Abstract
Characterization of prenatal exposure to hazardous chemicals most often relies upon the analysis of cord blood. However, human placenta is an appropriate tissue alternative with noteworthy advantages. Owing to analytical challenges, reports on placental levels of toxic chemicals are limited. The purpose of this study was to establish a reliable, cost effective, and relatively fast and simple method to extract polybrominated diphenyl ethers (PBDEs) from human placenta for analysis using gas chromatography coupled with mass spectrometry (GC/MS). The matrix solid phase dispersion (MSPD) method was optimized for the extraction and analysis of 43 PBDEs (including BDE209) from human placenta samples. Different sorbents, sample conditions, grinding methods, elution solvents, and single and repeated extractions were compared for their effects on the extraction efficiency. The performance of the optimized method was validated by analyzing spiked placenta samples and a standard reference material of fish tissue. Congener specific PBDE recovery ranged from 91to 114% for the spiked samples and 89 to 115% for a standard reference material (SRM) of fish tissue. The optimized MSPD procedure was compared with two conventional extraction methods. The extraction efficiency of MSPD was found to be comparable with that of the traditional Soxhlet method and superior to that using a liquid extraction method. Twenty two PBDEs were detected in all of the 5 samples collected in Chicago in 2008. This is the first description of PBDEs detected in human placentas reported in the U.S.
Keywords: Placenta, MSPD, PBDEs, Extraction
INTRODUCTION
During their development, humans may involuntarily take in toxic chemicals via maternal circulation as fetuses, via breast milk as infants, and via food as children and adults. Of particular concern is toxin intake during the pre- and neo-natal periods when defense mechanisms against toxic agents are poorly developed, and therefore the effects of such intake can be especially detrimental and long lasting. Adverse consequences of in utero exposure to environmental toxicants such as pesticides, polychlorinated biphenyls (PCBs), lead and mercury on fetal development have been documented.1,2 As an emerging pollutant, polybrominated diphenyl ethers (PBDEs) are found ubiquitously in the human environment. Similar to that of PCBs, one of the major concerns for PBDEs is their developmental toxicity. Studies have found correlations between elevated PBDE levels and adverse health outcomes such as low birth weight, length, chest circumference, and cryptorchidism in newborns.3,4
Cord blood is the most widely used matrix in the assessment of prenatal exposure to environmental toxicants. Though it has not been extensively used thus far, placenta can be a valuable substitute. The placenta is an ephemeral organ that grows with the developing fetus. It acts as a mediator in the selective exchange of materials between maternal and fetal blood and serves as a barrier to some xenobiotics, which get stored in the placental tissue.5 Therefore, the chemical concentration in the placenta at the time of child delivery may proportionally reflect levels of exposure during the entire pregnancy, especially for bioaccumulative chemicals such as PBDEs. The use of placenta has remarkable advantages over cord blood. The non-invasiveness of sample collection, the minimal risk to the mother and the infant, and the trouble-free sample acquisition in which professional expertise is not required are optimal for researchers. Furthermore, the bulky sample size provides sufficient tissue for multiple laboratory procedures, thus enabling the screening of various toxicants and allowing the investigation of chemical synergy. However, the placenta is a unique and complex organ. In addition to the common difficulties associated with environmental analysis, laboratory processing of placenta samples is rather tedious compared to those for homogeneous matrices such as plasma, serum, or breast milk. Applying an extraction technique that is vigorous enough to surface the analytes buried in the tissue is critical to reliably determining the concentration levels.
Matrix solid phase dispersion (MSPD) was developed in the late 1980s for the extraction of solid and semisolid samples. The principle of this method is to disrupt and disperse the sample in a solid phase sorbent that is present in excess quantity. The basic procedure, which comprises three major steps, is extensively illustrated by Barker.6 The first step involves sample grinding in the presence of excess amounts of sorbent. Then the ground mixture of sample and sorbent is loaded onto a chromatographic column, followed by elution with a suitable solvent. The quality of the MSPD performance depends on multiple factors, particularly the sorbent type and extraction solvent. A careful selection of a combination of factors specific for the analyte and the sample matrix is critical. The advantages of MSPD include its simplicity, efficacy, low cost, and the possibility of simultaneous extraction and cleanup. It has been successfully used to extract trace level organic pollutants, including PBDEs, from different environmental and biological matrices.7-10
To date, only limited data on PBDEs in placenta are available;4,10-12 and to our knowledge, no study has reported PBDE concentrations in human placenta in the United States. In addition, a variety of sample pretreatment, extraction, cleanup, and instrumental analysis procedures have been used in the studies on placenta, making it difficult to compare the results. The purpose of this study was to establish a reliable, yet simple and cost-effective, method to extract PBDEs from human placenta. The MSPD method for PBDE extraction was optimized by comparing different sorbents, sample conditions, grinding methods, elution solvents, and single and repeated procedures. The efficiency of the optimized MSPD method for extracting 43 PBDEs was compared to two other widely used extraction methods, i.e. liquid extraction and Soxhlet extraction, by using placenta samples obtained in a local hospital. Method detection limits (MDL) were determined. Validation of the MSPD method was carried out by analyzing matrix spike samples and a fish tissue standard reference material (SRM) with certified PBDE concentrations.
