Abstract
ZW10 was initially identified as a mitotic checkpoint protein involved in chromosome segregation. It was subsequently implicated in targeting cytoplasmic dynein and dynactin to mitotic kinetochores, though the relationship between these functions remains incompletely understood. Recent studies have revealed that ZW10 performs important functions in nondividing cells as well. These include cytoplasmic dynein targeting to Golgi and other membranes, but also SNARE-mediated ER-Golgi trafficking. Identifying a unifying function for ZW10 in these diverse contexts has been elusive, but likely involves cytoplasmic dynein, as discussed here.
Keywords: ZW10, dynein, dynactin, targeting, vesicle, transport, trafficking, Golgi, ER, mitosis, checkpoint
Introduction
ZW10 is a fascinating protein that has been implicated in an increasing number of apparently disparate cellular functions. It was initially identified in Drosophila, where mutational analysis revealed it to be involved in chromosome segregation.1 During mitosis ZW10 is localized to kinetochores and kinetochore-associated microtubules, and was deduced to participate in mitotic checkpoint control. Subsequently, it was found to interact with a subunit of the dynactin complex, and through this link to participate in anchoring dynein to the kinetochore. Whether this function alone accounts for the mitotic phenotype observed in ZW10 mutant cells, or whether the dynein targeting and mitotic checkpoint roles are independent has remained an unresolved issue.
Curiously, ZW10 is expressed at constant levels throughout the cell cycle, suggesting that it might have yet further, nonmitotic functions. Recent results have indicated that ZW10 participates in recruitment of cytoplasmic dynein to diverse membranous sites during interphase in addition to its mitotic functions. ZW10 has been directly implicated in membrane motility, but also in ER-Golgi membrane sorting. The relationship between these functions poses another quandary for understanding the cellular role of ZW10.
How ZW10 may participate in such a wide range of functions is the subject of this article.
Role for ZW10 in Chromosome Segregation and Mitotic Checkpoint
Mitotic cells make use of a potent checkpoint control mechanism that ensures proper alignment of chromatid pairs at the metaphase plate before they are allowed to separate and migrate to opposite spindle poles. Mitotic defects that interfere with proper microtubule capture by kinetochores and subsequent chromosome alignment cause the checkpoint to remain activated and prevent anaphase onset.2,3 In contrast, defects in the checkpoint mechanism itself allow mitotic cells to ignore attachment and alignment defects and progress into anaphase prematurely.
Mutations in Drosophila zw10 were initially found to produce severe chromosome segregation defects.1 Lagging chromosomes during anaphase were common. Furthermore, separation of paired sister chromatids was observed in the presence of the microtubule-depolymerizing drug colchicine. Normally, this treatment arrests or delays cells in a “pseudoprometaphase” state (paired sister chromatids), and “precocious” separation was taken as a sign of a defective mitotic checkpoint mechanism. This conclusion has been supported by more recent data. Injection of antibodies against human ZW10 or its associated kinetochore protein Rod (Rough Deal) displaced the respective antigens from the kinetochore and interfered with proper chromosome segregation.4,5 Injected cells treated with nocodazole to depolymerize microtubules notably failed to accumulate in mitosis,4 a result which has also been obtained using a dominant negative ZW10 cDNA6 or RNAi.7 Cyclin levels were also found by immunocytochemistry to be decreased in colchicine-treated Drosophila zw10 and rod mutant neuroblasts.5 Thus, ZW10 has been implicated in mitotic checkpoint behavior using several independent criteria and inhibitory approaches. The effects of these treatments on disjunction of sister chromatids in vertebrate cells has not been used as a test for ZW10-dependent checkpoint defects because of the large numbers of chromosomes relative to Drosophila. Furthermore, time-lapse analysis of chromosome behavior in ZW10 or Rod deficient cells has not been performed in either invertebrates or vertebrates as a direct test of checkpoint behavior.
Role for ZW10 in Kinetochore Dynein Recruitment and Regulation
ZW10 was found to interact in a yeast two-hybrid screen with the 50 kDa dynamitin subunit of the dynactin complex.8 Overexpression of dynamitin was known to displace dynactin and cytoplasmic dynein from the kinetochore,9 suggesting that ZW10 could play an important role in anchoring dynactin, and indirectly dynein, to this site (Fig. 1A). To test this possibility the distribution of dynein and dynactin was examined in Drosophila zw10 mutants. Indeed, both complexes were observed by immunofluorescence microscopy to be missing from kinetochores in primary Drosophila spermatocytes and larval neuroblasts.8 This effect has since been tested using ZW10 RNAi, which is less effective in removing the protein from kinetochores.7 However, in favorable instances cytoplasmic dynein was observed to be depleted. Consistent with a loss of motor activity, the rate of poleward chromosome movement was found to be attenuated in Drosophila zw10 and rod mutant spermatocytes.10
Figure 1.
