Abstract
Purpose
We sought to determine whether survival of patients managed at a large community hospital improved after an affiliated facility opened and its associated programs were initiated.
Methods
Survival data for patients with invasive cancer was obtained from the Hoag Hospital tumor registry for the successive periods 1986-1991 and for 1992-1999 for historical intramural comparisons; national Surveillance, Epidemiology, and End Results (SEER) program data for the same periods were used for contemporary and historical extramural comparisons.
Results
We observed survival improved significantly during 1992-1999 compared with 1986-1991 for all patients with invasive cancers (P < .0001), and specifically for cancers of the breast (P = .026), lung (P = .012), prostate (P < .0001), stomach (P = .006), pancreas (P = .0001), and oral cavity (P = .024), with strong trends for improved survival for leukemia (P = .051) and rectal cancer (P = .063). Relative 5-year survival rates increased from 63% during 1986-1991 to 71% during 1992-1999, and were higher for 22 of 24 tumor types during the more recent period (P < .0001). Compared with SEER data, Hoag relative survival for all patients with invasive cancer was 63% versus 58% during 1986-1991, and 71% versus 64% during 1992-1999. Survival for Hoag patients was better than SEER rates for only 50% of malignancies (12 of 24) during 1986-1991 compared with 87% (21 of 24) during 1992-1999 (P = .013). In the most common tumor types, there were substantial improvements in survival for patients with regional disease at diagnosis. Improved survival was associated with earlier diagnosis and increased use of systemic treatment and combined modality therapy.
Conclusion
Patients with invasive cancer who were treated at an integrated community cancer center had better survival compared with historical survival and patients from the SEER registry. The findings are consistent with the hypothesis that the accelerated dissemination of new information resulted in earlier adoption of improved screening, diagnostic, and multidisciplinary treatment approaches, leading to higher survival rates.
Introduction
Little objective evidence supports the premise that cancer centers are associated with better patient outcomes. Phrases used to define cancer centers include “multidisciplinary group of research scientists and/or physicians,” “unity of purpose,” “share concepts, facilities, and other resources,” “organizational structure,” 1 “organization of diverse and complementary specialists who work on the cancer problem together,” “sufficient central authority to focus efforts and organize resources,” and “patient care and/or research.”2 Most early cancer centers were organized around basic and animal research because of limited treatment options.3 The concepts of comprehensive cancer centers and clinical cancer centers, to facilitate and centralize patient care, education, and research, evolved after passage of the National Cancer Act in 1971. Large federal construction grants and core grants were administered by the National Cancer Institute (NCI).4,5 Most NCI-designated and -funded cancer centers are involved in patient care, education, and research, but emphasis varies depending on mission and funding. Many community hospitals have created cancer centers that focus on patient care, since few have a mission for laboratory-based research or education.2,6 In as much as the vast majority of cancer care is delivered in the community by private practitioners, centers in this setting might enhance education and communication opportunities to accelerate transmission of new information and facilitate multidisciplinary management and treatment decisions.
We wished to determine whether the existence of our community hospital–based programs were associated with improvements in survival rates for patients with invasive cancer. The impetus for this study derived from questions regarding whether the investment in money, space, and operations for the center had been associated with improved survival for patients diagnosed with invasive cancer. We chose survival as an indicator of overall quality of cancer management, because it is influenced by early detection procedures, cancer-directed treatment by various cancer specialists, and medical management of comorbid medical conditions by practitioners. The date of diagnosis of invasive cancer can be ascertained from pathology reports and tumor registry abstracts, and date of death is a well-documented vital statistic. One measure of quality improvement is survival outcome compared with an earlier era, but historical comparisons can be misleading because of changes in patient populations. Because of limitations associated with historical populations, we also compared our observations to contemporary populations, which necessitated comparisons to external data. To determine whether the existence of our center was associated with improved outcomes, we measured survival rates for patients with invasive cancer for the time periods immediately preceding and following opening of the center, and made comparisons with intramural and extramural benchmarks.
Methods
Institution and Cancer Program
Hoag Cancer Center is both a facility and program of Hoag Hospital, a 400-bed, not-for-profit hospital, located in the coastal community of Newport Beach, in Orange County, California, which is bounded by the counties of Los Angeles to the north, San Diego to the south, Riverside to the east, and the Pacific ocean to the west. Hoag Hospital has a primary service area that includes about 1 million people living within a 10- to 20-mile radius. The hospital does not offer training for medical house staff. In the mid 1980s, hospital leadership decided to pursue oncology as a “center of excellence” for the treatment of adult patients with cancer. After successful fund-raising efforts, in late 1990 a 65,000 square foot structure, the Patty and George Hoag Cancer Center, was constructed with a tunnel connecting it to the main hospital. A medical director was recruited from outside the institution for a full-time position that included oversight of clinical quality of cancer care, oncology education, laboratory and clinical cancer research and administrative responsibilities. An administrative director with oncology experience was also recruited from outside the institution and she assembled a staff for various programs. Since the opening of the center in the winter of 1991, the annual numbers of new patients accessioned into the tumor registry has been among the highest in southern California, with an annual growth rate of 4.4% per year, and between 2,100 and 2,200 patients per year in recent years.
