is even higher when objectively tested (3,10,11). There are also some limitations concerning the SCIN. As a brief screening instrument, it did not address each symptom in detail, such as all classical signs of Raynaud phenomena (7). Therefore, some of the men who were classified as having Raynaud-like phenomena may include men who had cold-induced pallor but not all of the classical signs of Raynaud phenomena (7). Likewise, we cannot rule out that some men with reported paresthesias may have had pain in their hands or feet because of other conditions. Because this was a cross-sectional study, we do not have information about the existence of symptoms before diagnoses or whether they changed over time. Unequal access to health care was not likely to be a confounding factor in this study because both the primary health-care system and the follow-up routines at hospitals are well established and evenly distributed in Norway.
Raynaud-like phenomena were the most frequently reported major symptoms among chemotherapy-treated testicular cancer survivors in our study. Although Raynaud phenomenon is a common disorder in the general population, its prevalence varies by sex and climate zone (7). The 12% prevalence of major Raynaud-like phenomena in the surveillance group is similar to the male prevalence of Raynaud phenomenon in an area with mean January temperature of −0.4°C (34), which is somewhat warmer than the mean January temperature in most parts of Norway (35). Although the frequency of reported major Raynaud-like phenomena among all chemotherapy-treated testicular cancer survivors (39%) is similar to the typically reported long-term rates of Raynaud phenomena of 25%–45% (3–6,10,15,36,37), the frequency we observed among men who received five or more chemotherapy cycles—61%—is somewhat higher than the 44% rate reported by Hansen and Olsen (10) in germ cell cancer patients treated with six cycles of cisplatin, vinblastine, and bleomycin. Interestingly, they found that these patients had an exaggerated response to cold, even those without finger symptoms.
We found statistically significantly higher odds for increasing severity of Raynaud-like phenomena for men who received five or more chemotherapy cycles or dose-intensive chemotherapy than for men who received one to four cycles of chemotherapy. Although previous smaller studies did not detect any association between Raynaud-like phenomena and the cumulative cisplatin dose or the number of chemotherapy cycles (5,36–38), an association with cisplatin is likely because the incidence of Raynaud-like phenomena following chemotherapy for testicular cancer seemed to increase following the introduction of cisplatin (9). We found no association between Raynaud-like phenomena and bleomycin dose, in contrast to the study by Berger et al. (5). However, this lack of an association in our study is not inconsistent with bleomycin being the principal cause of Raynaud phenomenon (39) because all but 17 of our patients received bleomycin, mostly within a limited dose range (median dose was 300 000 IU, and 77% of patients received between 240 000 and 360 000 IU).
The neurotoxic effects of testicular cancer treatment are mainly cisplatin-induced sensory neuropathies that are chiefly caused by toxic effects on the dorsal root ganglia neurons; however, other mechanisms may be involved (40–42). The higher prevalence of reported major paresthesias following five or more cycles than following one to four cycles in this study is consistent with previous data suggesting that both the incidence and the severity of paresthesias are mainly determined by the cumulative cisplatin dose (3,41). Persisting paresthesias have previously been reported in 14%–43% of men following cisplatin-based chemotherapy for testicular cancer (3,4,11,13,15,37,43,44). This wide range is likely to be influenced by several factors, including the length of follow-up, chemotherapy regimen, and dose.
We found that radiotherapy was associated with paresthesias in the feet. Although neurological side effects following radiotherapy of the para-aortic and iliac areas are considered to be rare, postirradiation lower motor neuron syndrome with paresis as well as transient sensory symptoms have been described in testicular cancer patients treated with radiotherapy (24).
We observed that ototoxicity increased with treatment intensity and was particularly pronounced among men who had received dose-intensive chemotherapy. Chemotherapy-induced ototoxicity is mainly ascribed to cisplatin, which causes loss of outer hair cells in the organ of Corti, leading to tinnitus and hearing impairment (45,46). Although cisplatin-induced hearing loss initially involves the higher frequencies, it can eventually affect a broader range of frequencies (47,48). Both the total and the peak plasma dose of cisplatin are considered relevant measures associated with the ototoxic effects of this drug (49).