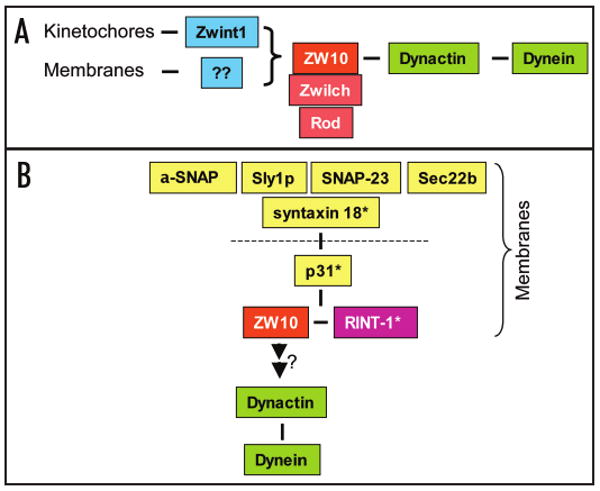
Diagrammatic representation of ZW10-containing complexes. (A) ZW10 exists as a coherent 20S complex, which contains Zwilch and Rod as components. ZW10 itself interacts with the dynamitin subunit of the dynactin complex, which in turn interacts through its p150Glued subunit with the base of the dynein molecule. ZW10 is shown anchored to the kinetochore through Zwint-1. Recent evidence indicates that ZW10 also participates in recruiting dynactin and dynein to membranous and cell cortical structures, though how ZW10 is anchored in these cases is unknown. (Other dynein/dynactin interactors involved in its association with membranes are not been indicated in this scheme for simplicity.) (B) An apparently independent complex has been identified associated with the t-SNARE syntaxin-18. This complex can be dissociated (dotted line) by NSF and α-SNAP to yield α subcomplex containing p31, ZW10 and RINT-1. Whether ZW10 participates in SNARE function, links dynein to membranes, or links SNAREs to dynein remains to be explored more fully. Although a ZW10 homologue has not been identified in yeast, potential orthologues of syntaxin-18 and others of its interactors are known in this organism (noted by *): syntaxin-18 = Ufe1p; p31 = Use1p; and RINT-1 = Tip20p (See text for details).
Cytoplasmic dynein has been independently implicated in the mitotic checkpoint mechanism. Its proposed function is to remove checkpoint proteins from the kinetochore, by transporting them away along the kinetochore fiber microtubules.11,12 Curiously, the role of dynein in checkpoint regulation seems to be opposite that of ZW10. Whereas altered ZW10 expression or function reduces checkpoint activity, dynein inhibition activates the checkpoint.12 The evidence for the latter point comes from several sources. Dynamitin overexpression (see above) causes an increase in mitotic index,9 with cells accumulating in prometaphase as judged by the failure of chromosomes to align at the metaphase plate and sister chromatids to separate. However, because dynein is involved in diverse aspects of mitotic behavior, including mitotic spindle orientation,13,14 spindle pole separation,15 spindle organization9,16,17 and chromosome segregation,10,18 it has been difficult to discriminate between direct and indirect effects of dynein inhibition on chromosome behavior. To address this issue, inhibitors of dynein or dynactin (anti-dynein antibody and purified recombinant dynamitin, respectively) were injected into cells following entry into prometaphase.12 Under these conditions normal bipolar mitotic spindle organization persisted, and chromosomes aligned at the metaphase plate, where they became arrested. The checkpoint protein Mad2 was reported to remain associated with the aligned kinetochores, suggesting that the block in mitotic progression beyond metaphase resulted from persistent checkpoint activity.
