Abstract
The thermal, photochemical, and photochemical/CuI-mediated cascade cyclizations of a range of substituted 1-(2-azidophenyl)-3-alkenylallenes are described. These reactions provide both 1,2- and 2,3-cyclopentennelated indole products in varying ratios. In most cases, high regioselectivity for the 2,3-annelated isomer can be achieved under the hv/CuI conditions. Computational studies of this multistep reaction support the intermediacy of indolidene intermediates whose electrocyclizations (with or without copper present) define the regioselectivity branch point in the sequence.
Introduction
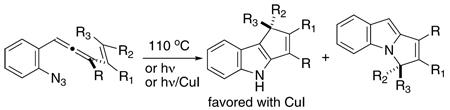
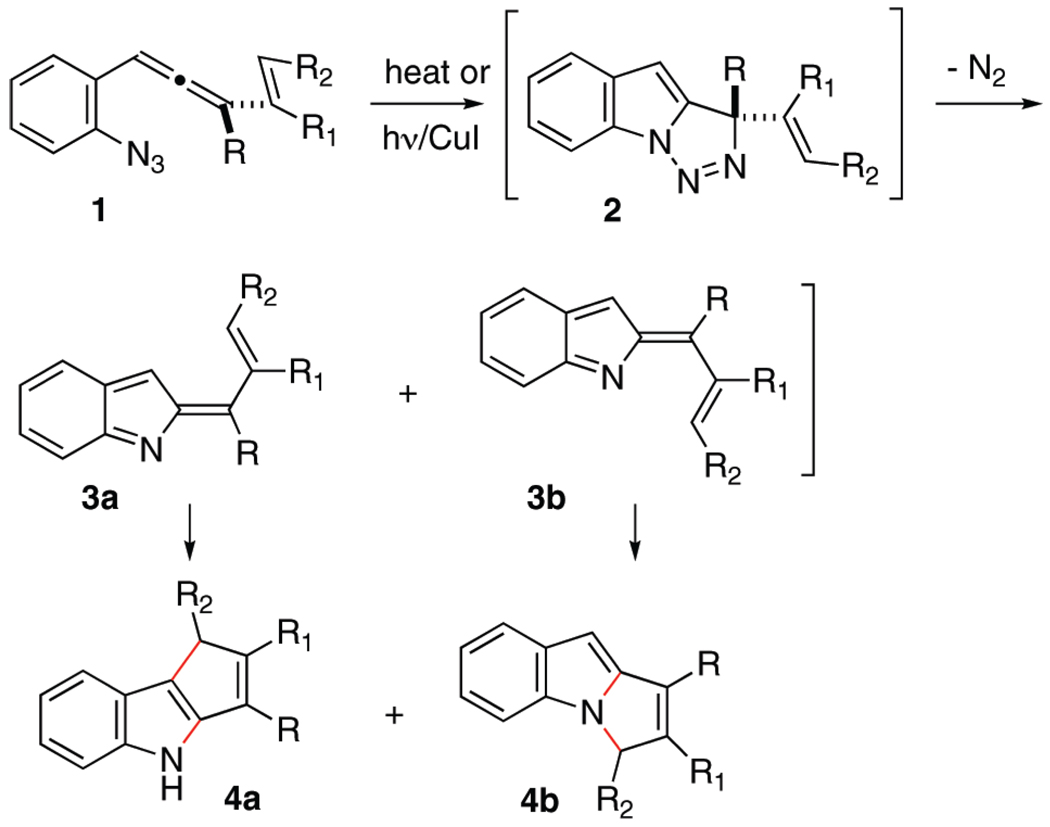
Cascade cyclization sequences offer great promise for the expeditious assembly of polycyclic products. To the extent that these processes convert readily accessible cascade precursors to polycyclic products with predictable control of relevant selectivity issues (i.e., chemoselectivity, regioselectivity, stereoselectivity, enantioselectivity), these transformations may impact favorably on total synthesis efforts directed toward complex polycyclic natural products. It is within this context that the allenyl azide cascade cyclization sequence was developed, as the potential for accessing cyclopentennelated indole products from simple ortho-positioned (3-alkenyl)allene phenylazide substrates was apparent, Scheme 1. Preliminary results documented that species 1 under diverse reaction conditions rapidly and cleanly proceeded to the regioisomeric indole products 4a and 4b.1a Furthermore, reaction conditions could be identified that led to high levels of regioselectivity for the C–C-bonded indole product 4a.1b Computational tools were brought to bear on this complex cascade sequence, and the initial mechanistic picture that emerged from these calculated models implicated indolidenes 3a and 3b as pivotal intermediates linking allenyl azide 1 to products 4a and 4b.2 In addition, these in silico models provided a rationale for the regioselectivity (or lack thereof in certain circumstances) observed.
SCHEME 1.
Cyclization Cascade of 1-(2-Azidophenyl)-3-vinylallenesa
aThe new bonds are indicated in red.
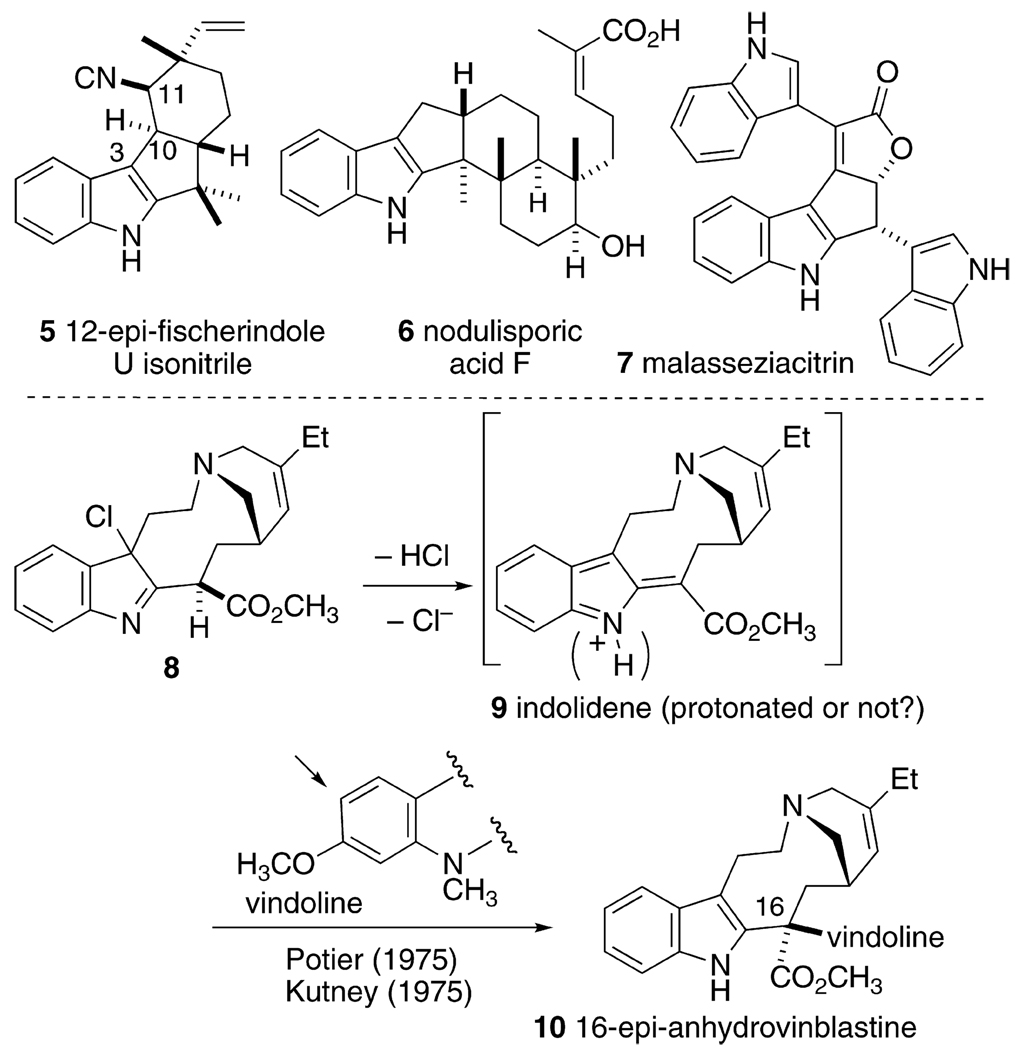
Potential natural product targets for this developing methodology are plentiful, provided that both stereochemical and regiochemical requirements can be met by the allenyl azide cascade cyclization. For example, the Fisher indoles (cf. 5),3 the indole diterpenes (cf. 6),4 and the unique triindole yeast isolate malasseziacitrin (7)5 all fall under this general structural class, Scheme 2. Reaction chemistry that evolves from reactive indolidene intermediates of the type 3 has not been well described.6 One notable exception involves the proposed intermediacy of indolidene (or protonated indolidene) species in the syntheses of ibogaine derivatives and dimeric alkaloids of the vinblastine series, Scheme 2.7 The fact that this putative intermediate is reactive (electrophilic) enough to efficiently trap the sterically hindered and modestly nucleophilic aryl ring of vindoline suggests that further development of the reaction chemistry of indolidenes has the potential for advances in several areas of indole synthesis/functionalization.
SCHEME 2.
Representative 2,3-Cyclopentennelated Indole Natural Products and the Historical Precedent for Invoking Indolidenes in Indole-Containing Natural Product Synthesis
In this full accounting of our work in this area, the scope of cyclopentennelated indole synthesis is investigated by examining several new allenyl azide substrates that probe the limits of the methodology. In addition, new calculational results that aid in elucidating mechanistic subtleties are presented; these calculations examine the “real system” and so extend beyond the model system calculations reported earlier.
Results and Discussion
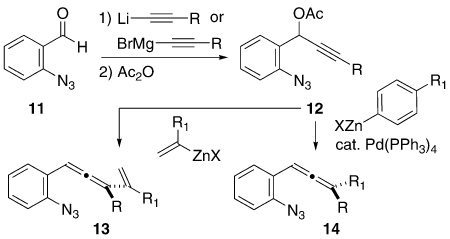
The exploratory studies commenced with the preparation of some simple 1-(2-azidophenyl)-3-alkenylallenes 13 and the analogous 1-(2-azidophenyl)-3-arylallenes 14, Table 1. The propargylic acetate displacement chemistry of Krause proved completely serviceable in this task, using either commercially available zinc nucleophiles or zinc species generated in situ from the corresponding Grignard reagent and ZnCl2.8 All of the allynyl azide substrates prepared in this study are racemic, and these species are a mixture of diastereomers when a second stereogenic element is present. The steric character of the substituent R directly attached to the allene was varied significantly (CH3 → CH2OTBS → Ph → TMS → t-Bu) in order to probe the consequences of steric bulk on the partitioning between 3a/3b and/or 4a/4b. As it transpired, an unexpected diversion of reactive intermediates with the R = TMS case (vide infra) limited the value of this entry in the steric comparison series. In this series of substrates, the 1-alkenyl position (i.e., R1) was subjected to some variation also (H → CH3 → Ph) with the expectation that R//R1 clashes (cf. 3a/3b) might also influence the regioselectivity profile of the reaction. The aryl series 14j–m was designed to probe for electronic effects on product formation (yield, regioselectivity), but the route illustrated by the conversion of 12 (R = CH3) into 14 did not afford products that survived attempts at chromatographic (SiO2) purification. Therefore, the crude allenyl azides 14j–m were subjected to thermal rearrangement without further purification. Subsequently, we determined that slightly modifying the sequence so that a methyl nucleophile was added to a phenyl-substituted propargyl acetate (12j, R = Ph) did furnish an allenyl azide 14j that tolerated passage over SiO2 and could be isolated as a pure, discrete compound. However, disappointing results upon thermolysis (vide infra, Scheme 5) did not encourage revisiting the syntheses of the other aryl substrates 14k–m.
TABLE 1.
Simple Vinyl- and Phenyl-Substituted (o-Azidophenyl)allene Synthesis
 | |||||
|---|---|---|---|---|---|
| entry | R | R1 | yield of 12a(%) |
yield of 13a(%) |
yield of 14a(%) |
| a | CH3 | H | 48 | 58 | |
| b | CH2OTBS | H | 91 | 55 | |
| c | (CH2)2OTBS | H | 73 | 57 | |
| d | t-Bu | H | 78 | 63 | |
| e | CH3 | Ph | 48 | 67 | |
| f | CH3 | CH3 | 48 | 59 | |
| g | Ph | CH3 | 79 | 69 | |
| h | TMS | CH3 | 96 | 62 | |
| i | H | CH3 | 82 | 49 | |
| j | Ph | CH3b | 79 | 81 | |
| k | CH3 | p-(CH3O)C6H4 | 48 | c | |
| l | CH3 | m-(CH3O)C6H4 | 48 | c | |
| m | CH3 | p-FC6H4 | 48 | c | |
Isolated, chromatographically purified material.
CH3MgBr/CuI/LiBr on 12j (R = Ph).
The allenes 14k–m were not isolated but rather carried forward to the thermolysis reaction directly. See Scheme 5 for details.
SCHEME 5.
Cascade Cyclization Attempts with Aryl-Substituted 1-(2-Azidophenyl)allene Substrates 14j–m
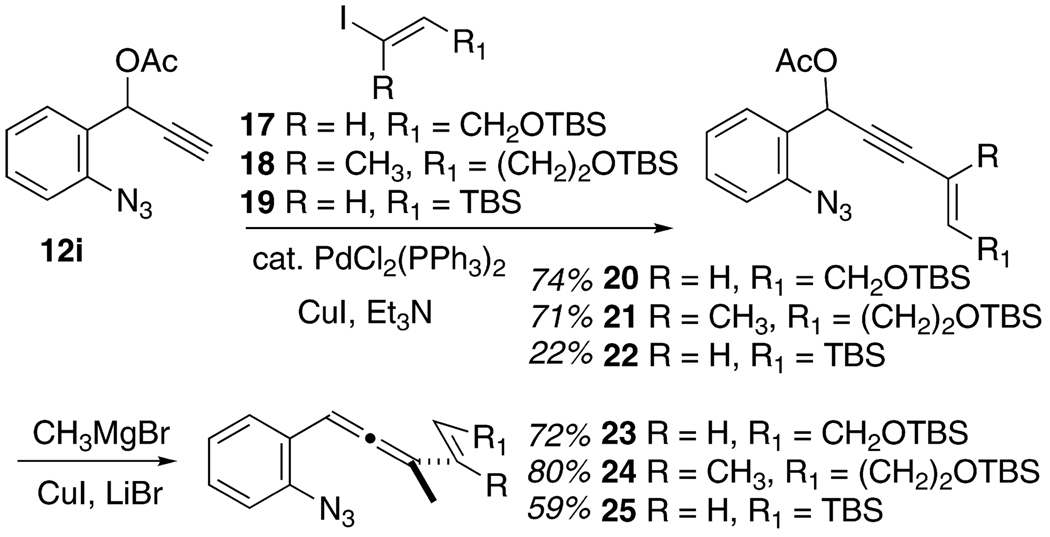
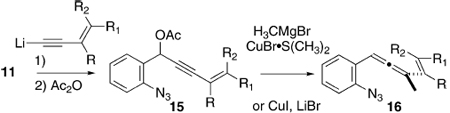
A related series of 1-(2-azidophenyl)-3-alkenylallenes 16 and 23–25 (Table 2 and Scheme 3, respectively) were prepared by slightly different routes than those described above. For the synthesis of 15, a preformed alkenyl acetylide anion was employed to introduce the propargyl acetate functionality, and then methyl cuprate was utilized as the allene-forming nucleophile. The preparation of 23–25 featured a Sonogashira coupling of the preformed propargyl acetate 12i with the appropriate alkenyl iodide fragment 17–19 to deliver the enyne acetates 20–22. The cuprate chemistry was relied upon again to deliver the allene product. These substrates introduced one (or two) substituents at the alkene terminus, a position destined to participate directly in C–C or C–N bond formation (cf. the red bonds in 4a/4b). In this way, the influence of steric effects (primarily) and possibly electronic effects can be assessed upon attempted cyclopentennelated indole formation. The substrate 16b in particular will test the premise that even very challenging all-carbon quaternary centers might be forged through this methodology.
TABLE 2.
Synthesis of More Complex 1-(2-Azidophenyl)-3-alkenylallenes
 | |||||
|---|---|---|---|---|---|
| entry | R | R1 | R2 | yield of 15a(%) |
yield of 16a(%) |
| a | H | Ph | H | 47 | 34 |
| b | Ph | Ph | Ph | 78 | 38 |
| c | –(CH2)3– | H | 49 | 30 | |
| d | –(CH2)4– | H | 31 | 37 | |
Isolated, chromatographically pure material.
SCHEME 3.