We found that ototoxicity was substantial in men who had received dose-intensive chemotherapy. However, there was also a statistically significant elevation of the odds of ototoxic symptoms with increasing number of chemotherapy cycles (none, one to four, and five or more cycles), as well as statistically significantly greater predicted hearing impairment in those who had received one to four cycles and dose-intensive chemotherapy compared with no chemotherapy. Bokemeyer et al. (28) reported persisting ototoxic symptoms in 17 (20%) of the 86 testicular cancer patients treated with cisplatin-based chemotherapy and that the cumulative cisplatin dose was a highly statistically significant predictor of ototoxicity. In that study, 12% of the patients received regimens that included high-dose cisplatin. An increased risk of ototoxicity has been described previously for a 3-day BEP regimen (etoposide administered for more than 3 days and cisplatin for more than 2 days with 50 mg/m2 cisplatin on days 1 and 2) vs the 5-day BEP regimen (20 mg/m2 cisplatin on days 1–5), particularly if four cycles was administered (12). Such an increased risk should also be considered if three cycles are planned (12).
The proportions of men in the three chemotherapy groups who reported major hearing impairment (18%–35%) and, to some degree, tinnitus (19%–42%), were somewhat higher than those previously reported for persisting ototoxic symptoms (4,15,28,50). Variation in the frequency of these symptoms may reflect differences in age at follow-up among these studies.
In this study, the testicular cancer survivors’ audiometric results were categorized into four groups according to age-specific quartiles of a large Norwegian reference population published in 2005 (29). We found that the proportion of men in the group corresponding to quartile 4 (most severe hearing loss) was 28% (one to four cycles), 37% (five or more cycles), and 69% (dose intensive) in the three chemotherapy groups. The predicted hearing impairment in decibels in these three groups was 3.2, 6.3, and 20.3, respectively. In previous studies of men treated with chemotherapy for testicular cancer that included audiometry (11,15,28,51), hearing threshold alterations were reported in 28%–77% of cases. Comparing our results of measured hearing impairment with the results of these previous studies is not feasible because the criteria for hearing threshold alterations used in these studies varied. The hearing threshold before treatment was not known for the men in this study. However, Hansen et al. (11), who compared the audiometry results of patients before and after six cycles of cisplatin, vinblastine, and bleomycin, found that treatment induced high-frequency hearing loss in 39% of the patients.
We found no statistically significant differences in any of the assessed symptoms, analyzed by regression models, between men exposed to etoposide (as in BEP) and those exposed to vinblastine (as in CVB). Others have also found that the long-term effects of the two regimens are similar, although the CVB regimen has been associated with more acute neurotoxicity and Raynaud phenomena compared with BEP (3,5,52). We have not assessed the impact of vincristine, which was administered to 40 of the men included in this study. However, neurotoxic symptoms were reported to be transient in the majority of patients following treatment with vincristine-containing regimens in previous studies (3,21,42), and the prevalence of persisting symptoms in those studies was less than that reported by men in this study who received one to four cycles of chemotherapy.
There was no statistically significant association between the length of follow-up and the severity of any of the self-reported symptoms or audiometric results, as analyzed by proportional odds and linear regression models. This finding may suggest that the symptoms had stabilized after a follow-up of 4–21 years. It is well recognized that improvements in symptoms such as tinnitus, paresthesias, and Raynaud-like phenomena may occur during the first months or year following treatment for testicular cancer (12), but data on long-term changes in symptoms are sparse. In a small longitudinal study of men treated for testicular cancer, a decline in the number of men who reported paresthesias followed by normalization of vibration perception was observed, even many years after treatment (11,43).