How inhibition of dynein/dynactin and their anchor ZW10 could have such divergent effects on the mitotic checkpoint is uncertain. One resolution to this seeming paradox is that ZW10 might independently participate in anchoring checkpoint proteins as well as dynein. This possibility was initially disputed in genetic experiments that found the checkpoint proteins Bub1 and Bub3 to retain their association with kinetochores in colchicine arrested mitotic neuroblasts in zw10 and rod mutant flies.19 Mad1, Mad2, and BubR1 were similarly retained at kinetochores of anti-ZW10 and anti-Rod-injected HeLa cells.4 However, more recent quantitative analysis of this problem in zw10 and rod mutant fly neuroblasts indicated that kinetochore levels of Mad2 in particular were uniquely reduced relative to other checkpoint proteins.20 Depletion of the ZW10-ROD complex in Xenopus extract also prevented the recruitment of Mad1, Mad2, and BubR1 in vitro.7 Whether the disparity between the earlier and later studies reflects underlying differences in biological system or in approach remains to be determined. Mad1 and Mad2 have not yet been reported to associate directly with ZW10 or its interaction partners, and the underlying molecular relationship between these proteins remains to be elucidated.
It is worth noting that at least one dynein regulatory protein, LIS1, which associates with kinetochores through dynein itself,21,22 has been implicated both in checkpoint activation and inactivation. Injection of anti-LIS1 antibodies into dividing rat NRK cells resulted in a prolonged metaphase—the dynein phenotype—due to unstable alignment of chromosomes at the metaphase plate.14 However, some cells ultimately proceeded through anaphase, leaving the unstably attached chromosomes behind—the ZW10/Rod phenotype. More recent analysis of lis1 mutant flies confirmed a delay in anaphase onset, which was rescued in crosses with zw10 and rod mutants,23 though evidence for a defective checkpoint in the lis1 mutants was not reported.
Role for ZW10 in Recruitment of Cytoplasmic Dynein to Interphase Structures
As noted above, ZW10 is expressed throughout the cell cycle, with no evidence to date for an increase or decrease in expression during mitosis.8 In addition, ZW10 cosediments with the particulate fraction from cell homogenates, and can be solubilized with nonionic detergents,6,8 clear signs of membrane association. Membrane flotation experiments have, indeed, supported an interaction with elements of the Golgi apparatus in one study6 and ER in another.24 Subcellular localization experiments have further supported an association with the Golgi apparatus and other pericentrosomal membranes6 and with the ER.24 Together, these results suggest that ZW10 is predominantly associated with membranes during interphase, a surprising feature which is so far unique among known kinetochore proteins.
In view of the substantial evidence for a role for ZW10 in dynein recruitment to kinetochores (see above), a related role at subcellular membranes was considered.6 Previous results had already revealed that dynactin served in anchoring dynein to kinetochores, as noted above, and to Golgi membranes.9,25,26 The Golgi apparatus was found to disperse in cells overexpressing the dynamitin subunit of dynactin, an effect correlated with inhibition of minus-end directed motility of Golgi elements along microtubules.25,27 Furthermore, immunocytochemical staining for cytoplasmic dynein revealed a substantial decrease in its association with Golgi elements.26 These results are similar to those for the kinetochore and suggest related dynein targeting mechanisms at the two subcellular sites.
Interference with ZW10 expression or function has subsequently supported a role in recruiting cytoplasmic dynein to membranous organelles via dynactin. This possibility was tested by a number of approaches, including antibody injection, oligonucleotide- and vector-based RNAi, and expression of dominant negative cDNAs. Each dispersed the Golgi apparatus.6 Endosomes and lysosomes, which are also known to be under dynein motor control, were dispersed by ZW10 RNAi as well.6 Further consistent with a cytoplasmic dynein phenotype, minus end-directed transport of Golgi, endosomal, and lysosomal vesicles along microtubules were each found to be inhibited by >70%. Finally, immunocytochemical staining for cytoplasmic dynein revealed a substantial decrease in its association with Golgi elements,6 providing direct support for a role for ZW10 in dynein recruitment to membranes.
A small but significant fraction of microtubule plus-end directed transport of each class of structure was also observed. Although this result argues against an exclusive effect on dynein motor function, it is consistent with a dynactin phenotype. Dynactin has been found to interact not only with cytoplasmic dynein but also with at least two forms of kinesin.28,29 The plus end-directed kinesin II, in particular, competes with cytoplasmic dynein for a common site within dynactin.29 Thus, whereas the predominant phenotype obtained by dynamitin overexpression is minus-end inhibition, some aspects of plus end vesicular transport are also affected.
Other aspects of ZW10 behavior are consistent with a dynein-related function at structures other than kinetochores.6 ZW10 was observed to accumulate within the cortex at the leading edge of migrating fibroblasts, a known localization and functional site for dynein and dynactin. Acute inhibition of directed cell migration was observed by injection of anti-ZW10 antibody into migrating fibroblasts, similar to results obtained for dynein, dynactin, and the dynein regulatory protein LIS1 (Stehman, Varma, Vallee, unpublished). Interference with ZW10 function, either acutely or by prolonged inhibition, also disrupted microtubule organization, another common phenotypic effect of dynein or dynactin inhibition.6
Role of ZW10 in Membrane Sorting
ZW10 has also been implicated in membrane sorting events between the ER and Golgi apparatus. Whether this function is independent of a role in dynein-mediated transport is uncertain, and the relationship between the proposed ZW10 roles has become an issue of interest.