MATERIALS AND METHOD
Chemicals
A standard mixture of 39 PBDEs (BDEs 1, 2, 3, 7, 8, 10, 11, 12, 13, 15, 17, 25, 28, 30, 32, 33, 35, 37, 47, 49, 66, 71, 75, 77, 85, 99, 100, 116, 118, 119, 126, 138, 153, 154, 155, 166, 181, 183, 190), PCB 204 and decabromobiphenyl (BB209) were purchased from AccuStandard (New Haven, CT). Individual PBDE standards (28, 47, 66, 85, 99, 100, 153, 154, 183, 196, 206, 207, 209) and 13C-labeled BDE118 (BDE 118L) were purchased from Cambridge Isotope Laboratories (Andover, MA).
GC-grade hexane, dichloromethane (DCM) and acetone were purchased from Fisher Scientific. Methyl tert-butyl ether (MTBE) with 99% purity (Acros Organics) was also purchased from Fisher. Bio-beads S-X3 (200 – 400 mesh) were purchased from Bio-Rad Laboratory (Richmond, CA). Bondesil C18 (40 μm) was purchased from Varian Inc. (Palo Alto, Ca). Anhydrous sodium sulfate, silica gel (100 – 200 mesh, Davisil Grade 644) and Florisil (60-100 mesh) were purchased from Fisher Scientific. Before use, the sorbents, silica gel and sodium sulfate, were cleaned by washing with acetone, dichloromethane and hexane, and dried overnight in an oven at 150 °C. A standard reference material (Lake Michigan fish tissue SRM 1947) was purchased from the National Institute of Standards and Technology (NIST, Gaithersburg, MD). It was stored in a −80 °C freezer upon receiving.
Placenta Collection and Pretreatment
Full term human placenta samples (N = 5) were collected from 5 pregnant women who were admitted to the University of Illinois at Chicago Medical Center for child delivery. They were asked to sign a written consent form approved by the university’s Institutional Review Board before donating their placentas. Collected placentas were immediately stored in a freezer at −20 °C.
Before extraction, excess blood from the placenta was wiped and the umbilical cord and other connective tissues were removed. Then, the placenta was cut into small pieces and homogenized in a commercial blender. Homogenate was freeze dried using a lyophilizer (Freezone 4.6L, Labconco, Kansas City, MO). Freeze dried samples were stored in tightly closed amber glass bottles and kept in a dessicator.
General MSPD Extraction Procedure
Approximately 4 grams of freeze dried sample was weighed into a solvent washed aluminum pan. To the same pan, extraction sorbent was added in a 1:2 sample:sorbent mass ratio and transferred into a glass mortar. After adding a known amount of surrogate (BDE118L), the sample- sorbent mixture was thoroughly ground using a glass pestle for approximately 5 minutes to become a fine powder. A glass column (13 mm id and 30 cm length) was packed from bottom to top with glass wool, pre-washed anhydrous sodium sulfate (~10 g), Florisil (~4 g), and the prepared sample-sorbent mixture. A photo of the MSPD column is presented in Figure S1 of the Supporting Information (SI). The packed column was gently tapped to remove the trapped air. The column was eluted with 100 mL of solvent. About half (50 mL) of this solvent was used to rinse the mortar and the pestle before it was used for elution. The elution was controlled at 1-2 drops/second using the Teflon clog and accomplished under gravitational flow. When the flow ceased, a gentle vacuum was applied to collect the remainder of the solution in the column.
Other Extraction Methods
The liquid extraction method developed by Jensen et al.13 was used with slight modifications. Briefly, a 50 mL glass centrifuge tube was filled with 4 g of the dried sample and mixed with 15 mL of acetone:hexane (7:2) mixture for 1 min using a vortex mixer. 10 mL of hexane:MTBE (9:1, v/v) were added and mixed for another 1 min, before the phase separation by centrifugation (Beckman Avanti 30 Centrifuge, Beckman Instruments, Fullerton, CA) at 3000 rpm for 30 minutes. The organic layer was pipetted out into another tube. The extraction was repeated by adding another 10 mL of hexane:MTBE (9:1) mixture. After repeated extraction, the combined organic extract was filtered through a hexane washed Whatman No. 1 filter paper. Then, the filtrate was washed with 5 mL of 0.1 M H3PO4 in 1% KCl solution by gentle shaking.
In the Soxhlet extraction, 4 g of freeze dried samples was transferred to a Whatman glass micro fiber thimble. Extraction flasks (250 mL) were filled with 150 mL of a solvent mixture (acetone, hexane, and dichloromethane in 45:45:10 ratio). The refluxing was adjusted at four cycles per hour, and the extraction continued for 22 hours.
Cleanup
The sample cleanup procedure was similar for all extraction methods. The extract was first transferred into a Kuderna-Danish (K-D) concentrator for solvent evaporation. Further volume reduction (down to ~2 mL) was achieved by a gentle N2 blow. Sample cleanup was accomplished by gel permeation chromatography (GPC) followed by silica gel chromatography. A glass GPC column (25 mm id × 400 mm L) was manually prepared by packing with S-X3 bio beads. The sample was eluted with 140 mL of hexane and dichloromethane (1:1) mixture. A multilayer silica gel column was prepared using (from bottom to top) 1 g of neutral silica, 1 g of basic silica, 4 g of acidic silica, 1 g of neutral silica and 5 g of anhydrous sodium sulfate. The preparation of the silica gel column followed the procedures described in EPA Method 1614.14 50 mL of hexane were used as the elution solvent. The final volume of the sample was reduced to 1 mL by K-D evaporation and N2 blow before the instrumental analysis.