We found that compared with never daily smoking, current daily smoking was statistically significant associated with Raynaud-like phenomena and paresthesias as well as with self-reported and measured impaired hearing. Paresthesias in feet were also statistically significantly associated with former daily smoking compared with never daily smoking. An increased risk of Raynaud phenomenon in male smokers has been reported in a population-based cohort (53); however, studies in the general population (54) and among testicular cancer patients (5,9,55) have shown conflicting results. To our knowledge, this is the first study to describe an association between smoking and peripheral neuropathy or impaired hearing following cancer treatment. However, associations have been described between smoking and neuropathies related to specific diseases (ie, diabetes and chronic obstructive pulmonary disease) (56–59) or hearing loss (60–64). Neurotoxic constituents of tobacco have been proposed to play a role in these associations with neuropathies, although an indirect effect of tobacco through the vasculature supplying the nerves, chronic hypoxemia, and other effects have also been discussed (56,59,60).
We were not able to assess any associations between the assessed symptoms and alcohol consumption because of insufficient data in our series. Nerves that have been previously damaged by alcohol exposure, diabetes mellitus, or inherited neuropathy seem to be predisposed to develop chemotherapy-induced neuropathy (42). However, in this study, there were no men with diabetes in the chemotherapy groups that were the most affected by the assessed symptoms (ie, five or more cycles or dose-intensive groups).
An important issue is whether the side effects of testicular cancer treatments can be prevented or the symptoms ameliorated. A major aim in the treatment of testicular cancer is to minimize toxic effects without compromising the high cure rate. Our data favor the use of chemotherapy regimens that contain 20 mg/m2 cisplatin per day to limit ototoxicity. We found no statistically significant difference between three and four cycles of BEP with respect to any of the assessed symptoms. However, as illustrated by the comparison of the one to four cycles group with the five or more cycles group, the total chemotherapy treatment burden is highly relevant with respect to side effects. Chemotherapy for testicular cancer should thus be chosen wisely according to guidelines and evidence-based medicine and should not be administered for longer duration than needed based on data from phase III trials (65–67). For men diagnosed with stage I nonseminomas, the recommendations regarding adjuvant treatment vary according to country and risk factors, with surveillance or one to two cycles of BEP being the most commonly used options. The total treatment burden, including potential salvage treatment, and possible side effects are important issues in these treatment decisions, as discussed by Tandstad et al. (68) in the context of a study that included 745 unselected men with stage I nonseminomas who were treated within the SWENOTECA collaboration.
To our knowledge, no drug is yet available that reliably prevents chemotherapy-induced neuropathy or effectively improves the symptoms of established neuropathies (42,69,70). Medications such as calcium channel antagonists can be tried to relieve symptoms of Raynaud phenomena, although such medications are probably less effective in treating Raynaud phenomena in patients after chemotherapy than in individuals with no underlying cause (7). Use of such medications was not assessed in this study. Preventing exposure to additional contributing factors, such as avoiding cold exposure of the digits in men with Raynaud phenomena, may be of benefit. When applicable, advice regarding lifestyle interventions, including cessation of smoking, should be given to testicular cancer survivors who might have an increased risk of cardiovascular morbidity. We found associations between smoking and several of the assessed symptoms in this study, which may imply yet another reason to motivate patients to quit smoking. In the future, individualized treatment of testicular cancer patients according to the functional polymorphisms they may harbor in genes that encode drug-detoxifying enzymes may be feasible (71,72).
In conclusion, testicular cancer survivors who were treated with up to four cycles of cisplatin-based chemotherapy were troubled more often by neuro- and ototoxic side effects and by Raynaud-like phenomena several years after treatment compared with those not treated with chemotherapy. Men who received more intensive chemotherapy were troubled even more by most of the assessed symptoms compared with those who received up to four cycles.
Funding
Western Norway Regional Health Authority (M.B.); Norwegian Health and Rehabilitation Fund (1998/207).