From 1992 to 1999, the Patty and George Hoag Cancer Center facility was a three-story building that included three linear accelerators and other radiation oncology equipment, examining rooms, and offices on the first floor. An outpatient treatment center, administrative offices, patient resource library, blood donor/pheresis center, and a multipurpose conference room occupied the second floor. Medical oncology physician offices and a 4,000-square-foot cell biology laboratory were on the third floor. Specific program changes to enhance patient care included establishing an inpatient oncology unit in the hospital, opening a cancer outpatient treatment center, facilitating advanced treatment programs including high-dose interleukin-2 therapy, high-dose chemotherapy with autologous hematopoietic stem cell rescue, intrahepatic chemoembolization, acquisition of state-of-the-art radiation therapy equipment, developing programs for cancer prevention, early detection, and hereditary cancer, and extensive patient support programs including physical fitness and psychosocial support programs. External-beam adapted stereotactic radiosurgery was introduced in 1994. Gamma knife therapy, prostate radioactive seed implant therapy, and sentinel lymph node staging were all introduced in 1997. For educational purposes, weekly mid-day oncology education conferences, weekly morning multidisciplinary tumor board case conferences, and a monthly educational newsletter with state-of-the art reviews on specific cancers or treatment modalities were initiated to facilitate dissemination of cancer information and to facilitate collegial interaction. The existing clinical trials program was expanded to include cooperative group trials supported by the NCI, clinical trials of promising new agents sponsored by pharmaceutical companies, and trials regulated by the U.S. Food and Drug Administration, which utilized patient-specific products developed in the local cell biology laboratory. Products developed in the cell biology laboratory have included various types of autologous lymphocyte therapy,7-9 and autologous tumor cell vaccines.10-13 The laboratory has also been responsible for processing and cryopreservation of autologous hematopoietic stem cells for an autologous transplant program.14
Historical Intramural Survival Benchmarks and Therapy
We used adjacent time periods, 1986-1991 and 1992-1999, before and after the opening of Hoag Cancer Center, for intramural survival comparisons. Those specific years were chosen because the center opened during 1991, and those were the same intervals used by the NCI's Surveillance, Epidemiology, and End Results (SEER) program in recent reports.15,16 The analysis focused on patients with invasive cancer. Per SEER methodology, basal cell and squamous cell carcinomas of the skin, and in situ carcinomas were excluded, except for in situ bladder cancer. Data for Hoag cancer patients was compiled from the Hoag tumor registry, which included follow-up clinical information during 2004 on more than 90% of patients in the registry dating back to 1980. Analyses were limited to “analytical cases” (i.e., patients who were diagnosed at the institution and/or who received cancer therapy at the institution within 4 months of diagnosis). Registry data included the following general treatment classifications: none, surgery, radiation therapy; chemotherapy; hormonal therapy; and biologic response modifiers (BRMs) such as interferon-alfa, interleukin-2, Bacille-Calmette-Guerin (BCG), tumor vaccines, and cell therapies.
Contemporary Extramural Survival Benchmarks
Because of eligibility restrictions in clinical trials and changing definitions of tumor stage, most medical publications are not useful for survival comparisons to the general population of cancer patients. Similarly, survival data from large referral centers can be misleading because patients who travel to such centers are not representative of the general population, and the make up of such populations may change over time because of reputation, or because of specific high-volume clinical trial programs. Data from the SEER program was selected for external benchmark comparisons because of consistency of stage definitions over time, the size and diversity of patients sampled, and the annual publication of survival data. Comparisons between Hoag and SEER relative survival figures were made for both of the two consecutive periods, 1986-1991 and 1992-1999. Historically, SEER data have been derived from a sample of about 10% of the cancer population, which did not include patients from Orange County, California. Because the proportion of African American patients at Hoag was less than 1% in both periods, comparisons were limited to the white population in the SEER data.
Statistical Considerations
For the Hoag patient populations in the two successive periods, estimates of observed survival were generated by the method of Kaplan and Meier and compared for significance using the two-tailed unadjusted log-rank test.18 The methodology used by SEER was adopted for comparisons to the SEER data; so, for those analyses survival data is reported as relative survival, the ratio of observed survival for cancer patients to the expected survival for the general population with adjustments for competing causes of mortality based on age, race, and sex.19 Relative 5-year survival rates were calculated using the SEER methodology with a computer software program designed specifically for this purpose (Electronic Registry Systems, Inc., Cincinnati, Ohio). Fisher's exact test was used for comparisons of proportions using two-tailed tests of probability. The two-tailed t test for paired samples was used to compare relative 5-year survivals for the 24 different tumor types.