GC/MS Analysis
Instrumental analyses of PBDEs were performed on an Agilent Model 6890 gas chromatograph (GC) with a Model 5973 mass spectrometer (MS) detector. A Rtx1614 capillary column (15 m × 0.25 mm i.d., 0.1 μm film thickness; Restek Inc.) was used with helium as the carrier gas. Internal standard CB204 was added to each sample before a GC injection to normalize the peak areas in the quantification of targeted PBDEs. Each sample was introduced into GC/MS through a programmable temperature evaporation (PTV) injection port. The operational parameters of the PTV inlet were optimized previously and have been described in detail elsewhere.15,16 In each run, 120 μL was injected using solvent vent mode. The initial oven temperature was 90°C, which lasted for 3 min, and then increased to 140°C at 10°C/min and further to 300°C at 5°C /min. The final temperature was kept for 15 min until the run was completed. Selected ion monitoring (SIM) was used in electron capture negative chemical ionization (ECNI) MS. The m/z values for the monitored ions of individual BDEs and BB209 were 79 and 81. CB204 was monitored using m/z 428, 430 and 432 and BDE209 with m/z 484 and 486.
A total of 43 PBDE congeners were analyzed. Identification was based on the retention time matching with the PBDE standards. For quantification, a six-point calibration curve for mono- to hepta-BDEs was prepared using the 39-PBDE mixture standard, with concentrations extended from 0.024 to 15 ng/mL. A five- point calibration curve with a concentration range from 1 to 38.4 ng/mL was used for the heavy congeners (BDEs 196, 206, 207 and 209). Good linearity was obtained for all the standard curves (r2 = 0.99 to 1.00). The standard curves were used to derive the response factors for individual congeners against internal standards. PCB204 was the internal standard for mono- to hepta- congeners, while BB209 was used for heavy congeners.
Lipid Measurement
The total extracted lipid was determined gravimetrically using an aliquot of 1 mL of the eluted extract solution, in order to determine the lipid removal efficiency by the MSPD and to examine whether the PBDE extraction depended on the extraction of lipid. Efficient lipid removal was desired in order to maximize the advantage of using MSPD procedure in combining the extraction and cleanup steps.
Quality Control and Method Validation
Procedural blanks were analyzed with each batch of extraction. The PBDE levels found in the blanks were deducted from those of the samples in that batch. Known amount of surrogate BDE118L was added into all the samples before extraction. The recovery of the surrogate was used to indicate the analytical accuracy and to correct the concentration values for each sample. All analyses were performed in duplicates.
The reliability of the optimized MSPD procedure was examined with matrix spike samples. Three aliquots of the same placenta samples were spiked with individual standard solutions of BDEs 28, 47, 66, 85, 99, 100, 153, 154, 183 and 209, and kept overnight in a refrigerator to allow proper penetration and mixing. These samples were extracted along with the non-spiked samples. The reliability of the procedure was further validated by analyzing SRM 1947. The fish tissue powder (1.00 g) was spiked with the surrogate, mixed with 5 g of sodium sulfate in a pre-cleaned aluminum pan, and kept covered in the refrigerator for 12 hours. Extraction of the matrix spike and the SRM samples was using the optimized MSPD method, and the cleanup and GC/MS analysis were carried out as described above.
Determination of the method detection limit (MDL) followed the standard procedure17. Briefly, the instrumental detection limit (IDL) was determined as the concentration capable of producing a GC/MS response 3 times greater than the noise (S:N = 3:1). Then, a homogenized freeze dried placenta sample, which had been pre-extracted, was divided into seven replicate samples, and each was spiked with PBDE standards at a level approximately equals to five times of the IDL. After the analyses, the MDL was calculated from the results by:
where SD is standard deviation of the seven replicate analyses, and t(n-1, α = 0.01) is the student’s t-value for 99% confidence level with n-1 degrees of freedom; it is 3.143 for n = 7.
Data Analysis
The Student’s t-test and two nonparametric statistical methods – paired sign test and signed rank test – were used to compare the experimental results between the extraction methods. The analysis was performed using SAS version 9.0, with the input experimental results expressed in ng/g dry weight (dw) for individual congeners and total PBDEs.
RESULTS AND DISCUSSION
MSPD Method Development
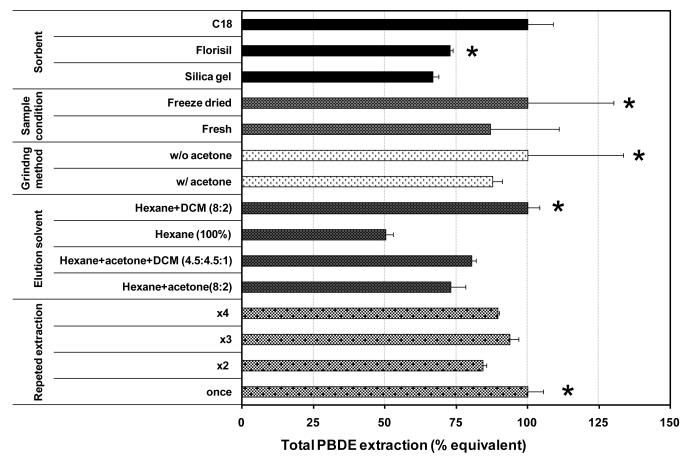
The MSPD optimization was carried out by sequential investigations of a number of factors, including the extraction sorbent, the sample condition, the grinding process, the elution solvent, and the number of elution cycles. To investigate each of these factors, different placenta samples were used. Aliquots of the same homogenized placenta were used to test different settings or conditions of each factor. The results are graphically summarized in Figure 1.