Footnotes
The study sponsors had no role in the design of the study, the collection of the data, the analysis or interpretation of the data, the decision to submit the manuscript for publication, or the writing of the manuscript.
This study is a joint national clinical study as part of the Norwegian Urological Cancer Group III project. Clinics outside the Norwegian Radium Hospital are members of the SWENOTECA Group.
References
- 1.Dearnaley D, Huddart R, Horwich A. Regular review: managing testicular cancer. BMJ. 2001;322(7302):1583–1588. doi: 10.1136/bmj.322.7302.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Registry of Norway. Cancer in Norway 2006—Cancer Incidence, Mortality, Survival and Prevalence in Norway. Oslo: Norway; Cancer Registry of Norway; 2007. [Google Scholar]
- 3.Bokemeyer C, Berger CC, Kuczyk MA, Schmoll HJ. Evaluation of long-term toxicity after chemotherapy for testicular cancer. J Clin Oncol. 1996;14(11):2923–2932. doi: 10.1200/JCO.1996.14.11.2923. [DOI] [PubMed] [Google Scholar]
- 4.Aass N, Kaasa S, Lund E, Kaalhus O, Heier MS, Fosså SD. Long-term somatic side-effects and morbidity in testicular cancer patients. Br J Cancer. 1990;61(1):151–155. doi: 10.1038/bjc.1990.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger CC, Bokemeyer C, Schneider M, Kuczyk MA, Schmoll HJ. Secondary Raynaud's phenomenon and other late vascular complications following chemotherapy for testicular cancer. Eur J Cancer. 1995;31A(13–14):2229–2238. doi: 10.1016/0959-8049(95)00460-2. [DOI] [PubMed] [Google Scholar]
- 6.Meinardi MT, Gietema JA, van der Graaf WTA, et al. Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J Clin Oncol. 2000;18(8):1725–1732. doi: 10.1200/JCO.2000.18.8.1725. [DOI] [PubMed] [Google Scholar]
- 7.Block JA, Sequeira W. Raynaud's phenomenon. Lancet. 2001;357(9273):2042–2048. doi: 10.1016/S0140-6736(00)05118-7. [DOI] [PubMed] [Google Scholar]
- 8.Teutsch C, Lipton A, Harvey HA. Raynaud's phenomenon as a side effect of chemotherapy with vinblastine and bleomycin for testicular carcinoma. Cancer Treat Rep. 1977;61(5):925–926. [PubMed] [Google Scholar]
- 9.Vogelzang NJ, Bosl GJ, Johnson K, Kennedy BJ. Raynaud's phenomenon: a common toxicity after combination chemotherapy for testicular cancer. Ann Intern Med. 1981;95(3):288–292. doi: 10.7326/0003-4819-95-3-288. [DOI] [PubMed] [Google Scholar]
- 10.Hansen SW, Olsen N. Raynaud's phenomenon in patients treated with cisplatin, vinblastine, and bleomycin for germ cell cancer: measurement of vasoconstrictor response to cold. J Clin Oncol. 1989;7(7):940–942. doi: 10.1200/JCO.1989.7.7.940. [DOI] [PubMed] [Google Scholar]
- 11.Hansen SW, Helweg-Larsen S, Trojaborg W. Long-term neurotoxicity in patients treated with cisplatin, vinblastine, and bleomycin for metastatic germ cell cancer. J Clin Oncol. 1989;7(10):1457–1461. doi: 10.1200/JCO.1989.7.10.1457. [DOI] [PubMed] [Google Scholar]
- 12.Fosså SD, de Wit R, Roberts JT, et al. Quality of life in good prognosis patients with metastatic germ cell cancer: a prospective study of the European Organization for Research and Treatment of Cancer Genitourinary Group/Medical Research Council Testicular Cancer Study Group (30941/TE20) J Clin Oncol. 2003;21(6):1107–1118. doi: 10.1200/JCO.2003.02.075. [DOI] [PubMed] [Google Scholar]