That ZW10 may be involved in membrane sorting was initially suggested from its identification in immunoprecipitates of syntaxin-18. Syntaxin-18 is weakly homologous to yeast Ufe1p, a t-SNARE implicated in transport between ER and Golgi.30 Overexpression of full-length syntaxin-18 and an N-terminal deletion mutant caused the formation of ER aggregates; more importantly, the exit of temperature-sensitive GFP-VSV-G from the ER was inhibited, a classic test for an involvement in ER-Golgi sorting. To test whether ZW10 played a related function, ZW10 RNAi, dominant negative cDNAs, and antibody injection were used.24 Each treatment led to dispersal of the Golgi apparatus. ZW10 RNAi was also used to test for effects on ER-Golgi sorting. Again, GFP-VSV-G secretion was inhibited. Together, these results were concluded to support a role for ZW10 in membrane sorting.
How might these results and those regarding dynein-mediated membrane transport be reconciled? Dynein is required for transport of ER-Golgi intermediate compartment (ERGIC) vesicles to the pericentrosomal region of the cell.25,27 These are the same vesicles involved in ER-Golgi sorting. Although this makes for a confusing situation, transport and sorting effects should, in principle, be distinguishable. For example, interference with ER-Golgi sorting should reduce the size of the Golgi apparatus and recycle Golgi components to the ER, as is observed with use of brefeldin A (BFA). In contrast, a transport defect should be manifested in reduced minus end-directed movement of Golgi elements or precursors along microtubules, as has been observed,6 resulting in the formation of dispersed Golgi stacklets. Large, dispersed structures positive for the Golgi markers γ-adaptin, 58 K protein, GM130, and NAGT-GFP were, indeed, observed under conditions of ZW10 inhibition,6,24 and Golgi stacklets were detected by electron microcopy.24 These results are comparable to the effects of dynamitin overexpression,25 suggesting that the Golgi dispersal phenotype could, indeed, be due predominantly to dynein inhibition.
The defect on VSV-G trafficking observed in cells transfected with ZW10 siRNAs would seem to be more readily interpreted by an effect on sorting.24,31 However, the effect was mild, resulting in a delay rather than a complete blockade in transit of VSV-G through the secretory pathway, though it was comparable in severity to the syntaxin-18 dominant negative phenotype.30 Whether other means of syntaxin-18 inhibition can produce more dramatic effects on sorting remains to be determined. It is also worth considering that redistribution of the elements of Golgi apparatus through any mechanism could, potentially, inhibit membrane sorting to the extent observed from ZW10 RNAi, though this possibility has not been tested directly.
Molecular Interactions
ZW10 has been implicated in diverse interactions, which shed light on the relationship between the several proposed functions of the protein. First, ZW10 has been found to fractionate as a ∼20 S complex in Drosophila32 and vertebrate cells33 (Dujardin, Varma, Vallee, unpublished). As ZW10 is, itself, 89 kDa, these observations indicate that it behaves as part of a large multimeric complex. Rod, which was initially identified with ZW10 in screens for mutations affecting cell division in Drosophila,34,35 was subsequently found to cofractionate with ZW10 as part of this complex.32 Zwilch (69 kDa) was identified as another component of the complex in ZW10 pull-downs from embryo and third instar larval extracts.7,36 The coherence of the complex during fractionation suggests that its components are tightly and stoichiometrically associated with each other.
Other ZW10 interactions appear to be weak and/or regulated. Despite the reported yeast two-hybrid interaction between ZW10 and the dynamitin subunit of the dynactin complex,8 cofractionation of ZW10 with the dynactin complex has been difficult to demonstrate biochemically. Together, these results indicate that dynein, dynactin, and ZW10 do interact, but are readily dissociated from each other in vitro. ZW10 has also been found to interact in yeast 2-hybrid and biochemical studies with the kinetochore protein Zwint-1.37,38 Zwint-1 has been characterized as a component of a distinct complex which is involved in anchoring ZW10 to the kinetochore.7,38 Together, these data identify a series of interactions which are involved in recruiting cytoplasmic dynein to kinetochores and membranous organelles (Fig. 1A). Although ZW10 has been implicated in both interphase and mitotic dynein functions,6 the range of activities in which the other components of the ZW10 complex participate remains to be tested.