Figure 1.
Relative extraction efficiency of total PBDEs under different conditions. The error bar represents one standard deviation of the duplicated analyses. The condition with an asterisk was chosen for the optimized MSPD procedure.
One of the major steps in the development of the MSPD method was to select the sorbent for sample dispersion. In this study, three sorbents including silica gel, C18, and Florisil were compared, all at a sample-to-sorbent ratio of 1:2 (dry weight). In the literature, ratios varying from 1:1 to 1:4 have been used7. For dry samples, the 1:2 ratio was found to be sufficient for proper dispersion of samples, and no improvement in extraction efficiency was observed upon increasing in the sorbent amount higher than twice the sample amount18. In this work, the highest PBDE extraction efficiency was observed for C18; while the extraction efficiency using Florisil and silica gel was 73% and 67%, respectively, of that using C18 (Figure 1). Surrogate recovery for both C18 and Florisil were similar (> 92%), but it was lower (60%) for silica gel. However, co-extracted lipid was also higher for C18, thus mandating an additional GPC procedure for lipid removal. Method blanks revealed that the C18 sorbent has the highest level of contamination, as seen from the elevated baseline and unidentified peaks appearing in the GC/MS total ion chromatogram, even after intensive washing with solvent prior to use. In addition, C18 is the most costly among the three sorbents. Based on the results and these considerations, Florisil was chosen as the dispersion sorbent. Compared with C18, Florisil may provide similar or even better abrasive shearing forces in the disruption of the tissue architecture, due to its larger particle size and sharp edges (Figure 2, A and B). As magnesium silica gel, Florisil particles may have a more polar surface than the C18-derivatized silica; nonetheless, the dispersion of the placental tissue on Florisil particles was evident (Figure 2, C and D) and similar to that shown by Barker for C18.6 Florisil has also been successfully used to extract environmental pollutants such as PCBs and pesticides with over 70% recovery.19-23
Figure 2.
Scanning Electron Micrographs of (A) pure Florisil particles, 100×; (B) pure Florisil particles 350×; (C) Florisil particle coated with dispersed placenta tissue, 500×; and (D) Florisil particle coated with dispersed placenta tissue, 3000×. The images were obtained using a Hitachi S-3000N scanning electron microscope (SEM).
Fresh and freeze-dried tissues from a homogenized placenta sample were extracted simultaneously to examine whether the sample moisture condition affects the MSPD extraction efficiency. An aliquot of 20 g of the fresh tissue was freeze dried and extracted using 8 g of Florisil. Another 20 g of the fresh sample was directly dispersed on 40 g of Florisil. The analytical results were not significantly different (Figure 1), although the total PBDE concentration of the freeze-dried sample was slightly higher than that of the fresh one. Surrogate recovery was similar for both samples, and the loss of any congener due to freeze drying was not detected. Lipid extraction appeared to be higher for the dry sample (0.08 g lipids / 100 mL solvent) compared to the fresh one (0.05 g lipids / 100 mL solvent). The major advantages of using freeze dried samples were the convenience in handling, low sorbent consumption, and the ability to treat a larger quantity of a sample if needed.
Dry grinding of the sample sorbent mixture was tested against the solvent assisted “wet” grinding. In wet grinding, 20 - 40 mL of acetone:hexane (3:1) mixture was added to the freeze dried sample prior to grinding in the glass mortar. In dry grinding, the freeze dried sample was ground with the sorbent without adding solvent. As shown in the Figure 1, the effect of the grinding method appears to be insignificant on extracted level of PBDEs. However, in contrast to PBDE extraction, the lipid extraction using wet grinding was five fold higher than using dry grinding (0.125 vs 0.025 g lipids per 100 mL solvent). This suggests that more efficient lipid extraction does not necessarily produce higher PBDE levels in the extract. The polar solvent acetone can denature the protein structure and thereby promote the extraction of polar lipids (phospholipids and cholesterol). However, highly hydrophobic compounds such as PBDEs may be more associated with nonpolar lipid (triglycerides) in placenta and other biological matrices.
Selection of a suitable elution solvent is critical in MSPD extraction.6 Hexane and three solvent mixtures were tested, and the resulted total PBDE concentrations are compared in Figure 1. It is obvious that solvent mixtures work better than hexane alone. Even though non-polar organic solvents like hexane are better solvents for hydrophobic compounds like PBDEs, they lack the ability to penetrate deep into the tissue. Hexane is commonly used in combination with a polar solvent to enhance the extraction efficiencies for biotic samples.24, 25 In this work, the binary solvent mixtures of hexane and dichloromethane (8:2) gave the highest extraction efficiency for PBDEs. An additional advantage of using this solvent mixture was that it extracted less lipid than the other two mixtures.
Elution of the MSPD extraction column is customarily conducted once. With an intention to increase the analyte extraction efficiency without increasing the total solvent consumption, the collected eluate was reintroduced to the top of the column after the initial 100 mL elution solvent mixture had passed through the MSPD column. The elution was then repeated for a total of 2, 3 or 4 cycles. As seen in Figure 1, a significant difference was not found; in fact, single elution resulted in higher PBDE concentration than repeated ones. Re-adsorption of the analyte to the packing material of the column could have occurred during the repeated elution.
Based on these results, the optimized MSPD method uses freeze dried sample in a 1:2 sample-to-sorbent ratio with Florisil as the sorbent, dry grinding, and 100 mL solvent mixture of hexane dichloromethane (8:2) in a single extraction and elution cycle (Figures 1 and S1).
