Abstract
Heart failure (HF) is accompanied by the upregulation of bioactive signaling molecules, known as cytokines, and a family of downstream proteases, matrix metalloproteinases (MMPs). It is now apparent that these molecules contribute to adverse myocardial remodeling during HF. Elevated levels of cytokines and MMPs exist in the myocardium and can subsequently spill over into the systemic circulation. The purpose of this article is to examine clinical studies of HF that have quantified levels of different types of cytokines, MMPs and endogenous tissue inhibitors of MMPs in relation to this disease process. HF is a complex syndrome that can develop from various etiologies and can be characterized into two distinct phenotypes: systolic and diastolic. This article will present recent clinical studies that have identified significant differences between the cytokine and MMP circulating profile of systolic and diastolic HF patients. In general, elevated levels of cytokines and MMPs exist in systolic HF patients when compared with diastolic HF patients, whereas diastolic HF patients have elevated levels of cytokines and MMPs when compared with controls. Therefore, future studies distinguishing between HF phenotypes may provide more consistent results in determining possible analytes to be used as biomarkers. Furthermore, this article will emphasize why standardization of analytical techniques and establishment of referent cytokine and MMP levels are necessary if these analytes are to be used as biomarkers for the diagnosis, prognosis and evaluation of treatment in the context of HF.
Keywords: chronic heart failure, cytokines, matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases
Heart failure & biomarkers
Heart failure (HF) can be classified into two separate phenotypes: systolic HF (SHF) and diastolic HF (DHF). SHF is characterized by a dilated and eccentrically hypertrophied left ventricle (LV) where ejection is impaired during systole [1]. Conversely, DHF is characterized by intrinsic abnormalities in LV relaxation and passive stiffness resulting in inadequate filling during diastole [2]. However, DHF patients exhibit normal systolic function and have a normal or preserved ejection fraction (EF) [3]. SHF and DHF are significantly different in demographic characteristics, underlying causal and pathophysiologyical mechanisms, LV remodeling and function and mortality rate [1,3]. Owing to these many differences, it is reasonable that the circulating biomarker profile, including cytokines and matrix metallo-proteinases (MMPs), may be significantly different between these HF phenotypes. The diagnosis of HF is a complex process, which is dependent on combinatorial results from medical history, physical examination and an array of functional tests, and can progress from various diseases including myocardial infarction, hypertrophy and cardiomyopathy. Therefore, the identification of biomarkers to assist in diagnosing and evaluating the severity and cause of HF could provide a more accurate and systematic method to improve evaluation and treatment. This article will examine several aspects of cytokines and MMPs:
Clinical studies that have quantified and identified relationships between cytokine and MMP levels with patient functional status or prognosis;
Differences between cytokine and MMP levels in SHF and DHF patients;
Interactions between cytokines and MMPs;
Analytical techniques used in the quantification of clinical studies.
Cytokines
Cytokines are a family of bioactive signaling molecules that regulate the inflammatory response and are involved in various cardiovascular diseases, including HF [4]. Although cytokines are pleiotropic, a simplified categorization of pro- and anti-inflammatory has been implemented. The prototypical proinflammatory cytokine, TNF, is expressed as a pro-TNF form and is subsequently inserted into the cellular membrane in various cell types [5]. Proteases then solublize and release the membrane-bound TNF to bind to the two TNF-receptor (TNFR) subtypes, TNFRI and TNFRII [6]. TNF can depress myocardial contractility by uncoupling regulated calcium handling through both sphingosine and nitric oxide pathways [7]. Furthermore, TNF can induce myocyte apoptosis in the myocardium [8]. Following TNF binding to the membrane-bound TNFR, a protease cleaves the extracellular domain and soluble TNFR can be detected in the systemic circulation [6,9–14]. Other proinflammatory cytokines include IL-1β, IL-2 and IL-6. These cytokines are involved in immune system activation and have been shown to have negative inotropic effects on the myocardium [15]. Interestingly, TNF and IL-1β have a synergistic relationship on inotropic effects and cytotoxicity [16]. In addition to proinflammatory cytokines, anti-inflammatory cytokines are also present in the myocardium [17]. The prototypical anti-inflammatory cytokine, IL-10, is expressed by monocytes and inhibits the expression of proinflammatory cytokines TNF, IL-1β and IL-6 [18]. Furthermore, IL-10 can block the contractility effects of other cytokines by inhibiting the production of nitric oxide [19]. Although there are currently numerous identified cytokines, this article will only examine a few that have been well characterized in clinical studies and have effects of the myocardium [6,7,9–14,16,19].
Matrix metalloproteinases
Matrix metalloproteinases are a family of zinc-dependent proteolytic enzymes that degrade all components of the extracellular matrix (ECM) [20]. The ECM is the structural component of the myocardium, and is continuously being synthesized and degraded [21]. However, during various cardiovascular diseases or following a myocardial infarction, a shift in the natural balance can result in myocardial remodeling. While myocardial remodeling may initially be compensatory, long-term effects on the myocardial structure and function may promulgate HF [22]. MMPs are categorized into subgroups depending on substrate specificity. The MMPs that have been measured in HF clinical studies include: collagenases (MMP-1 and -8), gelatinases (MMP-2 and -9) and stromelysins (MMP-3) [20]. MMPs are expressed as zymogens by a variety of cell types that are resident in the myocardium [22]. MMPs are not only capable of degrading the ECM components, but also play a role in regulating bioactive signaling pathways by selectively processing cytokines and their respective receptors [23]. Therefore, it is necessary not only for the synthesis of MMPs to be highly regulated, but also the MMP activity. MMP activity is regulated by endogenous tissue inhibitors of MMPs (TIMPs), which bind to MMP in a 1:1 stoichiometric ratio. There are currently four known TIMPs, which are expressed in various cell types and have been detected in the circulation [24,25]. However, most clinical studies in the context of HF have focused on TIMP-1 and -2 [26–30].
Cytokine & MMP interactions in cellular & animal models
While cytokines and MMPs have independent effects on the myocardium, past in vitro and animal studies have identified the ability of cytokines to regulate the transcription and synthesis of various MMPs [31–36]. For example, TNF over-expression in mice led to increased protein levels of MMP-2 and -9 and TIMP-1 [31,32]. Regulation of MMP synthesis includes several transcription factors that are downstream of cytokine signaling. Specifically, in fibroblasts, IL-1β stimulation has been reported to increase protein levels of MMP-2 and -9, which were attenuated with the inhibition of the transcription factor NF-κB [34]. Similarly, IL-6 can induce the expression of MMP-1 in macrophages mediated through transcriptional regulation of activator protein-1 and NF-κB [35]. By contrast, the anti-inflammatory cytokine IL-10 suppressed MMP-2 synthesis by signaling through the activating transcription factor 3 and binding to the cAMP-responsive element of the MMP-2 gene [36].
