Abstract
We investigated the geographic distribution and the relationship with neighborhood wealth of underweight and overweight in India. Using multilevel modeling techniques, we calculated state-specific smoothed shrunken state residuals of overweight and underweight, neighborhood and state variation of nutritional status, and the relationships between neighborhood wealth and nutritional status of 76,681 women living in 3204 neighborhoods in 26 Indian states. We found a substantial variation in overweight and underweight at the neighborhood and state levels, net of what could be attributed to individual-level factors. Neighborhood wealth was associated with increased levels of overweight and decreased levels of underweight, and was found to modify the relationship between personal living standard and nutritional status. These findings suggest that interventions to address the double burden of undernutrition and overnutrition in India must take into account state and neighborhood characteristics in order to be successful.
Keywords: Overweight, Underweight, Context, Neighborhood, Socioeconomic status, India
1. Introduction
Nutritional status is an important influence on health, with poor nutrition accounting for 12% of all deaths and 16% of all disability-adjusted life years lost globally (Murray and Lopez, 1997). While malnutrition encompasses a number of maladies, including micronutrient deficiencies, the most prevalent nutrition-related outcomes are those related to total energy intake, namely undernutrition, defined as having a body mass index (BMI) of less than 18.5 kg/m2, and overnutrition, defined as having a BMI greater than or equal to 25 kg/m2 (Shetty and James, 1994; WHO, 2003, 2000). Nutritional status, however, is unevenly distributed both within and between countries (Mendez et al., 2005; Monteiro et al., 2004). In developed countries, for instance in the United States, overweight and obesity disproportionately impact those having low incomes, having low education levels, and belonging to minority race/ethnicity (Drewnowski and Specter, 2004; USDHHS, 2001; Mokdad et al., 2003). In developing countries, such as India, nutritional status is related to levels of education, standard of living, and social status such that undernutrition is associated with low individual socioeconomic status (SES) and overnutrition is associated with high SES (Shukla et al., 2002; Subramanian and Davey Smith, 2006; Griffiths and Bentley, 2005; Osmani and Sen, 2003).
Although the social distribution of nutritional status is relatively well-described, the geographic distribution of nutritional status has received less empirical attention. In developed nations, the role of macro residential context, in recent years, has been implicated as a potential determinant of individual health status (Kawachi and Berkman, 2003; Kawachi and Subramanian, 2007), including nutritional status. For instance in the United States, southeastern and north-central regions of the country exhibit higher levels of obesity than the northeast or west even after accounting for individual-level socioeconomic and demographic characteristics (Mokdad et al., 2003, 1999). At the neighborhood level, obesity in the United States is associated with living in neighborhoods that have a high prevalence of residents living in poverty, even after accounting for one’s own SES (Mobley et al., 2006; van Lenthe and Mackenbach, 2002; Do et al., 2007). While this line of research is relatively new, initial investigations suggest that this relationship may be due in part to the lack of healthy food options in these neighborhoods that promote “obesogenic” eating patterns (Cummins and Macintyre, 2006; Ulijaszek, 2007) or to urban design characteristics that discourage physical activity (Booth et al., 2005).
The distinction between “contextual” and “individual” influences is complex. Underweight, overweight, and BMI are conceptualized as a function of individual characteristics since contextual characteristics cannot affect health unless the individual interacts with the environment in some way. To that extent any place variation is seen to be an artifact of the distribution of these individual factors (Subramanian, 2004; Subramanian et al., 2007a, 2003). Moreover, the individual influences that explain a contextual effect are simply mediators of the true effect of the environment on health (Subramanian, 2004; Subramanian et al., 2007a, 2003). Adding to this complexity is the fact that individual characteristics may interact with contextual characteristics such that a particular environment may be detrimental to the health of some individuals but benign or even beneficial to others. In this study, we test the extent to which neighborhoods and states influence nutritional status independent of individual factors.
While investigations pertaining to the role of neighborhood environment are emerging in the context of developed countries, we are not aware of any corresponding research in the context of developing countries that has systematically investigated the extent to which there are independent influences of neighborhood environments on nutritional status. To address this shortcoming in the existing literature we use a large, nationally representative dataset of women in India to investigate the geographic distribution of nutritional status, as measured by BMI (WHO, 2004, 1995), at the levels of neighborhoods and states, after accounting for individual-level factors associated with nutritional status. Additionally, to directly assess the role of states and neighborhoods in influencing the twin burden of over-and undernutrition in India, we also analyze the geographic patterning of the risk of being overweight and underweight.
2. Methods
2.1. Data
We utilized the 1998–1999 Indian National Family Health Survey (NFHS; available at http://www.nfhsindia.org), a nationally representative cross-sectional study of 92,447 households administered to investigate maternal and child health outcomes (IIPS and ORC-Macro, 2000).1 Trained data-collectors interviewed an adult member in each selected household to obtain socioeconomic and demographic information about the household and its family members, obtaining a household response rate of 98%. From these households, the data-collectors interviewed 90,303 ever-married women aged 15–49 in face-to-face interviews obtaining a response rate of 96%. We excluded 6508 women who were pregnant during the survey and 6200 women for whom plausible anthropometric measurements were not obtained. An additional 914 women who were missing information for covariates considered in this analysis were excluded, yielding a final analytic sample of 76,681 women. These women were located in 3204 primary sampling units in the 26 Indian states. In rural areas, these primary sampling units were villages or village clusters which are autonomous self-governing political units. In urban areas the primary sampling units were census enumeration districts which were created to be as demographically homogenous as possible. As political autonomy and demographic homogeneity provide a theoretical foundation for investigating the effect of these areas above and beyond that provided by geographic proximity, for the purposes of this study we operationalized the primary sampling unit as one realization of an individual’s neighborhood context.2
2.2. Outcome
Women were weighed using a solar-powered scale accurate to within 100 g, and height was measured using an adjustable measuring board designed to provide measurements in a field situation accurate to within 1 mm (IIPS and ORC-Macro, 2000). Body mass index was calculated in terms of kilograms per meter squared. Clinically relevant categories for underweight, normal weight, and overweight were created with the following respective cut-off points: <18.5, 18.5–24.9, and ≥25 kg/m2. While the World Health Organization (WHO) suggests that these cut-off points for nutritional status may have different clinical implications among Asian populations than they do among Caucasian populations, the organization asserts that the recommended cut-off points still provide important guidelines in these populations (WHO, 2004). Additionally, the results presented in this study do not substantially change if we adopt a more detailed classification of BMI as used in recent empirical studies on India (Subramanian and Davey Smith, 2006).
2.3. Individual-level covariates
For the multivariable analysis, we included several demographic, socioeconomic, and behavioral variables that included urban-rural status, age, religion, marital status, social caste, standard of living, employment status, education, number of children birthed, affliction by a major illness, use of oral contraceptives, tobacco chewing, tobacco smoking, and alcohol drinking (Table 1). Information from the 1991 Indian National Census was used to create categories defining whether each neighborhood was in an urban area of over one million people (large city), an urban area of between 100,000 and one million people (small city), an urban area of less than 100,000 people (town), or a rural area (village). Rural areas are defined as having at least 1 of 3 characteristics: (1) fewer than 5000 residents, (2) population density less than 1000 per square mile, or (3) at least 25% of the adult male population being employed in agriculture. Age ranged from 15 to 49 and was specified in five year categories. Religion was grouped into the categories of Hindu, Muslim, Christian, Sikh, or other. Marital status was classified as married, widowed, or divorced/separated. Caste was based on the identification of the head of the household as belonging to a scheduled caste, scheduled tribe, other backward class, or the general class, classifications which have been discussed elsewhere (Subramanian et al., 2006). Briefly, scheduled castes are those consisting of members in the lowest level of the caste system that have suffered the greatest burden of social and economic deprivation. Scheduled tribes consist of approximately 700 officially recognized social groups that are historically characterized by their geographic isolation and limited social and economic interaction with the rest of India. Other backward class is a legislatively defined group representing those who have historically suffered significant social deprivation, but not as severe as that endured by scheduled castes and tribes. The general class is thus a default group encompassing those who are not members of historically marginalized castes. Standard of living was defined in terms of living environment, and material possessions is a reliable and valid measure of household material well-being or wealth (Filmer and Pritchett, 2001). Each person was assigned a standard of living score that was based on a linear combination of the scores for 78 different household characteristics, such as the quality of the home, the type of fuel used for cooking, and the ownership of a bicycle or television, that were weighted according to a factor analysis procedure and standardized with a mean of 0 and a standard deviation of 1 (Rutstein and Johnson, 2004; Gwatkin et al., 2000). The analytic models used quintiles of these weighted scores. Employment was classified according to whether the women was not working, performing unpaid work, or working for pay in a manual, non-manual, or agricultural profession. Educational attainment of the women was specified as a categorical variable with each category representing significant milestones in the formal education system: 0 years, 1–5 years, 6–8 years, 9–10 years, 11–12 years, or 13 or more years. Number of children born included all live births, regardless of whether or not the children were alive at the time of the survey, and was categorized as 0, 1, 2, 3, or 4 or more births. A person was considered to be suffering from a major illness if they reported suffering from tuberculosis at the time of the survey, from malaria in the previous 3 months, or from jaundice in the previous 12 months. A woman was considered to be using oral contraceptives, chewing tobacco, smoked tobacco, or alcohol if she reported engaging in these activities at the time of the survey.
Table 1.