For the most prevalent cancers (lung, breast, colorectal, and prostate) subset comparisons were also made by stage. The staging systems of the American Joint Committee on Cancer (AJCC) have continually evolved; so they were not useful for these comparisons, and would have required retrospective efforts to redefine stage.20 Instead, comparisons were made using the general staging classifications defined by SEER, because they have utilized consistent definitions of local, regional, and distant metastatic disease stages, enabling comparisons between different eras.
Results
Characterization of Cancer Patients
During 1986-1991, the Hoag tumor registry accessioned 6,301 new diagnoses of cancer, 5,487 invasive (including 60 in situ bladder) and 814 in situ. Comparable figures for 1992-1999 were 11,803 new diagnoses of cancer, 10,548 invasive (including 223 in situ bladder) and 1,255 in situ malignancies. The proportions of nonbladder in situ cases in the successive eras decreased from 12.9% to 10.6%, respectively (P < .0001).
The characteristics of Hoag patients with invasive cancer (including in situ bladder) diagnosed during 1986-1991 and 1992-1999 are summarized in Table 1. There was a decline in numbers of patients classified as white, associated with an increase in patients classified as Asian. A similar majority of patients were female in both eras. During 1992-1999 there were higher proportions of patients ≥ 90 years of age, an increase in the proportion of patients with local disease at diagnosis, and an increase in the proportion diagnosed and treated at Hoag.
Table 1.
Characteristics of Hoag cancer patients diagnosed with invasive cancer* during 1986-1991 (n = 5,487) and 1992-1999 (n = 10,548)
| Category | 1986-1991 | 1992-1999 | P |
|---|---|---|---|
| % White | 97.2 | 95.8 | < .0001 |
| % Asian | 1.9 | 3.0 | .0001 |
| % African American | .38 | .36 | .171 |
| % female | 52.7 | 52.8 | .603 |
| Median age, years | 65.0 | 65.8 | — |
| Mean age, years | 62.3 | 63.4 | .928 |
| % < age 20 years | 0.49 | 0.39 | .171 |
| % ≥ age 90 years | 0.74 | 1.4 | .0009 |
| % local | 45.3 | 50.8 | < .0001 |
| % regional | 24.4 | 21.8 | .0004 |
| % distant | 22.7 | 21.8 | .190 |
| % unknown general stage | 7.6 | 5.4 | < .0001 |
| % diagnosed and treated at Hoag | 81.2 | 84.0 | < .0001 |
| % diagnosed elsewhere, treated at Hoag | 17.1 | 14.7 | .0001 |
| % diagnosed at Hoag, treated elsewhere | 1.7 | 1.3 | .032 |
| % diagnosed by histopathology | 93.1 | 91.2 | .0001 |
| % diagnosed by cytology only | 5.0 | 6.3 | .002 |
Includes tumors classified as “unknown stage”; excludes “in situ” except for bladder.
Treatment of Invasive Cancer
Initial treatment for Hoag cancer patients in the two periods is summarized in Table 2. There was a statistically significant increase in the use of systemic treatment in combination with other therapies, and in the use of biologic response modifiers alone or in combination with other therapy. The proportion of patients receiving systemic therapy nearly doubled. There was also an increase in the proportion of patients receiving no therapy. There were decreases in the use of local therapeutic modalities (surgery and/or radiation therapy) alone without systemic treatment.
Table 2.
Initial treatment for Hoag patients with invasive cancer (including in situ bladder) diagnosed 1986-1991 (n = 5487) and 1992-1999 (n = 10,548). Data shown are percentages of patients receiving such therapy
| Treatment | 1986-1991 | 1992-1999 | P |
|---|---|---|---|
| Surgery only | 36.9 | 31.0 | < .0001 |
| Surgery + radiation therapy | 9.0 | 8.3 | .095 |
| Radiation therapy only | 8.5 | 7.0 | .003 |
| Systemic therapy only* | 8.6 | 9.5 | .082 |
| Surgery + systemic therapy | 9.3 | 10.8 | .003 |
| Radiation + systemic therapy | 8.6 | 9.9 | .004 |
| Surgery, radiation & systemic therapy | 6.7 | 11.9 | .0008 |
| Received a biologic therapy | 1.7 | 6.7 | < .0001 |
| Received any systemic therapy | 24.6 | 42.1 | < .0001 |
| No anticancer therapy | 10.4 | 11.6 | .019 |
Chemotherapy, hormonal therapy, biologic response modifier.