Analytical detection of cytokines & MMPs
Many different techniques were used in past clinical studies to quantify circulating levels of cytokines and MMPs in HF patients (Table 1). The most common method utilized in clinical studies is ELISA [13,26,27,29,37–46]. Initial clinical studies using ELISAs were limited in cytokine and MMP analysis owing to categorical reporting: detectable versus nondetectable data [47,48]. The development of more sensitive ELISAs has overcome this challenge; however, measuring single analytes still requires larger volumes of sample. Therefore, a novel technique, multiplex suspension array, was developed to simultaneously quantify multiple analytes with greater sensitivity and has been validated with traditional ELISAs [49,50]. Multiplex suspension array uses flow cytometry for the identification and quantification of analytes by using primary antibodies conjugated to fluorescent microbeads and biotinylated secondary antibodies [49]. However, both ELISA and multiplex suspension arrays use antibodies that may not differentiate between the free forms of MMPs, the pro- or active form, or the inactive TIMP-bound MMPs. Some clinical studies have used gelatin zymography to distinguish between pro- and active forms of MMPs [51]. However, active MMPs do not circulate in the vasculature but are complexed to proteins such as albumin and α-macroglobulins, as well as TIMPs [22]. The use of electrophoresis can cause the disruption of these formed complexes, and results may not be indicative of the net proteolytic activity. Therefore, the measurement of MMP activity in the serum or plasma is problematic and presents difficulties in interpreting the data. However, the total levels of MMP and TIMP types may provide a reference value of relative abundance. Furthermore, gelatin zymography is difficult to analyze owing to the presence of multiple protein structures of an MMP type in the circulation. Variations in cytokine and MMP levels between clinical studies may also be due to the inconsistent analysis of serum or plasma [52,53]. Levels of cytokines, MMPs and TIMPs were elevated in serum when compared with plasma owing to the presence of polymorphonuclear neutrophils and platelets during the clotting process. These cells are capable of releasing both preformed cytokines and MMPs, which are not indicative of the disease state [52,53]. Previous studies have also demonstrated that the type of anticoagulants, such as citrate, ethylenediaminetetraacetic acid or heparin, can significantly alter MMP and TIMP levels measured in plasma [52]. Inconsistencies in techniques used to analyze cytokines and MMPs along with the lack of established referent levels have been problematic for the interpretation and direct comparison of clinical studies. However, these methods utilized in clinical HF studies can provide a directional change in cytokine and MMP levels. Individual analyte concentrations for the clinical studies are presented in the Supplementary Table 1 & Supplementary Table 2 (see online www.futuremedicine.com/toc/bmm/3/5).
Table 1.
Analytical methods for quantifying circulating cytokines and matrix metalloproteinases.
| Method (clinical study) |
Analytes | Advantage | Disadvantage | Sensitivity | Ref. |
|---|---|---|---|---|---|
| ELISA | ALL | Evaluate large number of patient samples |
Antibodies not specific for pro-, active or TIMP-bound MMPs |
Cytokines: 10–100 pg/ml MMPs: 0.1–100 ng/ml |
[9–14,26–30,37,38,40–43, 45–48,54,56–58,60,61,63,64] |
| Multiplex suspension array |
ALL | Simultaneous detection of multiple analytes |
Antibodies not specific for pro-, active or TIMP-bound MMPs |
Cytokines: 0.4 pg/ml MMPs: 10 pg/ml |
[50] |
| Zymography | MMP-2 and -9 |
Differentiate between pro- and active forms |
High variability Not indicative of MMP activity Difficult to analyze owing to the presence of multiple MMP-type structures Not ideal for large sample sizes |
100 pg | [51] |
MMP: Matrix metalloproteinase; TIMP: Tissue inhibitor of matrix metalloproteinase.
Clinical studies for profiling cytokine & MMPs
Cytokines in systolic heart failure
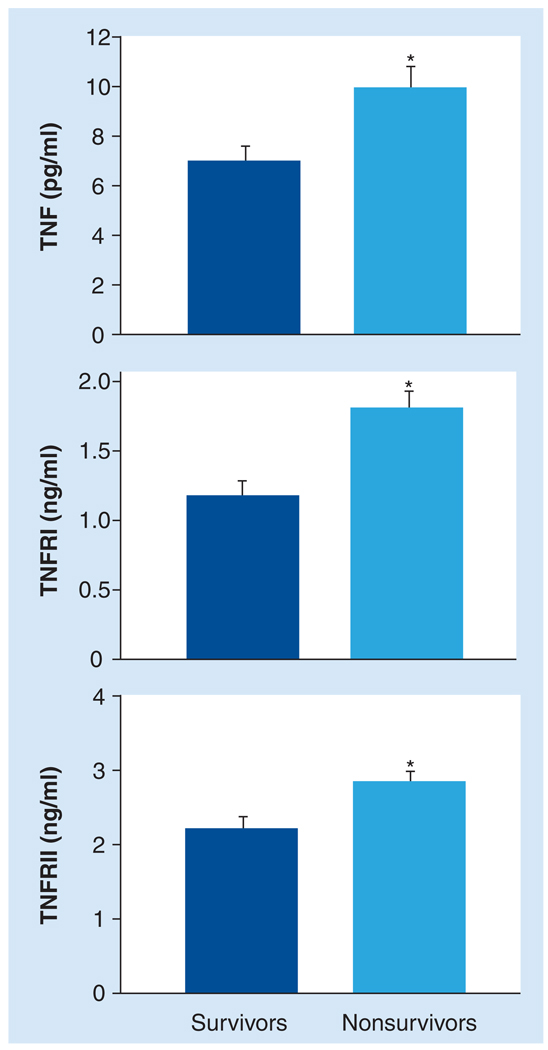
Many clinical studies have reported the circulating levels of cytokines in SHF patients (Table 2) [9,10,14,46,48,54–57]. The most consistently elevated cytokine is TNF and the respective TNFRs, TNFRI and TNFRII [9,10,14,46,54,55]. Furthermore, past studies have identified relationships between circulating levels of TNF, TNFRI and TNFRII, and clinical status. For example, circulating levels of TNFRI and TNFRII directly correlate with New York Heart Association (NYHA) functional class [11,58] and negatively correlate with cardiac index [9]. Other negative correlations in HF patients have been reported: TNFRI levels with EF [9], and TNF and TNFRII levels with 6-min walk distance [58]. In a large study by Deswal et al., the relationship between cytokine levels and all-cause mortality in HF patients was examined. Levels of TNF, TNFRI and TNFRII were significantly elevated in nonsurvivors when compared with survivors (Figure 1) and were independent predictors of mortality in HF patients [11–13]. Following a myocardial infarction, elevated levels of TNF, TNFRI and TNFRII were independent predictors of patient mortality or the development of new-onset HF [59]. However, some clinical studies have reported that elevated levels of TNF, TNFRI and TNFRII were only observed in severe HF patients (NYHA class III and IV) [13,48,60]. Munger et al. reported no statistical difference in serum TNF levels between HF patients and controls; however, TNF levels significantly correlated with LV fractional shortening [44]. This early study by Munger et al. utilized a cytotoxicity assay to determine serum TNF levels, which may explain the inconsistent results when compared with other studies that were analyzed by ELISA [44].