Lipid Extraction
It was found that a higher content of extracted lipids was not accompanied by significantly higher PBDE levels. This observation is in line with previous reports that lipid-normalized PCB concentration is negatively correlated with the content of the extracted polar lipid in tissues.25, 26 In the literature, chemical concentrations are often presented on the basis of lipid mass. However, the inconsistency in lipid measurement makes it difficult to compare among published data. As required by U.S. EPA,14 the PBDE concentrations should be reported on the basis of wet tissue mass, rather than on the basis of the lipid content.
Owing to the in-line lipid removal, the MSPD method presented in this paper is incapable of simultaneous lipid determination. The optimized method extracted negligible amount of lipids. Two major factors acted in the decrease of extracted lipid, including the use of an elution solvent mixture of hexane and dichloromethane (8:2) to minimize the extraction of polar lipids, and the layer of Florisil in the MSPD column that captured the extracted lipids. The dimension of the MSPD column was also found to be important; longer columns with smaller diameters are more efficient in lipid removal. When MSPD is used, lipid content may be determined by a separate laboratory procedure, such as the one developed by Bligh and Dyer.28
Comparison of MSPD with Other Extraction Methods
Five placenta samples were analyzed separately in duplicate for PBDEs using the optimized MSPD method as well as the liquid extraction and the Soxhlet extraction methods. The sum of major PBDE congeners (BDEs 28+33, 47, 66, 85, 99, 100, 153, 154, 183, 209) extracted using these methods is compared in Figure 3. Statistical analyses used the averages of duplicated measurements.
Figure 3.
Total PBDE concentration from 5 individual placenta samples and fish tissue SRM extracted using MSPD, liquid and Soxhlet extraction methods. SRM concentrations are 10 times diluted. The error bars represent one standard deviation of duplicated analyses.
The optimized MSPD method performed similarly to the conventional, robust Soxhlet method. The Student’s t-test indicated a non-significant difference between the two methods, with a p-value that ranged from 0.13 to 0.78 for individual congeners and p = 0.5 for total PBDEs. On the other hand, the efficiency of liquid extraction was significantly lower than that of MSPD (p < 0.05 for 7 out of 10 congeners as well as the total PBDEs). The same conclusion was derived from the results of the paired sign test and signed rank test, which compare medians of two repeated group measures.
The advantages of MSPD over Soxhlet extraction are multi-fold. First, the extraction time was shortened from 22 – 24 h with Soxhlet to 30 – 45 min with MSPD. Secondly, solvent consumption was reduced by 50 mL per sample. In addition, the optimized method extracted few lipids, compared to approximately 5% and 4% lipid (dw) extracted using the Soxhlet and liquid methods, respectively. Therefore, lipid removal using the GPC cleanup procedure was necessary for Soxhlet-extracted samples, but not required when the MSPD procedure was used. Nonetheless, it is recommended that further cleanup is carried out in order to maintain the lifetime of the capillary GC column, even though the MSPD extract is of GC-injectable quality.7, 29 In this study, the multi-layer silica gel column was used following the elution and concentration steps. This was necessary when large volume GC injection is involved.
Method Validation and the Detection Limits
The analytical accuracy is best evaluated by analyzing certified or standard reference material (CRM/SRM). However, methods are most often validated by using only standard spiked samples due to the limited availability of suitable reference material.30 In this study, the reliability of the optimized MSPD procedure was examined by analyzing both spiked placenta samples and NIST SRM 1947 with certified concentrations for BDEs 47, 66, 99, 100, 153 and 153 and a reference concentration for BDE28. As presented in Table 1, excellent recoveries of 91% - 114% were observed with a relative standard deviation (RSD) of 2.7% to 11.6% for the matrix spike samples. For SRM 1947, the results were within 89% to 115% of the certified values for all congeners except BDE28, for which 49% of the reference value was achieved (Table 2). The SRM was also analyzed using the Soxhlet and the liquid extraction methods, and the comparison among the three methods is similar to those using placenta samples (Figure 3).
Table 1.
PBDE recovery from the spiked placenta samples (ng/g dw) *
| Congener | Spike level |
Sp1 | Sp2 | Sp3 | Un- spiked |
Mean Recovery (%) |
RSD (%) |
|---|---|---|---|---|---|---|---|
| BDE28+33 | 1.32 | 1.22 | 1.16 | 1.22 | 0.0025 | 91 ± 0.03 | 2.71 |
| BDE47 | 1.52 | 1.55 | 1.47 | 1.57 | 0.0775 | 95 ± 0.06 | 3.62 |
| BDE66 | 1.43 | 1.52 | 1.31 | 1.42 | 0.0000 | 99 ± 0.1 | 7.35 |
| BDE100 | 1.60 | 1.57 | 1.43 | 1.52 | 0.0150 | 94 ± 0.07 | 4.71 |
| BDE99 | 1.55 | 1.74 | 1.47 | 1.57 | 0.0325 | 100 ± 0.14 | 8.58 |
| BDE118L | 1.39 | 1.72 | 1.36 | 1.51 | 0.0000 | 110 ± 0.18 | 11.59 |
| BDE85 | 1.44 | 1.79 | 1.47 | 1.71 | 0.0150 | 114 ± 0.16 | 9.93 |
| BDE154 | 1.57 | 1.72 | 1.43 | 1.55 | 0.0075 | 99 ± 0.15 | 9.37 |
| BDE153 | 1.54 | 1.78 | 1.51 | 1.62 | 0.0350 | 104 ± 0.14 | 8.31 |
| BDE183 | 1.58 | 1.87 | 1.69 | 1.81 | 0.0100 | 113 ± 0.09 | 5.10 |
| BDE209 | 1.53 | 1.98 | 2.06 | 1.84 | 0.4775 | 97 ± 0.11 | 5.64 |
Recovery = (concentration in spiked sample – concentration in unspiked sample) / spike level × 100%
Table 2.