Synthesis of Additional (o-Azidophenyl)allenes
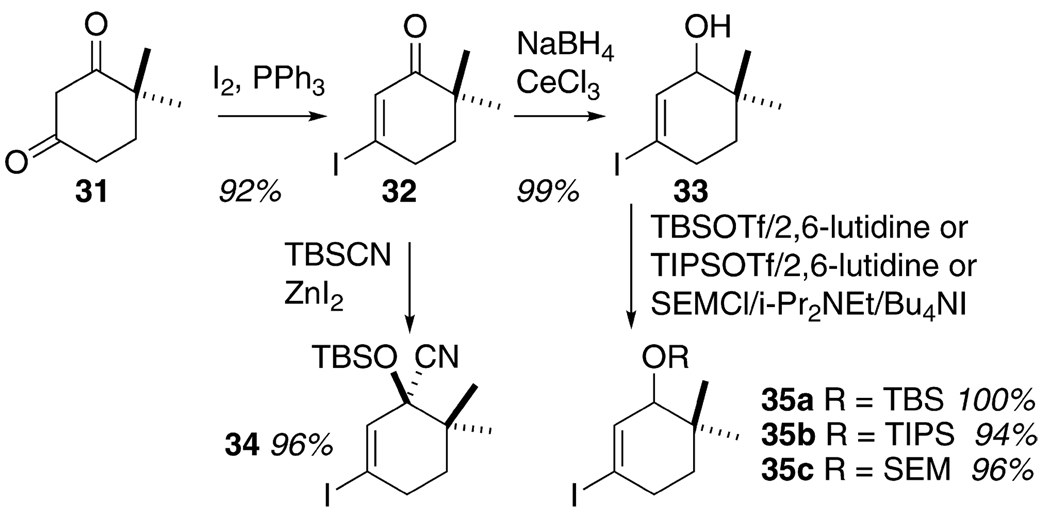
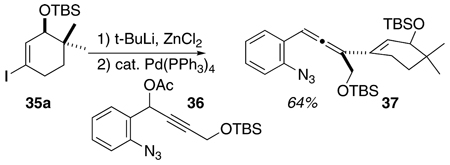
A series of cyclohexenyl alcohol-based substrates were prepared in order to test the feasibility of accessing the tetracyclic skeleton of the Fisher indoles via this methodology (cf. 5, Scheme 2, Table 3, and eq 1). The majority of these species were prepared by application of the chemistry developed for Scheme 3; Sonogashira coupling of 12i with the requisite cyclohexenyl iodide 26 followed by alkyl cuprate-mediated propargylic displacement of the acetate function in 27 to deliver the allene product 28. Since the cuprate required to prepare 37 (see eq 1) was not available, we resorted to the Krause chemistry with the highly functionalized cyclohexenyl zincate derived from 35a and the propargyl acetate 36, itself prepared from 11 and the TBS ether of propargyl alcohol. The various cyclohexenyl iodides 34 and 35a–c were synthesized from the commercially available dione 31 using routine and well-documented chemistry, Scheme 4.9 The free hydroxyl substrate 29 was conveniently available by desilylation of 28a. The ketone 30, prepared via oxidation of 29, was synthesized as well. The rationale behind the choice of these cyclohexenyl substrates was 2-fold; (1) the relationship between substrate structure and product regiochemistry was still underdeveloped at this early juncture of the research, and so probing the influence of allylic oxygen substitution (sterics, electronics?) on C–C vs C–N bond formation seemed appropriate, and (2) for the first time, the issue of relative stereochemistry, as is relevant to the C(10)/C(11) stereogenic centers in the Fisher indoles (cf. 5, Scheme 2) can be addressed–how will the preexisting C(11) stereogenic center (–OR) influence the stereochemical outcome at C(10) upon C(3)/C(10) bond formation during electrocyclization of an indolidene species related to 3a?
TABLE 3.
Preparation of Functionalized Cyclohexenyl-Substituted 1-(2-Azidophenyl)-3-(1-cyclohexenyl)allenes
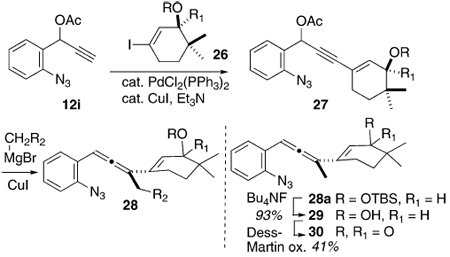 | |||||
|---|---|---|---|---|---|
| entry | R | R1 | R2 | yield of 27a(%) |
yield of 28a(%) |
| a | TBS | H | H | 79 | 92 |
| b | TIPS | H | H | 92 | 89 |
| c | SEM | H | H | 63 | 86 |
| d | TBS | CN | H | 76 | 81 |
| e | TBS | H | TMS | 79 | 85 |
Isolated, chromatographically pure material.
SCHEME 4.
Preparation of Functionalized Cyclohexenyl Iodides
 |
(1) |
The initial attempts at cascade cyclization of the 1-(2-azidophenyl)-3-alkenylallenes followed the experimental protocol identified previously for the successful reaction of the simple saturated-linker 1-azido-3,4,6-hepatrienes.10 Those processes furnished cyclopentennelated dihydropyrrole products via an azatrimethylenemethane intermediate, and at the outset of this work, there was no reason, a priori, to expect that the phenyl-linked analogues would proceed through an alternative pathway. In fact, this argument-by-analogy proved to be mechanistically misleading (vide infra), although it led to satisfactory results in a practical sense. Specifically, heating a 100 mM toluene solution of the parent allenyl azide 13a led efficiently to two new products in a 1.4:1 ratio following SiO2 chromatography, Table 4. Spectroscopic analysis of the minor component left little doubt that an indole product 38a was formed, presumably via formation of a C–C bond between C(3) and C(10) (Fisher indole numbering). However, the structure of the major product was a bit of a puzzle, as the methylene unit of this species appeared at a somewhat smaller value in the 1H NMR spectrum (3.77 ppm) than expected for the anticipated C–N cyclized product 39a. Adding to the mystery was the observation that examination of the crude thermosylate by 1H NMR spectroscopy did not reveal any of the signals for this compound; rather, a similar but still different species was present, with a methylene singlet at 4.53 ppm. The structure of this major isolated isomer tentatively was assigned as the pyrrole 40a after comparison with the spectroscopic data from the desmethyl analogue of 40a, a compound reported earlier by Kashulin and Nifant’ev11 and later secured by single-crystal X-ray analysis (see the Supporting Information for details). It appears that the indole isomer 39a was formed first as expected, but it suffered tautomerization to the pyrrole form 40a upon exposure to SiO2. Thus, this index example offered the hope that the cyclization cascade might be developed into a high-yielding route to cyclopentennelated indoles (96% yield overall), but it also suggested that modifications were necessary in order to meet the goal of regioselective formation of the C–C-bonded isomer 38.
TABLE 4.
Cyclization Cascade of Substituted Vinyl (o-Azidophenyl)allenes To Furnish Indole and Pyrrole Products
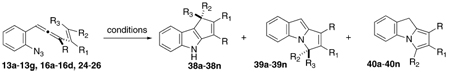 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 110 °Ca |
hvb |
hv/CuIc |
|||||||||||
| entry | allenyl azide | R | R1 | R2 | R3 | 38d(%) | 39d(%) | 40d(%) | 38d(%) | 39d(%) | 40d(%) | 38d(%) | 39d(%) |
| a | 13a | CH3 | H | H | H | 40 | 56 | 24 | 20 | 42 | |||
| b | 13c | (CH2)2OTBS | H | H | H | 52 | 43 | 24 | 16f | 36 | |||
| c | 13d | t-Bu | H | H | H | 57 | 20 | 53 | 38 | 59 | |||
| d | 13b | CH2OTBS | H | H | H | 22 | 29 | 24 | 33 | 55 | 3 | ||
| e | 13e | CH3 | Ph | H | H | 40 | 30 | 44 | 31 | 67 | |||
| f | 13f | CH3 | CH3 | H | H | 36 | 36 | 38 | 35 | 54 | |||
| g | 13g | Ph | CH3 | H | H | 47 | 39 | 23 | 11e,f | 69f | |||
| h | 23 | CH3 | H | CH2OTBS | H | 24 | 34 | decomp | decomp | ||||
| i | 25 | CH3 | H | TBS | H | 33 | 7 | 25 | 9 | 44f | |||
| j | 16a | CH3 | H | Ph | H | (33)e | 35 | decomp | decomp | ||||
| k | 16c | CH3 | –(CH2)3– | H | (36)e | 40 | not examined | not examined | |||||
| l | 16d | CH3 | –(CH2)4– | H | 36 | 51 | 32 | 27 | 54 | ||||
| m | 24 | CH3 | CH3 | (CH2)2OTBS | H | 7 | 40 | (20)e,g | (40)e,g | 42g | (42)e,g | ||
| n | 16b | CH3 | Ph | Ph | Ph | 45 | 21 | 51 | 31 | 53 | 26 | ||
100 mM in toluene.
254 nm in CH3CN (5 mM).
254 nm in CH3CN (5 mM), 150 mol % of CuI.
Isolated, chromatographically purified material.
Not isolated; estimated by integration of the 1H NMR spectrum of the crude reaction mixture.
Irradiated at 300 nm.
Irradiated at 350 nm.
One such modification might involve changing the size and/or electronic character of the substituents R, R1, and R2 in the hope that steric discrimination in the transition states for bond formation might translate into regioselectivity. Toward that end, the size of the allene substituent “R” was increased from methyl (entry a) to (CH2)2OTBS to t-Bu (entries b and c, respectively). Similar to the parent 13a, thermolysis of the substrates 13b and 13c furnished products in high chemical yield. The regioselectivity did respond to these steric changes, with the preference for the C–C bonded isomer 38 increasing as the size of the R unit increased (1:1.4 for CH3 to 1:1.2 for (CH2)2OTBS to 2.9:1 for t-Bu). The regioselectivity for the related R = CH2OTBS case (entry d) was similar to the R = CH3 case, but these results may be compromised by the overall lower yield.
Examination of steric effects at the R1 site of 13 extended to just two compounds, R1 = CH3 and R1 = Ph, entries f and e, respectively. Thermolysis of the R1 = CH3 compound 13f furnished the two products 38f and 40f in equal proportions (Table 4, entry f). Enlarging R1 to a phenyl substituent (Table 4, entry e) marginally increased the proportion of the C–C-bonded product 38e upon thermolysis of 13e. However, in neither case did the ratio of C–C to C–N bonded products vary significantly from the index case 13a (R1 = H). Thus, introducing steric bulk at the R1 site had little effect. As a variation on this theme, the R and R1 substituents were switched in entry g (R = Ph, R1 = CH3) in order to see if the same interaction (CH3//Ph) when approached from the “other” direction had any different consequence. In fact, the C–C/C–N ratio (38g/40g = 1.2) upon thermolysis of 13g fell in line with the earlier values of entry e (38e/40e = 1.3). Thus, there is no evidence to support the notion that a steric interaction between the R and R1 substituents has any material influence on the overall regiochemistry of product formation.
Variation in the steric bulk at the R2 position was expected to have the most profound effect on product regioselectivity since R2 was right at the bond-forming site. This expectation was partially supported experimentally, as thermolysis of allenyl azide substrates with R2 = CH2OTBS and R2 = Ph led to product regioisomer ratios that scarcely varied from 1:1, but when R2 = the larger TBS, the C–C bonded product was strongly favored (~ 5:1) over the C–N alternative. Note that the C–C-cyclized species 38j did not survive SiO2 chromatography, and so the reported yield derives from integration of characteristic signals in the 1H NMR spectrum of the crude thermolysate. The N-cyclized species featured an indole unit 39 and not the previously observed pyrrole moiety of isomer 40. Speculation about the absence of isomerization in this series might include the argument that planarization of the R2-bearing carbon upon pyrrole-forming isomerization would bring R2 and the peri-positioned aryl hydrogen in conflict, a steric clash that is absent in the R2 = H substrates. The structural assignment of 39j was confirmed by single crystal X-ray analysis of the derived hydrogenation product.1a The lack of correlation between steric bulk at R2 and regioselectivity of terminal bond formation was confounding at first, but a more thorough mechanistic analysis, guided by density functional calculations, provided a consistent rationale by placing the regioselectivity-determining event earlier on the reaction coordinate than the final C–C (→ 38) vs C–N (→39/40) bond closures (vide infra).
Three disubstituted alkene substrates, 16c, 16d, and 24, were examined in the thermolysis reaction in order to extend the scope of the transformation. The cyclic substrates 16c (cyclopentenyl) and 16d (cyclohexenyl) both participated in the cyclization cascade, but provided divergent types of products. The cyclohexenyl case (Table 4, entry l) delivered the expected C–C cyclized product 38l and the isomerized C–N-cyclized regioisomer 40l in a ratio slightly favoring the latter species. The cyclopentenyl substrate 16c, on the other hand (Table 4, entry k), provided only the unisomerized C–N bonded indole product 39k following SiO2 chromatography. Signals characteristic of the tentatively assigned C–C bonded product 38k could be detected in the 1H NMR spectrum of the crude thermolysate in nearly equal amounts with 39k, but this species did not survive the purification procedure. The differing stabilities of the similar tetracycles 38k and 38l to chromatography are surprising. Identifiable decomposition products from 38k (or 38j, another C-cyclized species intolerant to SiO2) were not isolated, and so the basis for this sensitivity remains unknown. The final disubstituted alkene substrate 24 was subjected to thermolysis, leading to a preponderance of the sensitive N-cyclized product 40m and only a trace of the C–C-bonded isomer 38m.
A further extension of this alkene substitution theme led to the trisubstituted alkene analogue 16b. This species was designed to test the premise that an all-carbon quaternary center might be accessible through this methodology. Thermally initiated cascade cyclization of 16b proceeded in good yield to afford an approximately 2:1 ratio of the C–C/C–N-bonded products 38n and 39n, respectively. The structural assignment of the C–N-bonded isomer 39n was based on single-crystal X-ray analysis (see the Supporting Information). Overall, the successful thermal cascade cyclization of the disubstituted alkene substrates 16c and 16d and the trisubstituted higher homologue 16b do extend the scope of the methodology in potentially useful ways, but they do not lend any insight into the factors that might influence the key issue of regioselectivity upon terminal bond formation. In the final analysis, all of the efforts to probe the relationship between steric bulk and reaction regioselectivity for the three conceivable substrate variables R, R1, and R2 did not provide either promising results or even enough encouraging information to craft a useful model for further developing a substrate-based strategy to favor the formation of the C–C-bonded product 38 over the C–N-bonded alternative 39 (or 40).
This discouraging state of affairs improved markedly when we turned to photochemistry to initiate the cascade process. Even then, however, success had to await one further chance discovery. The photochemistry experiments commenced without a clear notion of how light initiation might favorably influence the regiochemistry of the transformation, since a clear grasp of the mechanistic intricacies of the process still eluded us. Nevertheless, since ignorance should not tarnish hope, we proceeded to examine a few of the allenyl azide substrates under irradiation and found that, indeed, light at 254 nm did promote the cyclization cascade and furnish the expected suite of cyclopentennelated indole products (Table 4, entries a–g). However, no material improvement in the reaction regiochemistry followed from these examples–the usual unremarkable ratio of C–C 38 to C–N 39/40 bonded products were detected in yields comparable to the thermal cases. Thus, we learned that the sequence could be initiated with photons of appropriate energy, but it seemed likely that the same intermediate(s) were being accessed under either thermolysis or photolysis, led to the same nearly unbiased mixture of regioisomeric products.
During the course of these photolysis experiments, however, an unexpected observation was made. Every once in a while, a batch of allenyl azide substrate proceeded to indole product with remarkably high regioselectivity (>10:1) favoring the C–C-cyclized product 38. What distinguished these “exceptional” batches of substrate from the normal, disappointing ones? The key parameter was purity; the early nonregioselective runs utilized scrupulously purified and colorless allenyl azides that were obtained through careful SiO2 chromatography even at the inevitable expense of material loss due to the sensitivity of these species. In some cases, however, batches of allenyl azide were obtained that had a light pink color despite appearing to be impurity-free by 1H and 13C NMR spectroscopy. It was these colored batches that delivered indole product with high regioselectivity for the C–C-bonded isomer 38. Once this connection was made, the search for the apparently critical “impurity” in the pink samples led to examination of test case photolysis reactions with substrate 28a in the presence of various ingredients of the allenyl azide-forming reaction, which included CuI, LiBr, and Mg (see Table 5). Eventually, the key role of Cu(I) became apparent, and the salt CuI was chosen for its ready availability and solubility in the reaction solvent CH3CN. Furthermore, it is mechanistically noteworthy that the improvement in regioselectivity scaled with the amount of CuI present. Reproducibly high regioselectivity favoring the C–C-bonded product 42b was obtained when 150 mol % of CuI was included in the photolysis reaction mixture. None of the other additives tested, whether present in the allenyl azide synthesis or not, had any significant effect on the reaction regiochemistry. The role that copper plays in this complex process was still to be elucidated (vide infra), but this empirically derived solution to the regiochemical issue begged further study. In this light, reexamination of several of the allenyl azide substrates (Table 4) under the hv/CuI conditions led to favorable results overall, with a few notable exceptions. Simple unsubstituted-alkene substrates (Table 4, entries a–d) responded well to the new protocol, and the C–C-bonded regioisomer 38 was formed in good yield in ratios of at least 18:1 compared to the C–N-bonded isomer 40. The monosubstituted-alkene substrates tested, 13e–g, provided tricyclic indole product that appeared to consist exclusively of the C–C-bonded isomers 38e–g, respectively. The C–N bonded products 40e–g could not be detected in the 1H NMR spectrum of the crude photolysate. The cyclohexenyl-substituted allenyl azide 16d also provided only the C–C-bonded tetracycle 38l. Two exceptions to this dramatic improvement in reaction regioselectivity can be seen in Table 4, entries m and n. Both the disubstituted alkene substrate 24 and the trisubstituted analogue 16b did furnish good yields of tricyclic indole-containing products, but there was no apparent improvement in regioselectivity for 16b over the thermally initiated case. Disubstituted alkene substrate 24 did proceed to indole product with an enhanced selectivity for the C–C-bonded isomer 38m (C–C/C–N = 1:1) compared to the thermal case (C–C/C–N = 1:5), but the result was far short of the goal of strongly favoring the C–C-bonded isomer. Thus, it appears that these particular di- and trisubstituted alkene substrates can be induced to form indole products under the CuI/hv reaction conditions, but the high levels of regioselectivity for the C–C-bonded product experienced by simpler species do not translate for these entries.