Table 2.
Cytokines and matrix metalloproteinases included in clinical studies of heart failure.
| Systolic heart failure |
Diastolic heart failure |
Ref. | |
|---|---|---|---|
| Cytokines | |||
| IL-1β | ↑→ | N/A | [9,48] |
| IL-6 | ↑↑ | ↑↑ | [9–12,44,46,48,54,55,58,60,61,64] |
| IL-10 | ↑ | ↑→ | [9,45,55,57,64] |
| TNF | ↑↑ | → | [9–14,28,44,46–48,54,55,58,60,61,63,64,74] |
| Soluble cytokine receptors | |||
| IL-2R | ↑ | N/A | [44,48,56] |
| TNFRI | ↑↑ | ↑ | [9–14,64] |
| TNFRII | ↑↑ | ↑↑ | [9–13,48,58,64] |
| MMPs | |||
| Collagenase | |||
| MMP-1 | ↑↑ | ↑ | [26,30,43,65] |
| MMP-8 | ↓ | N/A | [37] |
| Gelatinase | |||
| MMP-2 | ↑ | ↑→ | [27,29,30,37] |
| MMP-9 | ↑ | ↑→ | [27–30] |
| Stromelysin | |||
| MMP-3 | ↑ | → | [27,42] |
| TIMPs | |||
| TIMP-1 | ↑ | ↑↑ | [26–30] |
| TIMP-2 | ↑ | → | [27,29] |
↑: Increase in analyte levels from referent controls; ↑↑: Robust increase in analyte levels from referent controls; ↑→: Slight increase in analyte levels from referent controls; →: No change in analyte levels from referent controls; ↓: Decrease in analyte levels from referent controls.
IL-2R: IL-2 receptor; MMP: Matrix metalloproteinase; N/A: Not applicable; TIMP: Tissue inhibitor of matrix metalloproteinase; TNFR: TNF receptor.
Figure 1. In chronic heart failure patients, circulating levels of TNF, TNFRI and TNFRII were compared between survivors (dark) and nonsurvivors (light).
TNF, TNFRI and TNFRII levels were significantly elevated in nonsurvivors when compared with survivors. Patients were followed up to 24 months following plasma collection and all-cause mortality was included in this study. Only systolic heart failure patients were included in this study. Nonsurvivors: n = 62; survivors: n = 90.
*p < 0.01.
TNFR: TNF receptor.
Adapted from [11].
Other proinflammatory cytokines have been quantified in HF patients, such as IL-1β, IL-2 receptor (IL-2R) and IL-6. IL-1β levels were elevated in patients with more severe HF (NYHA class III and IV) compared with controls in a study by Testa [48]. However, in a smaller study by Aukrust et al., IL-1β levels were not statistically different between HF and control groups [9]. Interestingly, the plasma range for IL-1β levels reported by Aukrust (0.3–2.8 pg/ml) were significantly lower than the range reported by Testa (4–50 pg/ml) [9,48], reiterating the need to establish cytokine referent levels. IL-2R levels were elevated in HF patients with dilated cardiomyopathy compared with ischemic heart disease, and correlated to LV volume and clinical course [56]. However, Testa et al. reported that circulating IL-2R levels were not statistically different between HF patients with ischemic heart disease and controls [48]. These results exemplify the importance of HF etiology in altering specific circulating cytokine levels. IL-2R levels also significantly correlated with NYHA functional class and exercise tolerance in HF patients [44]. Similarly, elevated levels of IL-6 have also been reported in HF patients compared with healthy controls [9,46,48,55]. IL-6 levels were negatively correlated with 6-min walk distance [58] and LVEF [44,58], and directly correlated with pulmonary artery resistance [9] and NYHA functional class [9,54,58]. In HF patients, IL-6 levels were also an independent predictor of mortality [11,12,60]. Maeda et al. measured IL-6 levels in HF patients following 3 months of optimized treatment and the presence of elevated IL-6 levels was an independent predictor of mortality [61]. Similarly, elevated IL-6 levels, following an acute myocardial infarction, were also an independent predictor for patient mortality or the development of new-onset HF [59,62]. Interestingly, the anti-inflammatory cytokine (IL-10) levels were elevated in HF patients when compared with healthy controls [9,55,57]. However, the increase in anti-inflammatory cytokine levels is not adequately proportional to the elevated proinflammatory cytokine levels to provide an overall beneficial effect in HF patients [9,57].
Cytokines in diastolic heart failure
While there are many clinical studies that have quantified the circulating cytokines in SHF, a cytokine profile in DHF has only recently been examined (Table 2) [28,63,64]. Niethammer et al. compared cytokine levels in HF patients with reduced EF and preserved EF (SHF and DHF, respectively) [64]. TNF levels were elevated in the SHF group, but there were no differences between the DHF group and controls. TNFRI levels were elevated in the SHF group when compared with the DHF group and controls, with greater levels in the DHF group than the controls. Furthermore, TNFRII and IL-6 levels were elevated in both HF groups when compared with the controls, with no differences between the SHF and DHF groups. IL-10 levels were elevated in the SHF group when compared with the DHF group and controls. Although not reaching significance, there was a trend of elevated IL-10 levels in the DHF group when compared with the controls [64]. A larger study by Dunlay et al. identified that elevated TNF levels were a predictor of HF mortality independent of patient categorization by HF phenotype: SHF or DHF [63]. Similar to Niethammer’s study, Dunlay observed a trend between the levels of TNF and LVEF, although this trend did not reach statistical significance. These studies have identified that the cytokine profile is different in DHF patients when compared with SHF patients and, generally, cytokine levels were observed to be greater in SHF patients.