Analytical results of SRM 1947 using optimized MSPD extraction (μg/kg ww)
| PBDE | (A) | (B) | Mean | RSD (%) | Certified | Recovery (%) |
|---|---|---|---|---|---|---|
| BDE28 | 1.26 | 0.94 | 1.10 ± 0.23 | 20.8 | 2.26 ± 0.46* | 49 |
| BDE47 | 70.17 | 67.38 | 68.77 ± 1.98 | 2.9 | 73.30 ± 2.9 | 94 |
| BDE66 | 1.84 | 1.46 | 1.65 ± 0.27 | 16.4 | 1.85 ± 0.13 | 89 |
| BDE100 | 14.82 | 15.50 | 15.16 ± 0.48 | 3.2 | 17.10 ± 0.6 | 89 |
| BDE99 | 23.15 | 20.91 | 22.03 ± 1.58 | 7.2 | 19.20 ± 0.8 | 115 |
| BDE154 | 6.49 | 6.66 | 6.57 ± 0.12 | 1.8 | 6.88 ± 0.52 | 96 |
| BDE153 | 4.16 | 4.27 | 4.22 ± 0.07 | 1.7 | 3.83 ± 0.04 | 110 |
The value for BDE28 is a reference value.
The detection limits of the MSPD procedure developed in this work are presented in Table S1 of the Supporting Information. MDLs for 39 out of the 43 spiked congeners ranged from 0.34 to 10.7 ng/kg of wet sample. BDEs 1, 2, 3, and 15 were not observed at the level of the spike.
PBDEs in Human Placenta
Table 3 summarizes the results of PBDE analyses in the five human placenta samples. The total PBDEs ranged from 385 to 2370 ng/kg (ww) among the five placenta samples. Among the 43 PBDE congeners quantitatively analyzed, 22 (Table 3) were detected in all five placenta samples analyzed in this study. The dominant congeners were BDE47 with a concentration range of 97-947 ng/kg (ww) and BDE99 with a range 166- 994 ng/kg (ww). The average concentration ratio of BDE47 to BDE99 was 5:4 and together these two congeners accounted for more than 75% of the total PBDE burden in human placenta. BDEs 85, 100, 153, 154 and 183 were also detected and quantified in all the samples, and they contributed approximately 15 % to the total PBDEs. The concentration of BDE209 in the 5 placenta samples ranged from < MDL to 29 ng/kg (ww). Although they did not contribute heavily to the total PBDE concentration, some infrequently reported PBDE congeners including BDEs 15, 17, 49, 71, 119, 155, 126, 196, 206, and 207 were also present in the placentas. A peak which highly likely represents BDE208 was also seen in all the samples analyzed. In addition, a number of unidentified brominated compounds were detected, based on the presence and the abundance ratio of the m/z 79 and m/z 81. Among them, U7, U8, U10, U11, U14, U16, U17, U18, and U20 appeared on all sample chromatograms with relatively high peaks.
Table 3.
Concentrations of PBDEs in placentas (ng/kg, ww)
| RT | Congener | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|---|
| 7.41 | BDE1 | nd | nd | nd | nd | nd |
| 7.55 | BDE2 | nd | nd | nd | nd | nd |
| 7.72 | BDE3 | nd | nd | nd | nd | nd |
| 7.98 | U1 | Y | Y | Y | Y | Y |
| 8.08 | U2 | Y | Y | Y | Y | nd |
| 8.18 | U3 | nd | nd | nd | Y | Y |
| 8.36 | U4 | Y | Y | nd | nd | Y |
| 8.69 | U5 | Y | Y | Y | Y | Y |
| 9.72 | BDE10 | nd | nd | nd | nd | nd |
| 10.46 | BDE7 | <MDL | nd | <MDL | nd | nd |
| 10.82 | BDE8+11 | nd | nd | nd | nd | nd |
| 11.04 | BDE12+13 | nd | nd | nd | nd | nd |
| 11.37 | BDE15 | 1.5 | 29.5 | 2 | 1.5 | 2.5 |
| 12.06 | U6 | Y | nd | Y | Y | nd |
| 12.22 | U7 | Y | Y | Y | Y | Y |
| 12.95 | BDE30 | nd | nd | nd | nd | nd |
| 14 | BDE32 | nd | nd | <MDL | nd | nd |
| 14.38 | BDE17* | 1 | <MDL | 2 | 1 | <MDL |
| 14.47 | BDE25* | 1 | 1 | 1 | <MDL | <MDL |
| 14.73 | U8 | Y | Y | Y | Y | Y |
| 14.89 | BDE28+33 | 5.3 | 2.3 | 4.8 | 9.8 | 3 |
| 15.23 | BDE35 | nd | nd | nd | nd | nd |