The relationship between the Rod-ZW10-Zwilch and the ZW10-syntaxin-18 complexes (Fig. 1B) is poorly understood. The former has been characterized primarily in embryonic and/or mitotic cells, whereas the latter was isolated from rat liver. Many components of the syntaxin-18 complex are known or expected to be involved in SNARE-mediated membrane fusion (e.g., Sly1, sec22, SNAP23, and α-SNAP). ZW10 was part of a subcomplex that was dissociated from syntaxin-18 by NSF in the presence of α-SNAP.24 The released ZW10 coimmunoprecipitated with two other polypeptides, p31 and RINT-1. p31 is an unconventional SNARE-like protein homologous throughout its length to the yeast SNARE Use1p, which is involved in retrograde Golgi-ER traffic.39 RINT-1 was initially identified as an interactor of the DNA repair protein Rad50, and has been functionally implicated in radiation-induced G2/M checkpoint control.40,41 RINT-1 was observed to interact with ZW10 by directed yeast 2-hybrid assay,24 and in pull-downs from both interphase and mitotic cells.31 RINT-1 dominant negative inhibition and RNAi interfere with the interaction of ZW10 with the syntaxin complex, and produce an ER-to-Golgi/Golgi-dispersal phenotype very similar to that for ZW10.31 Neither ZW10 not RINT-1 interacted with p31 using a directed yeast 2-hybrid assay.24 Nonetheless, the existing data appear to identify a novel subcomplex of ZW10, RINT-1, and p31. RINT-1, but not p31, was also identified at substantial levels in mitotic ZW10 pull-downs,7 though RINT-1 was not detected at kinetochores by immunocytochemistry31 (Varma and Vallee, unpublished observations), in contrast to ZW10, Rod, and Zwilch. These results suggest that the latter proteins are core components of a mitotic complex involved in kinetochore function, and independent of syntaxin-18 and p31 function.
Whether the ZW10, Rod, and Zwilch complex exists during interphase has not been explored in detail, though Rod and Zwilch both coimmunoprecipitated with ZW10 from extracts of Drosophila third instar larvae.36 The p31-ZW10-RINT-1 complex has, so far, been isolated from nonmitotic rat liver, and has been implicated solely in interphase function.24 p31 and syntaxin-18 were found to interact using a directed yeast two-hybrid assay, suggesting that p31 directly mediates the interaction with syntaxin-18 and its other binding partners.24 The overall size and composition of the p31 subcomplex remain to be defined, and its function is still under investigation. What, then, are the functions of ZW10 and RINT-1 in this complex? The latter protein may well participate in membrane fusion, as it is homologous throughout much of its length with yeast Tip20p, a yeast protein that has been implicated in Golgi-ER trafficking.42,43 Tip20p interacts genetically and can coimmunoprecipitate with Ufe1, the predicted yeast homologue of syntaxin-18.43
The role of ZW10, for which a yeast homologue has not been identified, is more uncertain. One possibility that has not been considered is that it may serve to link SNARE complexes to cytoplasmic dynein for their proper subcellular distribution. Perhaps, in this view, dynein carries SNAREs along microtubules toward the Golgi where they might be loaded for subsequent use in fusion of retrograde Golgi vesicles to the ER. An alternative hypothesis, that SNAREs are responsible for recruiting ZW10 and dynein to membranes seems unlikely, in view of the differential temporal involvement of motors and SNAREs with membranes.
Could the ZW10-Rod-Zwilch complex serve a role in recruiting cytoplasmic dynein to membranes? Whether this complex persists as such during interphase, how its properties change as a function of cell cycle stage, and how it may bind to membranes remain important issues for future investigation. Also, although RNAi and other means for inhibiting ZW10 result in the release of cytoplasmic dynein from both kinetochores and membranes, additional interactions have been reported to contribute to each of these functions.44-50 Understanding how these interactions are interrelated will ultimately be required to fully understand the diversity of ZW10 functions.
Conclusion
Existing data on ZW10 indicate that it participates in multiple physiological processes, but a unifying molecular function remains uncertain. However, a role in recruitment of dynein and dynactin to kinetochores and membranes seems to account for many ZW10 activities. Recent evidence for a role for ZW10 in linking Mad2 to kinetochores suggests that ZW10 has an additional function at these sites. Conceivably, these two functions both involve cytoplasmic dynein, though this issue remains largely unresolved at present.
