Abstract
Pyrethroid insecticides are one of the most commonly used residential and agricultural insecticides. Based on the increased use of pyrethroids and recent studies showing that pregnant women and children are exposed to pyrethroids, there are concerns over the potential for developmental neurotoxicity. However, there have been relatively few studies on the developmental neurotoxicity of pyrethroids. In this study, we sought to investigate the developmental toxicity of six common pyrethroids, three type I compounds (permethrin, resmethrin, and bifenthrin) and three type II compounds (deltamethrin, cypermethrin, and λ-cyhalothrin), and to determine whether zebrafish embryos may be an appropriate model for studying the developmental neurotoxicity of pyrethroids. Exposure of zebrafish embryos to pyrethroids caused a dose-dependent increase in mortality and pericardial edema, with type II compounds being the most potent. At doses approaching the LC50, permethrin and deltamethrin caused craniofacial abnormalities. These findings are consistent with mammalian studies demonstrating that pyrethroids are mildly teratogenic at very high doses. However, at lower doses, body axis curvature and spasms were observed, which were reminiscent of the classic syndromes observed with pyrethroid toxicity. Treatment with diazepam ameliorated the spasms, while treatment with the sodium channel antagonist MS-222 ameliorated both spasms and body curvature, suggesting that pyrethroid-induced neurotoxicity is similar in zebrafish and mammals. Taken in concert, these data suggest that zebrafish may be an appropriate alternative model to study the mechanism(s) responsible for the developmental neurotoxicity of pyrethroid insecticides and aid in identification of compounds that should be further tested in mammalian systems.
Keywords: pyrethroid, zebrafish, danio rerio, developmental toxicity, neurotoxicity, pesticide, sodium channel, diazepam; developmental neurotoxicity
Pyrethroid insecticides are one of the most widely used domestic and agricultural pesticides, comprising one-quarter of the world market as early as 1995 (Casida and Quistad, 1998), rising in popularity because they are generally more toxic to insects than nontarget species (Soderlund et al., 2002; Wolansky and Harrill, 2008). Pyrethroids have also been used extensively for mosquito control following outbreaks of the West Nile virus, and most recently, pyrethroids have become the pesticide of choice to combat bedbug infestations (Agency for Toxic Substances and Disease Registry [ATSDR], 2003). Following the removal of the organophosphate pesticide chlorpyrifos for household use, pyrethroids have become a top choice for household pest control. Indeed, a recent study demonstrated that biomarkers of pyrethroid pesticide exposure significantly increased, while biomarkers of chlorpyrifos exposure decreased, as its household uses were in the process of being phased out (Williams et al., 2008).
Although pyrethroids are often considered to be “safer” pesticides because of their low to moderate acute toxicity to nontarget species, their increased use raises concerns of potential adverse effects, particularly in sensitive populations such as children. This concern is intensified by recent studies indicating that children are exposed to pyrethroids during development. For example, pyrethroid metabolites have been found in the urine of pregnant women (Berkowitz et al., 2003; Whyatt et al., 2002). A recent study also found that 67% of a cohort of preschool children had detectable levels of the pyrethroid metabolite 3-phenoxybenzoic acid in their urine (Morgan et al., 2007). Lu et al. (2006, 2009) have also found pyrethroid metabolites in urine of elementary-age children that appear to be primarily the result of residential exposure.
The findings that the developing fetus and children are exposed to measurable levels of pyrethroid pesticides raises concern over the potential for developmental neurotoxicity because animal studies have demonstrated that some pyrethroids are more acutely toxic to developing animals than adults (Sheets, 2000). Furthermore, recent data demonstrate that developmentally expressed isoforms of voltage-gated sodium channels, the molecular target of pyrethroids, are uniquely susceptible to some pyrethroids (Meacham et al., 2008). Taken together, these studies suggest that developing animals are uniquely susceptible to pyrethroid toxicity. However, there are few published studies that have evaluated the developmental neurotoxicity of pyrethroid pesticides (reviewed by Shafer et al., 2005). Furthermore, the published studies that have been performed on in utero exposure to pyrethroids suffer from the use of commercial grade products that contain unidentified ingredients, which makes attribution of effects to the pesticide difficult (Aziz et al., 2001; Husain et al., 1992; Lazarini et al., 2001). One potential reason that there are not more published studies may be that developmental neurotoxicity studies in mammals are time consuming and expensive. Thus, there is a need to develop more rapid and economical models for assessing developmental neurotoxicity, which would aid in screening compounds that should be prioritized for mammalian testing.
The zebrafish model system has become one of the models of choice for research on developmental processes because of its fecundity and its genetic and physiological similarity to mammals. These advantages have led the use of the zebrafish model in drug discovery and toxicological screening (Peterson et al., 2008). In fact, zebrafish have recently been used as an alternative model to assess the teratogenic effects of a variety of pesticides (Cook et al., 2005; Haendel et al., 2004; Stehr et al., 2006; Tilton et al., 2006). In addition to the determination of classic teratogenic effects, zebrafish have recently been proposed and utilized as models for developmental neurotoxicology (Eddins et al., 2009; Linney et al., 2004). Zebrafish also provide the unique ability to test direct exposure of developing embryos to toxicants. This is an advantage compared to mammalian models that require administration of the toxicant to the mother, a protocol that makes it difficult to determine the precise dose that the embryo is exposed. Thus, the zebrafish model may provide a more rapid and cost-effective system for assessing the developmental neurotoxicity of pesticides.
The present study sought to determine the suitability of zebrafish embryos for assessing the developmental neurotoxicity of pyrethroids. Here, we examined the teratogenic effects of six commonly used pyrethroids and determined the relative potency of these compounds in causing lethality and developmental toxicity. Our data demonstrate that zebrafish respond to pyrethroids similar to mammals, in terms of potency and in teratogenicity. We also demonstrate that developmental exposure of zebrafish to pyrethroids produced neurotoxic effects reminiscent of those observed in mammals and that these effects are blocked by pharmacological agents that ameliorate pyrethroid neurotoxicity in animals and humans. Taken in concert, these data suggest that zebrafish may be an appropriate model to study the mechanism(s) responsible for the developmental neurotoxicity of pyrethroid insecticides and aid in prioritization of chemicals that should be further tested in mammalian systems.