MMPs in systolic heart failure
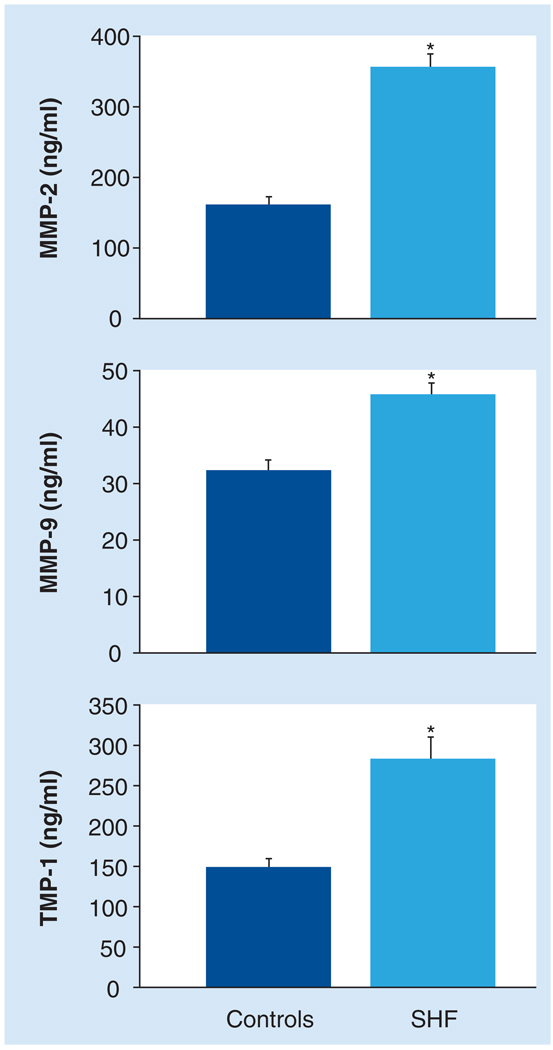
Past clinical studies have quantified various circulating levels of MMPs and TIMPs in SHF patients (Table 2) [26–28,37,38,41,43,45,50]. MMP-1 levels were increased in SHF patients when compared with controls in several studies [26,38,65]. Furthermore, elevated levels of MMP-1 were a negative independent predictor of 6-min walk distance [45]. Conversely, a few studies have reported MMP-1 levels were decreased compared with healthy controls and were an independent predictor of cardiac end points (i.e., death, hospitalization and transplant) [41,43]. MMP-1 levels can be altered with the presence of comorbidities, such as diabetes metallitus and atrial fibrillation, which have been associated with lower MMP-1 levels when compared with healthy controls [41,43,66,67]. MMP-8 levels were decreased in HF patients compared with controls [37]. The circulating levels of gelatinases, MMP-2 and -9, have also been well characterized in HF patients. MMP-2 levels were elevated in SHF when compared with healthy controls (Figure 2) [27,40,42,51]. MMP-2 levels were correlated with LV volume and fractional shortening, and NYHA classifications [27]. Furthermore, MMP-2 levels were an independent predictor of mortality in SHF patients [40]. Similarly, clinical studies have reported elevated circulating MMP-9 levels in SHF patients compared with healthy controls (Figure 2) [40,42,51]. Following a myocardial infarction, MMP-9 levels were greater in patients who developed HF [39]. By contrast, there are also reports that there are no differences between circulating levels of MMP-9 in SHF and control groups [27,28]. The discrepancies between these studies are not explained by variations in severity, HF etiology or age. Further studies are necessary to elucidate the MMP-9 profile in HF patients. MMP-3 levels were elevated in SHF secondary to dilated cardiomyopathy, negatively correlated with changes in the LV dimensions and independently predictive of cardiac events (death and hospitalization) [42]. Furthermore, MMP-3 levels were elevated in patients who developed HF following an acute myocardial infarction [50]. By contrast, MMP-3 levels were not statistically different between hypertrophic cardiomyopathy patients and controls [27]. The endogenous inhibitors of MMPs have also been quantified in the circulation of HF patients [27,40,41,51]. Specifically, patients with SHF had higher levels of TIMP-1 than healthy controls (Figure 2) [27,28,38,40,41,51]. Interestingly, elevated TIMP-1 levels were also an independent predictor of the development of HF in patients following an acute myocardial infarction [39]. The balance between the levels of TIMP-1 and other MMPs have been examined and related to functional status or disease prognosis. Likewise, the MMP-1: TIMP-1 ratio was also elevated in SHF patients when compared with controls, and correlated with LV volume and function [38]. Inversely, the TIMP-1: MMP-1 ratio correlated negatively with peak oxygen consumption (VO2) levels [41]. Fewer clinical studies have included the analysis of TIMP-2 levels. In hypertrophic cardiomyopathy, plasma TIMP-2 levels were elevated in SHF when compared with controls [27]; however, when comparing HF with any etiology, there was no difference between TIMP-2 levels between HF patients and controls [51].
Figure 2. Circulating levels of MMP-2, MMP-9 and TIMP-1 were compared between SHF patients (dark) and age-matched healthy controls (light).
MMP-2, MMP-9 and TIMP-1 levels from SHF patients were significantly elevated compared with the controls. SHF: n = 88; controls: n = 30.
*p < 0.05.
MMP: Matrix metalloproteinase; SHF: Systolic heart failure; TIMP: Tissue inhibitor of matrix metalloproteinase.
Adapted from [40].
MMPs in diastolic heart failure
A recent study has examined the use of circulating MMPs and TIMPs in the diagnosis of DHF in hypertensive patients [30]. MMP-2 and -9 levels were significant predictors of DHF. Using a cutoff level (1585 ng/ml), the predictive values of MMP-2 levels had 91% sensitivity and 76% specificity for DHF [30]. Similarly, Ahmed et al. reported that elevated levels of TIMP-1 (>1200 ng/ml) were predictive of DHF in hypertensive patients [29]. Lopez et al. compared the circulating levels of MMP-1 and TIMP-1 between SHF and DHF patients [26]. MMP-1 levels were elevated in the SHF and DHF groups when compared with controls, with greater levels in the SHF group than the DHF group. Similarly, elevated levels of TIMP-1 were observed in both SHF and DHF groups when compared with controls. However, TIMP-1 levels were greater in the DHF group than the SHF group. Thus, the ratio between MMP-1 and TIMP-1 levels was elevated in the SHF group and also inversely correlated with LVEF [26]. Noji et al. reported that MMP-2 levels were significantly elevated in SHF patients when compared DHF patients, and negatively correlated with fractional shortening [27]. Frantz et al. reported similar elevated levels of TIMP-1 in both SHF and DHF patients when compared with controls. Circulating TIMP-1 levels correlated with HF severity (Figure 3) and were an independent predictor of all-cause mortality risk [28]. TIMP-2 levels were greater in the SHF group when compared with the DHF group, but there was no difference between the DHF group and controls [27]. Furthermore, TIMP-2 levels correlated negatively with fractional shortening and positively with LV dimensions [27]. These studies have identified significant differences in the balance of circulating MMPs and TIMPs between the two phenotypes of HF, SHF and DHF. Overall, the MMP:TIMP ratio tends to be higher in SHF patients when compared with DHF patients.