ZW10 has also been implicated in at least two roles in membrane trafficking. First, it clearly interacts with syntaxin-18 and its associated proteins, whose apparent yeast homologues participate in ER-Golgi membrane sorting events. However, whether ZW10 itself functions directly in membrane fusion or budding remains to be further explored. Second and alternatively, it is quite possible that ZW10 serves in recruiting dynein to SNARE complexes. In this case the evidence for an interaction between syntaxin-18 and ZW10-containing complexes could represent the first indication for this interesting role. Dynactin has already been found to associate with the COPII complex48 and both dynein and dynactin to COPI vesicles.49 Thus, it seems likely that the motor protein serves not only to maintain the position of the major structural elements of the cell's endomembrane system, but transports diverse components of the sorting machinery toward microtubule minus ends as well.
Acknowledgments
We thank Brett Lauring, Janis Burkhardt, and Stephanie Stehman for helpful discussions. Supported by NIH Grant GM47434 to RBV and a Human Frontier Science Program fellowship to DLD.
Abbreviations
- ZW10
zeste white 10
- ER
endoplasmic reticulum
- RNAi
RNA interference
- NAGT
N-acetyl-glucosaminyltransferase
- cDNA
complementary DNA
- RINT-1
Rad50-interacting protein-1
- SNARE
SNAP receptors
Footnotes
Previously published online as a Cell Cycle E-publication: http://www.landesbioscience.com/journals/cc/abstract.php?id=3395
References
- 1.Williams BC, Karr TL, Montgomery JM, Goldberg ML. The Drosophila l(1)zw10 gene product, required for accurate mitotic chromosome segregation, is redistributed at anaphase onset. J Cell Biol. 1992;118:759–73. doi: 10.1083/jcb.118.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan GK, Liu ST, Yen TJ. Kinetochore structure and function. Trends Cell Biol. 2005;15:589–98. doi: 10.1016/j.tcb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Karess R. Rod-Zw10-Zwilch: A key player in the spindle checkpoint. Trends Cell Biol. 2005;15:386–92. doi: 10.1016/j.tcb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Chan GK, Jablonski SA, Starr DA, Goldberg ML, Yen TJ. Human Zw10 and ROD are mitotic checkpoint proteins that bind to kinetochores. Nat Cell Biol. 2000;2:944–7. doi: 10.1038/35046598. [DOI] [PubMed] [Google Scholar]
- 5.Basto R, Gomes R, Karess RE. Rough deal and Zw10 are required for the metaphase checkpoint in Drosophila. Nat Cell Biol. 2000;2:939–43. doi: 10.1038/35046592. [DOI] [PubMed] [Google Scholar]
- 6.Varma D, Dujardin DL, Stehman SA, Vallee RB. Role of the kinetochore/cell cycle checkpoint protein ZW10 in interphase cytoplasmic dynein function. J Cell Biol. 2006;172:655–62. doi: 10.1083/jcb.200510120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kops GJ, Kim Y, Weaver BA, Mao Y, McLeod I, Yates JR, IIIrd, Tagaya M, Cleveland DW. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J Cell Biol. 2005;169:49–60. doi: 10.1083/jcb.200411118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starr DA, Williams BC, Hays TS, Goldberg ML. ZW10 helps recruit dynactin and dynein to the kinetochore. J Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savoian MS, Goldberg ML, Rieder CL. The rate of poleward chromosome motion is attenuated in Drosophila zw10 and rod mutants. Nat Cell Biol. 2000;2:948–52. doi: 10.1038/35046605. [DOI] [PubMed] [Google Scholar]
- 11.Wojcik E, Basto R, Serr M, Scaerou F, Karess R, Hays T. Kinetochore dynein: Its dynamics and role in the transport of the Rough deal checkpoint protein. Nat Cell Biol. 2001;3:1001–7. doi: 10.1038/ncb1101-1001. [DOI] [PubMed] [Google Scholar]