Descriptive statistics of the sample
| Variable | Subjects | %a | Underweight | %b | Overweight | %c | Mean BMI | S.D. |
|---|---|---|---|---|---|---|---|---|
| Location | ||||||||
| Village | 52415 | 68.4 | 19560 | 37.3 | 3457 | 6.6 | 19.8 | 3.2 |
| Town | 10737 | 14.0 | 2557 | 23.8 | 2157 | 20.1 | 21.7 | 4.2 |
| Small city | 4964 | 6.5 | 1080 | 21.8 | 1209 | 24.4 | 22.2 | 4.6 |
| Large city | 8565 | 11.2 | 1393 | 16.3 | 2584 | 30.2 | 23.0 | 4.7 |
| Age | ||||||||
| 15–19 | 4919 | 6.4 | 1938 | 39.4 | 80 | 1.6 | 19.3 | 2.3 |
| 20–24 | 12200 | 15.9 | 4755 | 39.0 | 442 | 3.6 | 19.5 | 2.7 |
| 25–29 | 15230 | 19.9 | 5363 | 35.2 | 1229 | 8.1 | 20.1 | 3.3 |
| 30–34 | 14037 | 18.3 | 4296 | 30.6 | 1876 | 13.4 | 20.8 | 3.9 |
| 35–39 | 12441 | 16.2 | 3414 | 27.4 | 2141 | 17.2 | 21.3 | 4.2 |
| 40–44 | 10035 | 13.1 | 2766 | 27.6 | 1978 | 19.7 | 21.5 | 4.4 |
| 45–49 | 7819 | 10.2 | 2058 | 26.3 | 1661 | 21.2 | 21.7 | 4.5 |
| Religion | ||||||||
| Hindu | 59840 | 78.0 | 20393 | 34.1 | 6731 | 11.2 | 20.4 | 3.7 |
| Muslim | 8750 | 11.4 | 2663 | 30.4 | 1230 | 14.1 | 20.8 | 4.0 |
| Christian | 4280 | 5.6 | 860 | 20.1 | 576 | 13.5 | 21.3 | 3.6 |
| Sikh | 1875 | 2.5 | 316 | 16.9 | 578 | 30.8 | 23.0 | 4.8 |
| Other/missing religion | 1936 | 2.5 | 358 | 18.5 | 292 | 15.1 | 21.5 | 3.7 |
| Caste | ||||||||
| General | 31866 | 41.6 | 8476 | 26.6 | 5765 | 18.1 | 21.4 | 4.2 |
| Scheduled caste | 22572 | 29.4 | 7744 | 34.3 | 2265 | 7.2 | 20.3 | 3.6 |
| Scheduled tribe | 9220 | 12.0 | 3158 | 34.3 | 444 | 4.8 | 19.8 | 2.9 |
| Other backward class | 13023 | 17.0 | 5212 | 40.0 | 933 | 10.0 | 19.8 | 3.3 |
| Marital status | ||||||||
| Married | 71770 | 93.6 | 22890 | 31.9 | 8853 | 12.3 | 20.6 | 3.8 |
| Widowed | 3294 | 4.3 | 1119 | 34.0 | 407 | 12.4 | 20.5 | 3.9 |
| Divorced/separated | 1617 | 2.1 | 581 | 35.9 | 147 | 9.1 | 20.2 | 3.5 |
| Education | ||||||||
| No formal schooling | 38149 | 49.8 | 15117 | 39.6 | 2231 | 5.8 | 19.7 | 3.1 |
| 1–5 years | 12910 | 16.8 | 4154 | 32.2 | 1515 | 11.7 | 20.6 | 3.8 |
| 6–8 years | 9621 | 12.6 | 2522 | 26.2 | 1545 | 16.1 | 21.3 | 4.0 |
| 9–10 years | 8951 | 11.7 | 1847 | 20.6 | 1938 | 21.7 | 22.0 | 4.3 |
| 11–12 years | 3269 | 4.3 | 539 | 16.5 | 827 | 25.3 | 22.6 | 4.4 |
| 13 or more years | 3781 | 4.9 | 411 | 10.9 | 1351 | 35.7 | 23.7 | 4.5 |
| Employment | ||||||||
| Not working | 47753 | 62.3 | 13890 | 29.1 | 7021 | 14.7 | 21.0 | 4.0 |
| Unpaid | 7817 | 10.2 | 2933 | 37.5 | 429 | 5.5 | 19.8 | 3.0 |
| Paid non-manual | 3773 | 4.9 | 616 | 16.3 | 977 | 25.9 | 22.6 | 4.3 |
| Paid agricultural | 10502 | 13.7 | 4714 | 44.9 | 288 | 2.7 | 19.1 | 2.7 |
| Paid manual | 6836 | 8.9 | 2437 | 35.7 | 692 | 10.1 | 20.3 | 3.6 |
| Living standard | ||||||||
| 1st (lowest) quintile | 14113 | 18.4 | 6734 | 47.7 | 226 | 1.6 | 18.9 | 2.4 |
| 2nd quintile | 15080 | 19.7 | 6414 | 42.5 | 421 | 2.8 | 19.3 | 2.7 |
| 3rd quintile | 15685 | 20.5 | 5539 | 35.3 | 1016 | 6.5 | 19.9 | 3.1 |
| 4th quintile | 16024 | 20.9 | 3978 | 24.8 | 2355 | 14.7 | 21.2 | 3.8 |
| 5th (highest) quintile | 15779 | 20.6 | 1925 | 12.2 | 5389 | 34.2 | 23.6 | 4.6 |
| Oral contraceptive use | ||||||||
| No | 74730 | 97.5 | 24074 | 32.2 | 9198 | 12.3 | 20.6 | 3.8 |
| Yes | 1951 | 2.5 | 516 | 26.5 | 209 | 10.7 | 20.8 | 3.4 |
| Children | ||||||||
| 0 | 6554 | 8.6 | 2131 | 32.5 | 424 | 6.5 | 20.1 | 3.1 |
| 1 | 10516 | 13.7 | 3347 | 31.8 | 1079 | 10.3 | 20.4 | 3.5 |
| 2 | 16821 | 21.9 | 4918 | 29.2 | 2732 | 16.2 | 21.1 | 4.1 |
| 3 | 15614 | 20.4 | 4877 | 31.2 | 2243 | 14.4 | 20.9 | 4.1 |
| 4+ | 27176 | 35.4 | 9317 | 34.3 | 2929 | 10.8 | 20.4 | 3.8 |
| Illness | ||||||||
| No | 72313 | 94.3 | 22788 | 31.5 | 9153 | 12.7 | 20.7 | 3.8 |
| Yes | 4368 | 5.7 | 1802 | 41.3 | 254 | 5.8 | 19.6 | 3.3 |
| Tobacco chewing | ||||||||
| No | 67481 | 88.0 | 20998 | 31.1 | 8784 | 13.0 | 20.7 | 3.9 |
| Yes | 9200 | 12.0 | 3592 | 39.0 | 623 | 6.8 | 19.7 | 3.3 |
| Tobacco smoking | ||||||||
| No | 74615 | 97.3 | 23759 | 31.8 | 9312 | 12.5 | 20.6 | 3.8 |
| Yes | 2066 | 2.7 | 831 | 40.2 | 95 | 4.6 | 19.5 | 3.0 |
| Alcohol use | ||||||||
| No | 74487 | 97.1 | 23911 | 32.1 | 9307 | 12.5 | 20.6 | 3.8 |
| Yes | 2194 | 2.9 | 679 | 31.0 | 100 | 4.6 | 20.1 | 2.9 |
| Total | 76681 | 100.0 | 24590 | 32.1 | 9407 | 12.3 | 20.6 | 3.8 |
Indicates the percentage of women in the sample who have that characteristic.
Indicates the percentage of women with that characteristic who are underweight.
Indicates the percentage of women with that characteristic who are overweight.
2.4. Neighborhood-level predictor
We included a neighborhood wealth variable to capture the level of socioeconomic status of people’s residential context. Neighborhood wealth was calculated by averaging the weighted continuous household wealth scores by neighborhood and operationalized in tertiles.
2.5. Statistical analysis
Multilevel models (Goldstein, 2003; Raudenbush and Bryk, 2002), were estimated to distinguish the individual-, neighborhood-, and state-level sources of variation in nutritional status. The substantive and technical relevance of multilevel models to partitioning such variation is well described (Blakely and Subramanian, 2006; Subramanian, 2004; Subramanian et al., 2003). We estimated both linear models using BMI as a continuous measure as well as multinomial logistic models to model the risk of being underweight and overweight.
Three-level models were estimated, with a continuous response, BMI (y), for individual i living in neighborhood j in state k. The outcome yijk was related to a set of individual predictors, X, and a random effect for each level as yijk = β0 + βX + v0k + u0jk + e0ijk. The predictor on the right-hand side of the equation consisted of a fixed part (β0 + βX) estimating the conditional coefficients for the individual compositional variables, and three random effects attributable to individuals (e0ijk), neighborhoods (u0jk), and states (v0k), with each assumed to have an independent and identical distribution and variances (, and , respectively) estimated at each level. We also estimated the smoothed shrunken state residuals3 which represent the difference between the state mean and the national mean after accounting for the variance and the number of individuals in each state (Goldstein, 2003). These residuals were estimated from two models: a null variance component model (with no individual predictor variables in the fixed part), and a variance component model with individual compositional variables specified in the fixed part of the model, as indicated above. The standard errors of these state residuals estimates were used to determine whether the estimates were significantly different from the national mean (Goldstein, 2003). Estimates reported from the linear models are maximum likelihood based using the Iterative Generalized Least Squares algorithm as implemented within MLwiN (Rasbash et al., 2005).
Categorical BMI data was analyzed using multinomial multilevel modeling procedures (Goldstein, 2003). Three-level models were estimated with a multinomial response (y, underweight, normal weight, overweight) for individual i living in neighborhood j in state k. The probability πijk of being in response level s (overweight or underweight) given reference category t (normal weight) was related to a set of categorical predictors, X, and a random effect for each level, by a logit-link function as log . The linear predictor on the right-hand side of the equation consisted of a fixed part estimating the conditional coefficients for the individual variables, and two random intercepts attributable to neighborhoods and states , with each assumed to have an independent and identical distribution and variance estimated for each non-normal weight outcome response level (underweight and overweight) at each higher contextual level (neighborhoods and states). The strength of these variance estimates was compared to their standard errors to determine whether each outcome response category varied at each higher contextual level. Besides the variance estimates for overweight and underweight, each higher contextual level also contained a covariance estimate in the random part which described the relationship between overweight and underweight at the respective contextual level, neighborhoods or states. We also estimated the smoothed shrunken state-level residuals for the log odds of both underweight and overweight from a null variance component model, and from a variance component model with the individual compositional variables specified in the fixed part of the model (Goldstein, 2003). The multilevel logistic multinomial models estimates were based on penalized quasi-likelihood approximation with 2nd order Taylor linearization, as implemented within MLwiN (Goldstein and Rasbash, 1996; Rasbash et al., 2005).