TABLE 5.
Discovery and Optimization of the Cui-Mediated Photochemical Cascade Cyclization of 29a into 42b
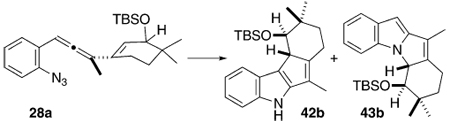 | |||||
|---|---|---|---|---|---|
| entry | irrada(nm) | heatb(°C) | conditions | yieldc(%) | 42b/43b ratiod |
| a | 110 | no additives | 80 | 1:1.6 | |
| b | 254 | no additives | 67 | 1:1 | |
| b | 254 | excess CuI, LiBr, Mg powder | 33 | >10:1 | |
| d | 254 | LiBr (1 equiv) | 68 | 1:1.1 | |
| e | 254 | Mg powder | (33)e | 1:1.2 | |
| f | 254 | LiBr (1 equiv), CuI (0.05 equiv) | f | 1:1.4 | |
| g | 254 | LiBr (1 equiv), CuI (1 equiv) | 21 | 5.4:1 | |
| h | 254 | CuBr·SMe2 (1 equiv) | f | 2.8:1 | |
| i | 254 | CuBr·SMe2 (0.25 equiv) | (17)e | 1.6:1 | |
| j | 254 | Cu(OAc)2 (1 equiv) | f | 5:1 | |
| k | 254 | CuI (0.5 equiv) | 67 | 4:1 | |
| l | 254 | CuI (0.8 equiv) | 72 | 6: 1 | |
| m | 254 | CuI (1.2 equiv) | 67 | 9:1 | |
| n | 254 | CuI (1.5 equiv) | 70 | 10:1 | |
| o | 110 | CuI (1.5 equiv), 5 mM | 75 | 1:1.6 | |
| p | 110 | CuI (1.5 equiv), 35 mM | 66 | 3.5:1 | |
| q | 110 | CuI (1.5 equiv), 51 mM | 65 | 5:1 | |
| r | 254 | [Rh(OAc)2]2 (1 equiv) | (48)e | 1:1 | |
Irradiated through a quartz vessel as a 5 mM CH3CN solution of 28a.
100 mM solution in toluene, unless otherwise noted.
Isolated, chromatographically purified material. The N-cyclized material had undergone elimination to furnish the diene 44b (see Table 6).
Ratio by integration of the 1H NMR spectrum of the crude reaction mixture prior to chromatography.
Only the N-cyclized material was isolated; 42b decomposed upon attempted chromatography.
Products were not isolated.
The positive effect of copper inclusion on reaction regioselectivity in the photochemical series could be extended to the thermal chemistry as well, Table 5, entries o–q. Substrate concentration appeared to be a key variable, as a thermolysis experiment run at the photochemical experiment’s concentration of allenyl azide did not show any influence of the copper additive. Upon increasing the substrate concentration 10-fold, however, the copper effect became manifest; the C–C-bonded product 42b was formed in a 5:1 ratio compared to the C–N-bonded isomer 43b (Table 5, entry q). These concentration effects, and our suspicion that both photochemistry and thermal chemistry proceeded through common intermediates (vide supra), formed the basis of speculation on the role of copper in this transformation. This argument will be elaborated with the aid of density function calculations as discussed with Scheme 9 below.
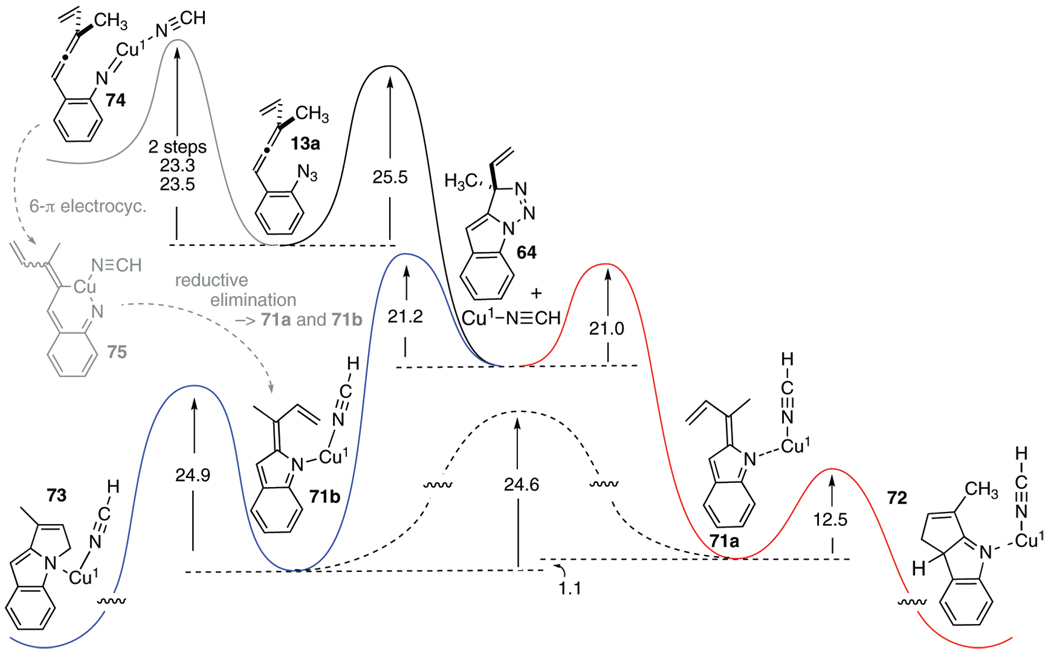
SCHEME 9.
Qualitative Representation of the Free Energy Profile for the Conversion of Allenyl Azide 13a into the C–C and C–N Cyclized Products 72 and 73, Respectively, in the Presence of Cu(I)•HCNa
aEnergies of ground-state structures and transition states were calculated using the B3LYP/6–311+G(d,p)//B3LYP/6–31G(d,p) methodology.
The final series of allenyl azide substrates examined in this study featured functionalized cyclohexenyl rings as model systems for the Fisher indoles, Table 6. The structural variables in this series included the allylic alcohol/ether substituent R, the carbinol group R1, and the alkyl residue appended to C(3) of the allene, R2. The reaction features that were tested by these structural probes comprised the overriding regioselectivity issue (C–C vs C–N terminal bond formation) and the relative stereochemistry emerging at C(10) under the influence of the adjacent C(11) stereogenic center. In addition, the sensitivity of the C(11) oxygen function to fulvene-forming elimination promoted by the potentially acidic C(10) cyclopentadiene proton was a concern. The preliminary CuI optimization studies with the OTBS variant 28a detailed in Table 5 suggested that this latter issue might not arise as a general problem, as 42b could be isolated without event following SiO2 chromatography. The data displayed in Table 6 indicate that all of the cyclohexenyl alcohol derivatives 28a–e, 29, and 37 performed satisfactorily under the new hv/CuI conditions to deliver good yields of the C–C-cyclized products 42a–g, respectively, accompanied by only trace amounts of the C–N-bonded regioisomer. The mildness of this protocol was evidenced by the functional group tolerance, which included a free alcohol, silyl ethers, and acid-sensitive alkyl ethers. The stereochemical outcome of all of the cyclizations examined was completely biased toward the C(10)/C(11) syn isomer shown as 42; no evidence for minor species bearing an alternative stereochemistry was detected in the 1H NMR spectrum of the crude photolysates. Surprisingly, even the cyanohydrin 42d, whose structure was determined by single-crystal X-ray analysis (see the Supporting Information), did not deviate from this trend, perhaps because of the steric distinction between the OTBS and CN groups (A values: CN 0.15 kcal/mol (298 K, t-BuOH);13a OSi(CH3)2-t-Bu 1.06 kcal/mol (179–188 K, CD2Cl2))13b–1.5 kcal/mol (193 K, CS2 solvent).13c
TABLE 6.
Cyclization Cascade of Substituted 1-(2-Azidophenyl)-3-cyclohexenylallenes To Furnish Indole Products
 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 110 °Ca |
hvb |
hv/CuIc |
|||||||||||
| entry | allenyl azide | R | R1 | R2 | 42d(%) | 43d(%) | 44d(%) | 42d(%) | 43d(%) | 44d(%) | 42d(%) | 43d(%) | 44d(%) |
| a | 29 | H | H | H | 34 | 25 | 21 | 24 | 60 | ||||
| b | 28a | TBS | H | H | 34 | 25 | 33 | 34 | 62 | 8 | |||
| c | 28b | TIPS | H | H | 51 | 31 | 45 | 64 | 5 | ||||
| d | 28d | TBS | CN | H | 27 | 48 | 27 | 43 | 62 | 7 | |||
| e | 28 | TBS | H | TMS | 35 | 41 (45) | (25)e | 50 | 11 (45) | f | |||
| f | 28c | SEM | H | H | 35 | 51 | 38 | 51 | 60 | ||||
| g | 37 | TBS | H | OTBS | not examined | 59 | |||||||
100 mM in toluene.
254 nm in CH3CN (5 mM).
254 nm in CH3CN (5 mM), 150 mol % CuI.
Isolated, chromatographically purified material.
Not isolated (unstable); estimated by integration of the 1H NMR spectrum of the crude reaction mixture.
A 1.6:1 ratio of 42e/45 was observed in the 1H NMR spectrum of the crude reaction mixture; chromatographic purification of this CuI-mediated reaction gave irreproducible isolation results.
In counterpoint, cyclohexenol-based allenyl azide cascade cyclizations performed under strictly thermal conditions afforded the usual mixture of the C–C- and C–N-cyclized regioisomers 42 and 43/44, respectively, with little preference for one mode of cyclization over the other. Similarly, photochemistry without copper furnished indole products with nearly identical regioisomer ratios as in the thermal cases. These contrasting results emphasize the importance of copper(I) inclusion in the photolysis solution and are consistent with our preliminary interpretation that this metal salt plays a critical role in steering the partitioning of a reactive intermediate(s) between the two regioisomeric products.
The two substrates that included functionality on the allenyl methyl unit proceeded to indole products as expected, but not to the same type of indole products, which was unexpected (Table 6). The R2 = TMS substrate 28e was the outlier within this series. Reaction under thermal conditions did not afford any of the C–C-bonded indole product 42e. Rather, the C–N-bonded regioisomer 43e (X-ray analysis; see the Supporting Information) and a new type of product, the alkene 45, predominated. 1H NMR analysis of the crude thermolysate suggested that the first-formed alkene product actually had a TMS moiety on the indole nitrogen but that labile group suffers protodesilylation upon SiO2 chromatography. The formation of this alkene can be rationalized by passage through an indolidene 3a (cf. Scheme 1), which engages in silyl transfer in preference to C–C bond-forming electrocyclization. Irradiation of 28e sans copper did furnish evidence for the C–C-bonded indole product 42e in the 1H NMR spectrum of the crude product mixture, but the C–N-bonded regioisomer 43e predominated. In the presence of copper(I), however, the photochemically derived product suite is entirely different, with the C–C-bonded regioisomeric indole 42e largely favored and the C–N regioisomer 43e absent. These observations provide further clues about the role of Cu(I) in influencing the regiochemical outcome of the cascade cyclization, a topic taken up in more detail below.
Using the Fisher indoles as a context for these studies introduces the need to convert sp2-hybridized C(16) of 42a into a quaternary carbon bearing two methyl groups, eq 2. Toward this end, we discovered that hydride addition to an intermediate fulvene 46, a species inadvertently derived from the alcohol 42a upon N-BOC protection conditions, provided a cyclopentadienylide anion intermediate that methylates exclusively at C(16). This intermediate anion could, in principle, methylate at C(10) as well, but an incipient 1,3 diaxial interaction between the incoming methyl electrophile and the axial CH3 of C(12) apparently thwarts this reaction trajectory. Of course, use of hydride as a nucleophile leaves C(11) in 47 devoid of functionality. Application of this methylation procedure in Fisher indole synthesis therefore will have to employ a nucleophilic species with fulvene 46 that preserves the opportunity to further functionalize C(11) as is required for 5.
 |
(2) |
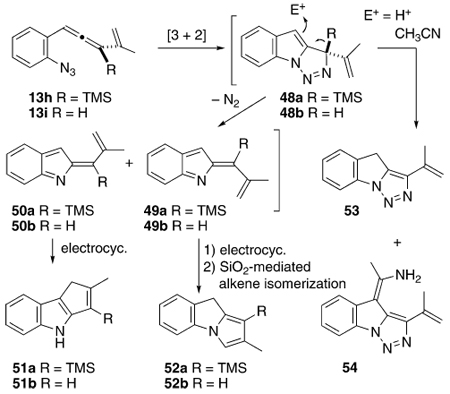
The TMS-substituted simple allenyl azide 13h was examined with the expectation that an uneventful reaction would deliver products of the type 51a and 52a, Table 7. Less certain was the fate of the single allenyl-H-substituted system 13i, given the propensity for H-substituted triazolines (cf. 2, R = H) to tautomerize into stable aromatic triazoles.14 Whereas the standard indole products 51a/b and 52a/b were produced under certain circumstances, the big surprise with 13h involved formation of the mechanistically informative triazoles 53 and 54. The triazole 53 also was formed as a minor component from 13i. The structural assignments of 51a/b, 52a/b, and 53 followed from their characteristic 1H NMR data, but the structure of 54 remained a puzzle until single-crystal X-ray analysis revealed it to be the formal acetonitrile adduct shown (see the Supporting Information for details). Under thermolytic conditions with or without copper, the triazoles were the major species formed from 13h. The formation of these compounds irrefutably implicates the intermediacy of a triazoline species 48a/b, previously posited but otherwise unconfirmed. Apparently, the elevated temperature of these reaction conditions with 13h provided enough energy for the nucleophilic capability of the allyl silane moiety within 48a to be expressed; trapping by either adventitious protons or, remarkably, by acetonitrile, led to 53 and 54, respectively. With 13i, simple tautomerization within 48b, presumably catalyzed by the same adventitious acid, delivers 53. Irradiation of 13h without copper(I) generates a reaction solution temperature of approximately 35 °C, and under this temperature regime the allyl silane moiety of 48a participates only slightly in triazole formation. In this instance, the major products formed are the C–C- and C–N-bonded indoles 51a and 52a, respectively, in a ratio similar to that seen earlier with related substrates (Table 4). Copper-mediated photolysis of 13h again produces a minor amount of the triazole 53, but the major product is the C–C-bonded regioisomer 51a, formed to the exclusion of the C–N-bonded alternative 52a. The H-substituted substrate 13i decomposed under irradiation and no further information was forthcoming from this species. The ± CuI photochemistry of 13h proceeded in a manner completely analogous to the other simple alkene-containing substrates. The anomalous behavior of the silyl-substituted substrate 13h occurs at higher temperatures, where an alternative pathway involving electrophilic desilylation intervenes, and in so doing, preserves evidence for the intermediacy of the triazoline 48a. Likewise, the thermal chemistry of 13i argues for reaction through triazoline 48b, but curiously, N2 loss is competitive with tautomerization for this intermediate.
TABLE 7.
Cyclization Cascade with the H-Substituted- and TMS-Substituted 1-(2-Azidophenyl)-3-(2-propenyl)allene Substrates 13h and 13i, Respectively
 | ||||||
|---|---|---|---|---|---|---|
| entry | allenyl azide | conditions (CH3CN) | 51a/ba(%) | 52a/ba(%) | 53a(%) | 54a(%) |
| a | 13h | 110 °C | 53 | 23 | ||
| b | 13h | 110 °C/150 mol % CuI | 3 | 40 | 6 | |
| c | 13h | 254 nm | 28 | 35 | 6 | |
| d | 13h | 254 nm/150 mol % CuI | 48 | 8 | ||
| e | 13i | 110 °C | 12b | 9 | ||
| f | 13i | 254 nm | decomp | |||
| g | 13i | 254 nm/150 mol % CuI | decomp | |||
Isolated, chromatographically purified material.
Observed in the 1H NMR spectrum of the crude reaction mixture, but decomposed upon attempted chromatographic isolation.