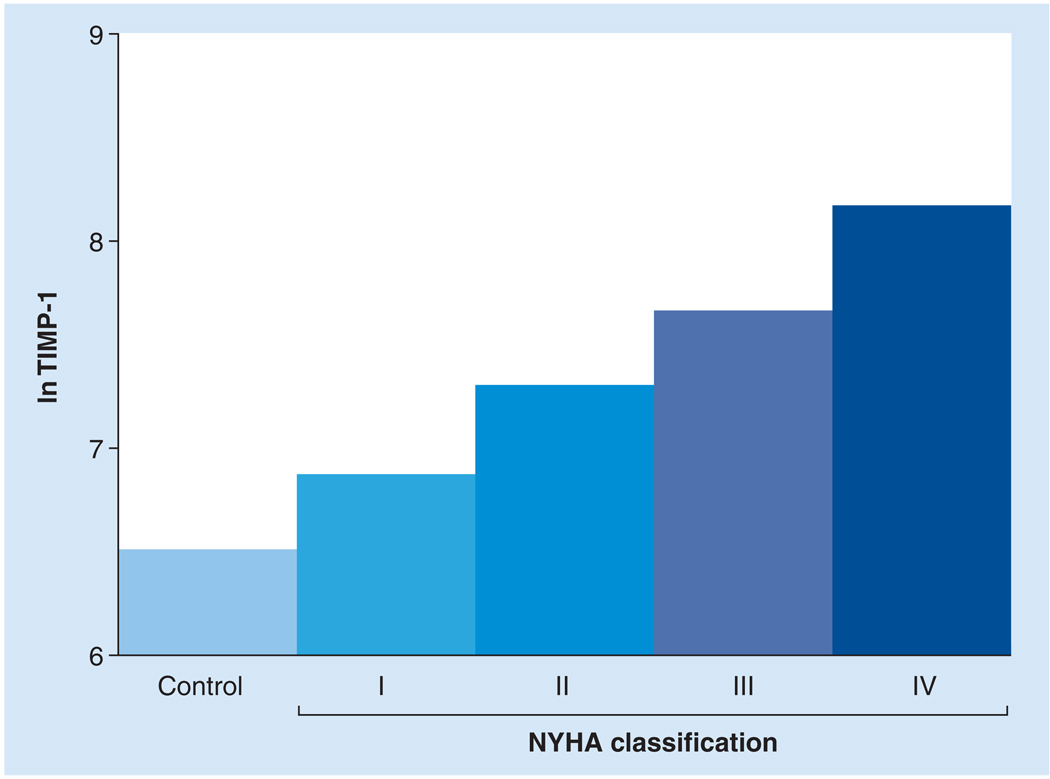
Figure 3. Circulating levels of TIMP-1 were compared between healthy controls and heart failure patients with NYHA classifications I, II, III and IV.
The log TIMP-1 levels, adjusted for age and gender, significantly increased with increasing NYHA classification. Both systolic and diastolic heart failure patients were included in this study. Controls: n = 74; NYHA I, II, III and IV: n = 35, 94, 98 and 22, respectively; p < 0.001.
NYHA: New York Heart Association; TIMP: Tissue inhibitor of matrix metalloproteinase.
Adapted from [28].
Conclusion & future perspective
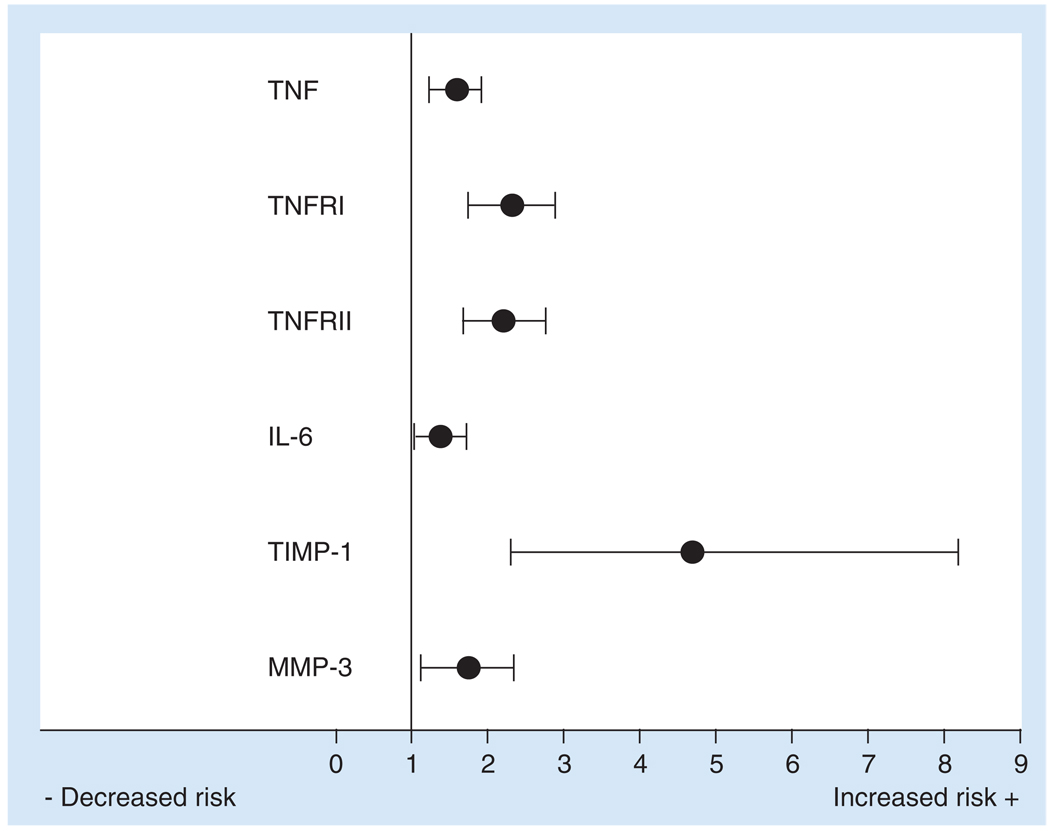
This article has focused on clinical studies that have observed directional changes of circulating cytokines and MMPs in HF patients. Furthermore, this article included correlations observed between clinical status and elevated levels of cytokines and MMPs. Specifically, TNFRI, IL-6, MMP-2 and TIMP-1 levels were elevated in HF patients and correlated with NYHA functional status [11,27,28,58]. Moreover, circulating levels of TNF, TNFRI, TNFRII, MMP-3 and TIMP-1 were independent predictors of patient mortality, and the relative risk ratios are presented in Figure 4 [11–13,28,39,59]. These clinical studies suggest that cytokines and MMPs may be used as biomarkers for the prognosis of HF. This article has also identified distinct differences in the circulating levels of cytokines and MMPs in DHF and SHF phenotypes. Generally, SHF patients have greater levels of circulating cytokines (TNF, TNFRI and IL-10) and MMPs (MMP-1 and -2) than DHF patients [26,27,64]. Results from these initial clinical studies comparing DHF with SHF patients suggest that prospective HF clinical studies should account for differences in HF phenotypes. Owing to the complexity of HF, a single biomarker may not provide adequate specificity or sensitivity in HF patients. However, a profile or specific cassette of cytokines and/or MMPs may exist, which would allow for further biomarker development. The addition of cytokines and MMPs to multivariate analysis, including other biomarkers involved in HF, such as neurohormones, collagen synthesis and degradation products, may optimize the diagnosis and prognosis of HF. Cytokines and MMPs are involved in various disease processes, such as arthritis and cancer, or are altered in the presence of other diseases, such as diabetes metallitus and atrial fibrillation [66–69]. Therefore, HF comorbidities need to be considered when cytokine and MMP profiles are analyzed in the clinical setting. Interestingly, both cytokines and MMPs can also be affected by conventional medications used for cardiovascular diseases and, therefore, may provide an index for a patient’s response to current HF treatment [46,70]. Past studies have attempted to inhibit the elevated cytokines and MMPs in HF; however, clinical trials were unsuccessful, and cytokine inhibition has resulted in increased patient mortality [71–73]. Therefore, while cytokines and MMPs may not currently be a viable target for HF treatment, the use of these analytes as biomarkers may assist in the diagnosis, prognosis and guidance of conventional therapy of HF. Before cytokines and MMPs can be considered for clinical application, four critical aspects must be addressed to optimize the direct comparison of future clinical studies: standardization of collection method, specifically, blood collected with ethylenediaminetetraacetic acid and plasma should be immediately separated; standardization of analytical technique, specifically, ELISA or multiplex suspension array; establishment of referent levels for age-matched individuals; and, most importantly, analysis of data that account for HF phenotype, severity and comorbidities. These results suggest that cytokines and MMPs can be potentially developed as biomarkers to diagnose, prognose or evaluate current HF treatment; however, these analytes have yet to be tested in a clinical prospective manner.