MATERIALS AND METHODS
Zebrafish husbandry.
AB strain zebrafish (Danio rerio), obtained from the Zebrafish International Resource Center, were bred and maintained in a recirculating aquatic habitat system on a 14-h light:10-h dark cycle. Fish system water, obtained by carbon/sand filtration of municipal tap water, was maintained at 28°C, < 0.05 ppm nitrite, < 0.2 ppm ammonia, and pH between 7.2 and 7.7. The AB strain fish were used for all experiments, and the husbandry protocol (#08-025) was approved by the Rutgers University Animal Care and Facilities Committee.
Chemicals.
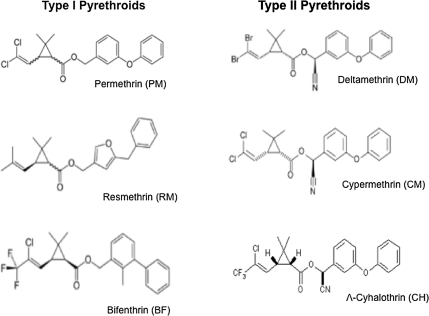
Pyrethroid insecticides were obtained from Chem Service (West Chester, PA) and stored dry in the dark at room temperature. Stock solutions of pyrethroids were made by dissolving pyrethroids in N,N-dimethylformamide (DMF; Sigma, St. Louis, MO). Three type I (permethrin [46% cis-52% trans], bifenthrin [99% purity, cis and trans], and resmethrin [99% purity, mix of isomers]) and three type II (deltamethrin [99% purity], cypermethrin [99% purity, mix of isomers], and λ-cyhalothrin [99.1% purity]) pyrethroids were used in this study (Fig. 1). Diazepam (7-chloro-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2(1H)-one) and MS-222 (tricaine methanesulfonate) were obtained from Sigma-Aldrich (St Louis, MO).
Pesticide exposures.
Male and female pairs of adult zebrafish (6 months–1 year) were placed in pairs (four to eight pairs per experiment) for breeding. Fertilized eggs were collected and randomly sorted into groups. For pyrethroid exposures, fertilized eggs were incubated in glass petri dishes in pyrethroid solutions (or control solution) beginning at 3-h postfertilization (hpf). Stock solutions of pyrethroids were made by dissolving pyrethroids in DMF (Sigma). Stock solutions were stored in complete darkness at 4°C for a maximum of 2 weeks and diluted to working concentrations in DMF. Pesticides were added to zebrafish system water in the proportion of 1 μl DMF with dissolved pesticide to 10 ml of system water (1:10,000 dilution, 0.01% final concentration). Control animals were treated with vehicle in the same proportion of 1 μl DMF/10 ml water.
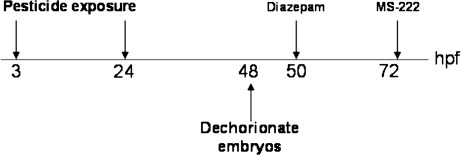
At 24 hpf, viable embryos were placed into 3.7-ml vials with 2 ml fresh treatment solution, one embryo per vial. The embryos were then immediately observed (day 1) using a dissecting microscope under low power. Embryos were observed every 24 h through day 6 (144 hpf), and the occurrence of lesions and mortality was recorded. Photographs of representative individuals were taken using a Scion CFW-1310C color digital camera mounted to either an Olympus IX51 inverted scope or an Olympus SZ4060 zoom stereo microscope under ×25 magnification (Olympus, America, PA). Exposure studies were repeated at least three times for each compound tested, with 5–25 fish per dose group. Doses presented for data are given as nominal calculated water concentrations. A schematic of the experimental design is provided in Figure 2.
FIG. 2.
Experimental timeline for dosing and pharmacological interventions.
Alcian blue staining.
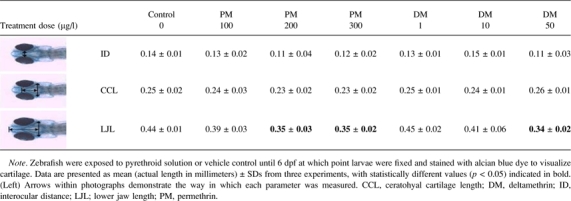
Embryos at 6-day postfertilization (dpf) were euthanized with an overdose of MS-222 (1 g/l; Sigma) and then placed into 4% paraformaldehyde overnight at 4°C for fixation. Embryos were then stained with alcian blue dye (Sigma) as described previously (Hillegass et al., 2008). Briefly, embryos were dehydrated in ethanol, bleached by incubation in 30% hydrogen peroxide, rinsed with a solution of 0.1% Tween-20 in PBS, and then stained overnight in alcian blue dye. Embryos were placed in acidified ethanol for 2–4 h to remove excess stain and photographed under ×40 magnification. The interocular distance (ID), lower jaw length (LJL), and ceratohyal cartilage length (CCL) were measured (Table 2) from the digital images using Adobe Photoshop software, and measurement values were converted to actual lengths (in millimeters) for reporting purposes.
TABLE 2.
Craniofacial Morphological Effects of Developmental Pyrethroid Exposure
 |
Pharmacological interventions.
For the diazepam treatments, embryos were exposed to deltamethrin as described above for the exposure studies. Stock solutions were made by dissolving diazepam in DMF. At 72 hpf, all vials were dosed with diazepam by adding 2 μl of DMF containing dissolved diazepam to the 2 ml of treatment solution contained in each vial. Vehicle control vials were treated only with DMF in the same proportion. The effect of the diazepam at each dose was tested on control animals (i.e., no deltamethrin exposure) as well as on animals that had been treated with deltamethrin until 72 hpf. All vials were observed at 72 hpf (before diazepam dosing), 2 h following diazepam dosing, and 24 h following diazepam dosing, and the incidences of spasms and curvature were recorded. Doses of diazepam tested were 5, 25, 40, 50, and 75μM. Spasms were defined as sudden and uncontrolled body movements, which were clearly different from normal swimming behaviors. Spasms were further characterized by visual observation noting the relative time between episodes and whether the spasms were accompanied by prolonged muscle contraction or arching of the body.
