1. Introduction
The basic steps of the infectious cycle of any pathogen—entry into, spread within, and exit from the host—offer crucial insights into how pathogens cause disease. As a result, a full understanding of the stages of a microorganism's life cycle may inform the rational design of therapeutics to prevent or ameliorate consequent disease.
Measles virus (MV) is one of the most transmissible microorganisms known; it continues to result in hundreds of thousands of infections annually throughout the world, many of which have serious pathogenic consequences that can result in death. Despite the tremendous progress that has been made in deciphering the basis of MV pathogenesis and in the creation of effective attenuated vaccines, how MV causes disease—including rare, but serious, central nervous system (CNS) complications--remains poorly understood. It is our view that defining the pathogenesis of MV in the CNS necessitates an understanding of the interaction of the virus with the host in trafficking to and spreading within the CNS. This is the focus of this review.
1.1 Measles virus: genome and proteins
MV is a member of the Paramyxoviridae, within the Morbillivirus genus. Its genome consists of ~16,000 bases of non-segmented, single-stranded negative sense RNA, meaning that the viral genome is transcribed immediately upon entry into the cell. Virions are spherical and enveloped, and the envelope is derived from the host cell as the viral particle buds from the plasma membrane. The viral genome encodes 8 proteins, the function of which are briefly noted below. Inserted into the envelope of the virion are the two MV glycoproteins, hemagglutinin (H) and fusion (F). These proteins mediate attachment to cellular receptors and fusion of the virion with the target cell or fusion of an infected cell with an adjacent uninfected cell. The matrix protein (M) lies immediately underneath the virion envelope and serves in virus assembly and budding. The two polymerase proteins, large (L) and phosphoprotein (P), are closely associated with the genome, which is encapsidated by the nucleocapsid (N) protein. The nonstructural proteins V and C, encoded within the P cistron, are also packaged within the virion; these proteins have recently been shown to play a role in counteracting host antiviral immune responses (reviewed in Griffin, 2001).
1.2 Measles virus pathogenesis
MV is a human-restricted pathogen that spreads among individuals by release of aerosol droplets. An infected individual will undergo a latent period of 10 to 14 days followed by a few days of fever, cough, coryza, and rash. Primary infection occurs in the upper respiratory tract, but MV will secondarily infect lymphoid cells (reviewed in Griffin, 2001; Rall, 2003; Schneider-Schaulies et al., 2003; Sips et al., 2007). Although the immunological response made by most infected, immunocompetent individuals is sufficient to clear the virus and provide life-long protection, a period of transient immunosuppression is a notorious characteristic of MV infection, and is likely the basis of most of the complications and the subsequent fatalities following acute infection (reviewed in Dhib-Jalbut and Johnson, 1994; Griffin et al., 2008; Rall, 2003). Additional consequences of acute infection include diarrhea, pneumonia, and encephalitis.
1.2.1 Immunosuppression
Immunosuppression following MV infection can last for weeks, extending beyond the classical MV symptoms. The chief risk of immunosuppression is that it renders infected individuals more susceptible to secondary infections, though precisely how this occurs is not fully understood. In humans, MV-induced immunosuppression is characterized by a loss of delayed type hypersensitivity responses to recall antigens [e.g., tuberculin (Tamashiro et al., 1987)], a limited response of lymphocytes to mitogens when cultured ex vivo (Hirsch et al., 1984), and impaired responses to new antigens (Coovadia et al., 1978). To date, a number of mechanisms have been proposed based on animal and cell culture studies, all of which could be pertinent in the natural infection. For example, Griffin and colleagues showed that MV infection of antigen-presenting cells suppresses interleukin-12 (IL-12) production, which then in turn skews the CD4+ T cell response toward a Th2 profile (Karp et al., 1996). This altered CD4+ T cell response leads to inappropriate priming of T cells and a failure of T cells to proliferate following interaction with MV-infected dendritic cells (Fugier-Vivier et al., 1997; Servet-Delprat et al., 2000). These studies correlate well with serum profiles in MV-infected macaques and humans that also show a skewing toward Th2-like cytokines (Atabani et al., 2001; Moss et al., 2002; Polack et al., 2000). In addition to influencing the Th1/Th2 balance, acute MV infection may precipitate immunosuppression by causing an overall lymphopenia, due to effects on T cell proliferation and progression through the cell cycle (Naniche et al., 1999; Niewiesk et al., 2000; Niewiesk et al., 1999; Schnorr et al., 1997), as well as specifically inhibiting immune function via the production of unidentified, immunosuppressive molecules from infected T cells (Sun, et al., 1998). A detailed discussion of MV-induced immunosuppression can be found in other chapters within this volume.
1.2.2 CNS Complications following Acute MV Infection
Approximately 1 in 100,000 acutely infected individuals later go on to develop CNS complications. These diseases differ in terms of immune status of the affected host, onset of symptoms, presence of MV within the CNS, host survival rate, and neuropathological findings. These are briefly discussed below.
1.2.2.1 Subacute sclerosing panencephalitis (SSPE)
is a slow, progressive disease that is invariably fatal, and can occur from 1 to 15 years following acute MV infection (Dubois-Dalcq et al., 1974). Children are far more likely to develop this complication than adults (reviewed in Johnson, 1998). The disease initially manifests as subtle cognitive losses, progressing to more overt cognitive dysfunction, followed by motor loss, seizures and eventual organ failure in virtually all affected individuals. The rate of SSPE occurrence ranges from 1 in 10,000-300,000 acute MV infections (reviewed in Rima and Duprex, 2005; Takasu et al., 2003). Neurons are predominantly infected, though at late times of infection, oligodendrocytes, astrocytes, and endothelial cells may also be involved (reviewed in Rima and Duprex, 2005). SSPE affects both gray and white matter and is histologically characterized by the presence of cellular inclusion bodies, inflammation, glial activation, loss of blood-brain barrier integrity, and neuronal loss (reviewed in Dhib-Jalbut and Johnson, 1994; Rall, 2003). A serologic hallmark of SSPE, as compared to the other CNS complications, is the elevation of measles-specific antibodies in the blood and cerebrospinal fluid (CSF)(Dubois-Dalcq et al., 1974).
Importantly, evidence from brain biopsies of SSPE patients indicates that infected neurons do not release budding virus (Paula-Barbosa and Cruz, 1981). Based on extensive sequencing studies of MV from these specimens and from cells persistently infected with MV isolates from SSPE patients, it has been proposed that the failure of infected neurons to produce complete extracellular virus may be due to defects in protein expression caused by extensive point mutations in the envelope associated genes, H, F and M (Cattaneo et al., 1988; reviewed in Dhib-Jalbut and Johnson, 1994; Rima and Duprex, 2005), though what role these viral proteins play in neuronal spread of MV—and how mutations may affect MV biology in infected neurons--are not known.
1.2.2.2 Post-infectious encephalomyelitis (PIE)
occurs more frequently than SSPE, affecting ~1 in 1,000 infected individuals. Symptoms of PIE normally appear 5 to 14 days after the characteristic MV rash but can predate the rash (reviewed in Johnson, 1998). This complication is thought to be an autoimmune reaction, perhaps to myelin basic protein (Johnson, et al., 1984). MV antigen and nucleic acids have not been detected in PIE brain biopsies by immunohistochemistry or in situ hybridization (Johnson, et al., 1984; reviewed in Dhib-Jalbut and Johnson, 1994; Norrby and Kristensson, 1997) supporting the notion that this is an autoimmune disease. Additional hallmarks of PIE include perivascular inflammation and demyelination (reviewed in Norrby and Kristensson, 1997). Unlike SSPE, intrathecal production of MV antibodies has only been found in a few cases of PIE. Affected individuals present with seizures, deafness, ataxia, and movement disorders. There is a ~25% mortality rate associated with PIE, and survivors are likely to suffer from frequent neurologic sequelae.
1.2.2.3 Measles inclusion body encephalitis (MIBE)
a rare CNS complication following acute MV infection, has been described in children and adults receiving immunosuppressive drugs and therefore is thought to chiefly affect immunocompromised hosts. The neurologic disease appears 3 to 6 months after the acute MV rash (reviewed in Dhib-Jalbut and Johnson, 1994; Johnson, 1998). As the name suggests, MIBE is characterized by inclusion bodies in both neurons and glia, with accompanying neuronal loss but an overall lack of inflammation (reviewed in Dhib-Jalbut and Johnson, 1994; Johnson, 1998; Norrby and Kristensson, 1997; Rall, 2003). Measles antigen is present in the brain, and virus has been isolated directly from the brains of affected individuals (Johnson, 1998). MIBE differs from SSPE in the absence of elevated serum and cerebrospinal fluid neutralizing antibodies (reviewed in Dhib-Jalbut and Johnson, 1994; Rima and Duprex, 2005). The disease course is relatively short, lasting from days to weeks, causing seizures, motor deficits, and stupor, often leading to coma and death (reviewed in Johnson, 1998).
Importantly, despite the fact that only a small percentage of acute MV infections will go on to develop CNS complications, a few studies have detected MV RNA in various organs, including brain, upon autopsy of elderly individuals who died of nonviral and non-CNS causes (Katayama et al., 1998; Katayama et al., 1995). These findings suggest that MV may persist in the brains (and other organs) of healthy individuals, and that the frequency with which MV invades the CNS cannot be determined by summing the occurrence of the above described CNS complications.
2. Understanding MV CNS complications: results from brains of infected individuals and persistently infected cell lines
The development of a robust live attenuated vaccine has profoundly decreased the infection rate in vaccinated populations. Vaccination has not been associated with SSPE, and only wild-type virus sequences have been isolated from SSPE tissues. However, vaccine strains have been isolated from the CNS of immunocompromised patients with MIBE (Bitnun et al., 1999; reviewed in Rima and Duprex, 2005), implying that the attenuated vaccine strain can traffic to the brain under conditions of poor immune surveillance.
Both SSPE and MIBE are characterized by mutations that apparently render the virus defective, though spread in neurons may still occur. As noted above, it has long been hypothesized that these mutations are the reason why infectious virus is not released from infected brain cells, especially since the mutations map to the envelope-associated proteins M, F and H (Baczko et al., 1988; Billeter et al., 1994; reviewed in Johnson, 1998). In SSPE, M protein expression is reduced, likely through one of two mechanisms: either a steeper gradient of transcription (Cattaneo et al., 1987a; Cattaneo et al., 1987b) or increased read-through across the P-M junction of the MV genome (reviewed in Rima and Duprex, 2005). In support of these data, hyperimmune antibody responses in SSPE are directed to all MV proteins with the key exception of M (reviewed in Rima and Duprex, 2005).
Mutations in F have been described for both SSPE and MIBE cases (Baczko et al., 1988; Billeter et al., 1994; reviewed in Johnson, 1998). Interestingly, in all SSPE cases, mutations in F characteristically consist of the loss of the C-terminal pentadecapeptide, a sequence that is strictly conserved among morbilliviruses and is therefore thought to play an essential role in F protein function. This region contains the basolateral sorting signal of F (Maisner et al., 1998; reviewed in Rima and Duprex, 2005), suggesting that mis-sorting of F may contribute to altered MV spread and lack of complete MV assembly in neurons of SSPE patients. Interestingly, a study of 10 SSPE cases by immunohistochemistry and in situ hybridization suggests that MV likely spreads transneuronally, an observation subsequently validated by in vitro model studies (Lawrence et al., 2000; Oldstone et al., 1999). The analysis of these infected brains revealed that MV infection in neuronal processes was predominantly dendritic, though there were signs of occasional axonal involvement as well (Allen et al., 1996). Whether mutations in viral proteins influence how MV is transported within neurons is currently under investigation.