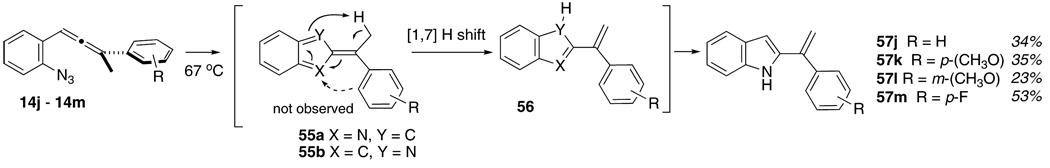
The final group of substrates examined feature an aryl substituent on the allenyl azide framework, 14j – 14m, Scheme 5. The challenge with this type of substrate lies in the competition within the indolidene intermediates 55a/55b between 1,7 H-shift and electrocyclization through the aryl ring. Whereas the H-shift has always been a potentially problematic side-reaction, the electrocyclization through an alkene partner apparently won out energetically in all of the previous cases. With 14j – 14m, however, breeching the aromaticity of the aryl group evidently is not competitive, and only the [1,7] H-shift products 57j – 57m are formed in modest yields. Photochemistry with and without CuI present was explored with 14j, although no evidence for redirection of the reaction course was detected. Thus, irradiation (254 nm) of 14j in CH3CN afforded only 57j in 56% yield, whereas similar reaction in the presence of 1.5 equivalents of CuI provided this same product in 41% yield.
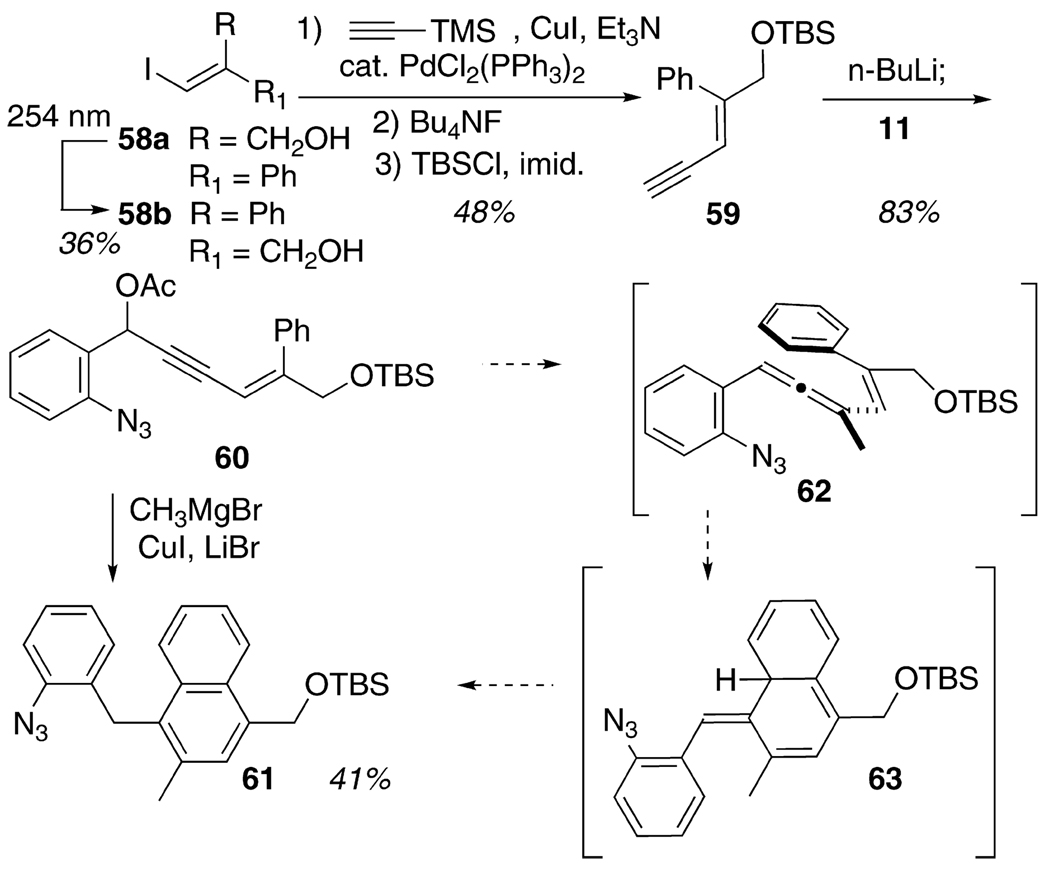
Finally, a discussion of the scope of the cascade cyclization would not be complete without acknowledging the 1-(2-azidophenyl)-3-alkenyl allene substrates that, for various reasons, did not participate productively in the transformation. For example, attempts to prepare a terminally disubstituted alkene substrate 62 were derailed by the facility of a 6π-electron electrocyclization that affords the naphthalene product 61 following tautomerization within 63 (Scheme 6). This example can be contrasted with the successful formation and unexceptional cascade cyclization of the triphenylalkenyl allenyl azide substrate 16b. A rationale for the lack of similar electrocyclization with this (Z)-phenyl-containing alkene might be traced to the additional phenyl group at position R1 (cf. 16b, Table 4), which might prevent access to the approximately planar conformation of the triene that should facilitate electrocyclization. Thus, the conformational consequences of the R//R1 steric interaction (= CH3//Ph in 16b, but only = H//CH3 in 62) may be the decisive factor in both the stability and the successful reaction of 16b and the failure of same with 62. A second irretrievable failure attended the attempted cascade cyclization of the enone 30 (cf. Table 3). Under any conditions examined (thermal, photochemistry, photochemistry with CuI), this substrate was consumed quickly without formation of any characterizable products. Presumably, the electronic activation conferred by the carbonyl functionality introduced lower energy competitive pathways that drained away allenyl azide faster than the desired [3 + 2] cycloaddition process.
SCHEME 6.
Attempt To Synthesize a (Z)-Phenyl-Substituted 1-(2-Azidophenyl)-3-alkenylallene Substrate 63
Mechanistic Analysis
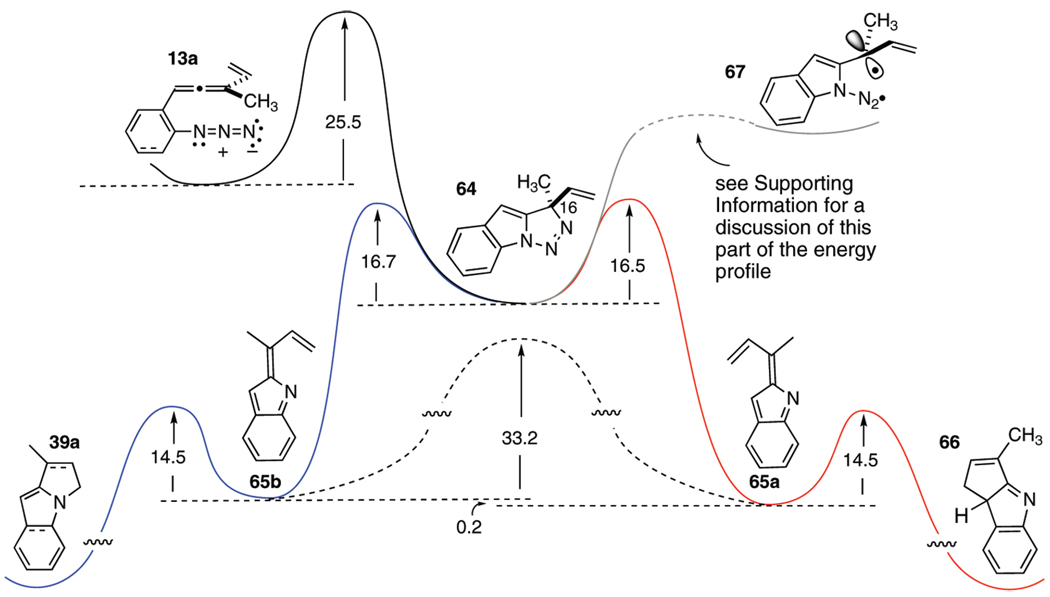
The accumulated experimental evidence to date can be framed within a unified mechanistic scenario featuring a sequence of discrete triazoline and then indolidene intermediates as hypothesized in Scheme 1. However, many subtleties remain unresolved in this crude picture; for example, (a) which step is the slow step? (b) is loss of N2 from the triazoline 2 concerted or stepwise? (c) what is the relationship between substituent R in 2 and the alkene geometry in 3a/3b?, (d) what is the relationship between the alkene geometry of 3a/3b and the regiochemistry of terminal bond formation, C–C (→4a) or C–N (→4b)?, and finally, (e) what is the role of Cu(I) in redirecting the regiochemistry of bond formation to strongly favor 4a? In order to gain more insight into the details of this complex process and address these questions, we turned to density functional theory in its Kohn–Sham formulation, using the B3LYP/6–311+G(d,p)//B3LYP/6–31G(d,p) computational approach.15 Second derivatives of the energy with respect to the Cartesian nuclear coordinates were computed for all stationary points and subsequent harmonic analysis confirmed the nature (minimum or transition state) of each structure. Unscaled frequency values obtained from the second derivatives were employed for thermochemical analysis. The calculated reaction profile for the noncopper mediated thermal conversion of allenyl azide 13a into the precursor 66 of the C–C bonded indole product 38a and the precursor 39a of the observed C–N bonded regioisomer 40a is detailed in Scheme 7.
SCHEME 7.
Qualitative Representation of the Free Energy Profile for the Conversion of Azidoallene 13a into the C–C and C–N Cyclized Products 66 and 39a, Respectivelya
aEnergies of ground-state structures and transition states were calculated using the B3LYP/6–311+G(d,p)//B3LYP/6–31G(d,p) functional. All energy values are in kcal/mol.
Interpretation of the energy values displayed on this reaction coordinate diagram provides an entry point for answering the mechanistic questions listed above. In order: (a) The rate-determining step appears to be the initial [3 + 2] cycloaddition to form triazoline 64. (b) The conversion of triazoline 64 into the indolidenes 65a and 65b can, in principle, occur through either concerted or stepwise loss of N2. The calculated barriers for concerted N2 loss, 16.5 kcal/mol for 65a and 16.7 kcal/mol for 65b, are likely to be smaller than the barrier for stepwise N2 loss, although a transition state for the conversion of triazoline 64 into the singlet diyl 67 could not be located by this computational approach (see the Supporting Information for details). This claim may seem surprising at first glance, since the concerted loss of N2 from 64 is equivalent to a formal suprafacial 12π-electron cycloreversion, a process that falls into the “forbidden” regime of the Woodward–Hoffmann (W–H) rules.16 How can these calculated results be reconciled with the otherwise infallibility of the W–H dogma? The key to understanding this apparent exception is appreciating the orthogonal relationship between the two π-systems (the indole and the N2). From this perspective, as the C–N and N–N bonds stretch during the approach to the transition state, the two π systems scarcely “communicate” and there is no appreciable passage of electron density between them. This supposition is supported by the results of ancillary ACID17 and NICS18 calculations of N2 loss from the desmethyl version of 64.2 These computational techniques provide “measurements” of electron density in off-atom regions of space, such as the region between the cleaving atoms in the transition state for N2 extrusion. These calculations clearly indicate that almost no electron density occupies this key locale in the transition state for N2 extrusion.2 It appears that as the C–N bond stretches in 64, the remaining N–N bond begins to stretch before the transition state to 67 is reached, and, in what might be characterized as a non-least-motion process, C(16) (cf. 64) begins to rotate until the planarity of 65a/65b is achieved. Overall, this N2 loss process appears to be accidentally concerted, and the lack of electronic communication between the two components removes it from the W–H regime of cycloadditions.
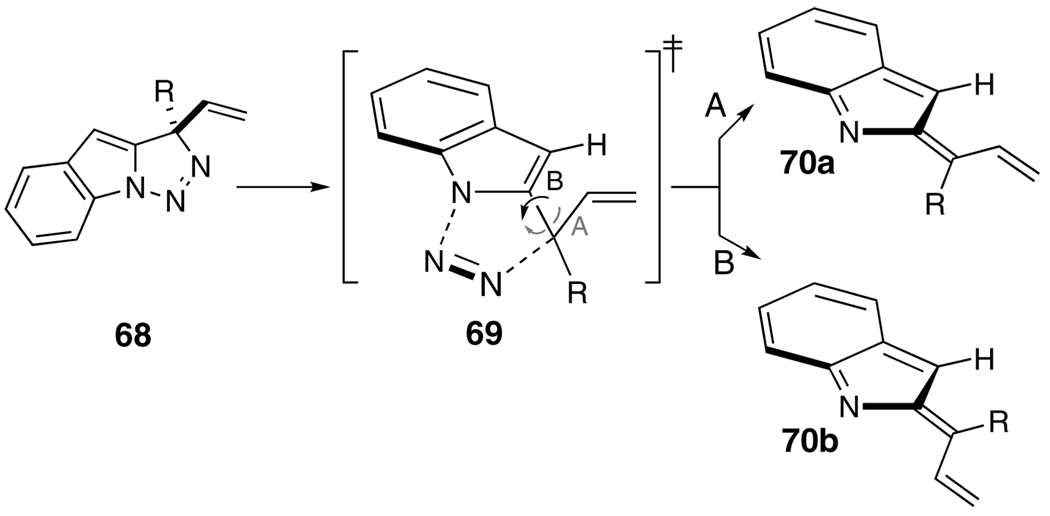
The relationship between indolidene alkene geometry and substituent R (question (c)) is illustrated in Scheme 8. The direction of rotation about the indicated bond in 69 determines the eventual alkene geometry; rotation in direction A as shown leads to the (E)-isomer 70a, whereas the alternative, rotation in direction B, delivers the (Z)-isomer 70b. These rotational directions engender different steric interactions between the proximate substituents, and it is reasonable to suppose that the energetic trade-offs between these interactions ultimately determine the alkene geometry ratio. Rotational direction A increases the vinyl//H clash but relieves the R//H interaction, whereas rotational direction B has the opposite effect. To the extent that the substituent R is larger than a vinyl group, rotation away from the R//H interaction as in 69-(rotation A) would be expected to be favored, leading to preferred formation of 70a over 70b. The limited series of substrates 13a–c (R = CH3, (CH2)2OTBS, t-Bu, respectively) provide results consistent with this analysis. As the R substituent increases in size, the C–C bonded product emerging from 70a is increasingly preferred; R = CH3 1:1.4 38a/40a, R = (CH2)2OTBS 1:1.2 38b/40b, and R = t-Bu 2.9:1 38c/40c (A values: CH3 = 1.7 kcal/mol, ethyl = 1.75 kcal/mol, vinyl = 1.35 kcal/mol, t-Bu ≈ 4 kcal/mol).13a DFT calculations largely support these conclusions. Thus, the calculated ratio of 65a/65b derived from 13a (R = CH3, R1 = H) is 55:45, a value that is not too dissimilar to the experimental ratio (42:58) of eventual isolable products 38a:40a (Table 4, entry a). The calculated/experimental values for the analogous tert-butyl case 13c (Table 4, entry c), remarkably, are completely coincident; 74:26 for 38c/40c. Finally, calculations of the barriers for 65a/65b formation from 13g (R = Ph, R1 = CH3) predict a 90:10 ratio of C- to N-cyclized material; the experimental ratio of 38g/40g is 55:45. The basis for this discrepancy has not been identified.
SCHEME 8.
Steric Interactions and Rotational Direction during Indolidene Formation
The importance of the alkene geometry of 65a and 65b on eventual product regiochemistry (question (d)) is highlighted as a result of these calculations. Each alkene isomer can proceed via an electrocyclization to give indole products, 65a → 66 and 65b → 39a, though concerted processes that require surmounting activation barriers of only 14.5 kcal/mol for each transformation. In contrast, the barrier for interconversion of these alkene isomers is much higher; 33.2 kcal/mol, and so a non-Curtin–Hammet situation arises in which 65a and 65b lead directly to product without equilibration. Thus, these calculations provide numerical support for a reactivity model in which the ultimate product regioisomer ratio (4a/4b, Scheme 1) replicates the indolidene alkene isomer population, which in turn reflects the differential steric interactions illustrated in Scheme 8.
The introduction of Cu(I) into this complex reaction sequence (question (e)) could, in principle, impact the chemistry at several different stages, Scheme 9. For example, the copper might play a defining role in the initial azide chemistry by forming a copper nitrene-type intermediate 74 by analogy with much prior copper-mediated amination chemistry.19 Alternatively, the copper might become involved in a much more passive capacity by providing a Lewis acidic binding site for various nitrogen donor ligands, as suggested by 71a/b. Initially, the nature of the Cu(I) ligation was explored through calculation. An assumption underlying all of the Cu–ligand models examined for Cu solvation was made; the I− was dissociated in the polar CH3CN solvent, and so the active species was a version of Cu(1+)(CH3CN)n. From n = 0 up to three coordinated HCN molecules (as a model for the CH3CN solvent used in the reactions) were explored computationally with several of the putative intermediates illustrated in Scheme 9. The structures and energies of species with no coordinating ligands (“naked copper(I)”) reflected the extreme electrophilicity of the metal, which was an unrealistic attribute for catalytic activity. Tricoordinated Cu(I) appeared to be too sterically hindered to participate in any productive interactions with the organic compounds present. Little energy difference was found between di- and monocoordinated copper with select intermediates of Scheme 9, but the singly coordinated Cu(I)•HCN complex consistently provided species with slightly lower energies, and so that motif was adopted for the calculational approach as a whole.