For the MS-222 treatments, embryos were exposed to deltamethrin (or vehicle control) beginning at 3 hpf. At 48 hpf, embryos were dechorionated and placed in vials (one embryo per vial) with fresh treatment solutions. These solutions contained either deltamethrin or vehicle control added to water containing dissolved MS-222. Embryos were observed at 2 and 24 h after placement into vials for the occurrence of spasms, body curvature, and any other lesions or mortality. Doses of MS-222 tested were 0.001% (38.3μM), 0.002% (76.5μM), 0.004% (153μM), and 0.006% (230μM). The response to mechanical stimulus was used to ensure that the doses of MS-222 employed did not cause anesthesia. Briefly, fish were lightly touched on their tails with a blunt needle and observed under a microscope for movement.
Statistics and data analysis.
LC50 and confidence interval (CI) values were calculated using the probit method of Miller and Tainter (1944). For compounds that caused less than 20% mortality at the highest dose, no LC50 was calculated. CIs were determined as the doses corresponding to the 16 and 84% effect level based on the best-fit line. For other outcome measurements, either a one-way ANOVA test with a Bonferroni-corrected t-test or a Mann-Whitney rank sum test was performed. Diazepam and MS-222 data were analyzed by chi-square tests to compare the percentage of fish exhibiting spasms and body curvature between groups. Groups for which the resulting p value was < 0.001 were considered significantly different from controls.
RESULTS
Type II Pyrethroids Are More Toxic Than Type I Pyrethroids
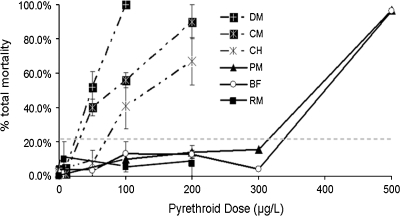
Exposure of zebrafish embryos (starting at 3 hpf) to type I pyrethroids (permethrin, resmethrin, and bifenthrin) and type II pyrethroids (deltamethrin, cypermethrin, and λ-cyhalothrin; Fig. 1) resulted in a dose-related increase in mortality, except for resmethrin (Fig. 3). Mortality data from all studies were combined and used to calculate LC50 values and CI for each pyrethroid (Table 1). Resmethrin was excluded from LC50 calculation because it did not cause significant mortality at any of the doses tested. The LC50 values were used to rank the six compounds based on relative toxicities. In order of decreasing toxicity (increasing LC50), the compounds are deltamethrin > cypermethrin > λ-cyhalothrin > bifenthrin > permethrin. In general, the type II compounds tested in this study were more toxic to developing zebrafish than the type I compounds, similar to the observation that type II pyrethroids are generally more toxic in rodents (Table 1).
FIG. 1.
Representative structures of pyrethroid pesticides tested for developmental toxicity.
FIG. 3.
Developmental exposure of zebrafish to pyrethroid pesticides results in a dose-related increase in mortality. Zebrafish were exposed to pyrethroid pesticides at various doses for 6 dpf and observed for mortality every 24 h. Data are presented as mean ± SEM. Percent mortality values greater than 20% (gray dotted line) were statistically significant from controls. Each experiment comprised 20–25 fish per treatment group, and each experiment was repeated at least three times. BF, bifenthrin; CH, ∧-cyhalothrin; CM, cypermethrin; DM, deltamethrin; PM, permethrin; RM, resmethrin.
TABLE 1.
Calculated LC50 Values for Pyrethroid Pesticides in Zebrafish
| Compound | Type | LC50 | CI | Rat LD50 values |
| Deltamethrin (DM) | II | 40 ± 0.08 | 19–100 | 67 |
| Cypermethrin (CM) | II | 65 ± 0.16 | 25–180 | 250 |
| Λ-Cyhalothrin (CH) | II | 110 ± 0.24 | 55–200 | 56 |
| Bifenthrin (BF) | I | 190 ± 0.45 | 90–350 | 55 |
| Permethrin (PM) | I | 300 ± 0.08 | 140–600 | 1200 |
| Resmethrin (RM) | I | n/a | n/a | 2000 |
Note. Values were obtained by plotting percent total mortality data for all trials for each compound against log (dose) and generating a best-fit line. Compounds are presented top to bottom in terms of highest to lowest lethality to zebrafish embryos. Type indicates whether the compound is a type I or II pyrethroid. No LC50 was calculated for resmethrin because the highest dose tested caused no significant (> 20%) mortality. CI represent the doses corresponding to the 16 and 84% mortality levels based on the best-fit line. Highest doses tested (micrograms per liter) were DM, 250; CM and CH, 500; PM, 800; and BF and RM, 3000. Values for rat LD50 data were taken from data cited in Wolansky et al. (2006). n/a, not applicable.
Type I and II Pyrethroids Cause Edema and Mild Craniofacial Abnormalities at High Doses
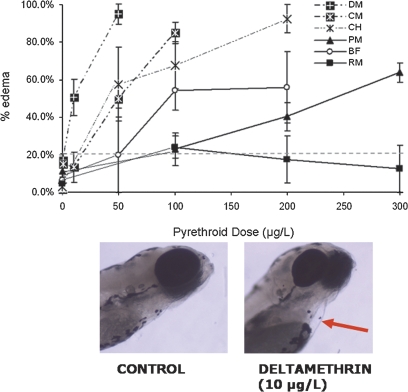
Pericardial edema and craniofacial malformations are teratogenic lesions commonly observed in zebrafish exposed to various toxins (Hillegass et al., 2008; McGrath and Li, 2008). Because pyrethroids are considered mildly to nonteratogenic in mammals (ATSDR, 2003; Presibella et al., 2005), pericardial edema and craniofacial structures were examined here to determine whether pyrethroids were similarly nonteratogenic to zebrafish. Pericardial edema was sometimes observed in control fish (0–11%) and those exposed to low doses of pyrethroids, although these were generally mild and tended to resolve within 24 h. Severe edemas were only associated with high doses of pyrethroids that approached the LC50 values (Fig. 4).
FIG. 4.
Developmental exposure of zebrafish to high doses of pyrethroid pesticides causes pericardial edema. Zebrafish were exposed to pyrethroid pesticides at various doses for 6 dpf and observed for edema every 24 h. Data are presented as mean ± SEM. Percent edema for control groups ranged from 0 to 11%. Percent lesion values greater than 20% (gray dotted line) were statistically significant from controls. Picture below shows representative pericardial edema. Each experiment comprised 20–25 fish per treatment group, and each experiment was repeated at least three times. BF, bifenthrin; CH, ∧-cyhalothrin; CM, cypermethrin; DM, deltamethrin; PM, permethrin; RM, resmethrin.