- 13.von Schlippe M, Fowler CJ, Harland SJ. Cisplatin neurotoxicity in the treatment of metastatic germ cell tumour: time course and prognosis. Br J Cancer. 2001;85(6):823–826. doi: 10.1054/bjoc.2001.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mykletun A, Dahl AA, Haaland CF, et al. Side effects and cancer-related stress determine quality of life in long-term survivors of testicular cancer. J Clin Oncol. 2005;23(13):3061–3068. doi: 10.1200/JCO.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 15.Boyer M, Raghavan D, Harris PJ, et al. Lack of late toxicity in patients treated with cisplatin-containing combination chemotherapy for metastatic testicular cancer. J Clin Oncol. 1990;8(1):21–26. doi: 10.1200/JCO.1990.8.1.21. [DOI] [PubMed] [Google Scholar]
- 16.Tierney DK, Facione N, Padilla G, Dodd M. Response shift: a theoretical exploration of quality of life following hematopoietic cell transplantation. Cancer Nurs. 2007;30(2):125–138. doi: 10.1097/01.NCC.0000265002.79687.af. [DOI] [PubMed] [Google Scholar]
- 17.Brydøy M, Fosså SD, Klepp O, et al. Paternity following treatment for testicular cancer. J Natl Cancer Inst. 2005;97(21):1580–1588. doi: 10.1093/jnci/dji339. [DOI] [PubMed] [Google Scholar]
- 18.Peckham MJ, Barrett A, McElwain TJ, Hendry WF. Combined management of malignant teratoma of the testis. Lancet. 1979;2(8137):267–270. doi: 10.1016/s0140-6736(79)90288-5. [DOI] [PubMed] [Google Scholar]
- 19.de Wit R, Roberts JT, Wilkinson PM, et al. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol. 2001;19(6):1629–1640. doi: 10.1200/JCO.2001.19.6.1629. [DOI] [PubMed] [Google Scholar]
- 20.Kaye SB, Mead GM, Fosså S, et al. Intensive induction-sequential chemotherapy with BOP/VIP-B compared with treatment with BEP/EP for poor-prognosis metastatic nonseminomatous germ cell tumor: a Randomized Medical Research Council/European Organization for Research and Treatment of Cancer study. J Clin Oncol. 1998;16(2):692–701. doi: 10.1200/JCO.1998.16.2.692. [DOI] [PubMed] [Google Scholar]
- 21.Dearnaley DP, Fosså SD, Kaye SB, et al. Adjuvant bleomycin, vincristine and cisplatin (BOP) for high-risk stage I non-seminomatous germ cell tumours: a prospective trial (MRC TE17) Br J Cancer. 2005;92(12):2107–2113. doi: 10.1038/sj.bjc.6602624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis CR, Fosså SD, Mead G, et al. BOP/VIP—a new platinum-intensive chemotherapy regimen for poor prognosis germ cell tumours. Ann Oncol. 1991;2(3):203–211. doi: 10.1093/oxfordjournals.annonc.a057906. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsen KD, Olsen DR, Fosså K, Fosså SD. External beam abdominal radiotherapy in patients with seminoma stage I: field type, testicular dose, and spermatogenesis. Int J Radiat Oncol Biol Phys. 1997;38(1):95–102. doi: 10.1016/s0360-3016(96)00597-4. [DOI] [PubMed] [Google Scholar]
- 24.Brydøy M, Storstein A, Dahl O. Transient neurological adverse effects following low dose radiation therapy for early stage testicular seminoma. Radiother Oncol. 2007;82(2):137–144. doi: 10.1016/j.radonc.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Oldenburg J, Fosså SD, Dahl AA. Scale for chemotherapy-induced long-term neurotoxicity (SCIN): psychometrics, validation, and findings in a large sample of testicular cancer survivors. Qual Life Res. 2006;15(5):791–800. doi: 10.1007/s11136-005-5370-6. [DOI] [PubMed] [Google Scholar]