The initial question of importance focuses on the competition between copper-mediated nitrene 74 formation and orthodox [3 + 2] cycloaddition (→ 64) within 13a to start the cascade cyclization off. The calculated activation barriers are similar, with a slight edge going to the nitrene mechanism. The subsequent electrocyclization of 74 and reductive elimination within the Cu(III) species 75 are essentially barrierless processes by these calculations. Since the numerical values for the two distinct pathways from 13a are within computational error, there is no basis to exclude either from further consideration. In fact, both starting points lead to the same intermediates, the Cu(I)-bound indolidenes 71a and 71b. The nitrene-based mechanism delivers these key species directly, whereas the [3 + 2] cycloaddition route requires a bimolecular step, Cu(I) ligation (not explicitly shown in Scheme 9; barrierless by these calculations), after the indolidenes 65a/65b are formed by the previously discussed N2 extrusion reaction. In neither chemistry is there yet any reason to link copper participation to a change in the regiochemistry of indole formation, the pivotal issue of this discussion.
The link between copper mediation and reaction regiochemistry comes at the next stage of the sequence, during which the indolidenes 71a and 71b electrocyclize to product tricycles 72 and 73, respectively. The inclusion of Cu(I) in this part of the transformation perturbs the system in two profound ways: (1) The barrier to electrocyclization to form the C–N bonded species (71b → 73) is now significantly raised compared to the noncopper case of Scheme 7 (24.9 kcal/mol for 71b vs 14.5 kcal/mol for 65b). (2) The barrier to interconversion between the two indolidenes is now lower than the same barrier in the copper-free case (23.5 kcal/mol for 71a → 71b, compared to 33.4 kcal/mol for 65a → 65b). The barrier to C–C bond forming electrocyclization (71a → 72) remains largely unchanged by copper ligation (12.5 kcal/mol vs 14.5 kcal/mol for 65a → 66). The confluence of these energy changes has significant consequences for the regioselectivity of the reaction. With Cu(I) present, the fate of the pre-C–N forming indolidene 71b is now different than its fate with Cu absent. In the former instance, isomerization of 71b to the pre-C–C bond forming indolidene 71a is calculated to be faster than electrocyclization to afford 73. Since the barrier to electrocyclization of 71a is largely unperturbed by copper, its closure is now the fastest option available to this (Z)-indolidene. Thus, in contradistinction to the noncopper-mediated chemistry of Scheme 7, a Curtin–Hammett situation appears to be at hand here; 71b interconverts to its geometrical isomer 71a faster than it proceeds to indole product 73, while 71a forms tricyclic product 72 faster than it returns to 71b. The sum of these factors then favors formation of the C–C bonded product 72. Thus, these calculations suggest that copper(I) inclusion in the cascade cyclization reaction may have two effects: (1) redirecting indolidene formation through a copper–nitrene intermediate and (2) ligating to, and retarding the rate of, electrocyclization of the pre-C–N bond forming indolidene. It is only this latter effect that influences reaction regioselectivity to favor the C–C bonded product 4a. The two substrates 16b and 24, whose regiochemistry upon photocyclization did not benefit from CuI incorporation, may have suffered from unfavorable steric interactions between the alkene substituents and Cu(L)n that mitigated against formation of the pivotal complex 71a.
The cascade cyclizations of 1-(2-azidophenyl)-3-alkenylallenes provide 2,3-annelated indole-containing products under a range of experimental conditions. These transformations likely commence through initial [3 + 2] cycloaddition of the azide to the allene and then proceed through loss of nitrogen gas and electrocyclization of the derived indolidene. Computational studies of the energy surface for this complex transformation suggest that the central issue of C–C vs C–N regiochemistry of ultimate bond formation appears to be related to the differences in the activation energies for indolidene alkene isomer interconversion compared with the barriers for final C–C or C–N bond-forming electrocyclization. Incorporation of a nitrogen binding metal (CuI) can influence these energetic tradeoffs and in favorable cases steer the reaction toward high levels of C–C-bonded cyclopentennelated indole product. One family of substrates bearing functionalized cyclohexene rings as the “alkene” portion were particularly suitable for C–C bond forming cyclization, and the derived tetracyclic structures may find use in the synthesis of cognate natural products such as the Fisher indoles, inter alia.
Experimental Section
Computational Methodology
All of the stationary points were located with the B3LYP20 density functional as implemented in Gaussian0321 in conjunction with the 6–31G(d,p) basis set. To further improve the electronic energy values, an energy refinement was computed with the larger triple-ζ quality 6–311+G(d,p) basis set. This scheme usually is noted as B3LYP/6–311+G(d,p)//B3LYP/6–32G(d,p).
Due to the potential diradical character of some of the structures considered in this work, the internal and external stability of the wave functions was computed via the Hermitian stability matrices A and B in all cases.22 For all of the structures exhibiting unstable restricted wave functions, the spin symmetry constraint of the wave function was released (i.e., expanding the SCF calculation to an unrestricted space, UB3LYP), leading to stable unrestricted wave functions.
This methodology has been tested extensively and compared against very robust and specialized methodology (CASSCF, CASPR2) for the study of oxa and aza derivatives of the trimethylenemethane motif.23
General Procedure 1. Allenyl Azide Formation
To a solution of ZnCl2 (2.5 equiv) in THF (0.3 M solution) was added the appropriate Grignard reagent (2.5 equiv), and the mixture was stirred at room temperature for 1 h. Pd(PPh3)4 (5 mol %) and the propargylic acetate (1 equiv) in THF (0.1 M solution) were added sequentially. The reaction mixture was allowed to stir at room temperature for 20 min (monitored by TLC for starting material consumption). After addition of an equal volume of a saturated NH4Cl solution, the organic layer was extracted into Et2O and washed with water and brine, dried over Na2SO4, and evaporated under reduced pressure maintaining a water bath temperature below 40 °C. The resulting oil was purified by flash chromatography (hexanes → 2% Et2O/hexanes).
General Procedure 2. Sonagashira Coupling
To a 0.5 M solution of alkenyl iodide in THF were added PdCl2(PPh3)2 (10 mol %), 1-(2-azidophenyl)prop-2-ynyl acetate (12i) (1.6 equiv), Et3N (7 equiv), and CuI (3 mol %). The mixture was stirred at room temperature under a nitrogen atmosphere for 16 h unless otherwise noted. The reaction solution was then diluted with an equivalent volume of Et2O, and an equivalent volume of saturated NH4Cl solution was added. The organic layer was washed (3 × H2O, 3 × brine), dried over MgSO4, and evaporated under reduced pressure. The resulting oil was purified by flash chromatography (1% Et2O/hexanes → 15% Et2O/hexanes).
General Procedure 3. Allenyl Azide Formation
CH3MgBr (3.0 M in Et2O, 10 equiv) was added dropwise to an ice-cold 0.1 M solution of CuI (10 equiv) and LiBr (10 equiv) in THF, and the resulting solution was stirred at that temperature for 30 min. A solution of the alkynyl azide in THF (0.1 M) was added slowly via cannula. The ice-cold solution was stirred for 30 min (monitored by TLC for starting material consumption). Ice-cold saturated NH4Cl solution was then added dropwise until gas evolution ceased, and then the reaction mixture was diluted with an equivalent volume of Et2O. The organic layer was washed (3 × ice-cold H2O, 3 × ice-cold brine), dried over MgSO4 and evaporated under reduced pressure maintaining a water bath temperature below 40 °C. The resulting oil was purified by flash chromatography (hexanes → 2% Et2O/hexanes).
General Procedure 4. Copper-Mediated Photolysis of Allenyl Azides
A flame-dried quartz vessel containing a 5 mM solution of allenyl azide and 1.5 equiv of CuI in CH3CN was vigorously purged with N2 or Ar for 5 min. The vessel was irradiated at the specified wavelength for a minimum of 1 h with monitoring by TLC for starting material consumption. The reaction solution was diluted with an equivalent volume of Et2O and washed 2× with ice-cold 10% NH4OH in saturated NH4Cl (1:4), 3× with ice-cold H2O (until the aqueous phase exhibited pH = 7), and 3× with brine, dried over Na2SO4, and evaporated under reduced pressure. An alternative aqueous workup also was found to be effective: ice-cold 1 M Na2S2O3, 2 × ice-cold 1 N H3PO4, 3 × ice-cold H2O, and 3 × ice-cold brine. The residue was purified by flash chromatography (2% Et2O/hexanes → 10% Et2O/hexanes).
General Procedure 5. Thermolysis of Allenyl Azides
A flame-dried round-bottomed flask or sealed tube containing a 0.1 M solution of allenyl azide in toluene was refluxed for a minimum of 30 min under a N2 atmosphere with monitoring by TLC for starting material consumption. The solvent was evaporated under reduced pressure and the residue was purified by flash chromatography (2% Et2O/hexanes → 10% Et2O/hexanes).
General Procedure 6. Photolysis of Allenyl Azides
A flame-dried quartz vessel containing a 5 mM solution of allenyl azide in CH3CN was vigorously purged with N2 or Ar for 5 min. The vessel was irradiated at the specified wavelength for a minimum of 1 h with monitoring by TLC for starting material consumption. The reaction solution was diluted with an equivalent volume of Et2O, washed 3× with ice-cold H2O and 3× with brine, dried over Na2SO4, and evaporated under reduced pressure. The residue was purified by flash chromatography (2% Et2O/hexanes → 10% Et2O/hexanes).
1-Azido-2-(4-methylpenta-1,2,4-trienyl)benzene (13i)
Following general procedure 1, isopropenylmagnesium bromide (0.5 M in THF, 4.6 mL, 2.3 mmol) and acetate 12i (200 mg, 0.929 mmol) produced 90 mg (49%) of 13i as a yellow oil: IR (neat) 2123, 1925 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.40 (dd, J = 7.8, 1.5 Hz, 1H), 7.24 (td, J = 7.7, 1.5 Hz, 1H), 7.13 (dd, J = 8.0, 1.1 Hz, 1H), 7.08 (td, J = 7.5, 1.1 Hz, 1H), 6.70 (d, J = 6.4 Hz, 1H), 6.34 (d, J = 6.5 Hz, 1H), 5.02 (d, J = 0.6 Hz, 1H), 4.93 (q, J = 1.5 Hz, 1H), 1.82 (t, J = 0.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 208.5, 138.5, 136.3, 128.2, 128.1, 125.5, 124.9, 118.4, 114.6, 101.5, 91.8, 19.7; EI+ m/z (relative intensity) 169.1 (M – N2, 70%); HRMS (EI+) calcd for C12H11N 169.0891, found 169.0973.
1-Azido-2-(3-phenylbuta-1,2-dienyl)benzene (14j)
Following general procedure 3, 400 mg (1.37 mmol) of acetate 12j produced 275 mg (81%) of 14j as a yellow oil: IR (neat) 2122, 1931 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.36–7.33 (m, 2H), 7.31 (dd, J = 8.5, 0.7 Hz, 1H), 7.25–7.20 (m, 2H), 7.15–7.10 (m, 2H), 7.03 (ddd, J = 8.1, 1.3, 0.4 Hz, 1H), 6.96–6.91 (m, 1H), 6.65 (q, J = 2.9 Hz, 1H), 2.12 (d, J = 3.0 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 207.5, 136.4, 136.1, 128.4, 128.2, 128.1, 127.0, 125.80, 125.77, 124.8, 118.5, 104.5, 91.0, 16.7; ES+ m/z (relative intensity) 220.2 (M − N2 + H, 20), 248.1 (M + H, 10); HRMS calcd for C16H14N 220.1126, found 220.1122.
tert-Butyl-(2-iodovinyl)dimethylsilane (19)
To an ice-cold stirring solution of Zr(Cp)2Cl2 (1.82 g, 6.22 mmol) in 20 mL of THF was added DIBAL-H (1.0 M in hexanes, 6.22 mL, 6.22 mmol). The reaction was stirred at that temperature for 30 min, and then a solution of (tert-butyldimethylsilyl)acetylene (1.06 mL, 5.66 mmol) in 3 mL of THF was added slowly and the reaction allowed to warm to room temperature. Upon warming, the reaction mixture turned green and then red. The reaction solution was cooled to −78 °C, a solution of I2 (1.87 g, 7.36 mmol) in 5 mL of THF was added slowly, and stirring was continued at that temperature for 1 h. The reaction solution was then allowed to warm to room temperature, stirring was continued for 2.5 h, ice-cold 1 M HCl (35 mL) was slowly added, and the mixture was diluted with Et2O (50 mL). The organic layer was washed with 1 M HCl (50 mL), 1 M Na2S2O3 solution (2 × 50 mL), saturated NaHCO3 solution (50 mL), H2O (3 × 50 mL), and brine (3 × 50 mL), dried over MgSO4, and concentrated under reduced pressure. The residue was purified by flash chromatography (hexanes) to provide 1.14 g (75%) of 19 as a pale yellow oil: 1H NMR (360 MHz, CDCl3) δ 7.04 (d, J = 16.1 Hz, 1H), 6.65 (d, J = 16.2 Hz, 1H), 0.86 (s, 9H), 0.02 (s, 6H); 13C NMR (90 MHz, CDCl3) δ 147.6, 90.1, 26.2, 16.6, −6.3; EI+ m/z (relative intensity) 141.1 (M – I, 20), 210.9 (M – t-Bu, 100), 268.0 (M+, 20); HRMS (EI+) calcd for C8H17SiI 268.0144, found 268.0146.
Acetic Acid 1-(2-Azidophenyl)-6-(tert-butyldimethylsilanyloxy)hex-4-en-2-ynyl Ester (20)
Following general procedure 2, with a reaction time of 4 h, 2.40 g (11.2 mmol) of 12i and 2.08 g (6.97 mmol) of tert-butyldimethylsilyl-(E)-3-iodo-2-propenyl ether (17)24 produced 1.99 g (74%) of 20 as a yellow oil: IR (neat) 2127, 1747 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.66 (dd, J = 7.6, 1.6 Hz, 1H), 7.37 (td, J = 7.7, 1.6 Hz, 1H), 7.17 (m, 2H), 6.73 (d, J = 1.8 Hz, 1H), 6.25 (dt, J = 15.8, 4.1 Hz, 1H), 5.79 (dq, J = 15.8, 2.2 Hz, 1H), 4.20 (ddd, J = 4.0, 2.2, 0.4 Hz, 1H), 2.07 (s, 3H), 0.89 (s, 9H), 0.03 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 169.3, 143.9, 137.7, 130.1, 129.2, 128.1, 124.9, 118.2, 107.5, 85.3, 85.0, 62.6, 61.2, 25.7, 20.9, 18.2, −5.5; ES+ m/z (relative intensity) 408.1 (M + Na, 100); HRMS (ES+) calcd for C20H27N3O3NaSi 408.1719, found 408.1729.
Acetic Acid 1-(2-Azidophenyl)-7-(tert-butyldimethylsilanyloxy)-4-methylhept-4-en-2-ynyl Ester (21)
Following general procedure 2, 605 mg (2.81 mmol) of alkyne 12i and 573 mg (1.76 mmol) of iodide 1824 produced 514 mg (71%) of 21 as a yellow oil: IR (neat) 2126, 1747 cm−1; 1H NMR (360 MHz, CDCl3) δ 7.66 (d, J = 7.6 Hz, 1H), 7.34 (td, J = 7.7, 1.4 Hz, 1H), 7.17–7.10 (m, 2H), 6.72 (s, 1H), 5.90 (t, J = 7.2 Hz, 1H), 3.59 (t, J = 6.8 Hz, 2H), 2.28 (app q, J = 6.9 Hz, 2H), 2.05 (s, 3H), 1.77 (s, 3H), 0.86 (s, 9H), 0.02 (s, 6H); 13C NMR (90 MHz, CDCl3) δ 169.2, 137.7, 135.8, 130.0, 129.3, 128.3, 124.8, 118.3, 118.1, 89.8, 81.6, 61.9, 61.2, 32.2, 25.8, 20.8, 18.2, 17.0, −5.5; ES+ m/z (relative intensity) 436.3 (M + Na, 100); HRMS (ES+) calcd for C22H31N3O3SiNa 436.2032, found 436.2029.
Acetic Acid 1-(2-Azidophenyl)-5-(tert-butyldimethylsilanyl)-pent-4-en-2-ynyl Ester (22)
Following general procedure 2, using 20 mol % of PdCl2(PPh3)2, 1.14 g (4.25 mmol) of iodide 19, and 1.46 g (6.80 mmol) of alkyne 12i produced 332 mg (22%) of 22 as a yellow oil: IR (neat) 2127, 1746 cm−1; 1H NMR (360 MHz, CDCl3) δ 7.67 (dd, J = 7.6, 1.4 Hz, 1H), 7.37 (td, J = 7.8, 1.5 Hz, 1H), 7.19–7.13 (m, 2H), 6.75 (d, J = 1.7 Hz, 1H), 6.50 (d, J = 19.4 Hz, 1H), 5.98 (dd, J = 19.3, 1.8 Hz, 1H), 2.08 (s, 3H), 0.86 (s, 9H), 0.01 (s, 6H); 13C NMR (90 MHz, CDCl3) δ 169.3, 144.9, 137.7, 130.2, 129.2, 128.0, 124.9, 123.5, 118.2, 87.0, 85.1, 61.2, 26.3, 20.9, 16.5, −6.5; ES+ m/z (relative intensity) 295.1 (M − TBS + MeOH + Na, 100), 378.2 (M + Na, 10); HRMS (ES+) calcd for C14H14N3O3Na 295.0933, found 295.0941.