- 12.Howell BJ, McEwen BF, Canman JC, Hoffman DB, Farrar EM, Rieder CL, Salmon ED. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J Cell Biol. 2001;155:1159–72. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busson S, Dujardin D, Moreau A, Dompierre J, De Mey JR. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr Biol. 1998;8:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- 14.Faulkner NE, Dujardin DL, Tai CY, Vaughan KT, O'Connell CB, Wang Y, Vallee RB. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat Cell Biol. 2000;2:784–791. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- 15.Vaisberg EA, Koonce MP, McIntosh JR. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J Cell Biol. 1993;123:849–58. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quintyne NJ, Reing JE, Hoffelder DR, Gollin SM, Saunders WS. Spindle multipolarity is prevented by centrosomal clustering. Science. 2005;307:127–9. doi: 10.1126/science.1104905. [DOI] [PubMed] [Google Scholar]
- 17.Merdes A, Heald R, Samejima K, Earnshaw WC, Cleveland DW. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J Cell Biol. 2000;149:851–62. doi: 10.1083/jcb.149.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharp DJ, Rogers GC, Scholey JM. Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nat Cell Biol. 2000;2:922–30. doi: 10.1038/35046574. [DOI] [PubMed] [Google Scholar]
- 19.Basu J, Logarinho E, Herrmann S, Bousbaa H, Li Z, Chan GK, Yen TJ, Sunkel CE, Goldberg ML. Localization of the Drosophila checkpoint control protein Bub3 to the kinetochore requires Bub1 but not Zw10 or Rod. Chromosoma. 1998;107:376–85. doi: 10.1007/s004120050321. [DOI] [PubMed] [Google Scholar]
- 20.Buffin E, Lefebvre C, Huang J, Gagou ME, Karess RE. Recruitment of Mad2 to the kinetochore requires the Rod/Zw10 complex. Curr Biol. 2005;15:856–61. doi: 10.1016/j.cub.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 21.Tai CY, Dujardin DL, Faulkner NE, Vallee RB. Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J Cell Biol. 2002;156:959–968. doi: 10.1083/jcb.200109046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coquelle FM, Caspi M, Cordelieres FP, Dompierre JP, Dujardin DL, Koifman C, Martin P, Hoogenraad CC, Akhmanova A, Galjart N, De Mey JR, Reiner O. LIS1, CLIP-170's key to the dynein/dynactin pathway. Mol Cell Biol. 2002;22:3089–102. doi: 10.1128/MCB.22.9.3089-3102.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siller KH, Serr M, Steward R, Hays TS, Doe CQ. Live imaging of Drosophila brain neuroblasts reveals a role for Lis1/dynactin in spindle assembly and mitotic checkpoint control. Mol Biol Cell. 2005;16:5127–40. doi: 10.1091/mbc.E05-04-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirose H, Arasaki K, Dohmae N, Takio K, Hatsuzawa K, Nagahama M, Tani K, Yamamoto A, Tohyama M, Tagaya M. Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi. EMBO J. 2004;23:1267–78. doi: 10.1038/sj.emboj.7600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roghi C, Allan VJ. Dynamic association of cytoplasmic dynein heavy chain 1a with the Golgi apparatus and intermediate compartment. J Cell Sci. 1999;112(Pt 24):4673–85. doi: 10.1242/jcs.112.24.4673. [DOI] [PubMed] [Google Scholar]
- 27.Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualizaed in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 28.Blangy A, Arnaud L, Nigg EA. Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin-related motor HsEg5 to the dynactin subunit p150. J Biol Chem. 1997;272:19418–19424. doi: 10.1074/jbc.272.31.19418. [DOI] [PubMed] [Google Scholar]
- 29.Deacon SW, Serpinskaya AS, Vaughan PS, Lopez Fanarraga M, Vernos I, Vaughan KT, Gelfand VI. Dynactin is required for bidirectional organelle transport. J Cell Biol. 2003;160:297–301. doi: 10.1083/jcb.200210066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatsuzawa K, Hirose H, Tani K, Yamamoto A, Scheller RH, Tagaya M. Syntaxin 18, a SNAP receptor that functions in the endoplasmic reticulum, intermediate compartment, and cis-Golgi vesicle trafficking. J Biol Chem. 2000;275:13713–20. doi: 10.1074/jbc.275.18.13713. [DOI] [PubMed] [Google Scholar]
- 31.Arasaki K, Taniguchi M, Tani K, Tagaya M. RINT-1 regulates the localization and entry of ZW10 to the Syntaxin 18 complex. Mol Biol Cell. 2006;17(6):2780–8. doi: 10.1091/mbc.E05-10-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scaerou F, Starr DA, Piano F, Papoulas O, Karess RE, Goldberg ML. The ZW10 and Rough Deal checkpoint proteins function together in a large, evolutionarily conserved complex targeted to the kinetochore. J Cell Sci. 2001;114:3103–14. doi: 10.1242/jcs.114.17.3103. [DOI] [PubMed] [Google Scholar]