- 26.Fosså SD, Moynihan C, Serbouti S. Patients’ and doctors’ perception of long-term morbidity in patients with testicular cancer clinical stage I. A descriptive pilot study. Support Care Cancer. 1996;4(2):118–128. doi: 10.1007/BF01845761. [DOI] [PubMed] [Google Scholar]
- 27.Von Hoff DD, Schilsky R, Reichert CM, et al. Toxic effects of cis-dichlorodiammineplatinum(II) in man. Cancer Treat Rep. 1979;63(9–10):1527–1531. [PubMed] [Google Scholar]
- 28.Bokemeyer C, Berger CC, Hartmann JT, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77(8):1355–1362. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engdahl B, Tambs K, Borchgrevink HM, Hoffman HJ. Screened and unscreened hearing threshold levels for the adult population: results from the Nord-Trøndelag Hearing Loss Study. Int J Audiol. 2005;44(4):213–230. doi: 10.1080/14992020500057731. [DOI] [PubMed] [Google Scholar]
- 30.Harrell FE., Jr . Regression Modeling Strategies. Chapter 13 Ordinal Logistic Regressions. Springer-Verlag New York, NY; 2001. [Google Scholar]
- 31.Haugnes HS, Aass N, Fossa SD, et al. Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann Oncol. 2007;18(2):241–248. doi: 10.1093/annonc/mdl372. [DOI] [PubMed] [Google Scholar]
- 32.Haugnes HS, Aass N, Fosså SD, et al. Predicted cardiovascular morbidity in testicular cancer survivors. J Cancer Surviv. 2009;2(3):128–137. doi: 10.1007/s11764-008-0054-1. [DOI] [PubMed] [Google Scholar]
- 33.Sagstuen H, Aass N, Fossa SD, et al. Blood pressure and body mass index in long-term survivors of testicular cancer. J Clin Oncol. 2005;23(22):4980–4990. doi: 10.1200/JCO.2005.06.882. [DOI] [PubMed] [Google Scholar]
- 34.Maricq HR, Carpentier PH, Weinrich MC, et al. Geographic variation in the prevalence of Raynaud's phenomenon: a 5 region comparison. J Rheumatol. 1997;24(5):879–889. [PubMed] [Google Scholar]
- 35.The Norwegian Meteorological Institute. Været i Norge - Klimatologisk månedsoversikt, January 2009. http://www.met.no/Forskning/Publikasjoner/metno_info/2009/filestore/2009-01.pdf. [Google Scholar]
- 36.Vogelzang NJ, Torkelson JL, Kennedy BJ. Hypomagnesemia, renal dysfunction, and Raynaud's phenomenon in patients treated with cisplatin, vinblastine, and bleomycin. Cancer. 1985;56(12):2765–2770. doi: 10.1002/1097-0142(19851215)56:12<2765::aid-cncr2820561208>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Bissett D, Kunkeler L, Zwanenburg L, et al. Long-term sequelae of treatment for testicular germ cell tumours. Br J Cancer. 1990;62(4):655–659. doi: 10.1038/bjc.1990.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roth BJ, Einhorn LH, Greist A. Long-term complications of cisplatin-based chemotherapy for testis cancer. Semin Oncol. 1988;15(4):345–350. [PubMed] [Google Scholar]
- 39.Chaudhary UB, Haldas JR. Long-term complications of chemotherapy for germ cell tumours. Drugs. 2003;63(15):1565–1577. doi: 10.2165/00003495-200363150-00004. [DOI] [PubMed] [Google Scholar]
- 40.Meijer C, de Vries EG, Marmiroli P, Tredici G, Frattola L, Cavaletti G. Cisplatin-induced DNA-platination in experimental dorsal root ganglia neuronopathy. Neurotoxicology. 1999;20(6):883–887. [PubMed] [Google Scholar]
- 41.Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33(1):15–49. doi: 10.1053/j.seminoncol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 43.Petersen PM, Hansen SW. The course of long-term toxicity in patients treated with cisplatin-based chemotherapy for non-seminomatous germ-cell cancer. Ann Oncol. 1999;10(12):1475–1483. doi: 10.1023/a:1008322909836. [DOI] [PubMed] [Google Scholar]