3. The big questions
Years of research on MV CNS complications have provided key insights into MV neuronal spread, and have identified future areas of study concerning the relationship between viral spread and pathogenesis. For example: how does MV move from the site of entry to the perinuclear space and then again to the site of viral egress? Once present at the synaptic membrane, what cellular and viral proteins mediate MV trans-synaptic spread? Is the mechanism of interneuronal spread related to the pathogenesis of MV within the brain? The establishment of permissive animal and cell culture models, coupled with advances in manipulating the viral genome, and the advent of cell biology resources to explore intracellular trafficking have been essential for key developments over the past few years. A discussion of these recent observations serves as the basis for the remainder of this review. We first describe some of these important technical advances, and then discuss how they have been applied to MV neuronal spread.
4. Tools
4.1 Transgenic mouse models
To date, two human receptors for MV have been identified: CD46 (Dorig et al., 1993; Naniche et al., 1993) and signaling lymphocyte activation molecule (SLAM; Erlenhoefer et al., 2001; Hsu et al., 2001; Tatsuo et al., 2000). While this is still an evolving field, and many believe that other receptors will be identified in the future, the general consensus is that CD46 is principally a receptor for vaccine strains, such as Edmonston, whereas SLAM, though restricted to hematogenous cells, permits entry of wild type MV. As the mouse and rat homologues of CD46 and SLAM do not confer susceptibility to MV infection (Dorig et al., 1993; Manchester et al., 1994; Naniche et al., 1993; Ono et al., 2001b), transgenic mice expressing human CD46 or SLAM were established, with the hypothesis that expression of these human proteins would overcome the initial barrier to viral entry (Mrkic et al., 1998; Oldstone et al., 1999; Rall et al., 1997; Sellin et al., 2006; reviewed in Manchester and Rall, 2001). Important for neuron-focused studies, these transgenic mice also provide a source of primary neurons for ex vivo experiments to complement observations made in vivo (Lawrence et al., 2000; Makhortova et al., 2007). A brief introduction to some of the transgenic model systems follows.
NSE-CD46 mice, developed in the laboratory of Michael Oldstone, were engineered to express the BC1 isoform of human CD46 under the control of the neuron-specific enolase (NSE) promoter, restricting CD46 expression to CNS neurons. These mice can be infected intranasally (i.n.) or intracranially (i.c.) with a vaccine strain of MV (e.g., Edmonston); as predicted, only CD46-expressing neurons are initially permissive for infection. Adult immunocompetent mice mount an aggressive T cell response and survive infection, whereas NSE-CD46+ neonatal mice, or adults on an immunodeficient background succumb to CNS disease. CD46+ primary hippocampal neurons can be cultured from transgenic embryos, providing a parallel in vitro culture system to corroborate and extend the in vivo observations (Lawrence et al., 1999; Lawrence et al., 2000; Makhortova et al., 2007; Patterson et al., 2002; Patterson et al., 2003; Rall et al., 1997).
Another CD46 transgenic model that was developed in Oldstone's lab is the YAC-CD46 mouse, in which CD46 is expressed from its own promoter, more closely mirroring expression in humans. Indeed, CD46 distribution is found throughout the mouse, and this model has been used for both CNS and immunosuppression studies. Importantly, in this model, all four major isoforms of CD46 are expressed to levels and in locations similar to those seen in humans. Furthermore, overall expression of CD46 was shown to be greater than that previously reported for other transgenic mouse models, including the NSE-CD46 mice (Oldstone et al., 1999; Patterson et al., 2001).
Mrkic et al. engineered mice to express CD46 on a mouse background lacking the interferon-α receptor [IFNAR−/−; (Duprex et al., 2000; Ludlow et al., 2007; Mrkic et al., 1998)]. IFNAR−/− mice can be infected intranasally with MV, though replication of MV is limited. Engineering mice to express the MV receptor CD46 on the IFNAR−/− background resulted in higher levels of MV infection in the respiratory tract with subsequent inflammation, providing a more relevant model for the acute MV infection seen in humans.
A perplexing observation from the establishment of a number of other CD46 transgenic mice was that, despite CD46 expression and ability to infect such cells when cultured ex vivo, little if any infection was observed in vivo. For example, Blixenkrone-Moller et al. generated a CD46 transgenic mouse that expressed a genomic copy of CD46 on a Bl/6 x SJL mouse background (Blixenkrone-Moller et al., 1998). Despite apparently robust CD46 expression, MV replication could not be detected following intraperitoneal (IP) or IN inoculation, though kidney and lung cell cultures from these mice were permissive. An important observation made by these authors was that, in these cultures, MV did not reach full replication levels as compared to MV-infected Vero cells. Furthermore, while IC challenge of CD46 transgenic mice did result in limited CNS infection, infection was also observed in non-transgenic mice, suggesting that factors other than CD46 play a role in mediating MV uptake into murine cells, and that key intracellular factors are needed to achieve optimal levels of MV replication. This work was supported by the findings of Horvat et al. and Evlashev et al. who found that cells from CD46 transgenic mice showed cell-type specific susceptibility to MV infection, indicating a role for host factors other than CD46 in mediating productive MV infection (Evlashev et al, 2001; Horvat et al., 1996).
Thus far, four different SLAM-expressing transgenic mouse models have been created (Ohno et al., 2007; Sellin et al., 2006; Shingai et al., 2005; Welstead et al., 2005). However, to date, only one group has used these mice to look at MV CNS infections. Sellin et al. engineered mice to ubiquitously express SLAM. Wild-type and vaccine strains of MV could infect transgenic mice, either by IC or IN routes, but vaccine strains were less virulent (Sellin et al., 2006).
4.2 Other animal models
Other investigators have focused on rodent-adapted MVs to infect non-transgenic (i.e., wild-type) mice, rats, or hamsters (Castro et al., 1972; Duprex et al., 1999a; Griffin et al., 1974; Moeller-Ehrlich et al., 2007; Schubert et al., 2006; Johnson and Swoveland, 1977; Johnson and Norrby, 1974; Roos et al., 1978). Such studies have been ongoing since the 1970s and have allowed researchers to study MV in a small animal model prior to the identification of the human MV receptors. Importantly, despite the mutations that occurred in adapting MV to rodents, the pathogenesis of such strains is remarkably similar to that seen following MV infection of humans or transgenic mouse models.
Efforts to create animal models to study MV infection and pathogenesis have not been limited only to mice. For example, MV-infected cotton rats recapitulate the immunosuppression seen in MV-infected humans (Niewiesk, 1999; Niewiesk et al., 1999; Wyde et al., 1992), even with wild-type (“clinical” ) isolates (Wyde et al., 1999). Furthermore, studies on rat brain slices grown in culture and infected with MV have revealed important clues about how MV spreads through an infected brain (Ehrengruber et al., 2002). Interestingly, the generation of a transgenic CD46-expressing rat demonstrated that, although MV was taken up by CD46-expressing cells, subsequent intracellular blocks in MV replication prevented robust infection of the animal (Niewiesk et al., 1997). Ferret models have also been used by some researchers as an SSPE model of MV infection in the CNS (Brown et al., 1985; Brown et al., 1987; Mehta and Thormar, 1979; Thormar et al., 1983).
Finally, while rhesus macaques have been chiefly used to study immune responses to MV, immune suppression induced by MV, and efficacy of possible vaccines against MV (de Swart et al., 2007; Pan et al., 2005; Pasetti et al., 2007; Polack et al., 2000), some investigators have used tissues collected from infected monkeys to model human CNS diseases (Albrecht et al., 1977; Steele et al., 1982).
How results from all of these model systems have been used to advance our understanding of MV inter-neuronal spread and neuropathogenesis will be discussed in Section 7.
4.3 In vitro culture systems and techniques in cellular transport studies
4.3.1 Primary neuron cultures and neuron-like cell lines
The ability to culture primary neurons from various CNS substructures (e.g., hippocampus, cortex, dorsal root ganglia, cerebellum) of transgenic mice has been a powerful tool in dissecting aspects of intra- and inter-neuronal MV transport. These cultures are validated as neurons in their expression of characteristic neuronal markers such as MAP-2 and NeuN, their ability to form synapses in culture, and the fact that, once plated, these cells are mitotically inactive. While in general quite pure neuron cultures can be obtained (90-95%), contaminating glial cells, culture-to-culture variability, difficulty in establishing these cultures and a fairly short lifespan in culture (10-14 days, typically), are some of the disadvantages.
Cells lines such as NT2 (human teratocarcinoma cells that can be terminally differentiated into neuronal cells by retinoic acid treatment), human astrocytoma cells, and mouse neuroblastoma cells offer an alternative that is free of the complexities and challenges of primary neuron cultures (Duprex et al., 2000; Duprex et al., 1999b; Lawrence et al., 2000; Ludlow et al., 2005; McQuaid et al., 1998). The ease of using these established lines of CNS cells is balanced by concerns about whether these cells accurately reflect primary CNS cell biology.
4.3.2 Slice cultures
The introduction of organotypic monolayer cultures of nervous tissue has added an “intermediate” technique to the neurologist's toolbox, falling between the complexity of a whole animal model and the culturing of cell lines or pure primary cultures that do not recapitulate the cellular heterogeneity of the CNS. Advantages to organotypic brain slice cultures are the ability to take cells from a defined region of the brain, the ability to use phase microscopy on the monolayer of resulting brain cells, the relevance of culturing primary cells without performing single cell dissociation, and the power of studying heterogeneous cell populations and how such diverse cells impact viral spread (Gahwiler, 1981). This approach has been used to assess the spread of rMV-EGFP through neurons of cultured rat brain slices (Ehrengruber et al., 2002) as well as the spread of SSPE isolates through hamster cerebellum slices (Sheppard et al., 1975).
4.3.3 Reagents to study the role of motor proteins in viral spread
The constant advancement of our understanding of the cellular cytoskeleton and how organelles, signaling complexes, and proteins are transported within cells has greatly advanced our understanding of how viruses are transported within cells (Jouvenet et al., 2004; reviewed in Leopold and Pfister, 2006; Mackenzie et al., 2006; reviewed in Ploubidou and Way, 2001; Rietdorf et al., 2001; Ward and Moss, 2004). For example, the development of dominant negatives to genetically manipulate the cellular transport system, as well as the increased availability of genetically altered mice with defined motor protein deficiencies, have enabled a dissection of what cellular proteins are utilized by viruses to enable transport. Although pharmaceutical approaches to ablate specific microtubule motors or microtubules themselves are broadly used (e.g., vanadate, colchicine, cytochalasin, nocodazole, etc.), the harsh and somewhat nonspecific consequences of using these cytotoxic reagents was always a limitation that can now be obviated with more precise and less caustic genetic strategies.
For example, the generation and characterization of mice via ENU-mutagenesis containing a single point mutation in dynein heavy chain has helped to define the role of dynein in the spread of mouse-adapted scrapie prions from the periphery to the CNS of infected mice (Hafezparast et al., 2004). These loa (“legs at odd angles”) mice are viable as heterozygotes, and are currently being used by a number of neurovirology labs to assess the role of dynein in virus transport (Ahmad-Annuar et al., 2003; Hafezparast et al., 2004; Hafezparast et al., 2003).