Figure 4. Hazard ratios for major adverse cardiac events for circulating levels of cytokines and matrix metalloproteinases.
TNF, TNFRI, TNFRII and IL-6 levels were independent predictors of patient mortality and new-onset heart failure following a myocardial infarction (n = 184; p < 0.001). Similarly, TIMP-1 levels were an independent predictor of heart failure following a myocardial infarction (n = 404; p < 0.001). Furthermore, MMP-3 levels were independent predictors of cardiac death or hospitalization in patients with systolic heart failure (n = 71; p < 0.0001). Each hazard ratio is presented as mean ± 95% CI.
MMP: Matrix metalloproteinase; TIMP: Tissue inhibitor of matrix metalloproteinase; TNFR: TNF receptor.
Executive summary.
Heart failure & biomarkers
Numerous clinical studies have identified relationships between cytokines and matrix metalloproteinases (MMPs) with patient status. However, cytokines and MMPs are yet to be used as biomarkers for heart failure (HF).
Possible discrepancies in clinical results may be due to differences in the patient population, including systolic HF (SHF) and diastolic HF (DHF).
SHF is the impaired ability of the left ventricle to eject blood during systole while DHF is the impaired ability of the left ventricle to fill, owing to intrinsic abnormalities in relaxation and passive stiffness.
Cytokines
Cytokines are a family of bioactive signaling molecules that regulate the inflammatory process and effect myocardial contractility.
Proinflammatory cytokines include TNF, IL-1β, IL-2 and IL-6.
Anti-inflammatory cytokines include IL-10.
Matrix metalloproteinases
MMPs are a family of zinc-dependent enzymes that degrade the extracellular matrix of the myocardium.
Endogenous tissue Inhibitors of MMPs (TIMPs) inactivate MMPs.
Cytokine & MMP Interactions
In vitro and animal models have demonstrated that cytokines can regulate the transcription and synthesis of MMPs.
Analytical detection of cytokines & MMPs
Current clinical studies are also difficult to directly compare owing to variations in techniques used to quantify circulating levels.
Reference levels of cytokines and MMPs remain to be established.
Cytokines in clinical studies
TNF receptors (TNFRs) and IL-6 are elevated in HF patients.
TNF and IL-6 levels have been associated with HF severity and mortality.
Cytokines in SHF versus DHF
TNF, TNFRI and IL-10 levels are elevated in SHF when compared with DHF.
TNFRII and IL-6 levels are similar between SHF and DHF.
MMPs in clinical studies
MMP-2 and -9 and TIMP-1 levels are elevated in HF patients.
TIMP-1 levels have been associated with HF severity and mortality.
MMPs in SHF versus DHF
MMP-1 and -2 and TIMP-2 levels are elevated in SHF when compared with DHF patients, although TIMP-1 levels were similar.
Future perspective
- Prior to cytokines and MMPs being applied in the clinical setting, certain aspects need to be addressed:
- Standardization of collection and analytical technique
- Establishment of referent controls
- Data analysis, which accounts for HF phenotype, severity and comorbidities
Supplementary Material
Footnotes
Financial & competing interests disclosure.
This article was made possible through research funding from the NIH Grant HL-59165 and Merit Review Funding from the Ralph H Johnson Veterans’ Association Medical Center. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.van Heerebeek L, Borbely A, Niessen HW, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 2.Zile MR, Baicu CF, Bonnema DD. Diastolic heart failure: definitions and terminology. Prog. Cardiovasc. Dis. 2005;47:307–313. doi: 10.1016/j.pcad.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure – abnormalities in active relaxation and passive stiffness of the left ventricle. N. Engl. J. Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 4.Mehra VC, Ramgolam VS, Bender JR. Cytokines and cardiovascular disease. J. Leukoc. Biol. 2005;78:805–818. doi: 10.1189/jlb.0405182. [DOI] [PubMed] [Google Scholar]
- 5.Meldrum DR. Tumor necrosis factor in the heart. Am. J. Physiol. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 6.Jansen J, van der Poll T, Levi M, et al. Inhibition of the release of soluble tumor necrosis factor receptors in experimental endotoxemia by an anti-tumor necrosis factor-α antibody. J. Clin. Immunol. 1995;15:45–50. doi: 10.1007/BF01489489. [DOI] [PubMed] [Google Scholar]
- 7.Prabhu SD. Cytokine-induced modulation of cardiac function. Circ. Res. 2004;95:1140–1153. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 8.Krown KA, Page MT, Nguyen C, et al. Tumor necrosis factor α-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J. Clin. Invest. 1996;98:2854–2865. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aukrust P, Ueland T, Lien E, et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1999;83:376–382. doi: 10.1016/s0002-9149(98)00872-8. [DOI] [PubMed] [Google Scholar]
- 10.Dibbs Z, Thornby J, White BG, Mann DL. Natural variability of circulating levels of cytokines and cytokine receptors in patients with heart failure: implications for clinical trials. J. Am. Coll. Cardiol. 1999;33:1935–1942. doi: 10.1016/s0735-1097(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 11. Rauchhaus M, Doehner W, Francis DP, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–3067. doi: 10.1161/01.cir.102.25.3060. • Clinical study of heart failure patients that identified the cytokine levels of TNF, TNF receptor (TNFR)I, TNFRII and IL-6 to predict a 24-month patient mortality.
- 12. Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. • Large clinical study identified elevated levels of the cytokines TNF, TNFRI, TNFRII and IL-6 to be associated with patient mortality.