3. Results
3.1. Patterning of BMI, overweight, and underweight
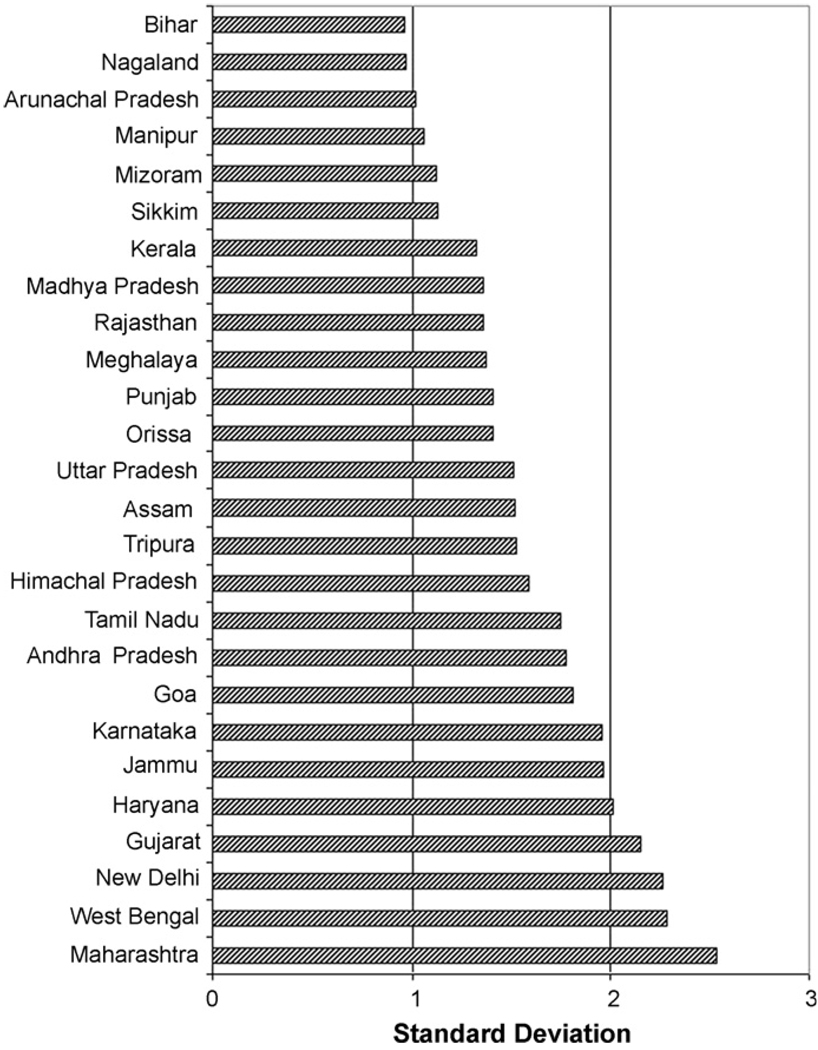
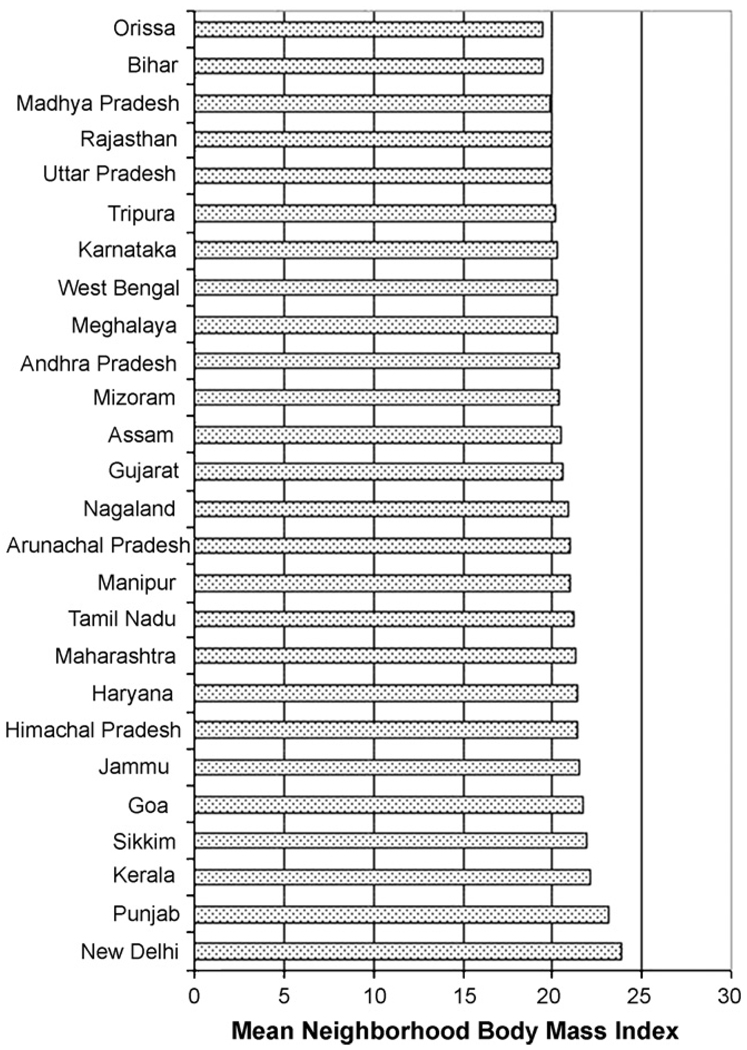
The mean BMI for the sample was 20.6. Approximately, 32% of the women in the sample were underweight and 12.3% were overweight. The variation between states was evident in the wide range of state mean BMI with low BMI in Orissa (19.3) and Bihar (19.4) and high BMI in Punjab (23.1) and New Delhi (23.6). The variation within states was apparent in the substantial standard deviation of BMI within each state, which ranged from a low of 2.3 in Arunachal Pradesh to a high of 4.9 in Punjab. The distribution of neighborhood mean BMI also showed substantial variation within and between states with the standard deviation in neighborhood mean BMI ranging from 0.96 in Bihar to 2.5 in Maharashtra (Fig. 1). The mean neighborhood BMI ranged from 19.4 in Orissa to 23.8 in New Delhi (Fig. 2).
Fig. 1.
Standard deviation of unadjusted neighborhood BMI by state.
Fig. 2.
Mean unadjusted neighborhood BMI by state.
In models that did not adjust for the individual variables, state and neighborhood effects accounted for significant proportions of the variation in BMI, underweight, and overweight, respectively (Table 2). In the unadjusted models, states accounted for 7.1%, 7.3% and 11.3% of the total variation, and neighborhoods accounted for 15.7%, 8.6%, and 17.3% of the total variation in BMI, underweight, and overweight, respectively. Although the proportion of total variation attributed to context declined after adjusting for all the individual variables, variation at the state and neighborhood levels for all three outcomes remained highly statistically significant (p < 0.001), suggesting a high degree of clustering within neighborhoods based on individual-level covariates. In the models adjusted by individual variables, states accounted for 2.1%, 5.7% and 4.0% of the total variation, and neighborhoods accounted for 4.9%, 5.3%, and 3.8% of the total variation in BMI, underweight, and overweight, respectively. Thus, this adjustment by all individual variables reduced the variability in underweight by 26% and 40%, and reduced the variability in overweight by 73% and 83% at the state and neighborhood levels, respectively. The inclusion of the household standard of living alone to the null model explained 94% of the between-state variation and 89% of the between-neighborhood variation in BMI.
Table 2.
Variance in BMI, underweight, and overweight present at higher contextual levels
| Body mass indexa |
Underweightb |
Overweightb |
||||
|---|---|---|---|---|---|---|
| Variance (S.E.) | Percentage of variance | Variance (S.E.) | Percentage of variance | Variance (S.E.) | Percentage of variance | |
| Unadjustedc | ||||||
| State | 1.059 (0.303) | 7.1 | 0.287 (0.082) | 7.3 | 0.523 (0.150) | 11.3 |
| Neighborhood | 2.325 (0.072) | 15.7 | 0.336 (0.014) | 8.6 | 0.795 (0.035) | 17.3 |
| Adjustedd | ||||||
| State | 0.231 (0.067) | 2.1 | 0.211 (0.060) | 5.7 | 0.141 (0.041) | 4.0 |
| Neighborhood | 0.550 (0.025) | 4.9 | 0.199 (0.011) | 5.3 | 0.134 (0.015) | 3.8 |
Note: All variances are significantly different from 0 at p < .001.
Modeling the variation in linear body mass index in units of kg/m2.
Modeling the variation of log odds of under/overweight status in relation to the reference normal weight group.
Unadjusted models are null models with no predictors.
Models are adjusted for age, religion, marital status, education, occupation, caste, oral contraceptive use, number of children, recent major illness, use of chewing tobacco, smoking, and alcohol use.
3.2. Geography of BMI
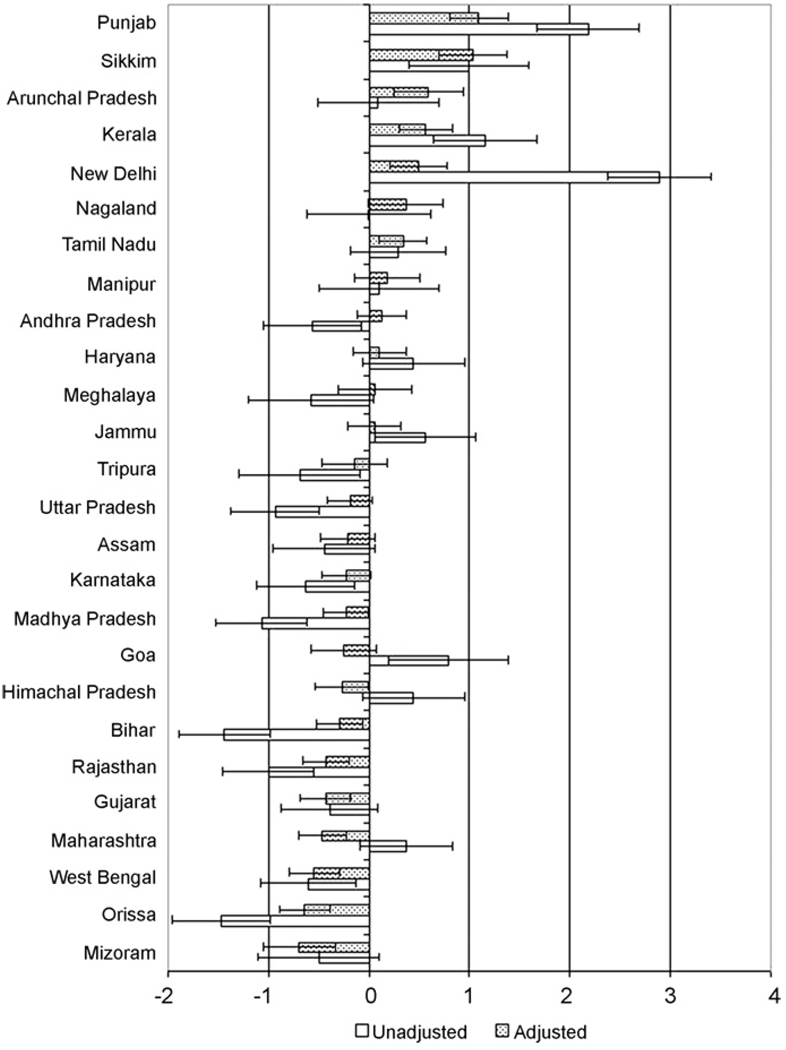
Fig. 3 shows the state-specific residuals in BMI before and after accounting for individual compositional factors. States with the highest BMI in unadjusted models (e.g., Punjab, New Delhi), as well as those states with the lowest BMI in the unadjusted models (e.g., Orissa, Bihar), had the most substantial attenuation toward the national mean. Adjusting for sociodemographic characteristics changed the signs of the residuals in several states, such as Goa, whose mean BMI was significantly above the national average before adjusting, but was below the national average after accounting for individual characteristics. Additionally, the adjusted models revealed significant contextual effects in a few states that had been hidden in the unadjusted models, such as Arunachal Pradesh and Tamil Nadu which had adjusted mean BMI significantly higher than the national average, and Mizoram and Gujarat which had adjusted mean BMI significantly lower than the national average.