Survival Comparisons With Intramural and Extramural Benchmarks
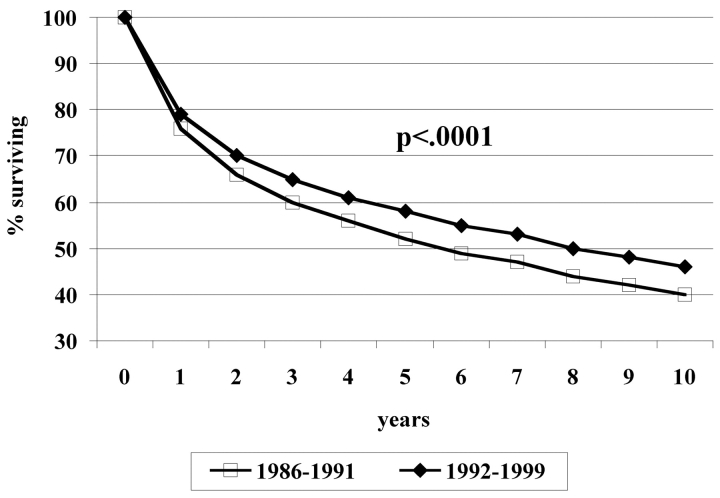
Table 3 shows the number of patients and observed 5-year survival rates and median survivals for Hoag patients diagnosed during 1986-1991 compared with 1992-1999. Median follow-up was more than 5 years in both groups. As shown in Figure 1, for all patients, actuarial 5-year survival rates increased from 52% to 58% and median survival increased by more than 2 years from 70 to 96 months (P < .0001). For individual cancer types, there was significant improvement in survival for cancers of the breast (P = .026), lung (P = .012), prostate (P < .0001), stomach (P = .006), pancreas (P = .0001), and oral cavity (P = .024), with strong trends for improved survival for leukemia (P = .051), and rectal cancer (P = .063). There were no tumor types for which there was a statistically significant decrease in survival over time.
Table 3.
Observed 5-year survival rates by Kaplan-Meier estimate for cancer patients at Hoag Hospital during 1986-1991 vs. 1992-1999
| Tumor Type | No. of Hoag Patients | Hoag 5-Year Survival (%) | Hoag Median Survival (months) | Log-Rank Test | ||||
|---|---|---|---|---|---|---|---|---|
| 1986-1991 | 1992-1999 | 1986-1991 | 1992-1999 | 1986-1991 | 1992-1999 | P | ||
| Breast | 917 | 1986 | 79 | 84 | NR | NR | .026 | |
| Colon | 430 | 573 | 51 | 53 | 65 | 71 | .575 | |
| Rectal | 147 | 211 | 55 | 65 | 74 | 107 | .063 | |
| Esophagus | 46 | 77 | 2 | 12 | 11 | 11 | .430 | |
| Liver | 27 | 60 | 4 | 12 | 5 | 4 | .184 | |
| Melanoma | 228 | 362 | 78 | 79 | NR | NR | .984 | |
| Pancreas | 137 | 202 | 1 | 5 | 6 | 6 | .0001 | |
| Stomach | 62 | 130 | 12 | 23 | 9 | 18 | .006 | |
| Thyroid | 49 | 133 | 90 | 93 | NR | NR | .303 | |
| Cervix | 150 | 183 | 66 | 68 | NR | NR | .849 | |
| Uterus | 198 | 297 | 78 | 75 | NR | NR | .497 | |
| Ovary | 191 | 270 | 39 | 49 | 36 | 57 | .276 | |
| Bladder | 203 | 389 | 64 | 64 | 103 | 101 | .453 | |
| Kidney | 104 | 185 | 54 | 55 | 89 | 91 | .646 | |
| Prostate | 702 | 1539 | 73 | 85 | NR | NR | < .0001 | |
| Testis | 68 | 100 | 88 | 95 | NR | NR | .308 | |
| Lung | 807 | 1455 | 16 | 19 | 11 | 13 | .012 | |
| Larynx | 44 | 75 | 68 | 67 | 107 | 110 | .944 | |
| Oral cavity | 151 | 213 | 49 | 59 | 54 | 98 | .024 | |
| Brain | 127 | 267 | 25 | 22 | 13 | 13 | .682 | |
| Hodgkin's | 47 | 73 | 90 | 80 | NR | NR | .849 | |
| Leukemia | 97 | 373 | 24 | 34 | 13 | 16 | .051 | |
| Lymphoma | 210 | 453 | 54 | 54 | 71 | 72 | .912 | |
| Myeloma | 23 | 108 | 30 | 28 | 34 | 32 | .834 | |
| All patients | 5,487* | 10,548* | 52 | 58 | 70 | 96 | < .0001 | |
Excludes in situ cancer, except for bladder cancer; includes unknown stage at diagnosis, and other less common tumor types that are not listed in the table; so columns do not add up to the numbers on the bottom line. NR, not reached.
Figure 1.