Another tool to define viral-cytoskeletal interactions are dominant negative constructs. For example, overexpression of p50 dynamitin, a member of the multi-protein complex dynactin which acts as an accessory factor to dynein, specifically disrupts dynein function (Ahmad et al., 1998; Burkhardt et al., 1997; Echeverri et al., 1996). This approach was used by Dohner et al. to show that herpes simplex virus type 1 (HSV-1) utilizes dynein following its entry into a cell to travel to the nucleus (Dohner et al., 2002). Similar reagents have been established for kinesins, motors that govern intracellular retrograde transport (Verhey et al., 1998; Verhey et al., 2001).
4.4 Reverse genetics for MV
The establishment of a reverse genetics system for vaccine strains of MV has also furthered our understanding of MV replication in neurons, and in MV intra- and inter-neuronal spread (Devaux et al., 2007; Radecke et al., 1995; Schneider et al., 1997). Future studies using this approach will be key to identifying what roles each of the viral proteins play in neuronal infection, and will define whether some viral proteins may be dispensable for neuronal infection. Moreover, given the speculation that point mutations found in SSPE isolates might make these viruses more neuropathogenic, reverse genetics offers an opportunity to directly test these hypotheses in a controlled setting. Already, MV reverse genetics has been used to address the role of the H protein of rodent brain-adapted MV in conferring neurovirulence to MV Edmonston in non-transgenic mice (Duprex et al., 1999a). Moreover, Maisner's group engineered viruses with altered basolateral sorting signals in F and H and showed that these domains are important for MV propagation through lymphoid cells, in addition to their previously described role in MV spread through epithelial cells (Runkler et al., 2008). Furthermore, the ability of SSPE-associated viral genes to confer certain phenotypes on wild-type or vaccine strains of MV has been tested through reverse genetics approaches. For example, a recombinant MV expressing the M gene of an SSPE isolate was found to replicate less efficiently and led to a CNS infection in YAC-CD46 transgenic mice with a longer course than that seen following infection with MV Edmonston. This MV expressing SSPE M also showed defects in viral assembly and subsequent budding of progeny virus from infected cells (Patterson et al., 2001). Finally, the power of reverse genetics has provided us with a bit of experimental convenience in that we can now molecularly tag MV with marker proteins such as GFP to more easily follow MV infections in vivo, in brain slices, and in cell culture (de Swart et al., 2007; Duprex et al., 1999b; Duprex et al., 2000; Ehrengruber et al., 2002; Ludlow et al., 2007; Plumb et al., 2002; Schubert et al., 2006).
5. Access to the CNS: Lessons from CDV, PV, and WNV
Despite this broad palette of tools that are available to study MV-neuron interactions, many of the reagents and strategies have been developed only recently, and—as a result—we have more questions than answers about how MV gets to the CNS, spreads within CNS cells, and mediates neurological disease. How MV gains access to the CNS from the periphery is not known, though it has been previously proposed that MV spreads into the brain by infecting endothelial cells during secondary viremia (Esolen et al., 1995). Alternatively, there is speculation that MV may infect the CNS by infiltration of infected lymphoid cells, e.g., via the turnover of microglial cells by infected infiltrating macrophages. Despite a lack of certainty regarding MV neuroinvasion, clues about how MV spreads to the CNS can be gained by looking at the spread of other neurotropic viruses from the periphery to the CNS.
Two routes of CNS infection have been proposed for a related morbillivirus, canine distemper virus (CDV)—the hematogenous route and anterograde trafficking via the olfactory nerve. Like MV, CDV can infect lymphocytes during the systemic phase of infection, thus providing an opportunity for infected lymphocytes to traffic across the blood-brain barrier and release infectious virus to initiate infection within the brain parenchyma. Importantly, the work by Rudd et al. suggested that entry of CDV into the CNS can also occur via the olfactory bulb and suggested that this may be a common mechanism of entry for neurotropic paramyxoviruses (Rudd et al., 2006).
Similar portals of entry have been proposed and tested for both polio virus (PV) and West Nile virus (WNV). One hypothesis is that these virus cross the BBB; the second is that PV or WNV is transmitted via peripheral nerves (Ohka et al., 1998; Samuel et al., 2007). Yang et al. demonstrated that circulating PV crosses the BBB at a high rate that is independent of polio virus receptor (PVR) expression (Yang et al., 1997). More recently, studies in transgenic PVR-expressing mice have shown that PV is transported through the sciatic nerve by fast retrograde axonal transport. Furthermore, PV accesses the sciatic nerve from its intramuscular inoculation site by a process dependent on PVR (Ohka et al., 1998), and the mechanism by which PV is transported retrogradely along the sciatic nerve is thought to be an interaction of the PVR cytoplasmic domain with dynein light chain Tctex1 (Ohka et al., 2004). As with polio, axonal transport of WNV can also occur, mediating entry into the CNS. However, WNV can spread to the CNS even when the sciatic nerve has been transected, suggesting that WNV likely uses multiple routes to access the brain (Samuel et al., 2007). One of these routes of WNV entry has been shown to be dependent on Toll-like receptor 3 (TLR3)-mediated inflammation and subsequent opening of the blood-brain barrier (Wang et al., 2004). Thus, as for CDV (Rudd et al., 2006), multiple non-mutually exclusive routes of spread are available for PV and WNV entry into the CNS. How MV penetrates into the CNS—and perhaps more importantly—how often this occurs in a human population, remain to be determined.
6. MV spread and transport in non-neuronal cells
6.1 MV spread in non-neuronal cells
MV infection is intiated when H binds to one of its cellular receptors, CD46 or SLAM (Dorig et al., 1993; Naniche et al., 1993; Tatsuo et al., 2000). The receptor used correlates with the source of the MV H protein: wild type isolates typically use SLAM (Erlenhoefer et al., 2001; Ono et al., 2001a; Ono et al., 2001b; Tatsuo et al., 2000), whereas attenuated vaccine strains, such as Edmonston and the strains derived from it, preferentially use CD46. Receptor selection is not exclusive, however: SLAM has been shown to be an effective receptor for MV entry for vaccine strains, as well, and conversely a few wild type strains have the ability to bind to CD46 (Erlenhofer et al., 2002). It has been suggested that the receptor usage of a given MV isolate may depend on the cell line used to isolate and amplify the virus rather than the wild-type or vaccine strain status of the MV isolate (Manchester et al., 2000). Furthermore, genetic and structural studies of MV H proteins have illustrated that the H binding domains to CD46 and SLAM are spatially distinct (Colf et al., 2007; Erlenhofer et al., 2002), providing support for the idea that H can interact with both CD46 and SLAM, albeit with different affinities for each. In any case, given that SLAM expression is limited to lymphoid cells (McQuaid and Cosby, 2002), it is likely that other MV receptors await discovery.
In non-neuronal cells, MV buds from the apical surface, resulting in the release of free virus or in cell fusion (reviewed in Griffin, 2001; Figure 1A). Transport of the viral nucleocapsid to the plasma membrane is dependent on levels of M protein and its accumulation at the cell surface (Runkler et al., 2007). Apical budding occurs despite the preferential homing of MV glycoproteins F and H to the basolateral membrane through a tyrosine-based sorting signal (Blau and Compans, 1995; Maisner et al., 1998; Moll et al., 2001). Appropriate budding is achieved by restricted expression of M at the apical surface which retargets some of F and H in polarized cells (Naim et al., 2000; Riedl et al., 2002). The interaction of the cytoplasmic tails of F and H with M mediates virus assembly (Cathomen et al., 1998a; Cathomen et al., 1998b). However, the predominant presence of the glycoproteins F and H at the basolateral membrane has been hypothesized to play a key role in MV spread through an infected individual. The tyrosine-based sorting signals in F and H are not only required for basolateral sorting but are also required for the fusogenicity of the F/H complex in polarized epithelial cells (Maisner et al., 1998; Moll et al., 2001). Consequently, it has been proposed that the basolaterally expressed F/H complex promotes cell-cell fusion, thus allowing the virus to spread to underlying tissues and facilitating systemic MV dissemination (Moll et al., 2004). This hypothesis emphasizes that the two modes of MV spread in non-neuronal cells—apically released budding virus and basolaterally-mediated cell-cell fusion—are both important components of MV systemic dissemination.
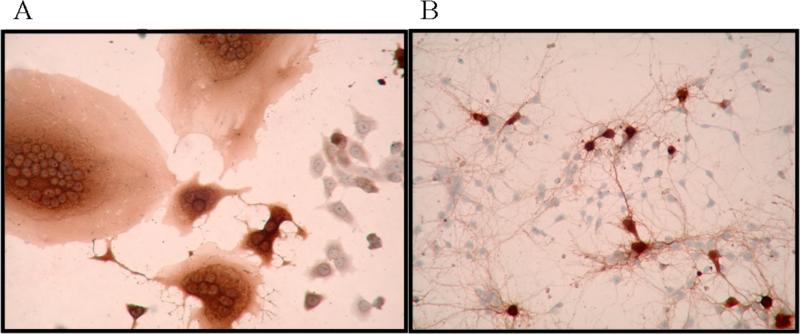
Figure 1.
Immunohistochemistry for MV antigen with hematoxylin counterstain. A) MV-infected Vero cells form syncytia, or multi-nucleated giant cells. B) MV-infected primary hippocampal neurons from NSE-CD46 mice do not form syncytia.
It should be noted that the “classical” MV spread in nonneuronal cells (extracellular progeny virus budding and cell-cell fusion) is dependent on MV glycoproteins. Thus, MV H interaction with its receptor likely triggers F protein fusogenic activity, initiating viral infection (Griffin, 2001; Lamb and Kolakofsky, 2001). As we point out later, these events may be substantially altered upon MV infection of neurons.
STOP
6.2 Use of microtubules and their associated motor proteins to achieve viral transport within an infected cell
6.2.1 Introduction to microtubules
How are the glycoproteins and RNP complexes shuttled through a cell to achieve appropriate assembly and egress? Microtubules are part of the cellular cytoskeleton and play a critical role in mediating movement of cellular proteins and even organelles to their proper destinations. In neurons, their role is even more critical, as neurons rely on microtubules for neurite extension, synaptic vesicle trafficking, and synapse formation (Zhai and Bellen, 2004). Microtubules are long, hollow cylindrical polymers of tubulin that assemble with a head-to-tail orientation (reviewed in Henry et al., 2006). The plus-end of microtubules typically extends to the plasma membrane and away from the nucleus, or towards the axon or dendrite and away from the cell body in neurons. In nonneuronal cells, microtubule minus-ends are bound and stabilized at the microtubule organizing center (MTOC) near the nucleus, whereas in neurons the minus-ends are oriented to the cell body, but lack an MTOC. The protein motors that move cargo along these microtubules are grouped into two families: the predominately plus-end directed kinesin family of motors, which move cargo away from the cell body (anterograde transport), and the minus-end directed dynein family of motors, which move cargo to the cell body (retrograde transport), often as part of the lysosomal pathway. Cytoplasmic kinesin (or conventional kinesin) consists of 2 heavy chains and 2 light chains. The heavy chains contain the motor domains which allow for the generation of force and subsequent movement along the microtubule. Furthermore, the heavy chains facilitate dimerization and cargo-binding. The kinesin light chains may also facilitate binding to cargo (reviewed in Mandelkow and Mandelkow, 2002). Cytoplasmic dynein binds to microtubules via its 2 heavy chains, but other components of this complex (including 6 light chains, 2 light intermediate chains, and 2 intermediate chains) mediate cargo binding and contribute to the specificity of the cargo that is transported. Furthermore, cytoplasmic dynein associates with another protein complex dynactin, which acts as a cofactor to facilitate cargo binding and processivity along the microtubule (reviewed in Leopold and Pfister, 2006; Radtke et al., 2006).