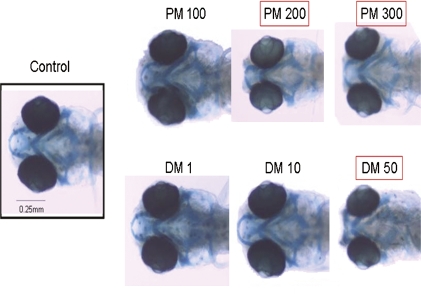
Because we observed only minor effects of pyrethroid exposure on pericardial edema, permethrin (type I) and deltamethrin (type II) were chosen as representative compounds from each class for determination of craniofacial abnormalities. Control and pyrethroid-treated larvae (6 dpf) were fixed, stained with alcian blue dye, and photographed. The ID, LJL, and CCL were measured from photographs. ID and CCL were not significantly altered by pyrethroid exposure at any dose tested (Table 2, Fig. 5). LJL was significantly lowered (p < 0.05) by exposure to 50 μg/l deltamethrin and by both 200 and 300 μg/l permethrin. Taken in concert, these data demonstrate that only high doses of pyrethroids produce teratogenic effects in developing zebrafish, similar to the mild effects observed in mammalian models.
FIG. 5.
Developmental exposure of zebrafish to high doses of pyrethroid pesticides alters crainiofacial structure. Pictures show representative craniofacial appearance of vehicle control and permethrin- or deltamethrin-treated zebrafish under ×40 magnification, with doses in units of micrograms per liter. Zebrafish were exposed beginning 3 hpf. Larvae were fixed at 6 dpf and stained with alcian blue dye to allow visualization of cartilage. Images shown are from the same experimental run and show the typical appearances of fish exposed to permethrin or deltamethrin. Alcian blue staining was performed on three experiments for both compounds and significantly different groups are indicated with boxes. Each experiment comprised 20–25 fish per treatment group, and each experiment was repeated at least three times. DM, deltamethrin; PM, permethrin.
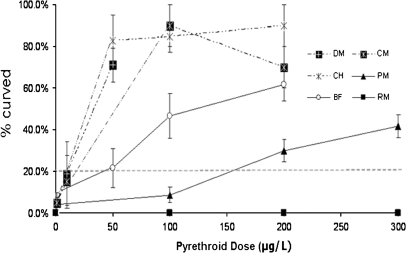
Developmental Exposure to Both Classes of Pyrethroids Causes Curvature of the Body Axis and Spasms
The data presented above demonstrate that the pyrethroid pesticides tested produced teratogenic lesions commonly observed in zebrafish exposed to xenobiotics and drugs only at the highest concentrations employed. However, we did observe a peculiar phenotype in zebrafish exposed to most of the pyrethroids. The phenotype presented as a body axis curvature and was observed at fairly low doses in zebrafish embryos exposed to type II pyrethroids (Fig. 6). Curvature was also observed in embryos exposed to the type I pyrethroids, bifenthrin and permethrin. The potency of the different pyrethroids in producing curvature was similar to that observed for the mortality curves. The curvature did not appear to be the result of a morphological effect on either the spinal cord or the musculature, as alcian blue staining and histopathology did not reveal any overt changes (data not shown).
FIG. 6.
Developmental exposure of zebrafish to various pyrethroids causes curvature of the body axis. Zebrafish were exposed to pyrethroid pesticides at various doses for 6 dpf and observed for curvature. Data are presented as mean ± SEM. Percent lesion values greater than 20% (gray dotted line) were found to be statistically greater than control. Each experiment comprised 20–25 fish per treatment group, and each experiment was repeated at least three times. BF, bifenthrin; CH, ∧-cyhalothrin; CM, cypermethrin; DM, deltamethrin; PM, permethrin; RM, resmethrin.
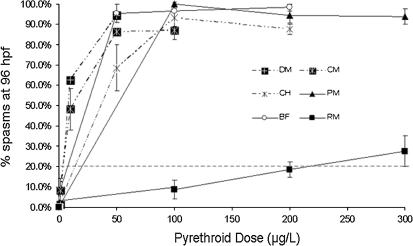
We also observed neurobehavioral effects following pyrethroid exposure at lower doses. These behavioral effects were presented as spastic movements, defined as sudden and uncontrolled body movements that were clearly different from normal swimming behaviors, and were reminiscent of the tremors and spasms observed in mammals following pyrethroid exposure (Fig. 7). The lowest tested dose that produced spasms lowest observable effect level was (in micrograms per liter) 1 for deltamethrin and cypermethrin, 10 for cyhalothrin, 100 for bifenthrin, 50 for permethrin, and 100 for resmethrin. Although both classes of pyrethroids induced spastic movements, visual observation of these spasms revealed differences between type I and type II compounds. The type I pyrethroids tested (permethrin, resmethrin, and bifenthrin) caused fish to exhibit tremors with little time between episodes. In contrast, the type II pyrethroids used in this study (deltamethrin, cypermethrin, and λ-cyhalothrin) caused tetanic spasms characterized by prolonged muscle contraction and arching of the body. The release of these muscular contractions was often followed by a period of 5–15 s of immobility. Although further study is required to characterize the spasms, these differences are reminiscent of the classical type I and type II syndromes of pyrethroid toxicity observed in mammals (Ray and Forshaw, 2000).
FIG. 7.
Developmental exposure to pyrethroid pesticides causes a dose-dependent increase in spasms. Zebrafish were exposed to pyrethroid pesticides at various doses beginning at 3 hpf and observed for spasms at 96 hpf. Data are presented as mean ± SEM. Percent lesion values greater than 20% (gray dotted line) were statistically significant from control. Each experiment comprised 20–25 fish per treatment group, and each experiment was repeated at least three times. BF, bifenthrin; CH, ∧-cyhalothrin; CM, cypermethrin; DM, deltamethrin; PM, permethrin; RM, resmethrin.