Observed survival for Hoag patients with invasive cancer for 1986-1991 vs. 1992-1999. For 1986-1999, n = 5,487, median age 65.0 years, mean age 62.3 years, 60% deceased. For 1992-1999, n = 10,548, median age 65.8 years, mean age 63.4 years, 47% deceased
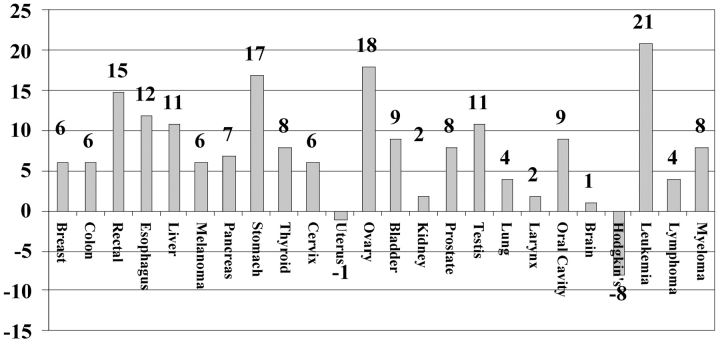
Table 4 shows the number of patients and relative 5-year survival rates for Hoag patients for 1986-1991 and 1992-1999, and relative 5-year survival rates for white patients from SEER data for those periods. For the Hoag cancer patient populations, differences in survival between the two eras by tumor type are shown in Figure 2. There was an eight-percentage-point increase in the relative 5-year survival rate from 63% to 71% for Hoag patients between eras. During the more recent era, the 5-year relative survival rates for Hoag patients were higher for 22 of the 24 specific cancer types (P < .0001, paired t test). The national SEER data during these two time periods also showed an six-percentage-point improvement in relative 5-year survival from 58% to 64%, and survival also was higher for 22 of 24 tumor types (P = .0001, paired t test).
Table 4.
Relative 5-year survival rates for patients with invasive cancer: Hoag and national SEER data for different time periods
| Hoag Relative 5-Year Survival (%) | SEER Relative 5-Year Survival (%) | Hoag Relative 5-year Survival (%) | SEER Relative 5-Year Survival (%) | |
|---|---|---|---|---|
| 1986-1991 | 1986-1991 | 1992-1999 | 1992-1999 | |
| Breast | 89 | 84 | 95 | 88 |
| Colon | 66 | 62 | 72 | 63 |
| Rectal | 68 | 60 | 83 | 62 |
| Esophagus | 3 | 11 | 15 | 15 |
| Liver | 4 | 11 | 15 | 7 |
| Melanoma | 86 | 87 | 92 | 90 |
| Pancreas | 0 | 3 | 7 | 4 |
| Stomach | 12 | 19 | 29 | 21 |
| Thyroid | 90 | 95 | 98 | 96 |
| Cervix | 70 | 71 | 76 | 73 |
| Uterus | 90 | 85 | 89 | 86 |
| Ovary | 39 | 44 | 57 | 52 |
| Bladder | 78 | 82 | 87 | 83 |
| Kidney | 64 | 59 | 66 | 63 |
| Prostate | 92 | 87 | 100 | 98 |
| Testis | 88 | 95 | 99 | 96 |
| Lung | 20 | 14 | 24 | 15 |
| Larynx | 83 | 68 | 85 | 67 |
| Oral cavity | 58 | 55 | 67 | 60 |
| Brain | 25 | 28 | 26 | 32 |
| Hodgkin's | 92 | 81 | 84 | 85 |
| Leukemia | 24 | 41 | 45 | 48 |
| Lymphoma | 55 | 52 | 59 | 57 |
| Myeloma | 30 | 28 | 38 | 31 |
| All patients | 63 | 58 | 71 | 64 |
Excludes in situ cancer, except for bladder cancer; includes unknown stage at diagnosis, and other less common tumor types that are not listed in the table; so columns do not add up to the numbers on the bottom line.
Figure 2.
Percentage point differences in relative 5-year survival rates for Hoag cancer patients diagnosed during 1986-1991 vs. 1992-1999
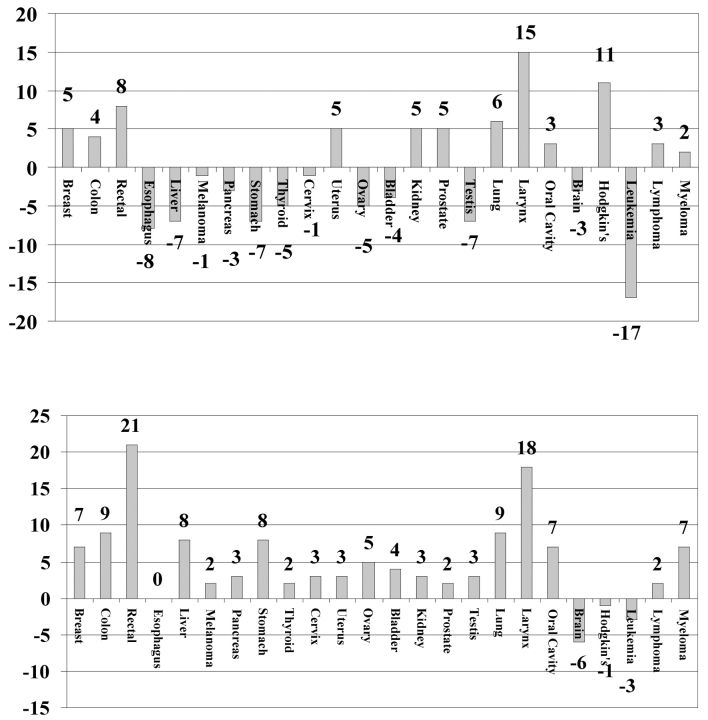
Differences in survival for Hoag compared with contemporary SEER data are shown in Figure 3 for 1986-1991 and for 1992-1999. During the pre–cancer center era, relative survival rates for Hoag patients were the same as or higher than SEER in only 12 (50%) of 24 malignancies compared with 21 (87%) of 24 after opening of the cancer center (P = .013, Fisher's exact test). The relative 5-year survival rates for the 24 different tumor types did not differ significantly between the SEER and Hoag populations during 1986-1991 (P = .85), but during 1992-1999 the relative 5-year survival rates for the 24 different tumor types were higher in the Hoag population compared with the SEER population (P = .001, Fisher's exact test).