Fig. 3.
Unadjusted and adjusted state BMI residuals and 95% confidence intervals.
3.3. Geography of underweight and overweight
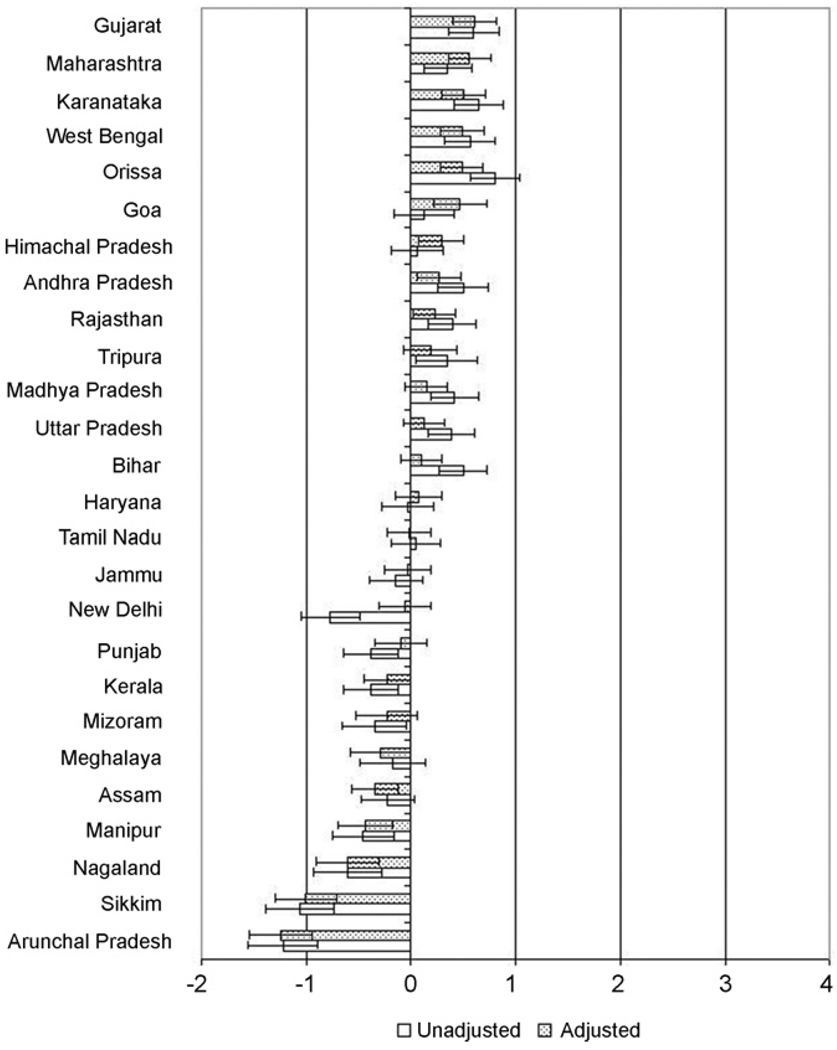
Fig. 4 presents the state-specific residuals for the risk of being underweight. Orissa and Karnataka had the highest unadjusted underweight residuals, while Gujarat and Maharashtra had the highest underweight residuals after adjusting for individual covariates. Arunachal Pradesh and Sikkim had the lowest underweight residuals in both the unadjusted and adjusted models. For most states the effects in the adjusted models were attenuated toward the national mean. For example, while underweight residuals in New Delhi and Punjab were significantly lower than the national mean, and those in Goa and Himachal Pradesh were significantly higher than the mean, none of these states were different from the national mean in the adjusted model. There were, nonetheless, several exceptions, such as Assam and Meghalaya which had significantly lower underweight residuals than the national average in adjusted models, and Himachal Pradesh and Goa which had overweight residuals significantly higher than that of the national average in adjusted models, none of which were significant in unadjusted models.
Fig. 4.
Unadjusted and adjusted state underweight residuals and 95% confidence intervals. Note: residuals indicate log odds of underweight compared to the national mean.
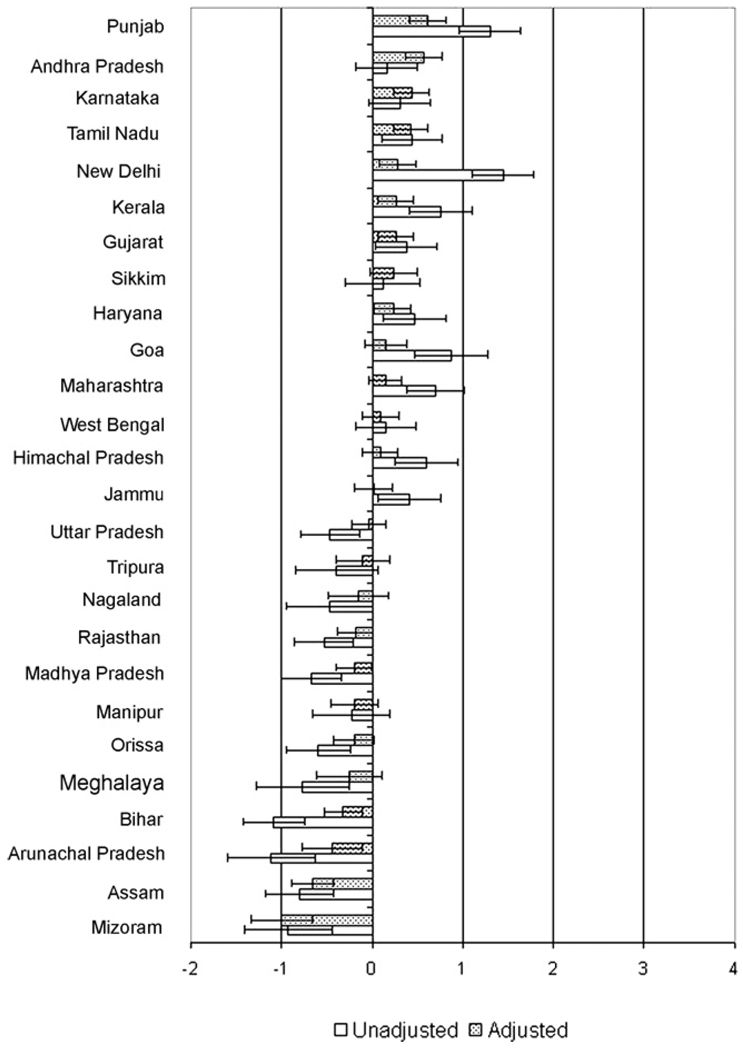
There was also a wide variation in state overweight residuals (Fig. 5). While New Delhi and Punjab had the highest unadjusted overweight residuals in the sample, Punjab and Andhra Pradesh had the highest underweight residuals in the adjusted model. Arunachal Pradesh and Bihar had the lowest overweight residuals in the unadjusted model while Mizoram and Assam had the lowest adjusted residuals. For most states, the effects in the adjusted models attenuated the overweight residuals towards the national mean, notably New Delhi and Goa for whom adjustment greatly reduced the observed residuals and Arunachal Pradesh and Bihar for whom adjustment strongly increased the observed residuals. Notable exceptions were Andhra Pradesh and Karnataka, which were similar to the state mean in unadjusted models but significantly higher than the mean in adjusted models.
Fig. 5.
Unadjusted and adjusted state overweight residuals and 95% confidence intervals. Note: residuals indicate log odds of overweight compared to the national mean.
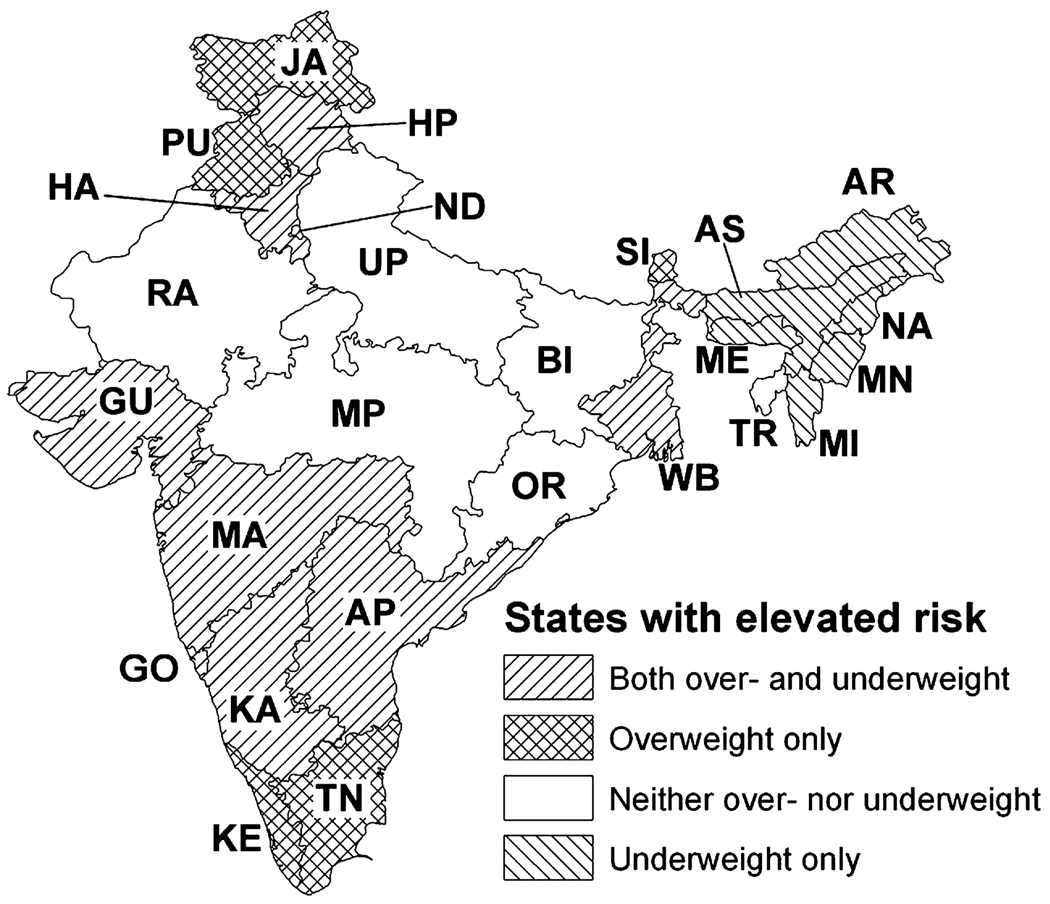
The patterning of overweight and underweight reveals regional clustering of states by nutritional status (Fig. 6). Groups of states in the far north and far south of the country had high prevalence of overweight, while a band of states in the north central region had high prevalence of underweight. A group of states covering substantial portions of the eastern and western coasts had high prevalence of both overweight and underweight, and a group of states in the northeast region had lower than average prevalence of both overweight and underweight.