Diazepam Ameliorates Spasms Induced by Deltamethrin, While Tricaine (MS-222) Ameliorates Spasms and Curvature
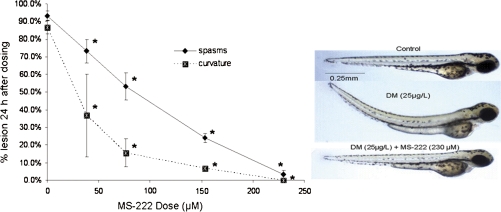
Based on our initial results, we sought to ascertain the mechanistic basis for the curvature and spasms elicited by developmental deltamethrin exposure. Examination of the histological sections of the embryo for muscle development did not indicate any gross physical abnormalities in either the muscle or the cartilage (data not shown); therefore, the curvature did not appear to be the result of a morphological defect. We hypothesized that the curvature and spasms were neuronal in origin. To test this hypothesis, we assessed the ability of the sodium channel antagonist MS-222 and the gamma-aminobutyric acid (GABA)A receptor antagonist diazepam to ameliorate these lesions. We chose these two treatments based on their use as treatments for pyrethroid intoxication in mammals (Gammon et al., 1982). Cotreatment of zebrafish with MS-222 and 25 μg/l deltamethrin at 48 hpf caused a decrease in the occurrence of both spasms and body curvature (Fig. 8) compared to animals exposed to deltamethrin without MS-222. This decrease was significantly different (p < 0.001) at 72 hpf for all doses of MS-222 tested. To determine whether the amelioration of spasms associated with MS-222 cotreatment was due merely to a general anesthetic effect, several fish from the highest dose (0.006%) MS-222 control group were tapped on their tails with the blunt edge of a needle and observed under the microscope. All the fish sampled were able to respond to the touch stimulus by swimming normally. This observation provides evidence that the absence of spasms in the deltamethrin-exposed groups was not the result of a nonspecific anesthetic effect. Furthermore, the data demonstrate that these effects are the result of pyrethroid interactions with sodium channels, similar to the accepted mechanism in mammals.
FIG. 8.
Sodium channel blockade with MS-222 prevents spasms and curvature induced by developmental deltamethrin exposure. Zebrafish were exposed to deltamethrin (25 μg/l) from 3 hpf until 72 hpf. Embryos were dechorionated at 48 hpf and dosed with MS-222. Data shown are from observations at 72 hpf or 24 h after MS-222 dosing. Significant differences (p < 0.001) from control are indicated with asterisks. Control animals exposed to MS-222 without deltamethrin demonstrated no lesions and, at the highest dose tested, were still able to respond to a touch stimulus at 72 hpf. (Right) Representative pictures taken at 72 hpf, at which point, fish had been coexposed to deltamethrin (25 μg/l) and MS-222 (or control) for 24 h. Each experiment comprised 20–25 fish per treatment group, and each experiment was repeated at least three times. DM, deltamethrin.
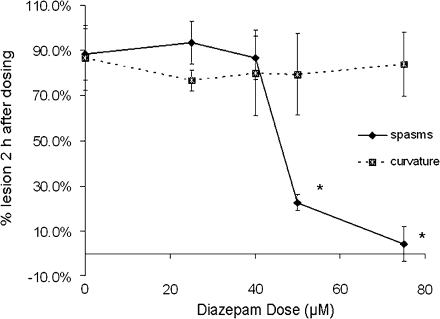
Diazepam is clinically used to ameliorate convulsions associated with acute pyrethroid intoxication and has been demonstrated to reduce convulsion in rodents exposed to pyrethroids (Gammon et al., 1982). Therefore, we sought to determine whether it could ameliorate the spasms observed in zebrafish developmentally exposed to deltamethrin. Embryos exposed to 25 μg/l deltamethrin beginning at 3 hpf exhibited spasms and body curvature. The incidence of spasms was significantly lowered (p < 0.001) by 50 and 75μM doses at 2 h after dosing (Fig. 9). None of the diazepam doses tested ameliorated the body curvature observed in the deltamethrin-treated animals. Taken in concert, these data suggest that exposure of zebrafish embryos produces neurotoxicity that appears to be similar to that observed in mammals.
FIG. 9.
The GABAA receptor antagonist diazepam ameliorates spasms but not body axis curvature induced by developmental deltamethrin exposure. Animals were exposed to 25 μg/l deltamethrin beginning at 3 hpf. Animals were dosed with diazepam at 72 hpf and reobserved 2 h after dosing. Significant differences (p < 0.001) are indicated by asterisks. Diazepam had no significant effect on the incidence of body axis curvature at any of the doses tested. Each experiment comprised 20–25 fish per treatment group, and each experiment was repeated at least three times.
DISCUSSION
The neurotoxicity of pyrethroid insecticides has been thoroughly studied in adult rodents, both in terms of acute lethality and behavioral endpoints (Bradberry et al., 2005; Soderlund et al., 2002; Wolansky and Harrill, 2008). However, less research has focused on examining the consequences of developmental pyrethroid exposure (reviewed in Shafer et al., 2005). Part of the reason for the lack of developmental toxicity studies with pyrethroids may stem from the time and cost associated with conducting these studies in standard rodent models. Zebrafish present a less-expensive less time-consuming alternative to rodent models, and the use of zebrafish as a model for the study of developmental toxicity has been steadily increasing (Parng et al., 2007). In addition, zebrafish have been demonstrated to be predictive models for evaluating the teratogenic and neurotoxic effects of many known toxins (McGrath and Li, 2008). Here, we present evidence that the zebrafish appear to be an ideal model to study the developmental neurotoxicity of pyrethroid insecticides, both in terms of standard toxicological parameters and mechanistic studies.
The toxicity of pyrethroids to developing zebrafish embryos has not been studied extensively. However, our data appear to be similar to that reported in other laboratories. For example, a recent study determined λ-cyhalothrin for which the calculated LC50 value for a 96-h exposure was 130 μg/l (Xu et al., 2008), which is similar to the LC50 of 110 μg/l found for a 6-day exposure in the present study. The other pyrethroids used in this study produce lethality in developing zebrafish, with a rank potency similar to that observed in rodents (Soderlund et al., 2002). Deltamethrin was found to be the most toxic, followed by cypermethrin, λ-cyhalothrin, bifenthrin, permethrin, and then finally resmethrin, which was only weakly toxic. A ranking of the same compounds according to their toxicities in mature rats would list bifenthrin as the most toxic, followed closely by λ-cyhalothrin, and then deltamethrin, cypermethrin, permethrin, and resmethrin (Soderlund et al., 2002; Wolansky and Harrill, 2008). Thus, with the exception of bifenthrin, the toxicity data in zebrafish embryos are similar to that observed in rodents, with the type II pyrethroids being more potent.