Figure 3.
Percentage point differences in relative 5-year survival rates for Hoag vs. SEER 1986-1991 (top) and 1992-1999 (bottom)
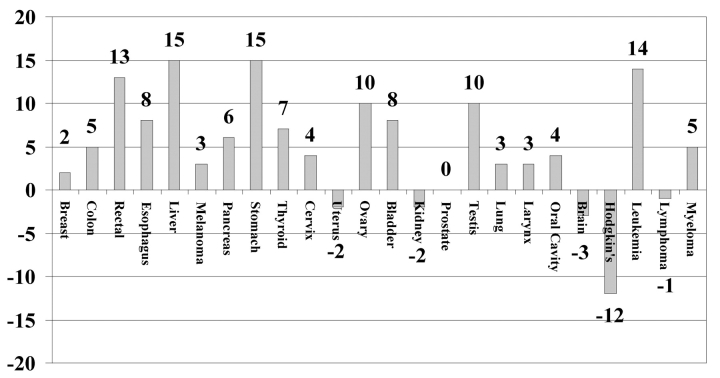
As shown in Figure 4, compared with 1986-1991, the difference in relative survival percentages (Hoag minus SEER) increased or stayed the same for 19 of 24 tumor types during 1992-1999 (P = .010, Fisher's exact test). The differences in prostate cancer were scored as 0 because Hoag relative 5-year survival was already 100% during 1986-1991. Thus, the rate of improvement in proportions of patients with a relative survival of at least 5 years, by individual tumor type, was faster for the Hoag cancer patients than nationally.
Figure 4.
Percentage point differences in relative 5-year survival rates for Hoag minus SEER for 1986-1991 compared to Hoag minus SEER for 1992-1999
Specific Tumor Stage Subset Comparisons
Table 5 shows the results for subset analyses that were carried out for the cancers for which there were more than 500 Hoag patients in both eras: lung, breast, colorectal, and prostate. In the intramural historical comparisons of proportions, in lung cancer during 1992-1999, more patients had local disease (P = .002) and fewer regional disease (P = .026) at the time of diagnosis. The proportion of lung cancer patients with a relative survival of more than 5 years increased (P = .032), although none of the differences by stage in the proportion with relative survival of 5 years was statistically significant. In breast cancer, there were no differences in stage distribution, but during 1992-1999, there was a higher proportion of patients with relative survival of more than 5 years for the group as a whole (P = .003), and for subsets of patients with local (P = .002) and regional (P = .006) extent of disease. In prostate cancer there was an increase in the proportion of patients with local and regional disease (P < .0001), a decrease in the proportion with distant metastases at diagnosis, and an increase in the proportion of patients with regional disease who had a 5-year relative survival, and for all prostate cancer patients as a group (P < .0001). In colorectal cancer, during 1992-1999 there were no significant changes in stage distribution, but there was an increased proportion of patients with 5-year relative survival for local (P < .0001) and regional stages of disease (P = .020), and for all patients collectively (P < .0001).
Table 5.
Stage distribution and relative 5-year survival by stage for the four most prevalent malignancies for Hoag patients 1992-1999 compared to 1986-1991
| % Stage 1986-1991 | % Stage 1992-1999 | P | % 5-Year Survival 1986-1991 | % 5-Year Survival 1992-1999 | P | |
|---|---|---|---|---|---|---|
| LUNG | n = 807 | n = 1,455 | n = 807 | n = 1,455 | ||
| Local | 19 | 25 | .002 | 61 | 60 | .669 |
| Regional | 26 | 22 | .026 | 20 | 23 | .459 |
| Distant | 48 | 50 | .365 | 5 | 6 | .516 |
| BREAST | n = 916 | n = 1,987 | n = 916 | n = 1,987 | ||
| Local | 65 | 66 | .620 | 99 | 100 | .002 |
| Regional | 28 | 29 | .595 | 81 | 88 | .006 |
| Distant | 5 | 4 | .165 | 20 | 18 | .620 |
| PROSTATE | n = 702 | n = 1,539 | n = 702 | n = 1,539 | ||
| Local | 63 | 78 | < .0001 | 100 | 100 | 1.00 |
| Regional | 19 | 12 | < .0001 | 89 | 100 | < .0001 |
| Distant | 9 | 3 | < .0001 | 37 | 42 | .480 |
| COLORECTAL | n = 538 | n = 931 | n = 538 | n = 931 | ||
| Local | 31 | 36 | .061 | 92 | 100 | < .0001 |
| Regional | 46 | 42 | .315 | 76 | 84 | .020 |
| Distant | 20 | 19 | .573 | 9 | 14 | .303 |
Some cases were classified as “stage unknown,” so percentages do not add up to 100%.