Fig. 6.
Map of state levels of overweight and underweight in adjusted models compared to national means. State name abbreviations: AP, Andhra Pradesh; AR, Arunachal Pradesh; AS, Assam; BI, Bihar; GO, Goa; GU, Gujarat; HA, Haryana; HP, Himachal Pradesh; JA, Jammu; KA, Karnataka; KE, Kerala; MA, Maharashtra; ME, Meghalaya; MI, Mizoram; MN, Manipur; MP, Madhya Pradesh; NA, Nagaland; ND, New Delhi; OR, Orissa; PU, Punjab; RA, Rajasthan; SI, Sikkim; TN, Tamil Nadu; TR, Tripura; UP, Uttar Pradesh; WB, West Bengal.
As women experiencing overweight and those experiencing underweight resided in every state and virtually every neighborhood studied, we investigated the extent to which these two aspects of nutritional status were correlated. A unique advantage of multinomial multilevel models is the ability to estimate the covariance in the random effects associated with underweight and overweight, which in turn allows an assessment of the extent of the double burden in nutritional status at the level of neighborhoods and states. In the adjusted model, we observed a very weak inverse relationship between the random effects associated with underweight and overweight at the neighborhood level (r = −0.13, p = 0.04). In other words, neighborhoods where individuals were at a greater risk of being underweight tended to be those where individuals were at a lower risk of being overweight. At the state level, however, we observed no significant association between high rates of underweight and overweight (r = 0.31, p = 0.14).
3.4. Neighborhood wealth and BMI, underweight, and overweight
Neighborhood wealth was independently associated with individual BMI, such that a change from the poorest tertile of neighborhood wealth to the richest tertile was associated with a 0.29 increase in BMI (p = 0.0001), even after adjusting for a range of individual-level demographic and socioeconomic factors, including household wealth. In adjusted multinomial models, while risk of being overweight increased in neighborhoods with moderate wealth (odds ratio [OR] = 1.19; 95% confidence interval [CI] = 1.06–1.34) and high wealth increased (OR = 1.39; 95% CI = 1.20–1.59) compared to low-wealth neighborhoods, no substantial association was observed between neighborhood wealth and the risk of being underweight.
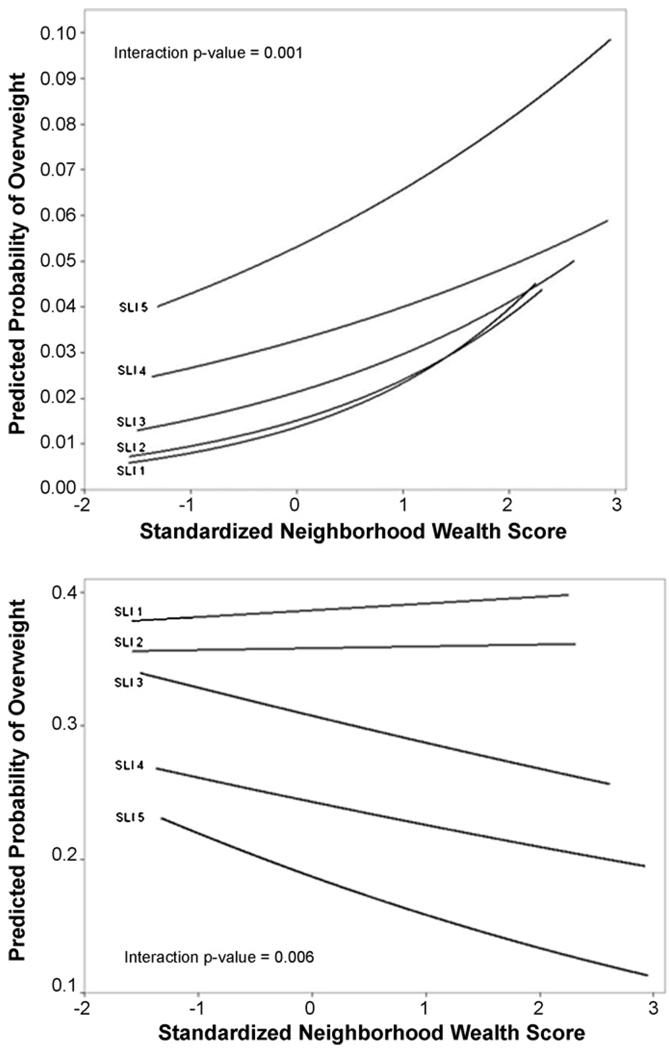
We additionally conducted tests of interaction between neighborhood wealth and household standard of living index. The interaction parameters were not substantial for BMI. The risk of being overweight, however, increased more quickly among those in the richest quintile of household compared to the rest of the sample, as neighborhood wealth increased (Fig. 7). In addition, while risk of underweight was reduced among women from more affluent households living in wealthier neighborhoods, no similar reductions occurred among women in poorer households. There also appears to be an interactive effect whereby an increase in neighborhood wealth by one standard deviation is associated with a decreased odds of underweight in large cities (OR = 0.85; 95% CI = 0.74–0.99) compared to the same increase in wealth in a rural village.
Fig. 7.
Predicted probability of overweight and underweight by the interaction of household standard of living index and neighborhood wealth.
4. Discussion
Our multilevel study, based on a large and nationally representative sample, identifies the following key findings that are relevant to understanding the role of geography in influencing nutritional status among women in India. First, even after accounting for a range of individual-level demographic and socioeconomic risk factors of nutritional status, we find a substantial contextual variation in nutritional status at the level of neighborhoods and states in India. Second, in terms of the relative importance of the two contextual levels, the level of neighborhoods was observed to be relatively more important as opposed to state in the determination of BMI, but the two levels were almost equally influential in determining the probability of experiencing underweight and overweight. Third, while both overweight and underweight are clearly prevalent in India, there appears to be no geographic patterning of this double burden at the state or neighborhood levels. Finally, neighborhood socioeconomic status, or more specifically wealth, was independently, and positively associated with BMI and the risk of being overweight. With increasing neighborhood wealth, the risk of being underweight decreases largely for women belonging to high SES groups, but not low SES groups.
The contextual variation of nutritional status between neighborhoods observed in this study is consistent with a number of proposed physical, social, and economic pathways between neighborhoods and impact health outcomes (Sampson et al., 2002). Research into urban/rural differences in BMI in India provides some insight into how neighborhoods in India may serve to pattern nutritional status (Singh et al., 1997; Venkatramana and Reddy, 2002). Compared to living in rural areas, urban residency has been found to be associated with higher consumption of refined sugars and dietary fat suggesting that there may be patterning in the ease of access to these calorie-dense foods (Shetty, 2002; Singh et al., 1995, 1996). Additionally, those living in rural areas report lower rates of sedentary behavior than urban dwellers, providing evidence that context may influence transportation, occupational, and recreational patterns in physical activity (Singh et al., 1995, 1996; Venkatramana and Reddy, 2002). Exposure to media has been found to be related to nutritional status among adults in developed countries, presumably through marketing of calorie-dense foods and the promotion of sedentary recreational behavior (Foster et al., 2006). As both media exposure and the strength of the relationship between media exposure and health behaviors have been found to be geographically patterned in India (Ghosh, 2006), this is another potential mechanism through which neighborhoods could affect nutritional status.
In addition to the variation between neighborhoods, there are several pathways through which state-specific characteristics could be responsible for variations in nutritional status. India is a country with a large, culturally diverse population (Dyson and Moore, 1983). These cultural differences may result in differences in eating patterns that serve to promote or suppress overeating. In addition, there is a wide variation in social policy between states (Peters et al., 2003). This difference in social policy may mean that while some state governments implement strong, well-funded policies to promote the distribution of food to those in need, other states may be less diligent in this regard (Subramanian et al., 2007b; Vijayaraghavan, 2002).
In regards to the distribution of nutritional status found in this study, several states merit specific mention. After adjusting for individual sociodemographic characteristics, Punjab was found to have the highest average BMI and the highest prevalence of overweight. This may be due, at least in part, to agricultural advances that have made the area a net food exporter (Tiwana et al., 2005), as well as cultural shifts in which sedentary behavior and a calorie-dense diet have gained wide appeal (Sidhu et al., 2006). Many states in India’s northeast, namely Arunachal Pradesh, Manipur, Mizoram, and Assam, had prevalences of both overweight and underweight which were below the national mean. This may be related to the fact that these areas are marked by having a high proportion of tribal communities which have been described as among the most egalitarian in India, placing less emphasis on the hierarchy of the Hindu caste system (Bhengra et al., 1999). While the western coastal states of Gujarat, Maharashtra, and Karnataka were found to have the highest adjusted prevalence of underweight in the country, the prevalence of overweight there was well above the national average. This simultaneous burden of overweight and underweight may result from higher levels of inequality that could disproportionately influence the distribution of food according to economic status in these states (Subramanian et al., 2007b; Subramanian and Davey Smith, 2006).
The nutrition transition is marked by the rapid rise in rates of overweight and obesity and the consequent rise in non-infectious disease in nations that undergo economic development typically accompanied by commensurate economic globalization and demographic urbanization (Caballero and Popkin, 2002; Popkin, 1998). This transition is also marked by the inverse relationship between SES and overweight in developed countries, and the direct relationship between SES and overweight in developing countries (Monteiro et al., 2004). The results of this study indicate that in terms of the clinically relevant standards created by the World Health Organization for this population, the prevalence of overweight is much lower than that of underweight in India, and much lower than that of most developed countries. This fact, combined with the direct relationship between household wealth and overweight indicate that India is in the initial stages of the nutrition transition. Nevertheless, the prevalence of overweight in India is not inconsequential, and in some areas, namely in New Delhi and Punjab, does achieve levels similar to those of many developed countries (Prentice, 2006). While the mortality attributed to elevated body mass index is currently 2.0% in India, this figure is expected to climb dramatically in the coming years as the prevalence of overweight among adult women in India is projected to climb to 30% by the year 2015 (WHO, 2005).