One potential factor that could have affected the relative toxicity of bifenthrin to zebrafish compared to rats is that different sodium channel isoforms and channel composition are known to vary in their susceptibility to pyrethroid action (Meacham et al., 2008; Soderlund et al., 2002). Thus, zebrafish might respond differently to pyrethroids because the distribution or function of their sodium channels differs from that of rats. However, Novak et al. (2006) have shown that the expression patterns of voltage-gated sodium channel genes in zebrafish are surprisingly similar to those of mammals. A more likely explanation for the observed differences in relative toxicities is based on the racemic mixture of bifenthrin utilized in our studies. The toxicity of bifenthrin has been demonstrated to depend on the relative proportion of the (+) and (−) enantiomers, with the (−) enantiomer being an order of magnitude more potent than the (+) and the racemate being intermediate (Liu et al., 2005). Thus, it is possible that our data differ from the reported rodent data based on the composition of the compound used. Other important variables that affect mammalian toxicity values include dosing vehicle and route of exposure. Indeed, Wolansky et al. (2006) demonstrated that dosing vehicle and volume significantly affected the toxicity of bifenthrin. Given that the rest of our data closely follow what has been observed in rodents, it is likely that the difference in bifenthrin potency in zebrafish result from one or more of the discussed variables.
We also observed cypermethrin to be more toxic in developing zebrafish than in adult rats. A likely explanation for the observed differences in relative toxicities is supported by findings that the developing mammal is more susceptible to pyrethroid toxicity than the adult counterpart and more importantly that this increased susceptibility is more pronounced for type II pyrethroids than for type I (Bradberry et al., 2005; Sheets, 2000). These differences are likely the result of the pyrethroid-specific detoxification by cytochrome P450 and carboxylesterase enzymes, which are present at lower levels in the developing mammal (Atterberry et al., 1997; Ross et al., 2006). Although there are many fewer studies exploring the development of P450 and carboxylesterase enzymes in zebrafish (Kuster, 2005), it is likely that the developmental status of these enzymes play a role in susceptibility of zebrafish to the various pyrethroids used in this study. Future studies on both the developmental profiles of carboxylesterases, P450, and age-dependent susceptibility of zebrafish are required to answer these questions.
Pyrethroids are typically considered only mild or nonteratogenic compounds (ATSDR, 2003). However, the present study found that certain doses of permethrin and deltamethrin were capable of eliciting minor alterations cartilaginous structures of the developing zebrafish. Specifically, the average LJL was significantly lower (20–22%) than control in the 200 and 300 μg/l permethrin groups and in the 50 μg/l deltamethrin group. However, the doses required to elicit these effects were extremely high, even approaching the LC50 value for deltamethrin. Kavlock et al. (1979) found no evidence of fetotoxicity or teratogenicity resulting from exposure of rat or mouse dams to deltamethrin. However, it should be noted that the dosing method for teratology studies on rodents is fundamentally different from the exposure study reported here in that compounds are administered to the developing rodent via the pregnant female rather than direct exposure of the offspring. Dosing the pregnant female diminishes the amount of pyrethroid that reaches the embryo by providing an opportunity for maternal enzymes to detoxify the pyrethroid, which makes it difficult to equate doses used in rodent teratology studies to the doses employed here. However, the developing zebrafish embryos were likely exposed to significantly higher doses of pyrethroid than the highest delivered doses to rodents in utero since there is no opportunity for maternal detoxication. In light of these differences, the fact that zebrafish only experienced teratogenic effects from very high levels of pyrethroid exposure further supports the hypothesis that zebrafish may be an appropriate model to study pyrethroid toxicity.
In addition to the mild teratogenic effects (small reductions in jaw length) observed following high-dose exposure, body axis curvature was associated with most of the pyrethroids tested, with type II compounds producing the greatest effects. Curvature of the body axis could be interpreted as a sign of teratogenicity, although further experiments discussed later suggest otherwise. We also observed spasms in zebrafish exposed to both type I and type II pyrethroids. It is well established that acute exposure of rats to type I pyrethroids is characterized by the tremor (T) syndrome, whereas type II pyrethroids cause the choreoathetosis with salivation syndrome (Bradberry et al., 2005; Soderlund et al., 2002). Although spasms were observed in both type I- and type II-treated fish, the appearances of the episodes differed. Exposure to type I pyrethroids elicited a spastic response reminiscent of tremors, with little to no time separating episodes of spasms. In contrast, the type II pyrethroids tended to produce more intense and prolonged contractions, often seen as an arching of the back or a curling up of the tail.
In an attempt to determine the mechanistic basis for the spasms and curvature observed in zebrafish exposed to pyrethroids, we sought to determine the ability of diazepam and the sodium channel blocker and anesthetic MS-222 to ameliorate these effects. For these two experiments, we chose to focus on deltamethrin because it elicited the strongest effects on both spasms and body axis curvature. Benzodiazepines like diazepam are known to modify GABAA receptor activity in the brain and periphery in a positive allosteric manner and enhance the inhibitory effect of the GABA neurotransmitter (McLean and Macdonald, 1988). In this manner, sustained repetitive firing of neurons, such as might be expected following pyrethroid exposure, would be reduced. Although pyrethroids primarily target sodium channels, it has been shown that deltamethrin can inhibit GABA-dependent chloride channels at high doses (Bloomquist et al., 1986; Crofton et al., 1987; Lawrence and Casida, 1983), in which case diazepam’s role in potentiating the effects of GABA may work by blocking the interactions of deltamethrin with GABAA receptors. Diazepam has also been demonstrated to delay the appearance of toxicity in mice exposed to deltamethrin (Gammon et al., 1982) and is used clinically in the treatment of pyrethroid intoxication. The finding that diazepam cotreatment effectively ameliorates spasms in zebrafish exposed to deltamethrin provides further support that zebrafish may indeed be a good model for determination of pyrethroid effects in developing animals.