[6-(2-Azidophenyl)-4-methylhexa-2,4,5-trienyloxy]-tert-butyldimethylsilane (23)
Following general procedure 3, 263 mg (0.682 mmol) of acetate 20 produced 167 mg (72%) of 23 as a yellow oil: IR (neat) 2123, 1929 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.35 (dd, J = 7.7, 1.4 Hz, 1H), 7.24 (td, J = 7.2, 1.3 Hz, 1H), 7.13 (dd, J = 8.0, 1.0 Hz, 1H), 7.08 (td, J = 7.6, 1.0 Hz, 1H), 6.53 (s, 1H), 6.28 (dd, J = 15.6, 0.7 Hz, 1H), 5.78 (dtd, J = 15.6, 5.3,1.2 Hz, 1H), 4.31 (t, J = 1.4 Hz, 1H), 4.29 (t, J = 1.4 Hz, 1H), 1.96 (s, 3H), 0.95 (s, 9H), 0.12 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 209.7, 136.3, 128.9, 128.4, 128.0, 127.3, 126.0, 124.8, 118.4, 103.3, 88.6, 63.9, 25.9, 18.4, 15.3, −5.2; ES+ m/z (relative intensity) 314.2 (M – N2 + H, 80%); HRMS (AP+) calcd for C19H28NOSi: 314.1940, found 314.1936.
[7-(2-Azido-phenyl)-4,5-dimethyl-hepta-3,5,6-trienyloxy]-tert-butyl-dimethyl-silane (24)
Following general procedure 3, 512 mg (1.24 mmol) of acetate 21 produced 368 mg (80%) of 24 as a red oil: IR (neat): 2122, 1929 cm−1; 1H NMR (360 MHz, CDCl3) δ 7.31 (dd, J = 7.7, 1.4 Hz, 1H), 7.17 (td, J = 7.6, 1.2 Hz, 1H), 7.07 (dd, J = 7.6, 1.2 Hz, 1H), 7.02 (t, J = 7.5 Hz, 1H), 6.54 (s, 1H), 5.50 (t, J = 7.2 Hz, 1H), 3.66 (t, J = 6.9 Hz, 2H), 2.37 (app q, J = 6.9 Hz, 2H), 1.94 (d, J = 2.6 Hz, 3H), 1.75 (s, 3H), 0.89 (s, 9H), 0.06 (s, 6H); 13C NMR (90 MHz, CDCl3) δ 207.9, 136.1, 132.3, 128.0, 127.7, 126.6, 124.7, 122.6, 118.3, 107.6, 89.8, 62.7, 32.4, 25.9, 18.3, 16.2, 15.2, −5.3; ES+ m/z (relative intensity) 342.2 (M − N2 + H, 100%), 387.3 (M + NH4, 60%), 392.2 (M + Na, 30%); HRMS (ES+) calcd for C21H35N4OSi: 387.2580, found 387.2578.
[5-(2-Azido-phenyl)-3-methyl-penta-1,3,4-trienyl]-tert-butyl-dimethyl-silane (25)
Following general procedure 3, 66 mg (0.19 mmol) of acetate 22 produced 34 mg (59%) of 25 as a yellow oil: IR (neat): 2121, 1929 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.31 (d, J = 7.6 Hz, 1H), 7.22 (t, J = 7.7 Hz, 1H), 7.11 (d, J = 7.9 Hz, 1H), 7.06 (t, J = 7.3 Hz, 1H), 6.54 (d, J = 18.9 Hz, 1H), 6.51 (s, 1H), 5.84 (d, J = 19.0 Hz, 1H), 1.91 (d, J = 2.5 Hz, 1H), 0.88 (s, 9H), 0.04 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 210.8, 142.3, 136.3, 128.5, 128.0, 126.8, 125.9, 124.8, 118.4, 105.4, 88.6, 26.4, 16.7, 14.6, −6.0, −6.1; ES+ m/z (relative intensity) 283.2 (M - N2, 50%).
1-Azido-2-{3-[3-(tert-butyl-dimethyl-silanyloxy)-4,4-dimethyl-cyclohex-1-enyl]-4-trimethylsilanyl-buta-1,2-dienyl}-benzene (28e)
Following general procedure 3 using TMSCH2MgCl (1.0 M in Et2O), 115 mg (0.251 mmol) of 27e produced 115 mg (95%) of 28e as a yellow oil: IR (neat): 2123, 1919 cm−1; 1H NMR (300 MHz, C6D6) δ 7.45 (m, 1H), 6.81 (m, 3H), 6.71 (m, 1H), 5.68 (d, J = 1.2 Hz, 1H), 3.96 (s, 1H), 2.25 - 2.10 (m, 2H), 1.75 (m, 2H), 1.43 (m, 1H), 1.31 (m, 1H), 1.00 (s, 9H), 0.96 (m, 3H), 0.94 (s, 3H), 0.15 (m, 3H), 0.10 (s, 12H);13C NMR (75 MHz, CDCl3) δ 208.1, 207.9, 136.2, 133.2, 133.0, 128.0, 127.7, 127.6, 127.5, 126.7, 124.8, 124.7, 118.4, 108.21, 108.18, 90.71, 90.65, 75.8, 75.7, 34.3, 34.23, 34.18, 34.1, 27.72, 27.66, 25.9, 24.9, 19.9, 19.7, 18.2, 18.1, 17.6, −0.8, −4.2, −4.9; APCI+ m/z (relative intensity) 454.3 (M + H - N2, 100%), 482.3 (M + H, 15%); HRMS (AP+) calcd for C27H44NOSi2: 454.2961, found 454.2979.
3-[3-(2-Azido-phenyl)-1-methyl-propa-1,2-dienyl]-6,6-dimethyl-cyclohex-2-enol (29)
To a 0 °C stirring solution of 28a (429 mg, 1.05 mmol) in 10 mL of THF was added n-Bu4NF (1.0 M in THF, 10 mL, 10 mmol). The reaction mixture was stirred at 0 °C for 54 h (monitored by TLC for starting material consumption). Ice-cold saturated NaHCO3 solution (10 mL) was added and then the reaction mixture was diluted with Et2O (10 mL). The organic layer was washed (ice-cold brine × 2), dried over Na2SO4 and evaporated under reduced pressure maintaining a water bath temperature below 40 °C. The resulting oil was purified by flash chromatography (neutral alumina, 25% EtOAc/hexanes) to give 289 mg (93%) of 29 as an orange oil: IR (neat): 3393, 2124, 1930 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.31 (m, 1H), 7.21 (m, 1H), 7.11 (dd, J = 8.0, 0.9 Hz, 1H), 7.05 (t, J = 7.8 Hz, 1H), 6.57 (s, 1H), 5.68 (m, 1H), 3.89 (d, J = 7.9 Hz, 1H), 2.11 - 2.03 (m, 2H), 1.96 (d, J = 2.7 Hz, 3H), 1.73 (br s, 1H), 1.50 (m, 1H), 1.39 (m, 1H), 0.96 (m, 3H), 0.90 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 207.9, 207.8, 136.11, 136.09, 135.1, 135.0, 127.93, 127.91, 126.1, 125.0, 124.8, 124.7, 118.4, 105.6, 90.2, 74.9, 74.6, 33.6, 33.5, 33.3, 32.9, 26.5, 26.2, 24.6, 24.5, 21.2, 20.7, 15.9; ES+ m/z (relative intensity) 318.1 (M + Na, 100%), 296.2 (M + H, 10%); HRMS (ES+) calcd for C18H21N3ONa: 318.1582, found 318.1585.
3-[3-(2-Azido-phenyl)-1-methyl-propa-1,2-dienyl]-6,6-dimethyl-cyclohex-2-enone (30)
To a stirring solution of alcohol 29 (57 mg, 0.20 mmol) in 1 mL of CH2Cl2 was added a solution of Dess-Martin periodinane (170 mg, 0.40 mmol) in 2 mL of CH2Cl2 and the reaction was stirred for 1 min. Saturated NaHCO3 (3 mL) was added and the organic layer washed with H2O (3 × 5 mL), and brine (3 × 5 mL), dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash chromatography (5% EtOAc/hexanes) to provide 24 mg (41%) of 30 as a yellow oil: IR (neat): 2124, 1927, 1664 cm−1; 1H NMR (360 MHz, C6D6) δ 7.21 (dd, J = 7.3, 1.7 Hz, 1H), 6.84 - 6.76 (m, 2H), 6.70 - 6.66 (m, 2H), 6.07 (d, J = 1.1 Hz, 1H), 2.20 (app pent, J = 6.1 Hz, 2H), 1.68 (d, J = 2.7 Hz, 3H), 1.46 (t, J = 6.2 Hz, 2H), 1.06 (s, 3H), 1.04 (s, 3H); 13C NMR (90 MHz, C6D6) δ 210.4, 202.7, 153.5, 136.9, 129.0, 128.4, 125.2, 124.9, 123.3, 118.9, 106.4, 91.5, 40.6, 36.4, 24.9, 24.3, 15.5; ES+ m/z (relative intensity) 316.1 (M + Na, 100%); HRMS (ES+) calcd for C18H19N3ONa: 316.1426, found 316.1429.
[2-(3-Iodo-6,6-dimethyl-cyclohex-2-enyloxymethoxy)-ethyl]-trimethylsilane (35c)
To a stirring solution of alcohol 33 (355 mg, 1.41 mmol) in 5 mL of CH2Cl2 was added Bu4NI (624 mg, 1.69 mmol), N,N-diisopropylethylamine (dried over Na2SO4 and decolorized with charcoal) (279 µL, 1.69 mmol), and SEMCl (tech. grade, 499 µL, 2.82 mmol). The reaction mixture was stirred for 16 h and then the solvent was removed in vacuo. The resulting oil was dissolved in Et2O (10 mL) and washed (3 × 10 mL H2O, 3 × 10 mL brine), dried over MgSO4, and then the solvent was removed in vacuo. The resulting oil was purified by flash chromatography (hexanes → 10% Et2O/hexanes) to yield 515 mg (96%) of 35c as a colorless oil: IR (neat): 1635 cm−1;1H NMR (360 MHz, C6D6) δ 6.45 (m, 1H), 4.57 (d, J = 7.0 Hz, 1H), 4.47 (d, J = 7.0 Hz, 1H), 3.66 - 3.56 (m, 2H), 3.46 (m, 1H), 2.28 (m, 2H), 1.28 (m, 1H), 1.06 (m, 1H), 0.91 (m, 5H), 0.77 (s, 3H), −0.02 (m, 9H); 13C NMR (90 MHz, C6D6) δ 138.8, 99.3, 94.6, 81.4, 65.2, 37.7, 35.9, 32.8, 26.3, 21.8, 18.2, −1.2; EI+ m/z (relative intensity) 309.0 (M - SiMe3, 40%); HRMS (EI+) calcd for C11H18O2I: 309.0352, found 309.0360.
1-Azido-2-{4-(tert-butyl-dimethyl-silanyloxy)-3-[3-(tert-butyl-dimethyl-silanyloxy)-4,4-dimethyl-cyclohex-1-enyl]-buta-1,2-dienyl}-benzene (37)
To a −78 °C solution of iodide 35a (2.078 g, 8.243 mmol, 2.5 equiv) in 82 mL of THF was added dropwise t-BuLi (1.7 M in pentane, 10.7 mL, 18.1 mmol, 5.5 equiv) and stirring continued at that temperature for 30 min, during which time the solution turned deep yellow. A solution of ZnCl2 (1.124 g, 8.243 mmol, 2.5 equiv) in 40 mL of THF was then added slowly and the resulting solution warmed to room temperature during which time the solution turned colorless. Pd(PPh3)4 (190 mg, 0.165 mmol, 5 mol %) and a solution of acetate 36 (1.185 g, 3.297 mmol, 1 equiv) in 33 mL of THF were added sequentially and the reaction mixture was stirred for 45 min (as monitored by TLC). Ice-cold saturated NH4Cl solution (30 mL) was added slowly and the organic layer was diluted with Et2O (30 mL). The organic layer was washed with H2O (3 × 30 mL) and brine (3 × 30 mL), dried over MgSO4 and concentrated in vacuo. The residue was purified by flash chromatography (2% Et2O/hexanes) to produce 1.132 g (64%) of 37 as a yellow oil: IR (neat): 2123, 1929 cm−1; 1H NMR (360 MHz, C6D6) δ 7.41 – 7.49 (m, 1H), 6.85 (d, J = 5.8 Hz, 1H), 6.78 – 6.81 (m, 2H), 6.65 – 6.68 (m, 1H), 5.96 (s, 1H), 4.49 – 4.60 (m, 2H), 3.96 (s, 1H), 2.02 – 2.15 (m, 2H), 1.44 – 1.49 (m, 1H), 1.28 – 1.34 (m, 2H), 1.01 (m, 9H), 0.98 (s, 3H), 0.93 – 0.97 (m, 11H), 0.18 (s, 3H), 0.10 (s, 3H), 0.07 (m, 6H); 13C NMR (90 MHz, C6D6) δ 208.1, 208.0, 136.7, 136.6, 131.4, 131.3, 128.5, 128.40, 128.35, 128.1, 126.2, 126.1, 125.03, 124.95, 118.71, 118.67, 111.6, 92.54, 92.49, 76.0, 75.8, 62.7, 62.6, 34.4, 34.3, 34.0, 33.9, 27.7, 27.5, 26.2, 26.0, 25.4, 25.3, 20.8, 20.5, 18.4, −3.7, −4.6, −5.0, −5.1; ES+ m/z (relative intensity) 512.3 (M + H, 35%), 562.3 (M + Na, 100%); HRMS (ES+) calcd for C30H49N3O2Si2Na: 562.3261, found 562.3242.
2-Methyl-1-phenyl-8H-3a-aza-cyclopenta[a]indene (40g)
Method A: Following General Procedure 4, 48 mg (0.18 mmol) of 13g provided 30 mg (69%) of 38g1b as a yellow oil. Method B: Following General Procedure 5, 193 mg (0.71 mmol) of 13g provided 81 mg (47%) of 38g1b as a yellow oil and 67 mg (39%) of 40g as a white solid. Method C: Following General Procedure 6, 100 mg (0.37 mmol) of 13g provided 21 mg (23%) of 38g1b as a yellow oil. 40g: mp. 89 – 90 °C; 1H NMR (400 MHz, CDCl3) δ 7.40 (m, 2H), 7.31 (m, 2H), 7.26 (d, J = 7.4 Hz, 1H), 7.19 − 7.11 (m, 2H), 7.08 (d, J = 7.7 Hz, 1H), 6.95 (t, J = 7.3 Hz, 1H), 6.87 (s, 1H), 3.84 (s, 2H), 2.23 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 140.9, 136.2, 133.8, 132.9, 128.4, 127.5, 127.4, 125.7, 125.2, 122.7, 122.1, 117.5, 109.2, 109.1, 29.7, 12.6; ES+ m/z (relative intensity) 246.1 (M + H, 100%); HRMS (ES+) calcd for C18H16N: 246.1283, found 246.1286.
1-(tert-Butyl-dimethyl-silanyloxymethyl)-3-methyl-1,4-dihydro-cyclopenta[b]indole (38 h) and 3-(tert-Butyl-dimethyl-silanyloxymethyl)-1-methyl-3H-3a-aza-cyclopenta[a]indene (39 h)
Following General procedure 5, 95 mg (0.28 mmol) of 23 produced 21 mg of 38h (24%) and 30 mg of 39h (34%). 38h: (yellow oil): IR (neat): 3401 cm−1; 1H NMR (400 MHz, C6D6) δ 7.81 (d, J = 7.4 Hz, 1H), 7.26 (t, J = 7.0 Hz, 1H), 7.21 (m, 2H), 6.73 (br s, 1H), 6.28 (s, 1H), 4.06 (dd, J = 9.1, 7.2 Hz, 1H), 3.75 (t, J = 7.1 Hz, 1H), 3.62 (t, J = 8.7 Hz, 1H), 1.84 (s, 3H), 1.00 (s, 9H), 0.05 (s, 3H), 0.02 (s, 3H); 13C NMR (90 MHz, C6D6) δ 147.5, 140.4, 134.5, 132.9, 125.6, 122.4, 120.6, 120.3, 119.4, 112.2, 65.3, 48.5, 26.2, 18.6, 13.2, −5.1, −5.3; ES+ m/z (relative intensity) 314.2 (M + H), 100%); HRMS (ES+) calcd for C19H28NOSi: 314.1940, found 314.1944.