The growing prevalence of overweight-related non-communicable disease in the developing world is impacting populations that are still afflicted by high rates of infectious and nutrient deficiency diseases, a condition termed the “double burden” (Prentice, 2006; WHO, 2003). Although previous studies have documented the existence of a double burden of underweight and overweight in India (Singh et al., 1999; Subramanian et al., 2007b; Subramanian and Davey Smith, 2006), little has been written about how this double burden is distributed within India. While our investigation did find substantial levels of both underweight and overweight in the nation as a whole, we did not find significant patterning of nutritional status indicating that overweight and underweight do not tend to cluster together in the same states or neighborhoods. We did, however, find some states with prevalence of both underweight and overweight that was higher than the national mean.
One reason for the simultaneous coexistence of under- and overnutrition in some states and in the nation as a whole may be the increased production of processed foods, particularly animal products. Those Indians who have become wealthy in the past decades have increased the demand for animal-derived foods (Kaur et al., 2005) which uses up a significant portion of the cheaper raw unprocessed food grains (Gopinath et al., 1996). Use of these unprocessed grains to grow food animals diminishes the supply of unprocessed foods and increases the price of these staples that make up the diet of the disadvantaged groups. As such, it is possible that when the wealthier classes eat large amounts of calorie-dense animal-derived food and become overweight, those in the poorer classes have difficulty purchasing enough calories to meet their minimum daily necessities.
Our investigation into contextual correlates found that, while personal standard of living was more strongly related to nutritional outcomes, neighborhood wealth was associated with these nutritional outcomes as well. Specifically, neighborhood wealth was associated with both BMI and overweight, but not with underweight. Further analyses investigating the interaction between household living standard and neighborhood wealth revealed interesting relationships with nutritional status, particularly regarding the modeling of underweight. These results revealed that neighborhood wealth is associated with lower rates of underweight in wealthy families but had no association among poorer families. This interaction apparently hid the association between the main effect of neighborhood wealth and risk of underweight. Increases in neighborhood wealth were also associated with especially large increases in overweight risk among people living in wealthy households compared with modest increases in overeweight risk among people in poorer households. This interaction between neighborhood wealth and household living standard mirrors similar findings where improvements in state-level GDP only reduced the risk of underweight for high SES women in India (Subramanian et al., 2007b). These findings suggest that the benefits of economic progress are translating rather selectively in terms of raising the level of nutrition (Subramanian et al., 2007b).
Although this research investigated nutritional status in women, there is evidence to support the notion that these results may be applicable to men as well. Previous research indicates that distribution of nutritional status of men in India follow similar patterns to those detected in women regarding socioeconomic status and urban rural residency (Chhabra and Chhabra, 2007). However, the overall burden of disease due to abnormal nutritional status among men in India is likely to be lower, since women tend to have higher rates of both underweight and overweight compared to men in India (Chhabra and Chhabra, 2007; Sadhukhan et al., 2007; Sauvaget et al., 2008).
An important limitation of this study is the use of BMI as a measure of nutritional status. First, it is necessary to note that BMI is more purely a measure of energy intake, and it is possible to be overnourished in terms of energy intake and yet undernourished in terms of important micronutrients (Asfaw, 2007). BMI also has shortcomings as a measure of energy intake. Due to variations in body shapes and muscle mass, BMI has an imperfect relationship with other important risk factors related to nutritional status, such as body fat and abdominal obesity (Gill, 2001). As a result, the use of BMI as a predictor for clinical outcomes, such as cardiovascular disease, cancer, and diabetes has been called into question (Deurenberg-Yap et al., 2002). Other anthropomorphic measures, such as waist-to-hip ratio have been offered as better predictors of non-communicable disease (Cox and Whichelow, 1996). The utility of BMI as a measure of overweight has been further challenged by research that indicate an elevated risk of clinical outcomes for Asians compared to Caucasians at comparable BMI levels (Deurenberg-Yap et al., 2002). One very thorough multilevel study of nutrition and social inequality in Nepal found that anthropometric measures in general were severely limited in their ability to describe the nutritional status of members across ethnically distinct groups, even in a relatively small geographical area, as a result of the long term cross-generational physical adaptation of the members of these groups to their environments (Strickland and Tuffrey, 1997). Despite these shortcomings, BMI has been found to be related to clinical outcomes among Indians, prompting the World Health Organization to reiterate its support for the use of BMI as an important health metric among this population (WHO, 2004).
5. Conclusion
The findings from this study illustrate the important relationship between context and nutritional status in India. While the individual-level characteristics are clearly influential, the contextual effects remained after accounting for these covariates. These results indicate that interventions to address the double burden of undernutrition and overnutrition in India must take into account the characteristics of states and neighborhoods in order to be successful, and that further research to investigate the salient contextual influences on nutritional status are merited.
Appendix A.
Adjusted estimates and standard errors for body mass index, and odds ratios and 95% confidence intervals for overwieght and underweight for ever-married women age 15–49 in the 1998–1999 National Family Health Survey in India
| Fixed effect | Body mass index |
Underweight |
Overweight |
|||
|---|---|---|---|---|---|---|
| Estimate | S.E. | OR | 95% CI | OR | 95% CI | |
| Intercept | 18.99 | 0.12 | ||||
| Location | ||||||
| Village (reference) | 0.00 | 1.00 | 1.00 | |||
| Town | 0.45 | 0.06 | 1.41 | 1.26–1.57 | 1.31 | 1.21–1.43 |
| Small city | 0.67 | 0.08 | 0.94 | 0.87–1.01 | 1.64 | 1.49–1.81 |
| Large city | 1.19 | 0.07 | 0.75 | 0.68–0.83 | 0.91 | 0.82–1.02 |
| Age (years) | ||||||
| 15–19 (reference) | 0.00 | 1.00 | 1.00 | |||
| 20–24 | −0.30 | 0.06 | 1.11 | 1.03–1.20 | 1.02 | 0.94–1.10 |
| 25–29 | 0.15 | 0.06 | 0.85 | 0.78–0.93 | 0.75 | 0.69–0.82 |
| 30–34 | 0.83 | 0.06 | 0.77 | 0.70–0.84 | 0.74 | 0.67–0.82 |
| 35–39 | 1.26 | 0.07 | 1.36 | 1.06–1.74 | 2.79 | 2.20–3.55 |
| 40–44 | 1.48 | 0.07 | 4.80 | 3.77–6.10 | 6.48 | 5.08–8.25 |
| 45–49 | 1.65 | 0.07 | 7.86 | 6.16–10.04 | 8.62 | 6.73–11.03 |
| Religion | ||||||
| Hindu (reference) | 0.00 | 1.00 | 1.00 | |||
| Muslim | 0.26 | 0.05 | 1.02 | 0.95–1.09 | 0.79 | 0.70–0.89 |
| Christian | 0.28 | 0.08 | 0.96 | 0.80–1.15 | 0.76 | 0.66–0.88 |
| Sikh | 0.29 | 0.11 | 1.40 | 1.28–1.53 | 1.14 | 1.00–1.30 |
| Other/missing religion | 0.46 | 0.09 | 1.25 | 1.07–1.45 | 1.31 | 1.11–1.53 |
| Caste | ||||||
| General (reference) | 0.00 | 1.00 | 1.00 | |||
| Scheduled caste | −0.36 | 0.04 | 1.18 | 1.11–1.24 | 1.00 | 0.93–1.08 |
| Scheduled tribe | −0.05 | 0.06 | 1.06 | 1.01–1.11 | 0.83 | 0.76–0.91 |
| Other backward caste | −0.20 | 0.03 | 0.84 | 0.73–0.97 | 0.89 | 0.83–0.95 |
| Marital status | ||||||
| Married (reference) | 0.00 | 1.00 | 1.00 | |||
| Widowed | −0.19 | 0.06 | 1.05 | 0.97–1.14 | 1.14 | 1.02–1.28 |
| Divorced | −0.05 | 0.08 | 0.93 | 0.82–1.05 | 1.03 | 0.85–1.25 |
| Education (years) | ||||||
| 0 (reference) | 0.00 | 1.00 | 1.00 | |||
| 1–5 | 0.19 | 0.04 | 0.98 | 0.93–1.03 | 0.91 | 0.86–0.97 |
| 6–8 | 0.35 | 0.04 | 0.87 | 0.81–0.94 | 0.83 | 0.74–0.93 |
| 9–10 | 0.38 | 0.05 | 0.78 | 0.69–0.89 | 1.21 | 1.11–1.31 |
| 11–12 | 0.42 | 0.07 | 1.33 | 1.22–1.45 | 1.40 | 1.28–1.53 |
| Over 12 | 0.59 | 0.07 | 1.40 | 1.25–1.57 | 1.57 | 1.40–1.76 |
| Employment | ||||||
| Not working (reference) | 0.00 | 1.00 | 1.00 | |||
| Unpaid | −0.16 | 0.05 | 0.99 | 0.93–1.05 | 0.83 | 0.75–0.92 |
| Paid non-manual | 0.15 | 0.06 | 1.11 | 1.05–1.18 | 1.07 | 1.01–1.14 |
| Paid agricultural | −0.41 | 0.04 | 0.73 | 0.65–0.82 | 0.92 | 0.83–1.01 |
| Paid manual | −0.33 | 0.05 | 0.57 | 0.50–0.65 | 0.79 | 0.72–0.87 |
| Standard of living | ||||||
| First (lowest) quintile (reference) | 0.00 | 1.00 | 1.00 | |||
| Second quintile | 0.13 | 0.04 | 0.91 | 0.86–0.95 | 0.75 | 0.71–0.80 |
| Third quintile | 0.44 | 0.04 | 0.54 | 0.50–0.58 | 0.34 | 0.31–0.38 |
| Fourth quintile | 1.26 | 0.05 | 1.30 | 1.10–1.54 | 2.13 | 1.82–2.49 |
| Fifth (highest) quintile | 2.78 | 0.06 | 3.38 | 2.89–3.95 | 5.97 | 5.06–7.04 |
| Oral contraceptive use | ||||||
| No (reference) | 0.00 | 1.00 | 1.00 | |||
| Yes | −0.01 | 0.08 | 0.87 | 0.78–0.97 | 0.87 | 0.74–1.02 |
| Number of children | ||||||
| 0 (reference) | 0.00 | 1.00 | 1.00 | |||
| 1 | −0.14 | 0.05 | 1.19 | 1.11–1.28 | 1.21 | 1.12–1.30 |
| 2 | 0.03 | 0.05 | 1.21 | 1.12–1.31 | 1.26 | 1.17–1.36 |
| 3 | 0.02 | 0.05 | 1.05 | 0.92–1.19 | 1.15 | 1.02–1.30 |
| 4 or more | −0.18 | 0.06 | 1.10 | 0.97–1.25 | 1.06 | 0.93–1.21 |
| Illness | ||||||
| No (reference) | 0.00 | 1.00 | 1.00 | |||
| Yes | −0.39 | 0.05 | 1.23 | 1.15–1.32 | 0.79 | 0.69–0.92 |
| Tobacco chewing | ||||||
| No (reference) | 0.00 | 1.00 | 1.00 | |||
| Yes | −0.37 | 0.04 | 1.20 | 1.13–1.26 | 0.94 | 0.85–1.04 |
| Tobacco smoking | ||||||
| No (reference) | 0.00 | 1.00 | 1.00 | |||
| Yes | −0.64 | 0.08 | 1.41 | 1.27–1.56 | 0.73 | 0.58–0.91 |
| Alcohol drinking | ||||||
| No (reference) | 0.00 | 1.00 | 1.00 | |||
| Yes | 0.28 | 0.08 | 0.84 | 0.74–0.94 | 0.91 | 0.71–1.15 |
Appendix B.