Although diazepam effectively reduced the spasms observed in zebrafish exposed to deltamethrin, the amelioration of both spasms and body axis curvature by cotreatment with the sodium channel blocker MS-222 suggests that the mechanism for the occurrence of these lesions is primarily through deltamethrin's action on sodium channels. Furthermore, the finding that MS-222 prevents the occurrence of body curvature provides evidence that body curvature is not a morphological effect on the spine or trunk musculature but instead has a neurological basis via the modification of sodium channels by deltamethrin. Electrophysiological studies have demonstrated that type I pyrethroids, such as permethrin, produce repetitive firing, while type II compounds can produce a depolarizing block (Narahashi, 2000; Soderlund et al., 2002). Based on the data demonstrating that the type II pyrethroids produce much greater body axis curvature than the type I pyrethroids, it is tempting to conclude that the curvature observed is the result of a depolarizing block following the arching observed during the spasms. The ability of MS-222 to prevent the curvature would seem to support this, but future studies are needed to determine the electrophysiological effects of pyrethroids on zebrafish sodium channel function to confirm this hypothesis.
In general, the present study demonstrates that zebrafish embryos exposed to pyrethroid insecticides demonstrate similar signs of toxicity to those seen in rat and mouse models. Specifically, the relative toxicities of various type I and type II pyrethroids are similar to the relative toxicities seen in rodents allowing for adjustments for differences in developmental sensitivity. In addition, the absence of teratogenic effects at all but the highest doses of permethrin and deltamethrin, even at doses which caused overt signs of poisoning-like spasms, parallels the lack of teratogenicity observed in rodent studies. The incidence of spasms in zebrafish exposed to pyrethroids further supports the appropriateness of this model because the effects are reminiscent of the tremors caused by pyrethroid poisoning in mammals. These findings together demonstrate that the developing zebrafish may be not only a convenient but also an appropriate model for the toxicological study of pyrethroid insecticides.
FUNDING
National Institutes of Health's National Institute of Environmental Health Sciences (P30ES005022, R01ES015991 [J.R.R.], and R21ES013828 to [J.R.R.]).
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Pyrethrins and Pyrethroids. 2003. [PubMed] [Google Scholar]
- Atterberry TT, Burnett WT, Chambers JE. Age-related differences in parathion and chlorpyrifos toxicity in male rats: target and nontarget esterase sensitivity and cytochrome P450-mediated metabolism. Toxicol. Appl. Pharmacol. 1997;147:411–418. doi: 10.1006/taap.1997.8303. [DOI] [PubMed] [Google Scholar]
- Aziz MH, Agrawal AK, Adhami VM, Shukla Y, Seth PK. Neurodevelopmental consequences of gestational exposure (GD14-GD20) to low dose deltamethrin in rats. Neurosci. Lett. 2001;300:161–165. doi: 10.1016/s0304-3940(01)01543-9. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, Landrigan PJ, Wolff MS. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ. Health Perspect. 2003;111:79–84. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist JR, Adams PM, Soderlund DM. Inhibition of gamma-aminobutyric acid-stimulated chloride flux in mouse brain vesicles by polychlorocycloalkane and pyrethroid insecticides. Neurotoxicology. 1986;7:11–20. [PubMed] [Google Scholar]
- Bradberry SM, Cage SA, Proudfoot AT, Vale JA. Poisoning due to pyrethroids. Toxicol. Rev. 2005;24:93–106. doi: 10.2165/00139709-200524020-00003. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Golden age of insecticide research: past, present, or future? Annu. Rev. Entomol. 1998;43:1–16. doi: 10.1146/annurev.ento.43.1.1. [DOI] [PubMed] [Google Scholar]
- Cook LW, Paradise CJ, Lom B. The pesticide malathion reduces survival and growth in developing zebrafish. Environ. Toxicol. Chem. 2005;24:1745–1750. doi: 10.1897/04-331r.1. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Reiter LW, Mailman RB. Pyrethroid insecticides and radioligand displacement from the GABA receptor chloride ionophore complex. Toxicol. Lett. 1987;35:183–190. doi: 10.1016/0378-4274(87)90205-0. [DOI] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol. Teratol. 2009 doi: 10.1016/j.ntt.2009.02.005. Doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon DW, Lawrence LJ, Casida JE. Pyrethroid toxicology: protective effects of diazepam and phenobarbital in the mouse and the cockroach. Toxicol. Appl. Pharmacol. 1982;66:290–296. doi: 10.1016/0041-008x(82)90294-0. [DOI] [PubMed] [Google Scholar]
- Haendel MA, Tilton F, Bailey GS, Tanguay RL. Developmental toxicity of the dithiocarbamate pesticide sodium metam in zebrafish. Toxicol. Sci. 2004;81:390–400. doi: 10.1093/toxsci/kfh202. [DOI] [PubMed] [Google Scholar]
- Hillegass JM, Villano CM, Cooper KR, White LA. Glucocorticoids alter craniofacial development and increase expression and activity of matrix metalloproteinases in developing zebrafish (Danio rerio) Toxicol. Sci. 2008;102:413–424. doi: 10.1093/toxsci/kfn010. [DOI] [PubMed] [Google Scholar]
- Husain R, Malaviya M, Seth PK, Husain R. Differential responses of regional brain polyamines following in utero exposure to synthetic pyrethroid insecticides: a preliminary report. Bull. Environ. Contam. Toxicol. 1992;49:402–409. doi: 10.1007/BF01239644. [DOI] [PubMed] [Google Scholar]
- Kavlock R, Chernoff N, Baron R, Linder R, Rogers E, Carver B, Dilley J, Simmon V. Toxicity studies with decamethrin, a synthetic pyrethroid insecticide. J. Environ. Pathol. Toxicol. 1979;2:751–765. [PubMed] [Google Scholar]
- Kuster E. Cholin- and carboxylesterase activities in developing zebrafish embryos (Danio rerio) and their potential use for insecticide hazard assessment. Aquat. Toxicol. 2005;75:76–85. doi: 10.1016/j.aquatox.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Lawrence LJ, Casida JE. Stereospecific action of pyrethroid insecticides on the gamma-aminobutyric acid receptor-ionophore complex. Science. 1983;221:1399–1401. doi: 10.1126/science.6310756. [DOI] [PubMed] [Google Scholar]