Viruses of many families utilize the microtubule pathway during infection. Some viruses associate with microtubules and retrograde motors to move to the nucleus, including murine polyomavirus, adeno-associated virus, adenovirus, herpes simplex virus 1, and human immunodeficiency virus (reviewed in Greber and Way, 2006; Radtke et al., 2006). Other viruses are associated with anterograde transport, as has been shown for both Vaccinia virus and African swine fever virus transport to the plasma membrane through an interaction of viral cargo with kinesin-1 (Jouvenet et al., 2004; Rietdorf et al., 2001). Presumably, for these viruses, interaction with anterograde motors facilitates viral egress. Moreover, recent work with the alpha-herpesvirus pseudorabies, a large DNA virus, illustrated fast axonal transport through neurons, a process mediated by microtubule motors (Smith et al., 2001). However, little is known about how neurotropic RNA viruses, including MV, interact with molecular motors in neurons.
6.2.2 MV and the cytoskeleton
It has been known for some time that actin plays a key role in MV trafficking within cells. Actin is packaged within MV virions (Tyrrell and Norrby, 1978), and treatment of infected cells with the actin-disrupting agent cytochalasin B resulted in disruption of MV virion formation (Stallcup et al., 1983). Electron microscopy studies of cytoskeletal preparations of MV-infected cells showed that the growing end of actin filaments protruded into budding virions. The authors suggested that the vectorial action of actin promotes MV budding and may also be involved in the transport of nucleocapsids to the cell surface (Bohn et al., 1986). Furthermore, actin was associated with transcriptionally silent MV nucleocapsids in vitro, supporting a role of actin in the budding of mature (i.e., not transcriptionally active) MV nucleocapsids. The same study showed that tubulin promoted MV RNA synthesis in in vitro RNA synthesis assays and could be co-immunoprecipitated with MV L, implying a further requirement for tubulin in MV replication (Moyer et al., 1990). These points, in addition to the large body of work illustrating the essential role of microtubules in facilitating large-distance transport within cells (reviewed in Greber and Way, 2006), strongly support the notion that MV engages the microtubule network to be transported long distances within infected neurons. Ongoing work in a number of MV-focused laboratories is addressing this hypothesis, recognizing the fact that it is hard to imagine a neurotropic virus achieving transport from one end of a neuron to the other without hijacking the cell's railroad—the microtubule system.
6.2.3 Transport of other viruses in neurons
The best-studied viruses, with regard to neuronal transport and spread, are the alphaherpesviruses, including herpes simplex virus 1 (HSV1) and pseudorabies virus (PRV). Cell imaging studies with both viruses have revealed by time-lapse fluorescence microscopy that these viruses travel retrogradely down the dendrite to the cell body (Bearer et al., 2000; Feierbach et al., 2007). This intracellular trafficking of the virus along microtubules can be disrupted by inhibitors of microtubule assembly, such as colchicine, vinblastine, or nocodazole (Sodeik et al., 1997; Topp et al., 1994). HSV-1 capsids entering a cell associate with the dynein complex, and overexpression of the dynactin component dynamitin (p50) blocks the transport of HSV1 to the nucleus (Dohner et al., 2002). Two somewhat contradictory models have emerged to explain virus transport away from the nucleus and subsequent viral egress. In the first model, viral capsids and glycoproteins are transported separately, and assembly takes place somewhere along the axon shaft or at the axon terminus. The second model proposes that fully assembled enveloped virions are transported intact down the axon in vesicles (reviewed in Diefenbach et al., 2008; Lyman et al., 2007). As of last count, five different laboratories—three for HSV, two for PRV—have demonstrated contradictory findings as to which model is correct, and the controversy continues as the differences between variables—viruses, types of neurons, kinetics, and technical differences—are sorted out (reviewed in Diefenbach et al., 2008). In either case, these two models provide the RNA virologist with an impetus for thought as to how smaller enveloped RNA viruses achieve egress from an infected neuron.
7. MV spread in neurons
7.1 MV movement within and among neurons
MV-infected CD46-expressing mice illustrated that, in vivo, neurons are the primary cell of the CNS infected by MV, even in animals where the expression of CD46 was not neuronally restricted (Oldstone et al., 1999). Similarly, neurons are the main target of MV infection in the brain of infected SLAM transgenic mice (Sellin et al., 2006), as is the case for infected human brains (Allen et al., 1996). The Edmonston strain of MV engineered to express EGFP (rMV-EGFP) spread through brains of infected CD46+/IFNAR−/− neonatal mice by neuronal processes. Furthermore, the authors detected EGFP-positive cells whose morphology was not neuronal and that the authors believed to be ependymal cells and neuroblasts (Duprex et al., 2000). This finding is of significance since it widens the population of cells susceptible to MV in the CNS of this animal model, and the result is different from the predominant neuronal infection described in other CD46-expressing animal models and in human cases of MV CNS complications. Interestingly, the first report describing the generation and characterization of CD46+/IFNAR−/− mice indicated that IC infection with MV Edmonston also resulted in infection of ependymal cells, oligodendrocytes, and neurons (Mrkic et al., 1998). One key difference among these studies and those of other CD46 transgenic mouse models and of infected humans is the absence of an intact type I interferon system, which may partially account for the altered MV tropism.
MV does not bud from infected neurons nor do MV-infected primary neurons form syncytia (Fig. 1B), but the presence of nucleocapsids in the axons and at the presynaptic membranes of infected neurons suggests a contact-dependent, trans-synaptic spread of MV (Lawrence et al., 2000; Oldstone et al., 1999). This finding is consistent with data from autopsy specimens of SSPE cases, where MV budding is not apparent (Paula-Barbosa and Cruz, 1981). Moreover, spread of MV-Edmonston in a neuronal population is independent of the receptor CD46 (Lawrence et al., 2000); thus, in this model, while CD46 was needed for viral entry, it was dispensable for neuron-to-neuron transmission.
MV spread via neuron-neuron contact has been confirmed in studies using rat organotypic hippocampal slices, as well as in tissue culture studies using differentiated human NT2 neurons (Lawrence et al., 2000; Ludlow et al., 2005; McQuaid et al., 1998). The spread of rMV-EGFP through hippocampal slices followed neuronal tracts, and no release of extracellular virus particles was observed. In this study, the interneuronal spread of rMV-EGFP was determined to be retrograde based on the spread of MV-EGFP from CA1 to CA3 pyramidal cells to granule cells within the slices and the known synaptic connectivity of these cells. Furthermore, the MV envelope proteins (F, H, and M) and P proteins were detected in dendrites of infected neurons (Ehrengruber et al., 2002).
The mutations that accumulate upon MV adaptation to rodents (Rima et al., 1997; Vanchiere et al., 1995) do not seem to affect neuronal spread, as these rodent-adapted MVs have yielded data consistent with observations in humans and data from MV-infected CD46-expressing mice and primary neurons cultured from such mice (Duprex et al., 1999a; Mrkic et al., 1998; Schubert et al., 2006). For example, the hamster-neurotropic strain (HNT) of MV was used to infect Balb/c mice, and the resulting infection was limited to neurons and was not cytolytic. No virus assembly was detected (Van et al., 1979). Other work with HNT supports the lack of virus assembly following infection of weanling or adult mice (Griffin et al., 1974). The infection of weanling hamsters with HBS, another rodent-adapted MV isolated from an SSPE case, also revealed no budding virions in infected brains (Johnson and Swoveland, 1977).
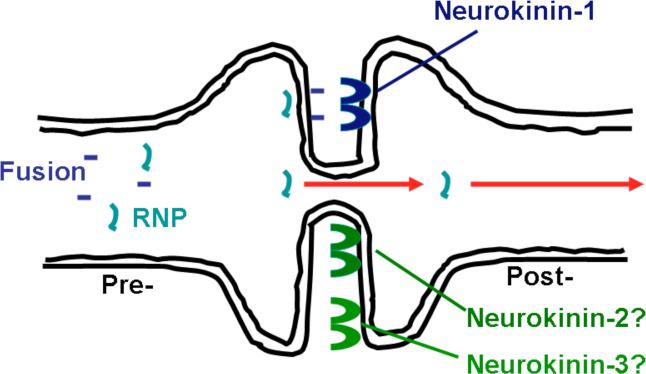
As mentioned earlier, MV neuronal spread can occur independent of the expression of CD46, suggesting that H may not be required for MV trans-synaptic spread. Recently, Makhortova et al. indirectly investigated the role of MV F in trans-synaptic spread by using FIP, fusion inhibitory peptide (Makhortova et al., 2007). FIP, a synthetic tripeptide (z-D-Phe-L-Phe-Gly), prevents fusion by multiple viruses, though its strongest activity is against MV (Norrby, 1971; Richardson et al., 1980; Richardson and Choppin, 1983). FIP reduced MV infection and subsequent spread through primary CD46-expressing neuron cultures, implicating a role for F in trans-neuronal transport. Furthermore, the neurotransmitter substance P, whose active site is identical to FIP, also blocked MV neuronal spread. Genetic deletion of the substance P receptor, neurokinin-1 (NK-1), or pharmacological inhibition of NK-1 decreased MV replication and subsequent disease in MV-infected mice. Overall, these data suggest that MV F may interact with NK-1 at the synapse to mediate trans-synaptic spread of MV (Makhortova et al., 2007). The idea that MV engages a cellular receptor other than SLAM or CD46 is not new (Andres et al., 2003; Blixenkrone-Moller et al., 1998; Takeda et al., 2007), though the potential that such a receptor may be bound by MV F, rather than H, broadens this original hypothesis. As a result of this work, the current model for MV trans-synaptic spread is a microfusion event at the synapse, illustrated in Fig. 2. In this model, at least the MV RNP and F glycoprotein are actively transported to the synapse. F engagement of NK-1 may then allow a membrane fusion event to permit movement of the RNP from one neuron to the next. The neurotransmitter receptors neurokinin-2 and -3 (NK-2 and NK-3) bind to neurotransmitters of the same family as Substance P and, therefore, may also play a role in MV trans-synaptic spread.
Figure 2.
Model for MV trans-synaptic spread. RNPs and F are transported to the synapse. The interaction of F with neurokinin-1 triggers a microfuison event, allowing the RNP to cross the synapse and infect the synaptically connected neuron. Neurokinin-2 and -3 may act as receptors for MV F, given their homology to neurokinin-1.
7.2 Effects of immune responses on MV spread in neurons
It has long been known that multiple host factors contribute to the ability of a virus to replicate in a given cell. The literature contains several examples in which the elicitation or removal of a host immune response can alter MV replication and subsequent spread. While most of these studies have measured the consequences of wholesale deletion of a particular immune cell type (e.g., recombinase activating gene (RAG) knockout (KO) mice that lack functional T and B cells), intrinsic responses of neurons can also influence the outcome of infection. For example, expression of the heat shock protein hsp72, which would be induced during a febrile illness, increased MV gene expression following infection of neonatal hsp72 transgenic mice. The increased MV RNA levels correlated with a higher mortality rate (Carsillo et al., 2006), providing an example wherein the cellular response to viral infection increases MV pathogenesis. Conversely, the removal of the adaptive immune response protein TAP1 (the transporter associated with antigen presentation) led to enhanced spread of MV into the brains of infected mice. The HNT strain of MV trafficked from the olfactory bulb into the limbic system of wild-type and TAP1−/− mice but showed a greater dissemination into the brains of TAP1−/− mice, as compared to that seen in wild-type mice (Urbanska et al., 1997).