- 13.Ferrari R, Bachetti T, Confortini R, et al. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation. 1995;92:1479–1486. doi: 10.1161/01.cir.92.6.1479. [DOI] [PubMed] [Google Scholar]
- 14.Tziakas D, Chalikias G, Parissis JT, et al. Prolonged activation of tumor necrosis factor (TNF)-α and its soluble receptors in chronic heart failure patients both in the compensated and decompensated state. Interplay between their levels and metalloproteinase-3. Eur. Cytokine Netw. 2004;15:231–239. [PubMed] [Google Scholar]
- 15.Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 16.Cain BS, Meldrum DR, Dinarello CA, et al. Tumor necrosis factor-α and interleukin-1β synergistically depress human myocardial function. Crit. Care Med. 1999;27:1309–1318. doi: 10.1097/00003246-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Frangogiannis NG, Mendoza LH, Lindsey ML, et al. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J. Immunol. 2000;165:2798–2808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- 18.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J. Immunol. 1994;153:811–816. [PubMed] [Google Scholar]
- 19.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 20.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J. Biol. Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 21.Tyagi SC. Proteinases and myocardial extracellular matrix turnover. Mol. Cell. Biochem. 1997;168:1–12. doi: 10.1023/a:1006850903242. [DOI] [PubMed] [Google Scholar]
- 22.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol. Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 23.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J. Leukoc. Biol. 2007;82:1375–1381. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- 24.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98:1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 25.Webb CS, Bonnema DD, Ahmed SH, et al. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation. 2006;114:1020–1027. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 26. Lopez B, Gonzalez A, Querejeta R, Larman M, Diez J. Alterations in the pattern of collagen deposition may contribute to the deterioration of systolic function in hypertensive patients with heart failure. J. Am. Coll. Cardiol. 2006;48:89–96. doi: 10.1016/j.jacc.2006.01.077. •• Reported distinct differences between the matrix metalloproteinase (MMP)-1/tissue inhibitor of MMP-1 levels of systolic and diastolic heart failure patients and identified correlations with heart volume and function.
- 27.Noji Y, Shimizu M, Ino H, et al. Increased circulating matrix metalloproteinase-2 in patients with hypertrophic cardiomyopathy with systolic dysfunction. Circ. J. 2004;68:355–360. doi: 10.1253/circj.68.355. [DOI] [PubMed] [Google Scholar]
- 28. Frantz S, Stork S, Michels K, et al. Tissue inhibitor of metalloproteinases levels in patients with chronic heart failure: an independent predictor of mortality. Eur. J. Heart Fail. 2008;10:388–395. doi: 10.1016/j.ejheart.2008.02.015. •• Large clinical study that compared systolic and diastolic heart failure patients, and identified a direct correlation between tissue inhibitor of MMP-1 levels and heart failure severity.
- 29.Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 30. Martos R, Baugh J, Ledwidge M, et al. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. • Study examined the potential of using MMP-2 as a diagnostic for diastolic heart failure.
- 31.Sivasubramanian N, Coker ML, Kurrelmeyer KM, et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104:826–831. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 32.Kawamura N, Kubota T, Kawano S, et al. Blockade of NF-κB improves cardiac function and survival without affecting inflammation in TNF-α-induced cardiomyopathy. Cardiovasc. Res. 2005;66:520–529. doi: 10.1016/j.cardiores.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Liacini A, Sylvester J, Li WQ, et al. Induction of matrix metalloproteinase-13 gene expression by TNF-α is mediated by MAP kinases, AP-1, and NF-κB transcription factors in articular chondrocytes. Exp. Cell Res. 2003;288:208–217. doi: 10.1016/s0014-4827(03)00180-0. [DOI] [PubMed] [Google Scholar]
- 34.Xie Z, Singh M, Singh K. Differential regulation of matrix metalloproteinase-2 and -9 expression and activity in adult rat cardiac fibroblasts in response to interleukin-1β. J. Biol. Chem. 2004;279:39513–39519. doi: 10.1074/jbc.M405844200. [DOI] [PubMed] [Google Scholar]
- 35.Sundararaj KP, Samuvel DJ, Li Y, Sanders JJ, Lopes-Virella MF, Huang Y. Interleukin-6 released from fibroblasts is essential for upregulation of matrix metalloproteinase-1 expression by u937 macrophages in coculture-crosstalking between fibroblasts and u937 macrophages exposed to high glucose. J. Biol. Chem. 2009;284:13714–13724. doi: 10.1074/jbc.M806573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stearns ME, Kim G, Garcia F, Wang M. Interleukin-10 induced activating transcription factor 3 transcriptional suppression of matrix metalloproteinase-2 gene expression in human prostate CPTX-1532 cells. Mol. Cancer Res. 2004;2:403–416. [PubMed] [Google Scholar]
- 37.Wilson EM, Gunasinghe HR, Coker ML, et al. Plasma matrix metalloproteinase and inhibitor profiles in patients with heart failure. J. Card. Fail. 2002;8:390–398. doi: 10.1054/jcaf.2002.129659. [DOI] [PubMed] [Google Scholar]
- 38.Schwartzkopff B, Fassbach M, Pelzer B, Brehm M, Strauer BE. Elevated serum markers of collagen degradation in patients with mild to moderate dilated cardiomyopathy. Eur. J. Heart Fail. 2002;4 doi: 10.1016/s1388-9842(02)00092-2. 439-434. [DOI] [PubMed] [Google Scholar]
- 39.Kelly D, Khan SQ, Thompson M, et al. Plasma tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9: novel indicators of left ventricular remodelling and prognosis after acute myocardial infarction. Eur. Heart J. 2008 doi: 10.1093/eurheartj/ehn315. doi: 10.1093/eurheartj/ehn315 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George J, Patal S, Wexler D, Roth A, Sheps D, Keren G. Circulating matrix metalloproteinase-2 but not matrix metalloproteinase-3, matrix metalloproteinase-9, or tissue inhibitor of metalloproteinase-1 predicts outcome in patients with congestive heart failure. Am. Heart J. 2005;150:484–487. doi: 10.1016/j.ahj.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Jordan A, Roldan V, Garcia M, et al. Matrix metalloproteinase-1 and its inhibitor, TIMP-1, in systolic heart failure: relation to functional data and prognosis. J. Intern. Med. 2007;262:385–392. doi: 10.1111/j.1365-2796.2007.01823.x. [DOI] [PubMed] [Google Scholar]
- 42.Ohtsuka T, Nishimura K, Kurata A, Ogimoto A, Okayama H, Higaki J. Serum matrix metalloproteinase-3 as a novel marker for risk stratification of patients with nonischemic dilated cardiomyopathy. J. Card. Fail. 2007;13:752–758. doi: 10.1016/j.cardfail.2007.06.730. [DOI] [PubMed] [Google Scholar]