(39 h). (yellow oil): 1H NMR (400 MHz, C6D6) δ 7.75 (d, J = 7.7 Hz, 1H), 7.47 (d, J = 8.0 Hz, 1H), 7.25 (t, J = 7.2 Hz, 1H), 7.19 (t, J = 7.6 Hz, 1H), 6.28 (s, 1H), 5.79 (s. 1H), 4.48 (t, J = 6.0 Hz, 1H), 3.78 (dd, J = 10.0, 6.0 Hz, 1H), 3.48 (dd, J = 10.0, 6.0 Hz, 1H), 1.87 (s, 3H), 0.89 (s, 9H), −0.05 (s, 3H), −0.12 (s, 3H); 13C NMR (100 MHz, C6D6) δ 148.5, 135.4, 133.7, 129.9, 122.1, 121.5, 119.6, 110.5, 90.9, 65.2, 64.3, 26.0, 18.4, 12.4, −5.3, −5.6; ES+ m/z (relative intensity) 314.2 (M + H, 100%), 627.4 (2 M + H, 25%); HRMS (ES+) calcd for C19H28NOSi: 314.1940, found 314.1951.
1-(tert-Butyl-dimethyl-silanyl)-3-methyl-1,4-dihydro-cyclopenta[b]indole (38i) and 3-(tert-Butyl-dimethyl-silanyl)-1-methyl-8H-3a-aza-cyclopent[a]indene (40i)
Method A: Following general procedure 4, with column chromatography performed at −78 °C, 84 mg (0.27 mmol) of allene 25 produced 34 mg (44%) of 38i as a yellow oil. Method B: Following general procedure 5, 75 mg (0.23 mmol) of allene 25 produced 22 mg (34%) of 38i as a yellow oil. Method B: Following general procedure 6, 1005 mg (0.30 mmol) of allene 25 produced 21 mg (25%) of 38i as a yellow oil and 7.4 mg (9%) of 40i as a light yellow oil. 38i: IR (neat): 3410 cm−1; 1H NMR (360 MHz, CDCl3) δ 7.95 (br s, 1H), 7.57 (m, 1H), 7.37 (m, 1H), 7.07 (m, 2H), 6.35 (app pent, J = 1.8 Hz, 1H), 3.43 (app pent, J = 1.8 Hz, 1H), 2.21 (t, J = 1.8 Hz, 3H), 0.87 (s, 9H), 0.24 (s, 3H), −0.36 (s, 3H);13C NMR (75 MHz, CDCl3) δ 147.6, 140.3, 133.2, 127.9, 124.6, 123.2, 119.9, 119.3, 119.2, 111.7, 38.0, 27.3, 18.0, 12.8, −4.5, −8.1; MS (ES+) m/z (relative intensity) 284.2 (M + H, 90%), 567.4 (2 M + H, 100%), 850.6 (3 M + H, 25%); HRMS (ES+) calcd for C18H26NSi: 284.1835, found 284.1837.
40iIR (neat): 2123, 1606 cm−1; 1H NMR (360 MHz, C6D6) δ ppm: 7.68 (d, 1H, J = 8.3 Hz), 7.21 (s overlap w solvent, 1H), 7.11 (d, 1H, J = 7.6 Hz), 6.74 (s, 1H), 3.34 (s, 2H), 2.18 (s, 3H), 1.11 (s, 9H), 0.50 (s, 6H); 13C NMR (90 MHz, C6D6) δ ppm: 143.5, 138.7, 135.6, 127.1, 126.1, 122.4, 122.4, 121.0, 111.9, 111.6, 27.6, 27.4, 18.2, 11.1, −3.1; MS (ES+) m/z (relative intensity) 284.2 (M + H, 100%); HRMS (ES+) calcd for C18H26NSi: 284.1835, found 284.1816.
1-[2-(tert-Butyl-dimethyl-silanyloxy)-ethyl]-2,3-dimethyl-1,4-dihydro-cyclopenta[b]indole (38m) and 3-[2-(tert-Butyl-dimethyl-silanyloxy)-ethyl]-1,2-dimethyl-8H-3a-aza-cyclopenta[a]indene (40m)
Method A: Following general procedure 4, with column chromatography performed at −78 °C and irradiation performed at 350 nm, 75 mg (0.20 mmol) of allene 24 produced 29 mg (42%) of 38m as a yellow oil. Method B: Following general procedure 5, with column chromatography on deactivated silica gel (2% H2O/SiO2), 100 mg (0.27 mmol) of allene 24 produced 6 mg (7%) of 38m as a yellow oil and 37 mg (40%) of 40m as a colorless oil. 38m: IR (neat): 3401 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.94 (br s, 1H), 7.53 (d, J = 7.7 Hz, 1H), 7.34 (dd, J = 7.0, 0.9 Hz, 1H), 7.08 (td, J = 7.1, 0.9 Hz, 1H), 7.01 (t, J = 7.2 Hz, 1H), 3.83 - 3.71 (m, 2H), 3.34 - 3.31 (m, 1H), 2.35 - 2.24 (m, 1H), 2.03 (d, J = 1.0 Hz, 3H), 2.01 (d, J = 0.7 Hz, 3H), 1.60 - 1.55 (m, 1H), 0.89 (s, 9H), 0.04 (s, 3H), 0.02 (s, 3H);13C NMR (90 MHz, CDCl3) δ 148.4, 145.5, 139.3, 125.0, 124.1, 120.7, 119.9, 119.2, 117.9, 111.7, 61.4, 44.7, 33.9, 26.0, 18.3, 12.9, 10.4, −5.15, −5.22; ES+ m/z (relative intensity) 342.2 (M + H, 100%); HRMS (AP+) calcd for C21H32NOSi: 342.2253, found 342.2239.
40m: IR (neat): 2857, 1607, 1098 cm−1; 1H NMR (300 MHz, C6D6) δ ppm: 7.32 (d, 1H, J = 3.9 Hz), 7.05 (t, 2H, J = 7.0 Hz), 6.86 (dt, 1H, J = 3.7, 0.9 Hz), 3.81 (t, 2H, J = 7.5 Hz), 3.32 (bs, 2H), 3.13 (t, 2H, J = 7.4 Hz), 2.07 (s, 3H), 2.02 (s, 3H), 0.93 (s, 9H), −0.02 (s, 6H); 13 C NMR (C6D6) δ ppm: 145.0, 136.1, 130.9, 126.0, 121.8, 120.8, 119.6, 110.5, 110.4, 63.3, 29.4, 27.5, 26.1, 18.1, 9.9, 9.7, −5.4; ES+ m/z (relative intensity) 342.2 (M + H, 100%); HRMS (ES+) calcd for C21H32NOSi: 342.2253, found 342.2253.
6,9,9-Trimethyl-5,7,8,9,10,10a-hexahydro-indeno[2,1-b]indol-10-ol (42a) and 3,3,1,1-Trimethyl-2,3-dihydro-1H-indolo[1,2-a]indole (44)
Method A: Following general procedure 4, 109 mg (0.369 mmol) of allene 29 produced 59 mg (60%) of 42a as a brown solid. Method B: Following general procedure 5, 350 mg (1.18 mmol) of allene 29 produced 107 mg (34%) of 42a as a brown solid and 72 mg (25%) of 441b as a brown solid. Method C: Following general procedure 6, 78 mg (0.26 mmol) of allene 29 produced 15 mg (21%) of 42a as a brown solid and 16 mg (24%) of 44 as a brown solid. 42a: mp. 76 °C (dec.); IR (neat): 3400, 3301 cm−1; 1H NMR (400 MHz, C6D6) δ 7.94 (d, J = 7.7 Hz, 1H), 7.29 - 7.20 (m, 3H), 6.99 (br s, 1H), 3.26 (d, J = 10.7 Hz, 1H), 2.56 (dd, J = 10.7, 4.8 Hz, 1H), 2.34 (dd, J = 14.2, 3.0 Hz, 1H), 2.16 (td, J = 14.2, 3.2 Hz, 1H), 1.80 - 1.75 (m, 1H), 1.77 (s, 3H), 1.44 (dd, J = 13.1, 3.2 Hz, 1H), 1.08 (s, 3H), 1.08 - 0.99 (m, 1H), 0.91 (s, 3H); 13C NMR (90 MHz, CDCl3) δ 148.9, 145.5, 139.3, 125.0, 122.5, 120.7, 120.1, 119.5, 118.3, 111.8, 82.6, 49.1, 40.7, 36.2, 28.0, 22.7, 17.5, 10.2; APCI+ m/z (relative intensity) 268.2 (M + H, 100%); HRMS (ES+) calcd for C18H22NO: 268.1701, found 268.1714.
4-(tert-Butyl-dimethyl-silanyloxy)-3,3-dimethyl-11-trimethyl-silanylmethyl-2,3,4,4a-tetrahydro-1H-indolo[1,2-a]indole (43e) and 2-{1-[3-(tert-Butyl-dimethyl-silanyloxy)-4,4-dimethyl-cyclohex-1-enyl]-vinyl}–1H-indole (45e)
Method A: Following General Procedure 5, 135 mg (0.28 mmol) of 28e produced 45 mg (35%) of 43e as a white solid and 44 mg (41%) of 45e as a brown oil. Method B: Following General Procedure 6, 60 mg (0.12 mmol) of 28e produced 28 mg (50%) of 43e as a white solid and 5 mg (11%) of 45e as a brown oil. Crystals of 43e suitable for X-ray crystallographic analysis were obtained by slow evaporation of an Et2O/hexanes solution of 43e over a period of 24 h at rt. mp. 105 − 106 °C. 43e: IR (neat): 1456 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.49 (m, 2H), 7.00 (m, 2H), 6.08 (s, 1H), 4.55 (d, J = 9.3 Hz, 1H), 3.10 (d, J = 9.4 Hz, 1H), 2.48 (dd, J = 14.2, 5.5 Hz, 1H), 2.31 (td, J = 12.9, 5.1 Hz, 1H), 1.87 (s, 2H), 1.63 (dd, J = 13.4, 4.0 Hz, 1H), 1.33 (m, 1H), 1.15 (s, 3H), 1.00 (s, 12H), 0.06 (s, 9H), −0.06 (s, 3H), −0.84 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 151.3, 139.6, 137.0, 132.3, 125.0, 120.7, 119.7, 118.8, 112.3, 90.8, 84.1, 65.6, 39.1, 37.8, 29.9, 26.6, 22.7, 18.5, 18.4, 15.5, −0.9, −2.3, −3.7; ES+ m/z (relative intensity) 454.3 (M + H, 100%); HRMS (ES+) calcd for C27H44NOSi2: 454.2961, found 454.2949.
45e: IR (neat): 3412 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.05 (br s, 1H), 7.56 (dd, J = 8.2, 1.1 Hz, 1H), 7.31 (dd, J = 8.0, 0.8 Hz, 1H), 7.16 (td, J = 7.0, 1.2 Hz, 1H), 7.08 (td, J = 7.1, 1.2 Hz, 1H), 6.52 (dd, J = 2.1, 0.9 Hz, 1H), 5.79 (pent, J = 1.6 Hz, 1H), 5.34 (s, 1H), 5.22 (s, 1H), 3.88 (dd, J = 4.9, 2.0 Hz, 1H), 2.25 (m, 2H), 1.65 (m, 1H), 1.45 (m, 1H), 0.96 (s, 3H), 0.92 (s, 3H), 0.90 (s, 9H), 0.05 (s, 3H), 0.00 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 142.3, 137.4, 136.4, 136.0, 131.2, 128.6, 122.2, 120.6, 119.9, 111.9, 110.6, 102.4, 74.7, 33.8, 33.3, 26.8, 25.9, 25.4, 21.4, 18.2, −4.1, −4.9; ES+ m/z (relative intensity) 382.2 (M + H, 100%); HRMS (ES+) calcd for C24H36NOSi: 382.2566, found 382.2555.
6,9,9-Trimethyl-10-(2-trimethylsilanyl-ethoxymethoxy)-5,7,8,9,10,10a-hexahydro-indeno[2,1-b]indole (42f) and 3,3,1,1-Trimethyl-2,3-dihydro-1H-indolo[1,2-a]indole (44)
Method A: Following general procedure 4, 84 mg (0.20 mmol) of allene 28c produced 47 mg (60%) of 42f as a yellow solid. Method B: Following general procedure 5, 67 mg (0.16 mmol) of allene 28c produced 22 mg (35%) of 42f as a yellow solid and 20 mg (51%) of 441b as a yellow solid. Method C: Following general procedure 6, 57 mg (0.13 mmol) of allene 28c produced 20 mg (38%) of 42f as a yellow solid and 17 mg (51%) of 44 as a yellow solid.42f: mp. 139 – 140 °C; IR (neat): 3405 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.99 (br s, 1H), 7.55 (d, J = 7.8 Hz, 1H), 7.32 (d, J = 7.8 Hz, 1H), 7.09 (t, J = 7.1 Hz, 1H), 7.03 (t, J = 7.6 Hz, 1H), 4.91 (d, J = 6.8 Hz, 1H), 4.61 (d, J = 6.8 Hz, 1H), 3.87 (m, 1H), 3.52 (m, 1H), 3.38 (d, J = 10.5 Hz, 1H), 2.73 (d, J = 10.7 Hz, 1H), 2.54 (m, 1H), 2.36 (m, 1H), 2.01 (s, 3H), 1.66 (m, 1H), 1.30 (m, 1H), 1.21 (s, 3H), 1.08 (s, 3H), 0.91 (m, 1H), 0.00 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 149.2, 145.9, 139.3, 125.3, 122.3, 120.1, 120.0, 119.4, 118.6, 111.7, 96.8, 89.7, 65.8, 48.7, 41.3, 36.5, 28.7, 22.7, 18.7, 18.0, 10.2, −1.5; ES+ m/z (relative intensity) 398.7 (M + H, 100%), 420.7 (M + Na, 40%); HRMS (ES+) calcd for C24H36NO2Si: 398.2515, found 398.2521.
10-(tert-Butyl-dimethyl-silanyloxy)-6-(tert-butyl-dimethyl-silanyloxymethyl)-9,9-dimethyl-5,7,8,9,10,10a-hexahydro-indeno[2,1-b]indole (42 g)
Following general procedure 4, allene 37 (99 mg, 0.18 mmol) produced 54 mg (59%) of 42g as a tacky yellow solid: IR (neat): 3483 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.20 (br s, 1H), 7.65 (m, 1H), 7.34 (m, 1H), 7.01 – 7.10 (m, 2H), 4.73 (d, J = 13.2 Hz, 1H), 4.64 (d, J = 13.2 Hz, 1H), 3.41 (d, J = 10.3 Hz, 1H), 3.07 (d, J = 10.3 Hz, 1H), 2.52 (ddd, J = 14.2, 5.6, 1.8 Hz, 1H), 2.36 – 2.47 (m, 1H), 1.67 (ddd, J = 13.1, 5.3, 1.8 Hz, 1H), 1.36 (dd, J = 13.1, 5.6 Hz, 1H), 1.19 (s, 3H), 1.07 (s, 9H), 1.02 (s, 3H), 0.95 (s, 9H), 0.13 (s, 3H), 0.11 (s, 3H), −0.02 (s, 3H), −0.45 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 148.5, 146.2, 139.2, 127.2, 126.0, 121.8, 120.4, 119.5, 118.7, 111.4, 82.9, 58.6, 50.0, 40.3, 37.7, 29.9, 26.6, 26.0, 23.4, 18.6, 18.4, 18.3, −1.9, −3.0, −5.1, −5.2; ES+ m/z (relative intensity) 512.2 (M + H, 100%); HRMS (ES+) calcd for C30H50NO2Si2: 512.3380, found 512.3379.
6,9,9-Trimethyl-8,9-dihydro-7H-indeno[2,1-b]indole-5-carboxylic Acid tert-Butyl Ester (46)
To a 0 °C stirring solution of alcohol 42a (198 mg, 0.741 mmol) in 7.5 mL of CH2Cl2 was added DMAP (904 mg, 7.41 mmol) and Boc2O (403 mg, 1.85 mmol) sequentially and then the reaction was warmed to rt and stirred for 1 h. The solvent was then removed under reduced pressure and the residue was dissolved in Et2O (15 mL) and washed with H2O (3 × 15 mL), and brine (3 × 15 mL), dried over MgSO4, and concentrated under reduced pressure. The residue was purified by flash chromatography (5% Et2O/hexanes) to provide 150 mg (58%) of 46 as an orange oil: IR (neat): 1736 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.01 (dd, J = 8.3, 0.8 Hz, 1H), 7.51 (d, J = 7.6 Hz, 1H), 7.17 (td, J = 7.5, 1.0 Hz, 1H), 7.10 (ddd, J = 8.5, 7.3, 1.4 Hz, 1H), 6.58 (s, 1H), 2.64 (td, J = 6.0, 1.3 Hz, 2H), 2.23 (s, 3H), 1.75 (t, J = 6.7 Hz, 2H), 1.69 (s, 9H), 1.20 (s, 6H);13C NMR (100 MHz, CDCl3) δ 150.2, 148.7, 141.9, 139.7, 133.6, 132.4, 125.5, 124.8, 123.0, 121.7, 117.6, 116.8, 116.3, 83.9, 38.6, 33.7, 28.4, 28.3, 20.5, 14.0; ES+ m/z (relative intensity) 350.2 (M + H, 100%); HRMS (ES+) calcd for C23H28NO2: 350.2120, found 350.2124.