Finally, an association was found between promoter polymorphisms of the innate immunity protein MxA and the occurrence of SSPE in Japan. The most frequent polymorphisms caused the MxA promoter to be more responsive to type I interferon than the wild-type promoter sequence. Therefore, the increased activity of MxA in response to type I interferon positively correlated with SSPE occurrence, suggesting that MxA may play a role in the establishment of persistent MV infections in neurons (Torisu et al., 2004), though these studies have been somewhat controversial (Pipo-Deveza et al., 2006). The analysis of SSPE lesions of affected individuals revealed that the cells staining positive for MxA were mainly astrocytes located in a belt around the region of MV antigen-positive cells in affected areas of the brain. Similar to the conclusions drawn from the Japanese cases of SSPE, the authors suggested that MxA plays an important role in slowing down viral spread in SSPE and therefore may contribute to the persistent nature of this MV CNS infection (Ogata et al., 2004; Torisu et al., 2004).
8. Remaining questions
It should be noted that molecular mechanisms of MV neuronal spread are only recently becoming defined. Ehrengruber's study on the spread of rMV-EGFP in cultured rat hippocampal slices found that MV spreads through the rat hippocampus in a retrograde direction, with the MV F, H, M, and P proteins localizing to the dendrite of infected neurons (Ehrengruber et al., 2002). Lawrence et al. demonstrated by electron microscopy the presence of MV nucleocapsids at the presynaptic membrane of infected primary hippocampal neurons, suggesting that MV may, in fact, spread in an anterograde direction, or from the axon of an infected neuron to the dendrite of a neighboring neuron (Lawrence et al., 2000). However, these studies could not distinguish between nucleocapsids that were exiting or nucleocapsids that were entering the neuron. Furthermore, there have been reports in support of both anterograde and retrograde trafficking of MV in neurons (McQuaid et al., 1998; Urbanska et al., 1997), which may not be unexpected as the virus must travel to the cell body to replicate and then back to the periphery of the neuron for egress, regardless of which neuronal process it uses (the dendrite or the axon) for entry and for exit. While a crystallized view of how the unique environment of the neuron affects MV replication, spread and, ultimately, neuropathogenesis awaits further study, the tools and ideas are in place for exciting advances in the coming years.
9. Acknowledgements
We gratefully acknowledge the assistance of Christine Matullo and Lauren O'Donnell in the preparation of this review. V.A. Young is supported by T32 NS007180-25 from the University of Pennsylvania. G. R. is supported by NIH grants NS40500, MH56951, and CA006927, as well as generous support from the F.M. Kirby Foundation.
Abbreviations
- BBB
blood-brain barrier
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EGFP
enhanced green fluorescence protein
- F
MV fusion
- FIP
fusion inhibitory peptide
- H
MV hemagglutinin
- HSV
herpes simplex virus
- IC
intracerebral
- IFNAR
interferon alpha receptor
- IL
interleukin
- IN
intranasal
- IV
intravenous
- KO
knockout
- L
MV large (viral polymerase)
- M
MV matrix
- MAP-2
microtubule associated protein 2
- MIBE
myelin inclusion body encephalitis
- MV
measles virus
- N
MV nucleoprotein
- NK
neurokinin
- P
MV phosphoprotein
- PIE
post-infectious encephalomyelitis
- PV
poliovirus
- PrV
pseudorabies virus
- RAG
recombinase activating gene
- RNP
ribonucleoprotein
- SLAM
signaling lymphocyte activation molecule
- SSPE
subacute sclerosing panencephalitis
- WNV
West Nile virus
- YAC
yeast artificial chromosome
Literature Cited
- Ahmad FJ, Echeverri CJ, Vallee RB, Baas PW. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. J Cell Biol. 1998;140:391–401. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad-Annuar A, Shah P, Hafezparast M, Hummerich H, Witherden AS, Morrison KE, Shaw PJ, Kirby J, Warner TT, Crosby A, Proukakis C, Wilkinson P, Orrell RW, Bradley L, Martin JE, Fisher EMC. No association with common Caucasian genotypes in exons 8, 13 and 14 of the human cytoplasmic dynein heavy chain gene ( DNCHC1 ) and familial motor neuron disorders. Amyotrophic Lateral Sclerosis & Other Motor Neuron Disorders. 2003;4:150. doi: 10.1080/14660820310011737. [DOI] [PubMed] [Google Scholar]
- Albrecht P, Burnstein T, Klutch MJ, Hicks HT, Ennis FA. Subacute sclerosing panencephalitis: experimental infection in primates. Science. 1977;195:64–66. doi: 10.1126/science.831255. [DOI] [PubMed] [Google Scholar]
- Allen IV, McQuaid S, McMahon J, Kirk J, McConnell R. The significance of measles virus antigen and genome distribution in the CNS in SSPE for mechanisms of viral spread and demyelination. J Neuropathol Exp Neurol. 1996;55:471–480. doi: 10.1097/00005072-199604000-00010. [DOI] [PubMed] [Google Scholar]
- Andres O, Obojes K, Kim KS, ter Meulen V, Schneider-Schaulies J. CD46- and CD150-independent endothelial cell infection with wild-type measles viruses. J Gen Virol. 2003;84:1189–1197. doi: 10.1099/vir.0.18877-0. [DOI] [PubMed] [Google Scholar]
- Atabani S, Byrnes A, Jaye A, Kidd IM, Magnusen A, Whittle H, Karp C. Natural measles causes prolonged suppression of Interleukin-12 production. J Infect Dis. 2001;184:1–9. doi: 10.1086/321009. [DOI] [PubMed] [Google Scholar]
- Baczko K, Liebert UG, Cattaneo R, Billeter MA, Roos RP, ter Meulen V. Restriction of measles virus gene expression in measles inclusion body encephalitis. J Infect Dis. 1988;158:144–150. doi: 10.1093/infdis/158.1.144. [DOI] [PubMed] [Google Scholar]
- Bearer EL, Breakefield XO, Schuback D, Reese TS, LaVail JH. Retrograde axonal transport of herpes simplex virus: Evidence for a single mechanism and a role for tegument. Proc Natl Acad Sci USA. 2000;97:8146–8150. doi: 10.1073/pnas.97.14.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter MA, Cattaneo R, Spielhofer P, Kaelin K, Huber M, Schmid A, Baczko K, ter Meulen V. Generation and properties of measles virus mutations typically associated with subacute sclerosing panencephalitis. Ann NY Acad Sci. 1994;724:367–377. doi: 10.1111/j.1749-6632.1994.tb38934.x. [DOI] [PubMed] [Google Scholar]
- Bitnun A, Shannon P, Durward A, Rota PA, Bellini WJ, Graham C, Wang E, Ford-Jones EL, Cox P, Becker L, Fearon M, Petric M, Tellier R. Measles inclusion-body encephalitis caused by the vaccine strain of measles virus. Clin Infect Dis. 1999;29:855–861. doi: 10.1086/520449. [DOI] [PubMed] [Google Scholar]
- Blau DM, Compans RW. Entry and release of measles virus are polarized in epithelial cells. Virology. 1995;210:91–99. doi: 10.1006/viro.1995.1320. [DOI] [PubMed] [Google Scholar]
- Blixenkrone-Moller M, Bernard A, Bencsik A, Sixt N, Diamond LE, Logan JS, Wild TF. Role of CD46 in measles virus infection in CD46 transgenic mice. Virology. 1998;249:238–248. doi: 10.1006/viro.1998.9301. [DOI] [PubMed] [Google Scholar]
- Bohn W, Rutter G, Hohenberg H, Mannweiler K, Nobis P. Involvement of actin filaments in budding of measles virus: studies on cytoskeletons of infected cells. Virology. 1986;149:91–106. doi: 10.1016/0042-6822(86)90090-5. [DOI] [PubMed] [Google Scholar]
- Bonami F, Rudd PA, von Messling V. Disease duration determines canine distemper virus neurovirulence. J Virol. 2007;81:12066–12070. doi: 10.1128/JVI.00818-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HR, Goller NL, Thormar H, Rudelli R, Tourtellotte WW, Shapshak P, Boostanfar R, Wisniewski HM. Measles virus matrix protein gene expression in a subacute sclerosing panencephalitis patient brain and virus isolate demonstrated by cDNA hybridization and immunocytochemistry. Acta Neuropathol. 1987;75:123–130. doi: 10.1007/BF00687072. [DOI] [PubMed] [Google Scholar]
- Brown HR, Thormar H, Barshatzky M, Wisniewski HM. Localization of measles virus antigens in subacute sclerosing panencephalitis in ferrets. Lab Anim Sci. 1985;35:233–237. [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsillo T, Traylor Z, Choi C, Niewiesk S, Oglesbee M. hsp72, a host determinant of measles virus neurovirulence. J Virol. 2006;80:11031–11039. doi: 10.1128/JVI.01438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro AE, Burnstein T, Byington DP. Properties in cell culture of a hamster brain-adapted subacute sclerosing panencephalitis virus. J Gen Virol. 1972;16:413–417. doi: 10.1099/0022-1317-16-3-413. [DOI] [PubMed] [Google Scholar]
- Cathomen T, Mrkic B, Spehner D, Drillien R, Naef R, Pavlovic J, Aguzzi A, Billeter MA, Cattaneo R. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J. 1998a;17:3899–3908. doi: 10.1093/emboj/17.14.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathomen T, Naim HY, Cattaneo R. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J Virol. 1998b;72:1224–1234. doi: 10.1128/jvi.72.2.1224-1234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Rebmann G, Baczko K, ter Meulen V, Billeter MA. Altered ratios of measles virus transcripts in diseased human brains. Virology. 1987a;160:523–526. doi: 10.1016/0042-6822(87)90031-6. [DOI] [PubMed] [Google Scholar]
- Cattaneo R, Rebmann G, Schmid A, Baczko K, ter Meulen V, Billeter MA. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 1987b;6:681–688. doi: 10.1002/j.1460-2075.1987.tb04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Schmid A, Eschle D, Baczko K, ter Meulen V, Billeter MA. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988;55:255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colf LA, Juo ZS, Garcia KC. Structure of the measles virus hemagglutinin. Nat Struct Mol Biol. 2007;14:1227–1228. doi: 10.1038/nsmb1342. [DOI] [PubMed] [Google Scholar]
- Coovadia HM, Wesley A, Henderson LG, Brain P, Vos GH, Hallett AF. Alterations in immune responsiveness in acute measles and chronic post-measles chest disease. Int Arch Allergy Appl Immunol. 1978;56:14–23. doi: 10.1159/000231998. [DOI] [PubMed] [Google Scholar]
- de Swart RL, Ludlow M, deWitte L, Yanagi Y, van Amerongen G, McQuaid S, Yuksel S, Geijtenbeek TB, Duprex WP, Osterhaus AD. Predominant infection of CD150(+) lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 2007;3:e178. doi: 10.1371/journal.ppat.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P, von Messling V, Songsungthong W, Springfeld C, Cattaneo R. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology. 2007;360:72–83. doi: 10.1016/j.virol.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Dhib-Jalbut S, Johnson KP. Measles virus diseases. In: McKendall RR, Stroop WG, editors. Handbook of neurovirology. Marcel Dekker, Inc.; New York: 1994. pp. 539–554. [Google Scholar]
- Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. Transport and egress of herpes simplex virus in neurons. Rev Med Virol. 2008;18:35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- Dohner K, Wolfstein A, Prank U, Echeverri C, Dujardin D, Vallee R, Sodeik B. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol Biol Cell. 2002;13:2795–2809. doi: 10.1091/mbc.01-07-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Coblentz JM, Pleet AB. Subacute sclerosing panencephalitis. Unusual nuclear inclusions and lengthy clinical course. Arch Neurol. 1974;31:355–363. doi: 10.1001/archneur.1974.00490420021001. [DOI] [PubMed] [Google Scholar]
- Duprex WP, Duffy I, McQuaid S, Hamill L, Cosby SL, Billeter MA, Schneider-Schaulies J, ter Meulen V, Rima BK. The H gene of rodent brain-adapted measles virus confers neurovirulence to the Edmonston vaccine strain. J Virol. 1999a;73:6916–6922. doi: 10.1128/jvi.73.8.6916-6922.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprex WP, McQuaid S, Hangartner L, Billeter MA, Rima BK. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol. 1999b;73:9568–9575. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprex WP, McQuaid S, Roscic-Mrkic B, Cattaneo R, McCallister C, Rima BK. In vitro and in vivo infection of neural cells by a recombinant measles virus expressing enhanced green fluorescent protein. J Virol. 2000;74:7972–7979. doi: 10.1128/jvi.74.17.7972-7979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrengruber MU, Ehler E, Billeter MA, Naim HY. Measles virus spreads in rat hippocampal neurons by cell-to-cell contact and in a polarized fashion. J Virol. 2002;76:5720–5728. doi: 10.1128/JVI.76.11.5720-5728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenhoefer C, Wurzer WJ, Loffler S, Schneider-Schaulies S, ter Meulen V, Schneider-Schaulies J. CD150 (SLAM) is a receptor for measles virus but is not involved in viral contact-mediated proliferation inhibition. J Virol. 2001;75:4499–4505. doi: 10.1128/JVI.75.10.4499-4505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenhofer C, Duprex WP, Rima BK, ter Meulen V, Schneider-Schaulies J. Analysis of receptor (CD46, CD150) usage by measles virus. J Gen Virol. 2002;83:1431–1436. doi: 10.1099/0022-1317-83-6-1431. [DOI] [PubMed] [Google Scholar]
- Esolen LM, Takahashi K, Johnson RT, Vaisberg A, Moench TR, Wesselingh SL, Griffin DE. Brain endothelial cell infection in children with acute fatal measles. J Clin Invest. 1995;96:2478–2481. doi: 10.1172/JCI118306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evlashev A, Moyse E, Valentin H, Azocar O, Trescol-Biemont MC, Marie JC, Rabourdin-Combe C, Horvat B. Productive measles virus brain infection and apoptosis in CD46 transgenic mice. J Virol. 2000;74:1373–1382. doi: 10.1128/jvi.74.3.1373-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evlashev A, Valentin H, Rivailler P, Azocar O, Rabourdin-Combe C, Horvat B. Differential permissivity to measles virus infection of human and CD46-transgenic murine lymphocytes. J Gen Virol. 2001;82:2125–2129. doi: 10.1099/0022-1317-82-9-2125. [DOI] [PubMed] [Google Scholar]
- Feierbach B, Bisher M, Goodhouse J, Enquist LW. In vitro analysis of transneuronal spread of an alphaherpesvirus infection in peripheral nervous system neurons. J Virol. 2007;81:6846–6857. doi: 10.1128/JVI.00069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firsching R, Buchholz CJ, Schneider U, Cattaneo R, ter Meulen V, Schneider-Schaulies J. Measles virus spread by cell-cell contacts: uncoupling of contact-mediated receptor (CD46) downregulation from virus uptake. J Virol. 1999;73:5265–5273. doi: 10.1128/jvi.73.7.5265-5273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan MC, Liu YJ, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Greber UF, Way M. A superhighway to virus infection. Cell. 2006;124:741–754. doi: 10.1016/j.cell.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Griffin DE. Measles virus. In: Knipe DM, Howley PM, editors. Fields virology. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 1401–1441. [Google Scholar]
- Griffin DE, Pan CH, Moss WJ. Measles vaccines. Front Biosci. 2008;13:1352–1370. doi: 10.2741/2767. [DOI] [PubMed] [Google Scholar]
- Griffin DE, Mullinix J, Narayan O, Johnson RT. Age dependence of viral expression: comparative pathogenesis of two rodent-adapted strains of measles virus in mice. Infect Immun. 1974;9:690–695. doi: 10.1128/iai.9.4.690-695.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezparast M, Brandner S, Linehan J, Martin JE, Collinge J, Fisher EMC. Prion disease incubation time is not affected in mice heterozygous for a dynein mutation. Biochemical and Biophysical Research Communications. 2004;326:18–22. doi: 10.1016/j.bbrc.2004.10.206. [DOI] [PubMed] [Google Scholar]
- Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, Morgan PJ, Oozageer R, Priestley JV, Averill S, King VR, Ball S, Peters J, Toda T, Yamamoto A, Hiraoka Y, Augustin M, Korthaus D, Wattler S, Wabnitz P, Dickneite C, Lampel S, Boehme F, Peraus G, Popp A, Rudelius M, Schlegel J, Fuchs H, de Angelis MH, Schiavo G, Shima DT, Russ AP, Stumm G, Martin JE, Fisher EMC. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- Henry T, Gorvel JP, Meresse S. Molecular motors hijacking by intracellular pathogens. Cell Microbiol. 2006;8:23–32. doi: 10.1111/j.1462-5822.2005.00649.x. [DOI] [PubMed] [Google Scholar]
- Hirsch RL, Griffin DE, Johnson RT, Cooper SJ, Lindo de Soriano I, Roedenbeck S, Vaisberg A. Cellular immune responses during complicated and uncomplicated measles virus infections of man. Clin Immunol Immunopathol. 1984;31:1–12. doi: 10.1016/0090-1229(84)90184-3. [DOI] [PubMed] [Google Scholar]
- Horvat B, Rivailler P, Varior-Krishnan G, Cardoso A, Gerlier D, Rabourdin-Combe C. Transgenic mice expressing human measles virus (MV) receptor CD46 provide cells exhibiting different permissivities to MV infections. J Virol. 1996;70:6673–6681. doi: 10.1128/jvi.70.10.6673-6681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu EC, Iorio C, Sarangi F, Khine AA, Richardson CD. CDw150(SLAM) is a receptor for a lymphotropic strain of measles virus and may account for the immunosuppressive properties of this virus. Virology. 2001;279:9–21. doi: 10.1006/viro.2000.0711. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Swoveland P. Measles antigen distribution in brains of chronically infected hamsters. An immunoperoxidase study of experimental subacute sclerosing panencephalitis. Lab Invest. 1977;37:459–465. [PubMed] [Google Scholar]
- Johnson KP, Norrby E. Subacute sclerosing panencephalitis (SSPE) agent in hamsters. III. Induction of defective measles infection in hamster brain. Exp Mol Pathol. 1974;21:166–178. doi: 10.1016/0014-4800(74)90087-2. [DOI] [PubMed] [Google Scholar]
- Johnson RT. Viral infections of the nervous system. Lippincott-Raven Publishers; Philadelphia: 1998. [Google Scholar]
- Jouvenet N, Monaghan P, Way M, Wileman T. Transport of african swine fever virus from assembly sites to the plasma membrane is dependent on microtubules and conventional kinesin. J Virol. 2004;78:7990–8001. doi: 10.1128/JVI.78.15.7990-8001.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp CL, Wysocka M, Wahl LM, Ahearn JM, Cuomo PJ, Sherry B, Trinchieri G, Griffin DE. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Hotta H, Nishimura A, Tatsuno Y, Homma M. Detection of measles virus nucleoprotein mRNA in autopsied brain tissues. J Gen Virol. 1995;76:3201–3204. doi: 10.1099/0022-1317-76-12-3201. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Kohso K, Nishimura A, Tatsuno Y, Homma M, Hotta H. Detection of measles virus mRNA from autopsied human tissues. J Clin Microbiol. 1998;36:299–301. doi: 10.1128/jcm.36.1.299-301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RA, Kolakofsky D. Paramyxoviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields virology. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 1305–1340. [Google Scholar]
- Lawrence DM, Vaughn MM, Belman AR, Cole JS, Rall GF. Immune response-mediated protection of adult but not neonatal mice from neuron-restricted measles virus infection and central nervous system disease. J Virol. 1999;73:1795–1801. doi: 10.1128/jvi.73.3.1795-1801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DM, Patterson CE, Gales TL, D'Orazio JL, Vaughn MM, Rall GF. Measles virus spread between neurons requires cell contact but not CD46 expression, syncytium formation, or extracellular virus production. J Virol. 2000;74:1908–1918. doi: 10.1128/jvi.74.4.1908-1918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold PL, Pfister KK. Viral strategies for intracellular trafficking: motors and microtubules. Traffic. 2006;7:516–523. doi: 10.1111/j.1600-0854.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- Ludlow M, Duprex WP, Cosby SL, Allen IV, McQuaid S. Advantages of using recombinant measles viruses expressing a fluorescent reporter gene with vibratome slice technology in experimental measles neuropathogenesis. Neuropathol Appl Neurobiol. 2007 doi: 10.1111/j.1365-2990.2007.00900.x. doi: 10.1111/j.1365-2990.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- Ludlow M, McQuaid S, Cosby SL, Cattaneo R, Rima BK, Duprex WP. Measles virus superinfection immunity and receptor redistribution in persistently infected NT2 cells. J Gen Virol. 2005;86:2291–2303. doi: 10.1099/vir.0.81052-0. [DOI] [PubMed] [Google Scholar]
- Lyman MG, Feierbach B, Curanovic D, Bisher M, Enquist LW. Pseudorabies virus Us9 directs axonal sorting of viral capsids. J Virol. 2007;81:11363–11371. doi: 10.1128/JVI.01281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie GG, Keen CL, Oteiza PI. Microtubules are required for NF-kappaB nuclear translocation in neuroblastoma IMR-32 cells: modulation by zinc. J Neurochem. 2006;99:402–415. doi: 10.1111/j.1471-4159.2006.04005.x. [DOI] [PubMed] [Google Scholar]
- Maisner A, Klenk HD, Herrler G. Polarized budding of measles virus is not determined by viral surface glycoproteins. J Virol. 1998;72:5276–5278. doi: 10.1128/jvi.72.6.5276-5278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhortova NR, Askovich P, Patterson CE, Gechman LA, Gerard NP, Rall GF. Neurokinin-1 enables measles virus trans-synaptic spread in neurons. Virology. 2007;362:235–244. doi: 10.1016/j.virol.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchester M, Eto DS, Oldstone MBA. Characterization of the inflammatory response during acute measles encephalitis in NSE-CD46 transgenic mice. J Neuroimmunol. 1999;96:207–217. doi: 10.1016/s0165-5728(99)00036-3. [DOI] [PubMed] [Google Scholar]