- 33.Emanuele MJ, McCleland ML, Satinover DL, Stukenberg PT. Measuring the stoichiometry and physical interactions between components elucidates the architecture of the vertebrate kinetochore. Mol Biol Cell. 2005;16:4882–92. doi: 10.1091/mbc.E05-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gatti M, Goldberg ML. Mutations affecting cell division in Drosophila. Methods Cell Biol. 1991;35:543–86. doi: 10.1016/s0091-679x(08)60587-7. [DOI] [PubMed] [Google Scholar]
- 35.Karess RE, Glover DM. Rough deal: A gene required for proper mitotic segregation in Drosophila. J Cell Biol. 1989;109:2951–61. doi: 10.1083/jcb.109.6.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams BC, Li Z, Liu S, Williams EV, Leung G, Yen TJ, Goldberg ML. Zwilch, a new component of the ZW10/ROD complex required for kinetochore functions. Mol Biol Cell. 2003;14:1379–91. doi: 10.1091/mbc.E02-09-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starr DA, Saffery R, Li Z, Simpson AE, Choo KH, Ten TJ, Goldberg ML. HZwint-1, a novel human kinetochore component that interacts with HZW10. J Cell Sci. 2000;113(Pt 11):1939–50. doi: 10.1242/jcs.113.11.1939. [DOI] [PubMed] [Google Scholar]
- 38.Lin YT, Chen Y, Wu G, Lee WH. Hec1 sequentially recruits Zwint-1 and ZW10 to kinetochores for faithful chromosome segregation and spindle checkpoint control. Oncogene. 2006 doi: 10.1038/sj.onc.1209687. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Dilcher M, Veith B, Chidambaram S, Hartmann E, Schmitt HD, Fischer von Mollard G. Use1p is a yeast SNARE protein required for retrograde traffic to the ER. EMBO J. 2003;22:3664–74. doi: 10.1093/emboj/cdg339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao J, Liu CC, Chen PL, Lee WH. RINT-1, a novel Rad50-interacting protein, participates in radiation-induced G2/M checkpoint control. J Biol Chem. 2001;276:6105–11. doi: 10.1074/jbc.M008893200. [DOI] [PubMed] [Google Scholar]
- 41.Kong LJ, Meloni AR, Nevins JR. The Rb-related p130 protein controls telomere lengthening through an interaction with a Rad50-interacting protein, RINT-1. Mol Cell. 2006;22:63–71. doi: 10.1016/j.molcel.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Sweet DJ, Pelham HR. The TIP1 gene of Saccharomyces cerevisiae encodes an 80 kDa cytoplasmic protein that interacts with the cytoplasmic domain of Sec20p. EMBO J. 1993;12:2831–40. doi: 10.1002/j.1460-2075.1993.tb05944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis MJ, Rayner JC, Pelham HR. A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO J. 1997;16:3017–24. doi: 10.1093/emboj/16.11.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holleran EA, Tokito MK, Karki S, Holzbaur EL. Centractin (ARP1) associates with spectrin revealing a potential mechanism to link dynactin to intracellular organelles. J Cell Biol. 1996;135:1815–29. doi: 10.1083/jcb.135.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680–5. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 46.Short B, Preisinger C, Schaletzky J, Kopajtich R, Barr FA. The Rab6 GTPase regulates recruitment of the dynactin complex to Golgi membranes. Curr Biol. 2002;12:1792–5. doi: 10.1016/s0960-9822(02)01221-6. [DOI] [PubMed] [Google Scholar]
- 47.Matanis T, Akhmanova A, Wulf P, Del Nery E, Weide T, Stepanova T, Galjart N, Grosveld F, Goud B, De Zeeuw CI, Barnekow A, Hoogenraad CC. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol. 2002;4:986–92. doi: 10.1038/ncb891. [DOI] [PubMed] [Google Scholar]
- 48.Watson P, Forster R, Palmer KJ, Pepperkok R, Stephens DJ. Coupling of ER exit to microtubules through direct interaction of COPII with dynactin. Nat Cell Biol. 2005;7:48–55. doi: 10.1038/ncb1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen JL, Fucini RV, Lacomis L, Erdjument-Bromage H, Tempst P, Stamnes M. Coatomer-bound Cdc42 regulates dynein recruitment to COPI vesicles. J Cell Biol. 2005;169:383–9. doi: 10.1083/jcb.200501157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papoulas O, Hays TS, Sisson JC. The golgin Lava lamp mediates dynein-based Golgi movements during Drosophila cellularization. Nat Cell Biol. 2005;7:612–8. doi: 10.1038/ncb1264. [DOI] [PubMed] [Google Scholar]