- 44.Roth BJ, Greist A, Kubilis PS, Williams SD, Einhorn LH. Cisplatin-based combination chemotherapy for disseminated germ cell tumors: long-term follow-up. J Clin Oncol. 1988;6(8):1239–1247. doi: 10.1200/JCO.1988.6.8.1239. [DOI] [PubMed] [Google Scholar]
- 45.Marco-Algarra J, Basterra J, Marco J. Cis-diaminedichloro platinum ototoxicity. An experimental study. Acta Otolaryngol. 1985;99(3–4):343–347. doi: 10.3109/00016488509108921. [DOI] [PubMed] [Google Scholar]
- 46.Kaltenbach JA, Rachel JD, Mathog TA, Zhang J, Falzarano PR, Lewandowski M. Cisplatin-induced hyperactivity in the dorsal cochlear nucleus and its relation to outer hair cell loss: relevance to tinnitus. J Neurophysiol. 2002;88(2):699–714. doi: 10.1152/jn.2002.88.2.699. [DOI] [PubMed] [Google Scholar]
- 47.van der Hulst RJ, Dreschler WA, Urbanus NA. High frequency audiometry in prospective clinical research of ototoxicity due to platinum derivatives. Ann Otol Rhinol Laryngol. 1988;97(2, pt 1):133–137. doi: 10.1177/000348948809700208. [DOI] [PubMed] [Google Scholar]
- 48.Biro K, Noszek L, Prekopp P, et al. Characteristics and risk factors of cisplatin-induced ototoxicity in testicular cancer patients detected by distortion product otoacoustic emission. Oncology. 2006;70(3):177–184. doi: 10.1159/000093776. [DOI] [PubMed] [Google Scholar]
- 49.Vermorken JB, Kapteijn TS, Hart AA, Pinedo HM. Ototoxicity of cis-diamminedichloroplatinum (II): influence of dose, schedule and mode of administration. Eur J Cancer Clin Oncol. 1983;19(1):53–58. doi: 10.1016/0277-5379(83)90398-x. [DOI] [PubMed] [Google Scholar]
- 50.Stoter G, Koopman A, Vendrik CP, et al. Ten-year survival and late sequelae in testicular cancer patients treated with cisplatin, vinblastine, and bleomycin. J Clin Oncol. 1989;7(8):1099–1104. doi: 10.1200/JCO.1989.7.8.1099. [DOI] [PubMed] [Google Scholar]
- 51.Osanto S, Bukman A, Van Hoek F, Sterk PJ, De Laat JA, Hermans J. Long-term effects of chemotherapy in patients with testicular cancer. J Clin Oncol. 1992;10(4):574–579. doi: 10.1200/JCO.1992.10.4.574. [DOI] [PubMed] [Google Scholar]
- 52.Williams SD, Birch R, Einhorn LH, Irwin L, Greco FA, Loehrer PJ. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. 1987;316(23):1435–1440. doi: 10.1056/NEJM198706043162302. [DOI] [PubMed] [Google Scholar]
- 53.Suter LG, Murabito JM, Felson DT, Fraenkel L. Smoking, alcohol consumption, and Raynaud's phenomenon in middle age. Am J Med. 2007;120(3):264–271. doi: 10.1016/j.amjmed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Palesch YY, Valter I, Carpentier PH, Maricq HR. Association between cigarette and alcohol consumption and Raynaud's phenomenon. J Clin Epidemiol. 1999;52(4):321–328. doi: 10.1016/s0895-4356(99)00005-0. [DOI] [PubMed] [Google Scholar]
- 55.Fosså SD, Lehne G, Heimdal K, Theodorsen L. Clinical and biochemical long-term toxicity after postoperative cisplatin-based chemotherapy in patients with low-stage testicular cancer. Oncology. 1995;52(4):300–305. doi: 10.1159/000227478. [DOI] [PubMed] [Google Scholar]
- 56.Gasnault J, Moore N, Arnaud F, Rondot P. Peripheral neuropathies during hypoxaemic chronic obstructive airways disease. Bull Eur Physiopathol Respir. 1987;(23) suppl 11:199s–202s. [PubMed] [Google Scholar]