In the extramural contemporary comparisons of proportions (data not shown), in lung cancer there were higher proportions of Hoag patients with local and distant disease, but a lower proportion with regional disease (all P < .0001), while Hoag patients had a higher proportion surviving 5 years in all stages of disease: local (P < .0001), regional (P = .015), and distant (P < .0001). In breast cancer there was no significant difference in proportions in various stages of disease, but Hoag patients had a higher proportion surviving 5 years for local and regional stages of disease (both P < .0001). In prostate cancer Hoag had a higher proportion with local/regional disease (P < .0001), but the survival differences in the subsets did not differ significantly. In colorectal cancer, a higher proportion of Hoag patients had regional disease at diagnosis (P = .011), and a higher proportion survived more than 5 years in each subset: local (P < .0001), regional (P < .0001), and distant (P = .016).
Discussion
Summary of Results
This study shows that the creation of a community hospital–based cancer center and its programs was associated with improvement in patient outcome, as measured by intramural comparisons of observed and relative 5-year survival rates for cancer patients diagnosed and/or managed at the center during 1992-1999 as compared with 1986-1991. Furthermore, survival rates were higher for the center's patients compared with a contemporary external comparator group, and the changes in relative survival between the two periods were more pronounced for the center's patients than the survivor improvement observed nationally.
Impact of Earlier Diagnosis and Systemic Therapy
On the basis of the treatment changes identified in Table 2, and subset analyses in the most prevalent cancers, the improved survival appears to be due to a combination of detection of disease during localized stage in a higher proportion of patients, increased application of multimodality approaches to regionally advanced cancer, and systemic therapy in patients with distant metastases. Earlier diagnosis due to increased use of screening procedures for cancers of the breast, prostate, and lung likely resulted in patients' having more limited local disease than in the earlier era, so that surgery alone had a greater chance of being potentially curative. In addition, there was improved survival in more advanced stages of disease in association with greater use of systemic therapy. Analysis of national survival data has also suggested that improved survival nationally has been due to a combination of both earlier diagnosis and more effective therapeutic interventions.21
Limitations of This Study
The major limitation of this study is the lack of certainty regarding the comparability of the center's patient populations in the two periods of observation. Unfortunately, there is insufficient information available in these databases regarding prognostic variables other than those related to sex, age, race, and stage of disease. Some of the apparent improvement in survival is probably due to lead- and length-time bias. Lead-time bias may also account for the increased proportions of patients with limited-stage disease for certain tumor types such as lung and prostate cancer. However, stage-to-stage comparisons showed improved survival for local and for regional breast cancer and for both local and regional colorectal cancer compared with the earlier period. There were corresponding trends toward increased survival in regionally advanced lung and prostate cancer.
Importance of This Study
It has been estimated that 85% to 90% of cancer care in the United States is delivered in community settings.5 One strength of this study is that it focuses on a community population of patients, most of whom receive their primary and cancer care through physicians on the medical staff at one hospital. This population has not experienced substantial changes in economic status, ethnic and racial mix, or age distribution during the 15 years covered in this analysis. Less than two percent of these patients were diagnosed at Hoag but went elsewhere for their primary cancer treatment, whereas more than 80% had their cancer diagnosed at Hoag and received their initial cancer treatment at Hoag. Our experience shows that a comprehensive community cancer center, with programs that enhance specialized patient care and promote and facilitate education, communication, and coordination of care among private practitioners, was associated with improved survival for cancer patients. Opening the Hoag Cancer Center facility was associated with both weekly multidisciplinary case conferences, as well as weekly oncology education programs. In addition, the physical proximity of offices for radiation and medical oncologists almost certainly facilitated communication among these specialists. We believe that Hoag Cancer Center outreach programs increased awareness of and compliance with cancer screening test recommendations in the lay population and also among primary care physicians.