Mean and standard deviation (SD) of the household characteristics used to calculate the standard of living score in the 1998–1999 Indian National Family Health Survey
| Characteristic | Mean | SD |
|---|---|---|
| Has electricity | 0.66530 | 0.47189 |
| Has a radio | 0.41640 | 0.49296 |
| Has a television | 0.38955 | 0.48765 |
| Has a refrigerator | 0.14346 | 0.35054 |
| Has a bicycle | 0.43583 | 0.49587 |
| Has a motorcycle | 0.12632 | 0.33221 |
| Has a car | 0.02432 | 0.15403 |
| Has a telephone | 0.10082 | 0.30109 |
| Uses a separate room as a kitchen | 0.54776 | 0.49772 |
| Household owns agricultural land | 0.49206 | 0.49994 |
| Acres of land under cultivation | 119.4 | 589.4 |
| Acres of irrigated land under cultivation | 4068.3 | 3413.1 |
| Household owns livestock | 0.46785 | 0.49897 |
| Has a mattress | 0.57765 | 0.49394 |
| Has a pressure cooker | 0.38259 | 0.48602 |
| Has a chair | 0.52696 | 0.49928 |
| Has a cot or bed | 0.83841 | 0.36808 |
| Has a table | 0.48950 | 0.49989 |
| Has a clock or watch | 0.71219 | 0.45274 |
| Has a fan | 0.48738 | 0.49984 |
| Has a bicycle | 0.43583 | 0.49587 |
| Has a sewing machine | 0.24008 | 0.42713 |
| Has a telephone | 0.10082 | 0.30109 |
| Has a refrigerator | 0.14346 | 0.35054 |
| Has a television (black and white) | 0.27052 | 0.44423 |
| Has a television (color) | 0.13270 | 0.33925 |
| Has a water pump | 0.08141 | 0.27346 |
| Has a bullock cart | 0.06047 | 0.23836 |
| Has a thresher | 0.01707 | 0.12954 |
| Has a tractor | 0.01634 | 0.12677 |
| Household has a domestic worker not related to head | 0.00043 | 0.02079 |
| Household works the family’s agricultural land | 0.19366 | 0.39517 |
| Number of members per sleeping room | 2.28628 | 1.88105 |
| Has piped drinking water in residence | 0.26282 | 0.44017 |
| Has piped drinking water in public tap | 0.17843 | 0.38287 |
| Has a well in residence | 0.01236 | 0.11048 |
| Gets water from a public well | 0.05867 | 0.23500 |
| Uses a spring for drinking water | 0.01941 | 0.13796 |
| Uses a river, a canal, or surface water for drinking | 0.03066 | 0.17241 |
| Uses rainwater for drinking | 0.00118 | 0.03431 |
| Uses a tanker truck for drinking water | 0.00219 | 0.04680 |
| Gets water from a covered public well | 0.01041 | 0.10151 |
| Gets water from a public open well | 0.09959 | 0.29946 |
| Uses a residential handpump | 0.13024 | 0.33656 |
| Uses a public handpump | 0.18927 | 0.39173 |
| Uses another source of drinking water | 0.00467 | 0.06819 |
| Has a private flush toilet | 0.22489 | 0.41751 |
| Has a public flush toilet | 0.02964 | 0.16958 |
| Uses a private pit latrine | 0.13684 | 0.34368 |
| Uses a public pit latrine | 0.00660 | 0.08095 |
| Uses a shared pit latrine | 0.02032 | 0.14108 |
| Uses a shared flush toilet | 0.03696 | 0.18866 |
| Uses the bush or a field as latrine | 0.54355 | 0.49810 |
| Uses another type of latrine | 0.00112 | 0.03351 |
| Uses clay kitchenware | 0.01023 | 0.10062 |
| Uses aluminum kitchenware | 0.36287 | 0.48083 |
| Uses kitchenware made of cast iron | 0.00271 | 0.05202 |
| Uses brass or copper kitchenware | 0.01379 | 0.11660 |
| Uses stainless steel kitchenware | 0.60984 | 0.48779 |
| Uses another kind of kitchenware | 0.00003 | 0.00570 |
| Uses electricity for lighting | 0.66530 | 0.47189 |
| Uses kerosene for lighting | 0.33061 | 0.47044 |
| Uses gas for lighting | 0.00055 | 0.02348 |
| Uses oil for lighting | 0.00130 | 0.03600 |
| Uses other lighting | 0.00158 | 0.03970 |
| Uses wood cooking fuel | 0.57660 | 0.49410 |
| Uses dung cooking fuel | 0.05214 | 0.22231 |
| Uses coal cooking fuel | 0.01582 | 0.12477 |
| Uses charcoal cooking fuel | 0.00302 | 0.05484 |
| Uses kerosene cooking fuel | 0.08719 | 0.28212 |
| Uses LPG cooking fuel | 0.21486 | 0.41073 |
| Uses biogas cooking fuel | 0.00434 | 0.06570 |
| Uses crop residual for cooking fuel | 0.03629 | 0.18700 |
| Uses electricity for cooking | 0.00790 | 0.08855 |
| Uses other cooking fuel | 0.00158 | 0.03970 |
| House is made from high quality materials | 0.35097 | 0.47728 |
| House is made from low quality materials | 0.29623 | 0.45660 |
| House is made from mixed quality materials | 0.35079 | 0.47722 |
Acknowledgements
We acknowledge the support of Macro International (www.measuredhs.com) for providing us access to the 1998–1999 Indian National Family Health Survey data. We also acknowledge Amy Cohen for invaluable assistance with programming and data management. LA completed this project while supported by the Harvard Education Program in Cancer Prevention and Control (NIH/NCI 5 R25 CA057711-12R). SVS is supported by the National Institutes of Health Career Development Award (NHLBI 1 K25 HL081275). No direct financial support was available for this study.
Footnotes
IIPS is the International Institute for Population Sciences.
Details of this study were approved by the Harvard School of Public Health Institutional Review Board Human Subjects Committee.
“Smoothed” or “shrunken” residuals are different from raw residuals in that account is taken of the reliability factor (i.e., the number of lower level units within each higher unit). At the state level, these residuals were calculated as: , where is the shrunken residual, is the state level variance, is the neighborhood level variance, is the variance between individuals, nk is the number of neighborhoods in state k, njk is the number of individuals in neighborhood j in state k, and Vk is the raw residual for state k. For more details see Goldstein (2003) and Subramanian et al. (2007a).
References
- Asfaw A. Micronutrient deficiency and the prevalence of mothers’ overweight/obesity in Egypt. Economics & Human Biology. 2007;5(3):471–483. doi: 10.1016/j.ehb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Bhengra R, Bijoy CR, Luithui S. The Adivasis of India. London: Minority Rights Group International; 1999. [Google Scholar]
- Blakely T, Subramanian SV. Multilevel studies. In: Oakes JM, Kaufman JS, editors. Methods in Social Epidemiology. San Fransisco: Jossey-Bass; 2006. [Google Scholar]
- Booth KM, Pinkston MM, Poston WS. Obesity and the built environment. Journal of the American Dietetic Association. 2005;105(5 Suppl 1):S110–S117. doi: 10.1016/j.jada.2005.02.045. [DOI] [PubMed] [Google Scholar]
- Caballero B, Popkin BM. Food Science and Technology International Series. Amsterdam/Boston: Academic Press; 2002. The nutrition transition: diet and disease in the developing world. [Google Scholar]
- Chhabra P, Chhabra SK. Distribution and determinants of body mass index of non-smoking adults in Delhi, India. Journal of Health Population and Nutrition. 2007;25(3):294–301. [PMC free article] [PubMed] [Google Scholar]
- Cox BD, Whichelow M. Ratio of waist circumference to height is better predictor of death than body mass index. British Medical Journal. 1996;313(7070):1487. doi: 10.1136/bmj.313.7070.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins S, Macintyre S. Food environments and obesity—neighbourhood or nation? International Journal of Epidemiology. 2006;35(1):100–104. doi: 10.1093/ije/dyi276. [DOI] [PubMed] [Google Scholar]
- Deurenberg-Yap M, Chew SK, Deurenberg P. Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obesity Reviews. 2002;3(3):209–215. doi: 10.1046/j.1467-789x.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- Do DP, Dubowitz T, Bird CE, Lurie N, Escarce JJ, Finch BK. Neighborhood context and ethnicity differences in body mass index: a multilevel analysis using the NHANES III survey (1998–1994) Economics & Human Biology. 2007;5(2):179–203. doi: 10.1016/j.ehb.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. American Journal of Clinical Nutrition. 2004;79(1):6–16. doi: 10.1093/ajcn/79.1.6. [DOI] [PubMed] [Google Scholar]
- Dyson T, Moore M. On kinship structure, female autonomy, and demographic behavior in India. Population and Development Review. 1983;9(1):35–60. [Google Scholar]
- Filmer D, Pritchett LH. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- Foster JA, Gore SA, West DS. Altering TV viewing habits: an unexplored strategy for adult obesity intervention? American Journal of Health Behavior. 2006;30(1):3–14. doi: 10.5555/ajhb.2006.30.1.3. [DOI] [PubMed] [Google Scholar]
- Ghosh D. Effect of mother’s exposure to electronic mass media on knowledge and use of prenatal care services: a comparative analysis of Indian states. Professional Geographer. 2006;58(3):278–293. [Google Scholar]
- Gill TP. Cardiovascular risk in the Asia-Pacific region from a nutrition and metabolic point of view: abdominal obesity. Asia Pacific Journal of Clinical Nutrition. 2001;10(2):85–89. doi: 10.1111/j.1440-6047.2001.00231.x. [DOI] [PubMed] [Google Scholar]
- Goldstein H. Multilevel Statistical Models. London: Arnold; 2003. [Google Scholar]
- Goldstein H, Rasbash J. Improved approximations for multilevel models with binary responses. Journal of the Royal Statistical Society. Series A. 1996;159(3):505–513. [Google Scholar]
- Gopinath M, Roe TL, Shane MD. Competitiveness of US food processing: benefits from primary agriculture. American Journal of Agricultural Economics. 1996;78(4):1044–1055. [Google Scholar]
- Griffiths P, Bentley M. Women of higher socio-economic status are more likely to be overweight in Karnataka, India. European Journal of Clinical Nutrition. 2005;59(10):1217–1220. doi: 10.1038/sj.ejcn.1602228. [DOI] [PubMed] [Google Scholar]
- Gwatkin DR, Rutstein S, Johnson K, Pande RP, Wagstaff A. HNP/Poverty Thematic Group. Washington, DC: World Bank; 2000. Socio-economic differences in health, nutrition, and population. [Google Scholar]