- 57.Faden A, Mendoza E, Flynn F. Subclinical neuropathy associated with chronic obstructive pulmonary disease: possible pathophysiologic role of smoking. Arch Neurol. 1981;38(10):639–642. doi: 10.1001/archneur.1981.00510100067011. [DOI] [PubMed] [Google Scholar]
- 58.Poza JJ, Marti-Masso JF. Peripheral neuropathy associated with chronic obstructive pulmonary disease [abstract] Neurologia. 1997;12(9):389–394. [PubMed] [Google Scholar]
- 59.Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 60.Cruickshanks KJ, Klein R, Klein BE, Wiley TL, Nondahl DM, Tweed TS. Cigarette smoking and hearing loss: the epidemiology of hearing loss study. JAMA. 1998;279(21):1715–1719. doi: 10.1001/jama.279.21.1715. [DOI] [PubMed] [Google Scholar]
- 61.Palmer KT, Griffin MJ, Syddall HE, Coggon D. Cigarette smoking, occupational exposure to noise, and self reported hearing difficulties. Occup Environ Med. 2004;61(4):340–344. doi: 10.1136/oem.2003.009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanbury M, Rafferty AP, Rosenman K. Prevalence of hearing loss and work-related noise-induced hearing loss in Michigan. J Occup Environ Med. 2008;50(1):72–79. doi: 10.1097/JOM.0b013e31815b568c. [DOI] [PubMed] [Google Scholar]
- 63.Mizoue T, Miyamoto T, Shimizu T. Combined effect of smoking and occupational exposure to noise on hearing loss in steel factory workers. Occup Environ Med. 2003;60(1):56–59. doi: 10.1136/oem.60.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pouryaghoub G, Mehrdad R, Mohammadi S. Interaction of smoking and occupational noise exposure on hearing loss: a cross-sectional study. BMC Public Health. 2007;7:137. doi: 10.1186/1471-2458-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classificationa prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15(2):594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 66.Krege S, Beyer J, Souchon R, et al. European consensus conference on diagnosis and treatment of germ cell cancer: a report of the second meeting of the European germ cell cancer consensus group (EGCCCG): part 1. Eur Urol. 2008;53(3):478–496. doi: 10.1016/j.eururo.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 67.Krege S, Beyer J, Souchon R, et al. European consensus conference on diagnosis and treatment of germ cell cancer: a report of the second meeting of the European germ cell cancer consensus group (EGCCCG): part 2. Eur Urol. 2008;53(3):497–513. doi: 10.1016/j.eururo.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 68.Tandstad T, Dahl O, Cohn-Cedermark G, et al. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: the SWENOTECA management program. J Clin Oncol. 2009;27(13):2122–2128. doi: 10.1200/JCO.2008.18.8953. [DOI] [PubMed] [Google Scholar]
- 69.Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27(1):127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 70.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44(11):1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 71.Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fosså SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol. 2007;25(6):708–714. doi: 10.1200/JCO.2006.08.9599. [DOI] [PubMed] [Google Scholar]
- 72.Oldenburg J, Kraggerud SM, Brydøy M, Cvancarova M, Lothe RA, Fosså SD. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med. 2007;5(1):70–77. doi: 10.1186/1479-5876-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]