| 15.56 | U9 | nd | nd | Y | Y | Y |
| 15.63 | BDE37 | <MDL | 2 | <MDL | <MDL | <MDL |
| 17.68 | BDE75 | nd | nd | <MDL | <MDL | nd |
| 17.9 | U10 | Y | Y | Y | Y | Y |
| 18.05 | BDE49* | 11 | 5 | 31.5 | 3.5 | 1.5 |
| 18.14 | BDE71* | 5 | 2.5 | 15.5 | 1.5 | 1 |
| 18.62 | BDE47 | 652.8 | 194 | 535.5 | 946.5 | 97 |
| 18.96 | U11 | Y | Y | Y | Y | Y |
| 19.14 | BDE66 | 6.3 | 1.3 | 6 | 10.5 | <MDL |
| 19.65 | BDE77 | nd | nd | nd | <MDL | nd |
| 21.26 | U12 | Y | Y | Y | Y | nd |
| 20.89 | U13 | nd | nd | Y | Y | Y |
| 21.4 | BDE100 | 57.8 | 48 | 118.5 | 192.8 | 47 |
| 21.68 | BDE119 | 4 | 46 | 13 | 39 | 6.5 |
| 22.13 | U14 | Y | Y | Y | Y | Y |
| 22.23 | BDE99 | 290 | 207.3 | 636.5 | 993.8 | 165.5 |
| 22.39 | BDE116 | # | # | # | # | # |
| 22.74 | U15 | nd | nd | nd | nd | Y |
| 23.59 | BDE85 | 29 | 10.5 | 32.8 | 48 | 5 |
| 23.84 | BDE155+126 | 18.5 | 1 | 6 | 3.5 | nd |
| 24.44 | U16 | Y | Y | Y | Y | Y |
| 24.46 | BDE154 | 34.8 | 13.3 | 36.3 | 55.3 | 12.5 |
| 25.55 | BDE153 | 74.5 | 23.3 | 58.5 | 37.3 | 18.3 |
| 25.93 | U17 | Y | Y | Y | Y | Y |
| 26.23 | U18 | Y | Y | Y | Y | Y |
| 26.93 | BDE138+166 | 4.5 | 4 | nd | nd | 2.5 |
| 27.68 | U19 | Y | nd | Y | Y | Y |
| 28.63 | BDE183 | 8 | 6.5 | 6.3 | 9.3 | 6.8 |
| 29.67 | U20 | Y | Y | Y | Y | Y |
| 30.16 | BDE181 | nd | nd | nd | nd | nd |
| 30.38 | BDE190 | nd | 2.5 | nd | nd | nd |
| 32.81 | BDE196 | <MDL | <MDL | <MDL | <MDL | <MDL |
| 35.58 | BDE208 | <MDL | <MDL | <MDL | <MDL | <MDL |
| 35.83 | BDE207 | <MDL | 2.5 | 6 | 2.5 | 3.5 |
| 36.57 | BDE206 | <MDL | <MDL | 8 | <MDL | <MDL |
| 39.54 | BDE209 | <MDL | 24 | 28.5 | 13.5 | 12 |
| ∑42BDEs | 1205 | 626.5 | 1548.7 | 2369.3 | 384.6 |
RT= retention time
nd = not detected
Y = present
<MDL = below the method detection limit
U = unidentified brominated compound
P = placenta
= peak bases not resolved
# = completely covered by BDE 99 Italicized numbers carry higher uncertainty
CONCLUSION
In this work, the matrix solid phase dispersion method was optimized to extract PBDEs from human placenta. Florisil is an efficient dispersion sorbent, and 100 ml of hexane/dichloromethane (8:2) solvent mixture is adequate to extract and elute the PBDEs from the MSPD column combining extraction and lipid removal. The MSPD method drastically reduces the extraction time and solvent consumption, in comparison with the traditional Soxhlet method. Moreover, the cleaner extract produced by the MSPD reduces the need for subsequent cleanup efforts. Overall, the optimized method is an efficient, reliable, easy to use, and cost effective procedure to extract PBDEs from human placenta tissue.
This paper reports for the first time the PBDE concentration levels in human placenta in the United States, even though such an investigation is not the major objective of this work, therefore the number of samples involved is small. We also found that the PBDE extraction efficiency of the MSPD method does not depend on the extracted lipid, which depends heavily on the use of polar solvent (acetone, in this work). This observation provides a rationale for the requirement of the U.S. EPA to report concentrations based on tissue mass.14 With the growing needs in identifying emerging toxic chemicals and using integrated risk assessment, the use of placenta in prenatal exposure studies is advantageous. The results of this work should be highly valuable in such endeavors.