- Manchester M, Eto DS, Valsamakis A, Liton PB, Fernandez-Munoz R, Rota PA, Bellini WJ, Forthal DN, Oldstone MBA. Clinical isolates of measles virus use CD46 as a cellular receptor. J Virol. 2000;74:3967–3974. doi: 10.1128/jvi.74.9.3967-3974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchester M, Rall GF. Model systems: transgenic mouse models for measles pathogenesis. Trends Microbiol. 2001;9:19–23. doi: 10.1016/s0966-842x(00)01903-x. [DOI] [PubMed] [Google Scholar]
- Mandelkow E, Mandelkow EM. Kinesin motors and disease. Trends Cell Biol. 2002;12:585–591. doi: 10.1016/s0962-8924(02)02400-5. [DOI] [PubMed] [Google Scholar]
- McQuaid S, Campbell S, Wallace IJ, Kirk J, Cosby SL. Measles virus infection and replication in undifferentiated and differentiated human neuronal cells in culture. J Virol. 1998;72:5245–5250. doi: 10.1128/jvi.72.6.5245-5250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid S, Cosby SL. An immunohistochemical study of the distribution of the measles virus receptors, CD46 and SLAM, in normal human tissues and subacute sclerosing panencephalitis. Lab Invest. 2002;82:403–409. doi: 10.1038/labinvest.3780434. [DOI] [PubMed] [Google Scholar]
- Mehta PD, Thormar H. Immunological studies of subacute measles encephalitis in ferrets: similarities to human subacute sclerosing panencephalitis. J Clin Microbiol. 1979;9:601–604. doi: 10.1128/jcm.9.5.601-604.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller-Ehrlich K, Ludlow M, Beschorner R, Meyermann R, Rima BK, Duprex WP, Niewiesk S, Schneider-Schaulies J. Two functionally linked amino acids in the stem 2 region of measles virus haemagglutinin determine infectivity and virulence in the rodent central nervous system. J Gen Virol. 2007;88:3112–3120. doi: 10.1099/vir.0.83235-0. [DOI] [PubMed] [Google Scholar]
- Moll M, Klenk HD, Herrler G, Maisner A. A single amino acid change in the cytoplasmic domains of measles virus glycoproteins H and F alters targeting, endocytosis, and cell fusion in polarized Madin-Darby canine kidney cells. J Biol Chem. 2001;276:17887–17894. doi: 10.1074/jbc.M010183200. [DOI] [PubMed] [Google Scholar]
- Moll M, Pfeuffer J, Klenk HD, Niewiesk S, Maisner A. Polarized glycoprotein targeting affects the spread of measles virus in vitro and in vivo. J Gen Virol. 2004;85:1019–1027. doi: 10.1099/vir.0.19663-0. [DOI] [PubMed] [Google Scholar]
- Moss W, Ryon J, Monze M, Griffin D. Differential regulation of interleukin-4 (IL-4), IL-5, and IL-10 during measles in Zambian children. J Infect Dis. 2002;186:879–887. doi: 10.1086/344230. [DOI] [PubMed] [Google Scholar]
- Moyer SA, Baker SC, Horikami SM. Host cell proteins required for measles virus reproduction. J Gen Virol. 1990;71:775–783. doi: 10.1099/0022-1317-71-4-775. [DOI] [PubMed] [Google Scholar]
- Mrkic B, Pavlovic J, Rulicke T, Volpe P, Buchholz CJ, Hourcade D, Atkinson JP, Aguzzi A, Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72:7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim HY, Ehler E, Billeter MA. Measles virus matrix protein specifies apical virus release and glycoprotein sorting in epithelial cells. EMBO J. 2000;19:3576–3585. doi: 10.1093/emboj/19.14.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D, Varior-Krishnan G, Cervoni F, Wild TF, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D, Reed SI, Oldstone MB. Cell cycle arrest during measles virus infection: a G0-like block leads to suppression of retinoblastoma protein expression. J Virol. 1999;73:1894–1901. doi: 10.1128/jvi.73.3.1894-1901.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiesk S, Schneider-Schaulies J, Ohnimus H, Jassoy C, Schneider-Schaulies S, Diamond L, Logan JS, ter Meulen V. CD46 expression does not overcome the intracellular block of measles virus replication in transgenic rats. J Virol. 1997;71:7969–7973. doi: 10.1128/jvi.71.10.7969-7973.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiesk S. Cotton rats (Sigmodon hispidus): an animal model to study the pathogenesis of measles virus infection. Immunol Lett. 1999;65:47–50. doi: 10.1016/s0165-2478(98)00123-0. [DOI] [PubMed] [Google Scholar]
- Niewiesk S, Gotzelmann M, ter Meulen V. Selective in vivo suppression of T lymphocyte responses in experimental measles virus infection. Proc Natl Acad Sci USA. 2000;97:4251–4255. doi: 10.1073/pnas.060012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiesk S, Ohnimus H, Schnorr JJ, Gotzelmann M, Schneider-Schaulies S, Jassoy C, ter Meulen V. Measles virus-induced immunosuppression in cotton rats is associated with cell cycle retardation in uninfected lymphocytes. J Gen Virol. 1999;80:2023–2029. doi: 10.1099/0022-1317-80-8-2023. [DOI] [PubMed] [Google Scholar]
- Norrby E. The effect of a carbobenzoxy tripeptide on the biological activities of measles virus. Virology. 1971;44:599–608. doi: 10.1016/0042-6822(71)90374-6. [DOI] [PubMed] [Google Scholar]
- Norrby E, Kristensson K. Measles virus in the brain. Brain Res Bull. 1997;44:213–220. doi: 10.1016/s0361-9230(97)00139-1. [DOI] [PubMed] [Google Scholar]
- Ogata S, Ogata A, Schneider-Schaulies S, Schneider-Schaulies J. Expression of the interferon-alpha/beta-inducible MxA protein in brain lesions of subacute sclerosing panencephalitis. J Neurol Sci. 2004;223:113–119. doi: 10.1016/j.jns.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Ohka S, Matsuda N, Tohyama K, Oda T, Morikawa M, Kuge S, Nomoto A. Receptor (CD155)-dependent endocytosis of poliovirus and retrograde axonal transport of the endosome. J Virol. 2004;78:7186–7198. doi: 10.1128/JVI.78.13.7186-7198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohka S, Yang WX, Terada E, Iwasaki K, Nomoto A. Retrograde transport of intact poliovirus through the axon via the fast transport system. Virology. 1998;250:67–75. doi: 10.1006/viro.1998.9360. [DOI] [PubMed] [Google Scholar]
- Ohno S, Ono N, Seki F, Takeda M, Kura S, Tsuzuki T, Yanagi Y. Measles virus infection of SLAM (CD150) knockin mice reproduces tropism and immunosuppression in human infection. J Virol. 2007;81:1650–1659. doi: 10.1128/JVI.02134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone MBA, Lewicki H, Thomas D, Tishon A, Dales S, Patterson J, Manchester M, Homann D, Naniche D, Holz A. Measles virus infection in a transgenic model: virus-induced immunosuppression and central nervous system disease. Cell. 1999;98:629–640. doi: 10.1016/s0092-8674(00)80050-1. [DOI] [PubMed] [Google Scholar]
- Ono N, Tatsuo H, Hidaka Y, Aoki T, Minagawa H, Yanagi Y. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J Virol. 2001a;75:4399–4401. doi: 10.1128/JVI.75.9.4399-4401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono N, Tatsuo H, Tanaka K, Minagawa H, Yanagi Y. V domain of human SLAM (CDw150) is essential for its function as a measles virus receptor. J Virol. 2001b;75:1594–1600. doi: 10.1128/JVI.75.4.1594-1600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CH, Valsamakis A, Colella T, Nair N, Adams RJ, Polack FP, Greer CE, Perri S, Polo JM, Griffin DE. Inaugural article: modulation of disease, T cell responses, and measles virus clearance in monkeys vaccinated with H-encoding alphavirus replicon particles. Proc Natl Acad Sci USA. 2005;102:11581–11588. doi: 10.1073/pnas.0504592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasetti MF, Resendiz-Albor A, Ramirez K, Stout R, Papania M, Adams RJ, Polack FP, Ward BJ, Burt D, Chabot S, Ulmer J, Barry EM, Levine MM. Heterologous prime-boost strategy to immunize very young infants against measles: pre-clinical studies in rhesus macaques. Clin Pharmacol Ther. 2007;82:672–685. doi: 10.1038/sj.clpt.6100420. [DOI] [PubMed] [Google Scholar]
- Patterson CE, Daley JK, Echols LA, Lane TE, Rall GF. Measles virus infection induces chemokine synthesis by neurons. J Immunol. 2003;171:3102–3109. doi: 10.4049/jimmunol.171.6.3102. [DOI] [PubMed] [Google Scholar]
- Patterson CE, Lawrence DM, Echols LA, Rall GF. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol. 2002;76:4497–4506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Cornu TI, Redwine J, Dales S, Lewicki H, Holz A, Thomas D, Billeter MA, Oldstone MBA. Evidence that the hypermutated M protein of a subacute sclerosing panencephalitis measles virus actively contributes to the chronic progressive CNS disease. Virology. 2001;291:215–225. doi: 10.1006/viro.2001.1182. [DOI] [PubMed] [Google Scholar]
- Paula-Barbosa MM, Cruz C. Nerve cell fusion in a case of subacute sclerosing panencephalitis. Ann Neurol. 1981;9:400–403. doi: 10.1002/ana.410090414. [DOI] [PubMed] [Google Scholar]
- Pipo-Deveza JR, Kusuhara K, Silao CLT, Lukban MB, Salonga AM, Sanchez BC, Kira R, Takemoto M, Torisu H, Hara T. Analysis of MxA, IL-4, and IRF-1 genes in Filipino patients with subacute sclerosing panencephalitis. Neuropediatrics. 2006;37:222–228. doi: 10.1055/s-2006-924724. [DOI] [PubMed] [Google Scholar]
- Ploubidou A, Way M. Viral transport and the cytoskeleton. Curr Opin Cell Biol. 2001;13:97–105. doi: 10.1016/S0955-0674(00)00180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb J, Duprex WP, Cameron CH, Richter-Landsberg C, Talbot P, McQuaid S. Infection of human oligodendroglioma cells by a recombinant measles virus expressing enhanced green fluorescent protein. J Neurovirol. 2002;8:24–34. doi: 10.1080/135502802317247785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Lee SH, Permar S, Manyara E, Nousari HG, Jeng Y, Mustafa F, Valsamakis A, Adams RJ, Robinson HL, Griffin DE. Successful DNA immunization against measles: neutralizing antibody against either the hemagglutinin or fusion glycoprotein protects rhesus macaques without evidence of atypical measles. Nat Med. 2000;6:776–781. doi: 10.1038/77506. [DOI] [PubMed] [Google Scholar]
- Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C, Christiansen G, Billeter MA. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke K, Dohner K, Sodeik B. Viral interactions with the cytoskeleton: a hitchhiker's guide to the cell. Cell Microbiol. 2006;8:387–400. doi: 10.1111/j.1462-5822.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- Rall GF. Measles virus 1998-2002: progress and controversy. Annu Rev Microbiol. 2003;57:343–367. doi: 10.1146/annurev.micro.57.030502.090843. [DOI] [PubMed] [Google Scholar]
- Rall GF, Manchester M, Daniels LR, Callahan EM, Belman AR, Oldstone MB. A transgenic mouse model for measles virus infection of the brain. Proc Natl Acad Sci USA. 1997;94:4659–4663. doi: 10.1073/pnas.94.9.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson CD, Choppin PW. Oligopeptides that specifically inhibit membrane fusion by paramyxoviruses: studies on the site of action. Virology. 1983;131:518–532. doi: 10.1016/0042-6822(83)90517-2. [DOI] [PubMed] [Google Scholar]