- Lazarini CA, Florio JC, Lemonica IP, Bernardi MM. Effects of prenatal exposure to deltamethrin on forced swimming behavior, motor activity, and striatal dopamine levels in male and female rats. Neurotoxicol. Teratol. 2001;23:665–673. doi: 10.1016/s0892-0362(01)00170-2. [DOI] [PubMed] [Google Scholar]
- Linney E, Upchurch L, Donerly S. Zebrafish as a neurotoxicological model. Neurotoxicol. Teratol. 2004;26:709–718. doi: 10.1016/j.ntt.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Liu W, Gan Y, Schlenk D, Jury WA. Enantioselectivity in environmental safety of current chiral insecticides. Proc. Natl. Acad. Sci. U.S.A. 2005;102:701–706. doi: 10.1073/pnas.0408847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Barr DB, Pearson M, Bartell S, Bravo R. A longitudinal approach to assessing urban and suburban children’s exposure to pyrethroid pesticides. Environ. Health Perspect. 2006;114:1419–1423. doi: 10.1289/ehp.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Barr DB, Pearson MA, Walker LA, Bravo R. The attribution of urban and suburban children's exposure to synthetic pyrethroid insecticides: a longitudinal assessment. J. Expo. Sci. Environ. Epidemiol. 2009;19:69–78. doi: 10.1038/jes.2008.49. [DOI] [PubMed] [Google Scholar]
- McGrath P, Li CQ. Zebrafish: a predictive model for assessing drug-induced toxicity. Drug Discov. Today. 2008;13:394–401. doi: 10.1016/j.drudis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- McLean MJ, Macdonald RL. Benzodiazepines, but not beta carbolines, limit high frequency repetitive firing of action potentials of spinal cord neurons in cell culture. J. Pharmacol. Exp. Ther. 1988;244:789–795. [PubMed] [Google Scholar]
- Meacham CA, Brodfuehrer PD, Watkins JA, Shafer TJ. Developmentally-regulated sodium channel subunits are differentially sensitive to alpha-cyano containing pyrethroids. Toxicol. Appl. Pharmacol. 2008;231:273–281. doi: 10.1016/j.taap.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Miller LC, Tainter ML. Graphical method for determination of LD50. Proc. Soc. Exp. Biol. Med. 1944;57:261–264. [Google Scholar]
- Morgan MK, Sheldon LS, Croghan CW, Jones PA, Chuang JC, Wilson NK. An observational study of 127 preschool children at their homes and daycare centers in Ohio: environmental pathways to cis- and trans-permethrin exposure. Environ. Res. 2007;104:266–274. doi: 10.1016/j.envres.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Neuroreceptors and ion channels as the basis for drug action: past, present, and future. J. Pharmacol. Exp. Ther. 2000;294:1–26. [PubMed] [Google Scholar]
- Novak AE, Taylor AD, Pineda RH, Lasda EL, Wright MA, Ribera AB. Embryonic and larval expression of zebrafish voltage-gated sodium channel alpha-subunit genes. Dev. Dyn. 2006;235:1962–1973. doi: 10.1002/dvdy.20811. [DOI] [PubMed] [Google Scholar]
- Parng C, Roy NM, Ton C, Lin Y, McGrath P. Neurotoxicity assessment using zebrafish. J. Pharmacol. Toxicol. Methods. 2007;55:103–112. doi: 10.1016/j.vascn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Nass R, Boyd WA, Freedman JH, Dong K, Narahashi T. Use of non-mammalian alternative models for neurotoxicological study. Neurotoxicology. 2008;29:546–555. doi: 10.1016/j.neuro.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presibella KM, Kita DH, Carneiro CB, Andrade AJ, Dalsenter PR. Reproductive evaluation of two pesticides combined (deltamethrin and endosulfan) in female rats. Reprod. Toxicol. 2005;20:95–101. doi: 10.1016/j.reprotox.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Ray DE, Forshaw PJ. Pyrethroid insecticides: poisoning syndromes, synergies, and therapy. J. Toxicol. Clin. Toxicol. 2000;38:95–101. doi: 10.1081/clt-100100922. [DOI] [PubMed] [Google Scholar]
- Ross MK, Borazjani A, Edwards CC, Potter PM. Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases. Biochem. Pharmacol. 2006;71:657–669. doi: 10.1016/j.bcp.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ. Health Perspect. 2005;113:123–136. doi: 10.1289/ehp.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets LP. A consideration of age-dependent differences in susceptibility to organophosphorus and pyrethroid insecticides. Neurotoxicology. 2000;21:57–63. [PubMed] [Google Scholar]
- Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology. 2002;171:3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- Stehr CM, Linbo TL, Incardona JP, Scholz NL. The developmental neurotoxicity of fipronil: notochord degeneration and locomotor defects in zebrafish embryos and larvae. Toxicol. Sci. 2006;92:270–278. doi: 10.1093/toxsci/kfj185. [DOI] [PubMed] [Google Scholar]
- Tilton F, La Du JK, Vue M, Alzarban N, Tanguay RL. Dithiocarbamates have a common toxic effect on zebrafish body axis formation. Toxicol. Appl. Pharmacol. 2006;216:55–68. doi: 10.1016/j.taap.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Camann DE, Kinney PL, Reyes A, Ramirez J, Dietrich J, Diaz D, Holmes D, Perera FP. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ. Health Perspect. 2002;110:507–514. doi: 10.1289/ehp.02110507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MK, Rundle A, Holmes D, Reyes M, Hoepner LA, Barr DB, Camann DE, Perera FP, Whyatt RM. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000-2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ. Health Perspect. 2008;116:1681–1688. doi: 10.1289/ehp.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolansky MJ, Harrill JA. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol. Teratol. 2008;30:55–78. doi: 10.1016/j.ntt.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Wolansky MJ, Jennings G, Crofton K. Relative potencies for acute effects of pyrethroids on motor function in rats. Toxicol. Sci. 2006;89:271–277. doi: 10.1093/toxsci/kfj020. [DOI] [PubMed] [Google Scholar]
- Xu C, Wang J, Liu W, Daniel Sheng G, Tu Y, Ma Y. Separation and aquatic toxicity of enantiomers of the pyrethroid insecticide lambda-cyhalothrin. Environ. Toxicol. Chem. 2008;27:174–181. doi: 10.1897/07-134.1. [DOI] [PubMed] [Google Scholar]