- IIPS AND ORC-Macro. National Family Health Survey, 1998–1999: India. Mumbai: International Institute for Population Sciences; 2000. [Google Scholar]
- Kaur S, Kapil U, Singh P. Pattern of chronic diseases amongst adolescent obese children in developing countries. Current Science. 2005;88(7):1052–1056. [Google Scholar]
- Kawachi I, Subramanian SV. Neighbourhood influences on health. Journal of Epidemiology and Community Health. 2007;61(1):3–4. doi: 10.1136/jech.2005.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Berkman LF. Neighborhoods and Health. Oxford/New York: Oxford University Press; 2003. [Google Scholar]
- Mendez MA, Monteiro CA, Popkin BM. Overweight exceeds underweight among women in most developing countries. American Journal of Clinical Nutrition. 2005;81(3):714–721. doi: 10.1093/ajcn/81.3.714. [DOI] [PubMed] [Google Scholar]
- Mobley LR, Root ED, Finkelstein EA, Khavjou O, Farris RP, Will JC. Environment, obesity, and cardiovascular disease risk in low-income women. American Journal of Preventive Medicine. 2006;30(4):327–332. doi: 10.1016/j.amepre.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Journal of the American Medical Association. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. Journal of the American Medical Association. 1999;282(16):1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- Monteiro CA, Conde WL, Lu B, Popkin BM. Obesity and inequities in health in the developing world. International Journal of Obesity and Related Metabolic Disorders. 2004;28(9):1181–1186. doi: 10.1038/sj.ijo.0802716. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet. 1997;349(9063):1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- Osmani S, Sen A. The hidden penalties of gender inequality: fetal origins of ill-health. Economics & Human Biology. 2003;1(1):105–121. doi: 10.1016/s1570-677x(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Peters DH, Rao KS, Fryatt R. Lumping and splitting: the health policy agenda in India. Health Policy and Planning. 2003;18(3):249–260. doi: 10.1093/heapol/czg031. [DOI] [PubMed] [Google Scholar]
- Popkin BM. The nutrition transition and its health implications in lower-income countries. Public Health Nutrition. 1998;1(1):5–21. doi: 10.1079/phn19980004. [DOI] [PubMed] [Google Scholar]
- Prentice AM. The emerging epidemic of obesity in developing countries. International Journal of Epidemiology. 2006;35(1):93–99. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- Rasbash J, Steele F, Browne W, Prosser B. A User’s Guide to MLwiN, Version 2.0. Bristol, UK: Centre for Multilevel Modelling; 2005. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods, 2nd ed. Advanced Quantitative Techniques in the Social Sciences. 2nd ed. Vol. 1. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Rutstein SO, Johnson K. The DHS Wealth Index: DHS Comparative Reports. Vol. 6. Calverton, MD: ORC Macro; 2004. [Google Scholar]
- Sadhukhan SK, Bose K, Mukhopadhyay A, Bhadra M. Age variations in overweight men and women in rural areas of Hooghly District, West Bengal. Indian Journal of Public Health. 2007;51(1):59–61. [PubMed] [Google Scholar]
- Sampson RJ, Morenoff JD, Gannon-Rowley T. Assessing neighborhood effects: social processes and new directions in research. Annual Review of Sociology. 2002;28:443–478. [Google Scholar]
- Sauvaget C, Ramadas K, Thomas G, Vinoda J, Thara S, Sankaranarayanan R. International Journal of Epidemiology. 2008. Body mass index, weight change and mortality risk in a prospective study in India. [DOI] [PubMed] [Google Scholar]
- Shetty PS. Nutrition transition in India. Public Health Nutrition. 2002;5(1A):175–182. doi: 10.1079/PHN2001291. [DOI] [PubMed] [Google Scholar]
- Shetty PS, James WPT. FAO Food and Nutrition Paper. Vol. 56. Rome: Food and Agriculture Organization of the United Nations; 1994. Body mass index: a measure of chronic energy deficiency in adults. [PubMed] [Google Scholar]
- Shukla HC, Gupta PC, Mehta HC, Hebert JR. Descriptive epidemiology of body mass index of an urban adult population in western India. Journal of Epidemiology and Community Health. 2002;56(11):876–880. doi: 10.1136/jech.56.11.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu S, Kaur N, Kaur R. Overweight and obesity in affluent school children of Punjab. Annals of Human Biology. 2006;33(2):255–259. doi: 10.1080/03014460600578631. [DOI] [PubMed] [Google Scholar]
- Singh RB, Beegom R, Mehta AS, Niaz MA, De AK, Mitra RK, Haque M, Verma SP, Dube GK, Siddiqui HM, Wander GS, Janus ED, Postiglione A, Haque MS. Social class, coronary risk factors and undernutrition, a double burden of diseases, in women during transition, in five Indian cities. International Journal of Cardiology. 1999;69(2):139–147. doi: 10.1016/s0167-5273(99)00010-8. [DOI] [PubMed] [Google Scholar]
- Singh RB, Ghosh S, Niaz AM, Gupta S, Bishnoi I, Sharma JP, Agarwal P, Rastogi SS, Beegum R, Chibo H, Zhu S. Epidemiologic study of diet and coronary risk factors in relation to central obesity and insulin levels in rural and urban populations of north India. Journal of Cardiology. 1995;47(3):245–255. doi: 10.1016/0167-5273(94)02186-m. [DOI] [PubMed] [Google Scholar]
- Singh RB, Niaz MA, Ghosh S, Beegom R, Rastogi V, Sharma JP, Dube GK. Association of trans fatty acids (vegetable ghee) and clarified butter (Indian ghee) intake with higher risk of coronary artery disease in rural and urban populations with low fat consumption. Journal of Cardiology. 1996;56(3):289–298. doi: 10.1016/0167-5273(96)02760-x. (discussion 299–300) [DOI] [PubMed] [Google Scholar]
- Singh RB, Sharma JP, Rastogi V, Raghuvanshi RS, Moshiri M, Verma SP, Janus ED. Prevalence of coronary artery disease and coronary risk factors in rural and urban populations of north India. European Heart Journal. 1997;18(11):1728–1735. doi: 10.1093/oxfordjournals.eurheartj.a015167. [DOI] [PubMed] [Google Scholar]
- Strickland SS, Tuffrey VR. Form and Function: A Study of Nutrition, Adaptation and Social Inequality in Three Gurung Villages of the Nepal Himalayas. London: Smith-Gordon; 1997. [Google Scholar]
- Subramanian SV. The relevance of multilevel statistical methods for identifying causal neighborhood effects. Social Science & Medicine. 2004;58(10):1961–1967. doi: 10.1016/S0277-9536(03)00415-5. [DOI] [PubMed] [Google Scholar]
- Subramanian SV, Davey Smith G. Patterns, distribution, and determinants of under- and overnutrition: a population-based study of women in India. American Journal of Clinical Nutrition. 2006;84(3):633–640. doi: 10.1093/ajcn/84.3.633. [DOI] [PubMed] [Google Scholar]
- Subramanian SV, Glymour MM, Kawachi I. Identifying causal ecologic effects on health: a methodological assessment. In: Galea S, editor. Macrosocial Determinants of Population Health. New York: Springer; 2007a. [Google Scholar]
- Subramanian SV, Jones K, Duncan C. Multilevel methods for public health research. In: Kawachi I, Berkman LF, editors. Neighborhoods and Health. New York: Oxford University Press; 2003. [Google Scholar]
- Subramanian SV, Kawachi I, Davey Smith G. Income inequality and the double burden of under- and over-nutrition in India. Journal of Epidemiology and Community Health. 2007b;61(9):802–809. doi: 10.1136/jech.2006.053801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian SV, Nandy S, Irving M, Gordon D, Lambert H, Davey Smith G. The mortality divide in India: the differential contributions of gender, caste, and standard of living across the life course. American Journal of Public Health. 2006;96(5):818–825. doi: 10.2105/AJPH.2004.060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwana NS, Jerath N, Saxena SK, Nangia P, Parwana HK. Punjab State Council for Science & Technology. Punjab, Chandigarh, India: 2005. State of Environment. [Google Scholar]
- Ulijaszek SJ. Frameworks of population obesity and the use of cultural consensus modeling in the study of environments contributing to obesity. Economics & Human Biology. 2007;5(3):443–445. doi: 10.1016/j.ehb.2007.08.006. [DOI] [PubMed] [Google Scholar]
- USDHHS. The Surgeon General’s call to action to prevent and decrease overweight and obesity. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001. [PubMed] [Google Scholar]
- van Lenthe FJ, Mackenbach JP. Neighbourhood deprivation and overweight: the GLOBE study. International Journal of Obesity and Related Metabolic Disorders. 2002;26(2):234–240. doi: 10.1038/sj.ijo.0801841. [DOI] [PubMed] [Google Scholar]
- Venkatramana P, Reddy PC. Association of overall and abdominal obesity with coronary heart disease risk factors: comparison between urban and rural Indian men. Asia Pacific Journal of Clinical Nutrition. 2002;11(1):66–71. doi: 10.1046/j.1440-6047.2002.00250.x. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan K. Control of micronutrient deficiencies in India: obstacles and strategies. Nutrition Reviews. 2002;60(5 Pt 2):S73–S76. doi: 10.1301/00296640260130786. [DOI] [PubMed] [Google Scholar]
- WHO. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. Geneva: World Health Organization; WHO Technical Report Series. 1995;854 [PubMed]
- WHO. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. Geneva: World Health Organization; WHO Technical Report Series. 2000;894 [PubMed]
- WHO. Diet, nutrition and the prevention of chronic diseases. Geneva: World Health Organization; WHO technical report series. 2003;916 [PubMed]
- WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed]
- WHO. Geneva: World Health Organization; Preventing chronic diseases: a vital investment. 2005