6,6,9,9-Tetramethyl-7,8,9,10-tetrahydro-6H-indeno[2,1-b]indole-5-carboxylic Acid tert-Butyl Ester (47)
To a −40 °C stirring solution of fulvene 46 (27 mg, 0.077 mmol) in 4 mL of THF was added LiAlH4 (1.0 M in THF, 120 µL, 0.12 mmol) and the reaction solution was allowed to warm to rt. CH3I (15 µL, 0.24 mmol) was then added and stirring was continued for 30 min, then ice-cold saturated NH4Cl (4 mL) was added slowly and the mixture was diluted with Et2O (15 mL). The organic layer was washed with H2) (3 × 15 mL) and brine (3 × 15 mL), dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash chromatography (5% Et2O/hexanes) to provide 24 mg (85%) of 47 as a yellow oil: IR (neat): 1729 cm−1; 1H NMR (360 MHz, CDCl3) δ 8.14 (m, 1H), 7.56 (m, 1H), 7.20 - 7.17 (m, 2H), 2.41 (t, J = 2.3 Hz, 2H), 2.15 - 2.12 (m, 2H), 1.71 (s, 9H), 1.54 (t, J = 6.2 Hz, 2H), 1.39 (s, 6H), 1.00 (s, 6H); 13C NMR (90 MHz, CDCl3) δ 151.8, 150.1, 145.0, 139.5, 127.4, 126.4, 124.4, 122.7, 122.5, 118.6, 116.4, 83.7, 48.5, 37.5, 35.9, 29.7, 28.4, 28.2, 20.6, 19.0; ES+ m/z (relative intensity) 366.3 (M + H, 100%); HRMS (ES+) calcd for C24H32NO2: 366.2433, found 366.2446.
(E)-3-Iodo-2-phenyl-prop-2-en-1-ol (58b)
A solution of (Z)-3-Iodo-2-phenyl-prop-2-en-1-ol (58a)25 (1.12 g, 4.31 mmol) in 160 mL of CH3CN was irradiated at 254 nm for 2 h and then concentrated under reduced pressure. 1H NMR analysis of the reaction mixture indicated that it contained a 5: 1 mixture of the E and Z isomers, respectively. The residue was purified by flash chromatography (hexanes → 4% EtOAc/hexanes → 8% EtOAc/hexanes) to provide 157 mg (14%) of the starting material 58a and 400 mg (36%) of 58b as a white solid: mp. 58 –59 °C; IR (neat): 3321 cm−1; 1H NMR (360 MHz, CDCl3) δ 7.28 - 7.21 (m, 3H), 7.11 - 7.09 (m, 2H), 6.49 (s, 1H), 4.19 (s, 2H), 2.27 (br s, 1H); 13C NMR (90 MHz, CDCl3) δ 151.7, 139.5, 128.5, 128.1, 128.0, 78.3, 67.3; EI+ m/z (relative intensity) 260.3 (M+, 100%); HRMS (EI+) calcd for C9H9OI 259.9698, found 259.9704.
(E)-tert-Butyldimethyl(2-phenylpent-2-en-4-ynyloxy)silane (59)
To a stirring solution of alcohol 58b (395 mg, 1.52 mmol) in 10 mL of Et3N were added trimethylsilylacetylene (433 µL, 3.04 mmol), PdCl2(PPh3)2 (21 mg, 0.30 mmol), and CuI (3 mg, 0.02 mmol), and the reaction mixture was stirred for 24 h at rt and then diluted with Et2O. The mixture was washed with saturated NH4Cl solution (3 × 50 mL), H2O (3 × 50 mL), and brine (3 × 50 mL), dried over MgSO4, and concentrated under reduced pressure. The crude residue was purified by flash chromatography (hexanes → 5% Et2O/hexanes → 25% Et2O/hexanes) to provide 215 mg (54%) of the corresponding TMS-alkyne. TBAF (1.0 M in THF, 1 mL, 1 mmol) was added slowly to a solution of the above TMS-alkyne (215 mg, 0.933 mmol) in 10 mL of THF and stirred for 20 min. Saturated NaHCO3 (10 mL) was added, and the organic layer was washed with H2O (3 × 30 mL) and brine (3 × 30 mL), dried over MgSO4, and concentrated under reduced pressure. The residue was dissolved in CH2Cl2 (6 mL). Imidazole (95 mg, 1.4 mmol) and TBSCl (211 mg, 1.40 mmol) were added sequentially, and the reaction solution was stirred for 20 min, then H2O (10 mL) was added slowly, and the reaction mixture was diluted with Et2O (50 mL). The organic layer was washed with H2O (3 × 50 mL) and brine (3 × 50 mL), dried over MgSO4, and concentrated under reduced pressure. The residue was purified by flash chromatography (hexanes → 5% Et2O/hexanes) to provide 228 mg (90%) of 59 as a yellow oil: IR (neat) 3294 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.49 - 7.46 (m, 2H), 7.39 - 7.29 (m, 3H), 5.94 (app q, J = 2.3 Hz, 1H), 4.45 (dd, J = 2.0, 0.7 Hz, 2H), 2.90 (d, J = 2.5 Hz, 1H), 0.94 (s, 9H), 0.10 (s, 6H); 13C NMR (90 MHz, CDCl3) δ 153.1, 137.0, 128.2, 128.1, 127.7, 103.5, 81.6, 80.6, 65.4, 25.8, 18.3, −5.4; ES+ m/z (relative intensity) 273.2 (M + H, 100%); HRMS (ES+) calcd for C17H25OSi: 273.1675, found 273.1664.
Acetic Acid 1-(2-Azidophenyl)-6-(tert-butyldimethylsilanyloxy)-5-phenylhex-4-en-2-ynyl Ester (60)
To a −30 °C stirring solution of alkyne 59 (278 mg, 0.837 mmol) in 8 mL of THF was added slowly n-BuLi (2.5 M in hexanes, 340 µL, 0.84 mmol). Stirring was continued for 1 h, and then a solution of 2-azidoben-zaldehyde (123 mg, 0.837 mmol) in 8 mL of THF was added. Stirring was continued for 20 min (as monitored by TLC for starting material consumption), and then Ac2O (119 µL, 1.26 mmol) was added, and the reaction solution was allowed to warm to rt. Ice-cold saturated NH4Cl (10 mL) was added dropwise, and the mixture was diluted with Et2O (25 mL). The organic layer was washed with H2O (3 × 25 mL) and brine (3 × 25 mL), dried over MgSO4, and concentrated under reduced pressure. The residue was purified by flash chromatography (5% Et2O/hexanes → 15% Et2O/hexanes) to provide 320 mg (83%) of 60 as a yellow oil: IR (neat) 2126, 1746 cm−1; 1H NMR (360 MHz, CDCl3) δ 7.35–7.32 (m, 2H), 7.30 (dd, J = 7.7, 1.1 Hz, 1H), 7.24 (td, J = 7.9, 1.4 Hz, 1H), 7.21–7.18 (m, 3H), 7.00–6.92 (m, 2H), 6.59 (d, J = 1.5 Hz, 1H), 5.91 (app q, J = 2.0 Hz, 1H), 4.37 (d, J = 1.7 Hz, 2H), 1.94 (s, 3H), 0.84 (s, 9H), 0.00 (s, 6H); 13C NMR (90 MHz, CDCl3) δ 169.3, 153.1, 137.6, 137.0, 130.0, 129.4, 128.02, 127.95, 127.8, 127.7, 124.7, 118.0, 103.6, 88.0, 85.3, 65.3, 61.4, 25.8, 20.8, 18.2, −5.5; ES+ m/z (relative intensity) 484.1 (M + Na, 80), 507.2 (M + 2Na, 70); HRMS (ES+) calcd for C26H31N3O3SiNa 484.2032, found 484.2033.
[4-(2-Azidobenzyl)-3-methylnaphthalen-1-ylmethoxy]-tert-butyldimethylsilane (61)
Following general procedure 3, 320 mg (0.693 mmol) of 60 produced 120 mg (41%) of 61 as a tacky yellow solid: IR (neat) 2120 cm−1; 1H NMR (360 MHz, CDCl3) δ 7.73 (dd, J = 7.5, 1.7 Hz, 1H), 7.54 (dd, J = 7.6, 1.8 Hz, 1H), 7.17–7.13 (m, 2H), 6.97–6.90 (m, 3H), 6.56 (td, J = 7.1, 2.0 Hz, 1H), 6.23 (d, J = 7.8 Hz, 1H), 4.96 (s, 2H), 4.08 (s, 2H), 2.17 (s, 3H), 0.74 (s, 9H), −0.08 (s, 6H); 13C NMR (90 MHz, CDCl3) δ 137.7, 135.2, 134.2, 132.9, 131.53, 131.48, 129.9, 129.0, 127.28, 127.25, 127.1, 125.9, 124.8, 124.6, 123.7, 117.6, 63.4, 28.9, 26.0, 20.4, 18.5, −5.2; ES+ m/z (relative intensity) 418.3 (M + H, 100), 435.3 (M + NH4, 70), 440.3 (M + Na, 70); HRMS (ES+) calcd for C25H32N3OSi 418.2315, found 418.2322.
Supplementary Material
Acknowledgment
Support from the National Institutes of Health, General Medical Sciences division (GM72572), and the National Science Foundation for the X-ray Crystallography Facility (CHE 0131112), is gratefully acknowledged. C.S.L. is grateful to CESGA for supercomputer time.
Footnotes
Supporting Information Available: General experimental procedures, copies of 1H and 13C NMR spectra for 13i, 14j, 19–25, 27c, 28c, 29, 30, 35c, 37, 38h,i,m, 39n, 40g, 42a,f,g, 43e, 45e, 46, 47, 58b, and 59–61, details of the X-ray crystallographic structural determinations of 39n, 40a, 42b,d, 43e, and 54, 2-D energy profiles for the 64 → 67 conversion and its desmethyl lower homologue, and Cartesian coordinates and SCF energies of all of the computed structures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Feldman KS, Iyer MR, Hester DK., II Org. Lett. 2006;8:3113–3116. doi: 10.1021/ol061216u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Feldman KS, Hester DK, II, López CS, Faza ON. Org. Lett. 2008;10:1665–1668. doi: 10.1021/ol800429j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López CS, Faza ON, Feldman KS, Iyer MR, Hester DK., II J. Am. Chem. Soc. 2007;129:7638–7646. doi: 10.1021/ja070818l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Park A, Moore RE, Patterson GML. Tetrahedron Lett. 1992;33:3257–3260. [Google Scholar]; (b) Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J. Am. Chem. Soc. 1994;116:9935–9942. [Google Scholar]
- 4.Ondeyka JG, Helms GL, Hensens OD, Goetz MA, Zink DL, Tsipouras A, Shoop WL, Slayton L, Dombrowski AW, Polishook JD, Ostlind DA, Tsou NN, Ball RG, Singh SB. J. Am. Chem. Soc. 1997;119:8809–8816. [Google Scholar]
- 5.Irlinger B, Bartsch A, Krämer H-J, Mayser P, Steglich W. Helv. Chim. Acta. 2005;88:1472–1485. [Google Scholar]
- 6.Gross G, Wentrup C. J. Chem. Soc. Chem. Commun. 1982;360 [Google Scholar]
- 7.(a) Büchi G, Manning RE. J. Am. Chem. Soc. 1966;88:2532. [Google Scholar]; (b) Kutney JP, Beck J, Bylsma F, Cretney WJ. J. Am. Chem. Soc. 1968;90:4504. doi: 10.1021/ja01018a085. [DOI] [PubMed] [Google Scholar]; (c) Potier P, Langlois Y, Gueritte F. J. Chem. Soc., Chem. Commun. 1975:670–2671. [Google Scholar]; (d) Kutney JP, Ratcliffe AH, Treasurywala AM, Wunderly S. Heterocycles. 1975;3:639–649. [Google Scholar]; (e) Schill G, Priester CU, Windhovel UF, Fritz H. Tetrahedron. 1987;43:3765–3786. [Google Scholar]; (f) Magnus P, Stamford A, Ladlow M. J. Am. Chem. Soc. 1990;112:8210–8212. [Google Scholar]; (g) Yokoshima S, Ueda T, Kobayashi S, Sato A, Kuboyama T, Tokuyama H, Fukuyama T. J. Am. Chem. Soc. 2002;124:2137–2139. doi: 10.1021/ja0177049. [DOI] [PubMed] [Google Scholar]; (h) Ishikawa H, Colby DA, Boger DL. J. Am. Chem. Soc. 2008;130:420–421. doi: 10.1021/ja078192m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen A, Krause N. Synthesis. 2002:1987–1992. [Google Scholar]
- 9.Piers E, Grierson JR, Lau CK, Nagakura I. Can. J. Chem. 1982;60:210–223. [Google Scholar]
- 10.Feldman KS, Iyer MR. J. Am. Chem. Soc. 2005;127:4590–4591. doi: 10.1021/ja050757w. [DOI] [PubMed] [Google Scholar]
- 11.Kashulin IA, Nifant’ev IE. J. Org. Chem. 2004;69:5476–5479. doi: 10.1021/jo049504w. [DOI] [PubMed] [Google Scholar]
- 12.Cambridge Crystallographic Date Centre deposition numbers: 39n, 649706; 40a, 619622; 42b, 650218, 42d, 652945; 43e, 650217; 54, 651276. The data can be obtained free of charge from the Cambridge Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
- 13.(a) Hirsch JA. Top. Stereochem. 1967;1:199–222. [Google Scholar]; (b) Eliel EL, Satici H. J. Org. Chem. 1994;59:688–689. [Google Scholar]; (c) Crich D, Jayalath P, Hutton TK. J. Org. Chem. 2006;71:3064–3070. doi: 10.1021/jo0526789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukai C, Kobayashi M, Kubota S, Takahashi Y, Kitagaki S. J. Org. Chem. 2004;69:2128–2136. doi: 10.1021/jo035729f. [DOI] [PubMed] [Google Scholar]
- 15.(a) Hohenberg P, Kohn W. Phys. Rev. 1964;136:B864–B871. [Google Scholar]; (b) Kohn W, Sham L. Phys. Rev. A. 1965;140:A1133–A1138. [Google Scholar]; (c) Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. J. Phys. Chem. 1994;98:11623–11637. [Google Scholar]; (d) Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]; (e) Lee C, Yang W, Parr RG. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 16.Woodward RB, Hoffmann R. The Conservation of Orbital Symmetry. Weinheim: VCH; 1970. [Google Scholar]
- 17.(a) Herges R, Geuenich D. J. Phys. Chem. A. 2001;105:3214–3220. [Google Scholar]; (b) Geuenich D, Hess K, Koehler F, Herges R. Chem. Rev. 2005;105:3758–3772. doi: 10.1021/cr0300901. [DOI] [PubMed] [Google Scholar]
- 18.Schleyer PvR, Maerker C, Dransfeld A, Jiao H, Hommes NJRvE. J. Am. Chem. Soc. 1996;118:6317–6318. doi: 10.1021/ja960582d. [DOI] [PubMed] [Google Scholar]
- 19.(a) Li Z, Quan RW, Jacobsen EN. J. Am. Chem. Soc. 1995;117:5889–5890. [Google Scholar]; (b) Brandt P, Södergren MJ, Andersson PG, Norrby P-O. J. Am. Chem. Soc. 2000;122:8013–8020. [Google Scholar]; (c) Bertz SH, Cope S, Murphy M, Ogle CA, Taylor BJ. J. Am. Chem. Soc. 2007;129:7208–7209. doi: 10.1021/ja067533d. [DOI] [PubMed] [Google Scholar]
- 20.(a) Hohenberg P, Kohn W. Phys. Rev. 1964;136:B864–B871. [Google Scholar]; (b) Kohn W, Sham L. Phys. Rev. A. 1965;140:A1133–A1138. [Google Scholar]; (c) Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. J. Phys. Chem. 1994;98:11623–11637. [Google Scholar]; (d) Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]; (e) Lee C, Yang W, Parr RG. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 21.Frisch MJ, et al. Gaussian03, revision C.02. Wallingford, CT: Gaussian, Inc; 2004. [Google Scholar]
- 22.Bauernschmitt R, Ahlrichs R. J. Chem. Phys. 1996;22:9047–9052. [Google Scholar]
- 23.(a) Hess BA, Jr, Eckart U, Fabian J. J. Am. Chem. Soc. 1998;120:12310–12315. [Google Scholar]; (b) Hess BA, Jr, Smentek L, Brash AR, Cha JK. J. Am. Chem. Soc. 1999;121:5603–5604. [Google Scholar]; (c) López CS, Faza ON, York DM, de Lera A. J. Org. Chem. 2004;69:3635–3644. doi: 10.1021/jo049620z. [DOI] [PubMed] [Google Scholar]; (d) Li J, Worthington SE, Cramer CJ. J. Chem. Soc., Perkin Trans. 2. 1998:1045–1052. [Google Scholar]
- 24.Huang Z, Negishi E-i. Org. Lett. 2006;8:3675–3678. doi: 10.1021/ol061202o. [DOI] [PubMed] [Google Scholar]
- 25.Duboudin JG, Jousseaume B, Bonakdar A. J. Organomet. Chem. 1979;168:227–232. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.