- Richardson CD, Scheid A, Choppin PW. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology. 1980;105:205–222. doi: 10.1016/0042-6822(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Riedl P, Moll M, Klenk HD, Maisner A. Measles virus matrix protein is not cotransported with the viral glycoproteins but requires virus infection for efficient surface targeting. Virus Res. 2002;83:1–12. doi: 10.1016/s0168-1702(01)00379-3. [DOI] [PubMed] [Google Scholar]
- Rietdorf J, Ploubidou A, Reckmann I, Holmstrom A, Frischknecht F, Zettl M, Zimmermann T, Way M. Kinesin-dependent movement on microtubules precedes actin-based motility of vaccinia virus. Nat Cell Biol. 2001;3:992–1000. doi: 10.1038/ncb1101-992. [DOI] [PubMed] [Google Scholar]
- Rima BK, Earle JA, Baczko K, ter Meulen V, Liebert UG, Carstens C, Carabana J, Caballero M, Celma ML, Fernandez-Munoz R. Sequence divergence of measles virus haemagglutinin during natural evolution and adaptation to cell culture. J Gen Virol. 1997;78:97–106. doi: 10.1099/0022-1317-78-1-97. [DOI] [PubMed] [Google Scholar]
- Rima BK, Duprex WP. Molecular mechanisms of measles virus persistence. Virus Res. 2005;111:132–147. doi: 10.1016/j.virusres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Roos RP, Griffin DE, Johnson RT. Determinants of measles virus (hamster neurotropic strain) replication in mouse brain. J Infect Dis. 1978;137:722–727. doi: 10.1093/infdis/137.6.722. [DOI] [PubMed] [Google Scholar]
- Rudd PA, Cattaneo R, von Messling V. Canine distemper virus uses both the anterograde and the hematogenous pathway for neuroinvasion. J Virol. 2006;80:9361–9370. doi: 10.1128/JVI.01034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runkler N, Dietzel E, Moll M, Klenk HD, Maisner A. Glycoprotein targeting signals influence the distribution of measles virus envelope proteins and virus spread in lymphocytes. J Gen Virol. 2008;89:687–696. doi: 10.1099/vir.0.83407-0. [DOI] [PubMed] [Google Scholar]
- Runkler N, Pohl C, Schneider-Schaulies S, Klenk HD, Maisner A. Measles virus nucleocapsid transport to the plasma membrane requires stable expression and surface accumulation of the viral matrix protein. Cell Microbiol. 2007;9:1203–1214. doi: 10.1111/j.1462-5822.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Wang H, Siddharthan V, Morrey JD, Diamond MS. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc Natl Acad Sci USA. 2007;104:17140–17145. doi: 10.1073/pnas.0705837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Spielhofer P, Kaelin K, Dotsch C, Radecke F, Sutter G, Billeter MA. Rescue of measles virus using a replication-deficient vaccinia-T7 vector. J Virol Methods. 1997;64:57–64. doi: 10.1016/s0166-0934(96)02137-4. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies J, ter Meulen V, Schneider-Schaulies S. Measles infection of the central nervous system. J Neurovirol. 2003;9:247–252. doi: 10.1080/13550280390193993. [DOI] [PubMed] [Google Scholar]
- Schnorr JJ, Seufert M, Schlender J, Borst J, Johnston IC, ter Meulen V, Schneider-Schaulies S. Cell cycle arrest rather than apoptosis is associated with measles virus contact-mediated immunosuppression in vitro. J Gen Virol. 1997;78:3217–3226. doi: 10.1099/0022-1317-78-12-3217. [DOI] [PubMed] [Google Scholar]
- Schubert S, Moller-Ehrlich K, Singethan K, Wiese S, Duprex WP, Rima BK, Niewiesk S, Schneider-Schaulies J. A mouse model of persistent brain infection with recombinant measles virus. J Gen Virol. 2006;87:2011–2019. doi: 10.1099/vir.0.81838-0. [DOI] [PubMed] [Google Scholar]
- Sellin CI, Davoust N, Guillaume V, Baas D, Belin MF, Buckland R, Wild TF, Horvat B. High pathogenicity of wild-type measles virus infection in CD150 (SLAM) transgenic mice. J Virol. 2006;80:6420–6429. doi: 10.1128/JVI.00209-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servet-Delprat C, Vidalain PO, Bausinger H, Manie S, Le Deist F, Azocar O, Hanau D, Fischer A, Rabourdin-Combe C. Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J Immunol. 2000;164:1753–1760. doi: 10.4049/jimmunol.164.4.1753. [DOI] [PubMed] [Google Scholar]
- Sheppard RD, Raine CS, Burnstein T, Bornstein MB, Feldman LA. Cell-associated subacute sclerosing panencephalitis agent studied in organotypic central nervous system cultures: viral rescue attempts and morphology. Infect Immun. 1975;12:891–900. doi: 10.1128/iai.12.4.891-900.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai M, Inoue N, Okuno T, Okabe M, Akazawa T, Miyamoto Y, Ayata M, Honda K, Kurita-Taniguchi M, Matsumoto M, Ogura H, Taniguchi T, Seya T. Wild-type measles virus infection in human CD46/CD150-transgenic mice: CD11c-positive dendritic cells establish systemic viral infection. J Immunol. 2005;175:3252–3261. doi: 10.4049/jimmunol.175.5.3252. [DOI] [PubMed] [Google Scholar]
- Sips GJ, Chesik D, Glazenburg L, Wilschut J, De Keyser J, Wilczak N. Involvement of morbilliviruses in the pathogenesis of demyelinating disease. Rev Med Virol. 2007;17:223–244. doi: 10.1002/rmv.526. [DOI] [PubMed] [Google Scholar]
- Smith GA, Gross SP, Enquist LW. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc Natl Acad Sci USA. 2001;98:3466–3470. doi: 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeik B, Ebersold MW, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup KC, Raine CS, Fields BN. Cytochalasin B inhibits the maturation of measles virus. Virology. 1983;124:59–74. doi: 10.1016/0042-6822(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Steele MD, Giddens WE, Jr, Valerio M, Sumi SM, Stetzer ER. Spontaneous paramyxoviral encephalitis in nonhuman primates (Macaca mulatta and M. nemestrina). Vet Pathol. 1982;19:132–139. doi: 10.1177/030098588201900204. [DOI] [PubMed] [Google Scholar]
- Sun X, Burns JB, Howell JM, Fujinami RS. Suppression of antigen-specific T cell proliferation by measles virus infection: role of a soluble factor in suppression. Virology. 1998;246:24–33. doi: 10.1006/viro.1998.9186. [DOI] [PubMed] [Google Scholar]
- Takasu T, Mgone JM, Mgone CS, Miki K, Komase K, Namae H, Saito Y, Kokubun Y, Nishimura T, Kawanishi R, Mizutani T, Markus TJ, Kono J, Asuo PG, Alpers MP. A continuing high incidence of subacute sclerosing panencephalitis (SSPE) in the Eastern Highlands of Papua New Guinea. Epidemiol Infect. 2003;131:887–898. doi: 10.1017/s0950268803008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Tahara M, Hashiguchi T, Sato TA, Jinnouchi F, Ueki S, Ohno S, Yanagi Y. A human lung carcinoma cell line supports efficient measles virus growth and syncytium formation via a SLAM- and CD46-independent mechanism. J Virol. 2007;81:12091–12096. doi: 10.1128/JVI.01264-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro VG, Perez HH, Griffin DE. Prospective study of the magnitude and duration of changes in tuberculin reactivity during uncomplicated and complicated measles. Pediatr Infect Dis J. 1987;6:451–454. doi: 10.1097/00006454-198705000-00007. [DOI] [PubMed] [Google Scholar]
- Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- Thormar H, Mehta PD, Lin FH, Brown HR, Wisniewski HM. Presence of oligoclonal immunoglobulin G bands and lack of matrix protein antibodies in cerebrospinal fluids and sera of ferrets with measles virus encephalitis. Infect Immun. 1983;41:1205–1211. doi: 10.1128/iai.41.3.1205-1211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp KS, Meade LB, LaVail JH. Microtubule polarity in the peripheral processes of trigeminal ganglion cells: relevance for the retrograde transport of herpes simplex virus. J Neurosci. 1994;14:318–325. doi: 10.1523/JNEUROSCI.14-01-00318.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torisu H, Kusuhara K, Kira R, Bassuny WM, Sakai Y, Sanefuji M, Takemoto M, Hara T. Functional MxA promoter polymorphism associated with subacute sclerosing panencephalitis. Neurology. 2004;62:457–460. doi: 10.1212/01.wnl.0000106940.95749.8e. [DOI] [PubMed] [Google Scholar]
- Tyrrell DLJ, Norrby E. Structural polypeptides of measles virus. J Gen Virol. 1978;39:219–229. doi: 10.1099/0022-1317-39-2-219. [DOI] [PubMed] [Google Scholar]
- Urbanska EM, Chambers BJ, Ljunggren HG, Norrby E, Kristensson K. Spread of measles virus through axonal pathways into limbic structures in the brain of TAP1 -/- mice. J Med Virol. 1997;52:362–369. doi: 10.1002/(sici)1096-9071(199708)52:4<362::aid-jmv3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Van PC, Rammohan KW, McFarland HF, Dubois-Dalcq M. Selective neuronal, dendritic, and postsynaptic localization of viral antigen in measles-infected mice. Lab Invest. 1979;40:99–108. [PubMed] [Google Scholar]
- Vanchiere JA, Bellini WJ, Moyer SA. Hypermutation of the phosphoprotein and altered mRNA editing in the hamster neurotropic strain of measles virus. Virology. 1995;207:555–561. doi: 10.1006/viro.1995.1116. [DOI] [PubMed] [Google Scholar]
- Verhey KJ, Lizotte DL, Abramson T, Barenboim L, Schnapp BJ, Rapoport TA. Light chain-dependent regulation of kinesin's interaction with microtubules. J Cell Biol. 1998;143:1053–1066. doi: 10.1083/jcb.143.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey KJ, Meyer D, Deehan R, Blenis J, Schnapp BJ, Rapoport TA, Margolis B. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J Cell Biol. 2001;152:959–970. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Ward BM, Moss B. Vaccinia virus A36R membrane protein provides a direct link between intracellular enveloped virions and the microtubule motor kinesin. J Virol. 2004;78:2486–2493. doi: 10.1128/JVI.78.5.2486-2493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welstead GG, Iorio C, Draker R, Bayani J, Squire J, Vongpunsawad S, Cattaneo R, Richardson CD. Measles virus replication in lymphatic cells and organs of CD150 (SLAM) transgenic mice. Proc Natl Acad Sci USA. 2005;102:16415–16420. doi: 10.1073/pnas.0505945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde PR, Ambrose MW, Voss TG, Meyer HL, Gilbert BE. Measles virus replication in lungs of hispid cotton rats after intranasal inoculation. Proc Soc Exp Biol Med. 1992;201:80–87. doi: 10.3181/00379727-201-43483. [DOI] [PubMed] [Google Scholar]
- Wyde PR, Moore-Poveda DK, Daley NJ, Oshitani H. Replication of clinical measles virus strains in hispid cotton rats. Proc Soc Exp Biol Med. 1999;221:53–62. doi: 10.1046/j.1525-1373.1999.d01-54.x. [DOI] [PubMed] [Google Scholar]
- Yang WX, Terasaki T, Shiroki K, Ohka S, Aoki J, Tanabe S, Nomura T, Terada E, Sugiyama Y, Nomoto A. Efficient delivery of circulating poliovirus to the central nervous system independently of poliovirus receptor. Virology. 1997;229:421–428. doi: 10.1006/viro.1997.8450. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Bellen HJ. Hauling t-SNAREs on the microtubule highway. Nat Cell Biol. 2004;6:918–919. doi: 10.1038/ncb1004-918. [DOI] [PubMed] [Google Scholar]