Supplementary Material
ACKNOWLEDGEMENT
We gratefully acknowledge the support of Dr. Xiaobin Wang of the Children’s Memorial Hospital in Chicago, and the helpful discussions with Dr. Amy Mucha of the School of Public Health, University of Illinois at Chicago. The project described was supported by Grant Number R03ES015608 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Supporting Information Available: This material is available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- (1).Schantz SL. Neurotoxicol. Teratol. 1996;18:217–227. doi: 10.1016/s0892-0362(96)90001-x. [DOI] [PubMed] [Google Scholar]
- (2).Myllynen P, Pasanen M, Pelkonen O. Placenta. 2005;26:361–371. doi: 10.1016/j.placenta.2004.09.006. [DOI] [PubMed] [Google Scholar]
- (3).Chao HR, Wang SL, Lee WJ, Wang YF, Papke O. Environ. Int. 2007;33:239–245. doi: 10.1016/j.envint.2006.09.013. [DOI] [PubMed] [Google Scholar]
- (4).Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, Vartiainen T, Skakkebmk NE, Toppari J. Environ. Health Persp. 2007;115:1519–1526. doi: 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Myren M, Mose T, Mathiesen L, Knudsen LE. Toxicol In Vitro. 2007;21:1332–1340. doi: 10.1016/j.tiv.2007.05.011. [DOI] [PubMed] [Google Scholar]
- (6).Barker SA. J. Chromatogr. A. 2000;885:115–127. doi: 10.1016/s0021-9673(00)00249-1. [DOI] [PubMed] [Google Scholar]
- (7).Kristenson EM, Ramos L, Brinkman UAT. Trends Anal. Chem. 2006;25:96–111. [Google Scholar]
- (8).Martinez A, Ramil M, Montes R, Hernanz D, Rube E, Rodriguez I, Torrijos RC. J. Chromatogr. A. 2005;1072:83–91. doi: 10.1016/j.chroma.2004.12.034. [DOI] [PubMed] [Google Scholar]
- (9).Gomara B, Herrero L, Bordajandi LR, Gonzalez MJ. Rapid Commun. Mass Sp. 2006;20:69–74. doi: 10.1002/rcm.2264. [DOI] [PubMed] [Google Scholar]
- (10).Gomara B, Herrero L, Ramos JJ, Mateo JR, Fernndez MA, García JF, Gonazlez MJ. Environ. Sci. Technol. 2007;41:6961–6968. doi: 10.1021/es0714484. [DOI] [PubMed] [Google Scholar]
- (11).Hirai T, Fujimine Y, Watanabe S, Nakamura Y, Shimomura H, Nagayama J. Organohalogen Compd. 2004;66:2451–2456. [Google Scholar]
- (12).Doucet J, Tague B, Arnold DL, Cooke GM, Hayward S, Goodyer CG. Environ. Health Persp. 2009;117:605–610. doi: 10.1289/ehp.0800205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Jensen S, Reutergardh L, Jansson B. Manual of methods in aquatic environment research, Part 9. FAO; Rome: 1983. FAO Fisheries Technical Paper 212 FIRI/T212. [Google Scholar]
- (14).United States Environmental Protection Agency [accessed August 5, 2009];Method 1614, Brominated diphenyl ethers in water, soil, sediment, and tissue by HRGC/HRMS. 2007 http://www.epa.gov/waterscience/methods/method/files/1614.pdf.
- (15).Wei H, Dassanayake PS, Li A. Int. J. Environ. An. Ch. doi: 10.1080/03067310902871299. (in print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Norlock F, Jang JK, Zou Q, Schoonover T, Li A. J. Air Waste Manage. 2002;52:19–26. doi: 10.1080/10473289.2002.10470752. [DOI] [PubMed] [Google Scholar]
- (17).United States Environmental Protection Agency [accessed August 5, 2009];Test Methods for Evaluating Solid Waste, Physical/Chemical Methods (SW-846) 2009 http://www.epa.gov/epawaste/hazard/testmethods/sw846/online/index.htm.
- (18).Valsamaki VI, Boti VI, Sakkas VA, Albanis TA. Anal. Chim. Acta. 2006;573:195–201. doi: 10.1016/j.aca.2006.03.050. [DOI] [PubMed] [Google Scholar]
- (19).Li ZY, Zhang ZC, Zhou QL, Wang QM, Gao RY, Wang QS. J. AOAC Int. 2003;86:521–528. [PubMed] [Google Scholar]
- (20).Morzycka B. J. Chromatogr. A. 2002;982:267–272. doi: 10.1016/s0021-9673(02)01505-4. [DOI] [PubMed] [Google Scholar]
- (21).Gomez-Ariza JL, Bujalance M, Giraldez I, Velasco A, Morales E. J.Chromatogr. A. 2002;946:209–219. doi: 10.1016/s0021-9673(01)01534-5. [DOI] [PubMed] [Google Scholar]
- (22).Lino CM, Azzolini CBF, Nunes DSV, Silva JMR, da Silveira MIN. J. Chromatogr. B. 1998;716:147–152. doi: 10.1016/s0378-4347(98)00284-9. [DOI] [PubMed] [Google Scholar]
- (23).Albero B, Sanchez-Brunete C, Tadeo JL. J. Agr. Food Chem. 2003;51:6915–6921. doi: 10.1021/jf030414m. [DOI] [PubMed] [Google Scholar]
- (24).de Boer J, Allchin C, Law R, Zegers B, Boon JP. Trends Anal. Chem. 2001;20:591–599. [Google Scholar]
- (25).Saito K, Sjodin A, Sandau CD, Davis MD, Nakazawa H, Matsuki Y, Patterson DG. Chemosphere. 2004;57:373–381. doi: 10.1016/j.chemosphere.2004.04.050. [DOI] [PubMed] [Google Scholar]
- (26).Ewald G, Bremle G, Karlsson A. Mar. Pollutl. Bull. 1998;36:222–230. [Google Scholar]
- (27).Booij K, van der Berg C. B. Environ. Contam. Tox. 1994;53:71–76. doi: 10.1007/BF00205141. [DOI] [PubMed] [Google Scholar]
- (28).Bligh EG, Dyer WJ. Can. J. Biochem. Phys. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- (29).Barker SA. J. Biochem. Biophys. Methods. 2007;70:151–162. doi: 10.1016/j.jbbm.2006.06.005. [DOI] [PubMed] [Google Scholar]
- (30).Garcia-Lopez M, Canosa P, Rodriguez I. Anal. Bioanal. Chem. 2008;391:963–974. doi: 10.1007/s00216-008-1898-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.