In the last 20 years there have been many major advances in the management of cancer patients. First, there has been increased emphasis on early detection and acceptance of screening for various malignancies. Second, there have been tremendous improvements in diagnostic technology including mammography, computed tomography, magnetic resonance imaging, and photon emission tomography. These have contributed to earlier diagnosis, and more sensitive detection of regional and distant metastatic disease. Third, there has been increased acceptance of systemic therapy in general, and specifically in the adjuvant setting. The decade of the 1990s saw the emergence of biologic therapies, such as cytokines, including the hematopoietic growth factors and interleukin-2, and monoclonal antibodies, such as rituximab and trastuzumab, as important systemic modalities for the treatment of many cancer patients.22 Existence of the Hoag Cancer Center likely accelerated adoption of many treatment advances and incorporation of new systemic agents into treatment regimens. We do not have specific data regarding which programs were most important in effecting these changes in practice, but it seems likely that changes did take place, at least in part, because of the transmission of new information in multidisciplinary case conferences and education programs, and the communication of survival outcomes and patterns of practice to the medical staff of Hoag Hospital. The improved survival associated with existence of Hoag Cancer Center may provide added impetus for other hospitals and communities to invest in creation of such physical facilities and comprehensive cancer programs.
Acknowledgment
We thank Ken Devane, PhD, for his review of the manuscript and statistical input, and Kanoe Allen, BSN, for her assistance in data entry.
Footnotes
Authors' Disclosure of Potential Conflicts of Interest
The following authors or their immediate family members have indicated a financial interest. No conflict of interest exists for drugs or devices used in a study if they are not being evaluated as part of the investigation. Employment relationship: Robert O. Dillman, Sherri D. Chico, Hoag Cancer Center.
References
- 1.Rusch HP: The beginnings of cancer research centers in the United States. J Natl Cancer Inst 74:391-403, 1985 [PubMed] [Google Scholar]
- 2.Yarbro JW, Newell GR: Cancer centers: Their relationship to the academic community. J Med Educ 51:487-495, 1976 [PubMed] [Google Scholar]
- 3.Steckel RJ: The NCI's Cancer Centers Program: Past, present, and future. Med Pediatr Oncol 13:59-64, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Shingleton WW: Cancer centers: Origins and purpose. Arch Surg 124:43-45, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Simone JV: Understanding cancer centers. J Clin Oncol 20:4503-4507, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Lokich JJ, Silvers S, Brereton H, et al: Free-standing cancer centers: Rationale for improving cancer care delivery. Am J Clin Oncol 12:402-406, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Dillman RO, Soori G, DePriest C, et al: Treatment of human solid malignancies with autologous activated lymphocytes and cimetidine: A phase II trial of the Cancer Biotherapy Research Group. Cancer Biother Radiopharm 18:727-733, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Dillman RO, Duma CM, Schiltz PM, et al: Intracavitary placement of autologous lymphokine-activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother 27:398-404, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Dillman RO, Schiltz PM, DePriest C, et al: Tumor-infiltrating lymphocytes and interleukin-2: Dose and schedules of administration in the treatment of metastatic cancer. Cancer Biother Radiopharm 19:730-737, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Dillman RO, DeLeon C, Beutel LD, et al: Short-term autologous tumor cell lines for the active specific immunotherapy of patients with metastatic melanoma. Crit Rev Oncol Hematol 39:115-123, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Dillman RO, Beutel LD, Barth NM, et al: Irradiated cells from autologous tumor cell lines as patient-specific vaccine therapy in 125 patients with metastatic cancer: Induction of delayed-type hypersensitivity to autologous tumor is associated with improved survival. Cancer Biother Radiopharm 17:51-66, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Dillman R, Barth N, VanderMolen L, et al: Autologous tumor cell line-derived vaccine for patient-specific treatment of advanced renal cell carcinoma. Cancer Biotherapy Radiopharm 19:570-580, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Dillman R, Selvan S, Schiltz P, et al: Phase I/II trial of melanoma patient-specific vaccine of proliferating autologous tumor cells, dendritic cells, and GM-CSF: Planned interim analysis. Cancer Biother Radiopharm 19:658-665, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Dillman RO, Barth NM, VanderMolen LA, et al: High-dose chemotherapy and autologous stem cell rescue for metastatic breast cancer: Superior survival for tandem transplants compared to single transplants. Am J Clin Oncol. (in press) [DOI] [PubMed]
- 15.Parker SL, Tong T, Bolden S, et al: Cancer statistics, 1996. CA Cancer J Clin 46:5-28, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Jemal A, Tiwari RC, Murray T, et al: Cancer statistics, 2004. CA Cancer J Clin 54:8-29, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Henson ED, Ries LA: The relative survival rate. Cancer 76:1687-1688, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Kaplan G, Meier P: Non-parametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 19.Gloeckler Ries LA, Reichman ME, Lewis DR, et al: Cancer survival and incidence from the Surveillance, Epidemiology and End Results (SEER) program. Oncologist 8:541-552, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Fleming ID, Cooper JS, Henson DE, et al: AJCC Cancer Staging Manual, ed 5, Philadelphia, PA, Lippincott Raven Publishers, 1997
- 21.Jemal A, Clegg LX, Ward E, et al: Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer 101:3-27, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Oldham RK: Principles of Cancer Biotherapy, ed 4, The Netherlands, Kluwer Academic Publishers, 2003