- 43.Alla F, Kearney-Schwartz A, Radauceanu A, Das Dores S, Dousset B, Zannad F. Early changes in serum markers of cardiac extra-cellular matrix turnover in patients with uncomplicated hypertension and type II diabetes. Eur. J. Heart Fail. 2006;8:147–153. doi: 10.1016/j.ejheart.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Munger MA, Johnson B, Amber IJ, Callahan KS, Gilbert EM. Circulating concentrations of proinflammatory cytokines in mild or moderate heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1996;77:723–727. doi: 10.1016/s0002-9149(97)89206-5. [DOI] [PubMed] [Google Scholar]
- 45.Radauceanu A, Ducki C, Virion JM, et al. Extracellular matrix turnover and inflammatory markers independently predict functional status and outcome in chronic heart failure. J. Card. Fail. 2008;14:467–474. doi: 10.1016/j.cardfail.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Mohler ER, 3rd, Sorensen LC, Ghali JK, et al. Role of cytokines in the mechanism of action of amlodipine: the PRAISE heart failure trial. Prospective Randomized Amlodipine Survival Evaluation. J. Am. Coll. Cardiol. 1997;30:35–41. doi: 10.1016/s0735-1097(97)00145-9. [DOI] [PubMed] [Google Scholar]
- 47.Matsumori A, Yamada T, Suzuki H, Matoba Y, Sasayama S. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. Br. Heart J. 1994;72:561–566. doi: 10.1136/hrt.72.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Testa M, Yeh M, Lee P, et al. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J. Am. Coll. Cardiol. 1996;28:964–971. doi: 10.1016/s0735-1097(96)00268-9. [DOI] [PubMed] [Google Scholar]
- 49.Prabhakar U, Eirikis E, Davis HM. Simultaneous quantification of proinflammatory cytokines in human plasma using the LabMAP assay. J. Immunol. Methods. 2002;260:207–218. doi: 10.1016/s0022-1759(01)00543-9. [DOI] [PubMed] [Google Scholar]
- 50.Kelly D, Khan S, Cockerill G, et al. Circulating stromelysin-1 (MMP-3): a novel predictor of LV dysfunction, remodelling and all-cause mortality after acute myocardial infarction. Eur. J. Heart Fail. 2008;10:133–139. doi: 10.1016/j.ejheart.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altieri P, Brunelli C, Garibaldi S, et al. Metalloproteinases 2 and 9 are increased in plasma of patients with heart failure. Eur. J. Clin. Invest. 2003;33:648–656. doi: 10.1046/j.1365-2362.2003.01187.x. [DOI] [PubMed] [Google Scholar]
- 52.Rossignol P, Cambillau M, Bissery A, et al. Influence of blood sampling procedure on plasma concentrations of matrix metalloproteinases and their tissue inhibitors. Clin. Exp. Pharmacol. Physiol. 2008;35:464–469. doi: 10.1111/j.1440-1681.2008.04897.x. [DOI] [PubMed] [Google Scholar]
- 53.Wu CY, Wu MS, Chiang EP, et al. Plasma matrix metalloproteinase-9 level is better than serum matrix metalloproteinase-9 level to predict gastric cancer evolution. Clin. Cancer Res. 2007;13:2054–2060. doi: 10.1158/1078-0432.CCR-06-2299. [DOI] [PubMed] [Google Scholar]
- 54.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J. Am. Coll. Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Zhou Y, Meng L, Lu X, Ou N, Li X. Inflammatory mediators in Chinese patients with congestive heart failure. J. Clin. Pharmacol. 2009;49:591–599. doi: 10.1177/0091270009333265. [DOI] [PubMed] [Google Scholar]
- 56.Limas CJ, Hasikidis C, Iakovou J, Kroupis C, Haidaroglou A, Cokkinos DV. Prognostic significance of soluble interleukin-2 receptor levels in patients with dilated cardiomyopathy. Eur. J. Clin. Invest. 2003;33:443–448. doi: 10.1046/j.1365-2362.2003.01111.x. [DOI] [PubMed] [Google Scholar]
- 57.Yamaoka M, Yamaguchi S, Okuyama M, Tomoike H. Anti-inflammatory cytokine profile in human heart failure: behavior of interleukin-10 in association with tumor necrosis factor-α. Jpn. Circ. J. 1999;63:951–956. doi: 10.1253/jcj.63.951. [DOI] [PubMed] [Google Scholar]
- 58.Lommi J, Pulkki K, Koskinen P, et al. Haemodynamic, neuroendocrine and metabolic correlates of circulating cytokine concentrations in congestive heart failure. Eur. Heart J. 1997;18:1620–1625. doi: 10.1093/oxfordjournals.eurheartj.a015142. [DOI] [PubMed] [Google Scholar]
- 59. Valgimigli M, Ceconi C, Malagutti P, et al. Tumor necrosis factor-α receptor 1 is a major predictor of mortality and new-onset heart failure in patients with acute myocardial infarction: the Cytokine-Activation and Long-Term Prognosis in Myocardial Infarction (C-ALPHA) study. Circulation. 2005;111:863–870. doi: 10.1161/01.CIR.0000155614.35441.69. • Included patients following a myocardial infarction and identified the cytokine levels of TNF, TNFRI, TNFRII and IL-6 as predictors of death or new-onset heart failure.
- 60.Tsutamoto T, Hisanaga T, Wada A, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J. Am. Coll. Cardiol. 1998;31:391–398. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 61.Maeda K, Tsutamoto T, Wada A, et al. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J. Am. Coll. Cardiol. 2000;36:1587–1593. doi: 10.1016/s0735-1097(00)00912-8. [DOI] [PubMed] [Google Scholar]
- 62.Hartford M, Wiklund O, Mattsson Hulten L, et al. C-reactive protein, interleukin-6, secretory phospholipase A2 group IIA and intercellular adhesion molecule-1 in the prediction of late outcome events after acute coronary syndromes. J. Intern. Med. 2007;262:526–536. doi: 10.1111/j.1365-2796.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- 63.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Tumor necrosis factor-α and mortality in heart failure: a community study. Circulation. 2008;118:625–631. doi: 10.1161/CIRCULATIONAHA.107.759191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Niethammer M, Sieber M, von Haehling S, et al. Inflammatory pathways in patients with heart failure and preserved ejection fraction. Int. J. Cardiol. 2008;129:111–117. doi: 10.1016/j.ijcard.2007.05.061. •• Identified many distinct differences in the cytokine profile of systolic and diastolic heart failure patients.
- 65.Naito Y, Tsujino T, Lee-Kawabata M, et al. Matrix metalloproteinase-1 and -2 levels are differently regulated in acute exacerbation of heart failure in patients with and without left ventricular systolic dysfunction. Heart Vessels. 2009;24:181–186. doi: 10.1007/s00380-008-1100-7. [DOI] [PubMed] [Google Scholar]
- 66.Portik-Dobos V, Anstadt MP, Hutchinson J, Bannan M, Ergul A. Evidence for a matrix metalloproteinase induction/activation system in arterial vasculature and decreased synthesis and activity in diabetes. Diabetes. 2002;51:3063–3068. doi: 10.2337/diabetes.51.10.3063. [DOI] [PubMed] [Google Scholar]
- 67.Marin F, Roldan V, Climent V, Garcia A, Marco P, Lip GY. Is thrombogenesis in atrial fibrillation related to matrix metalloproteinase-1 and its inhibitor, TIMP-1? Stroke. 2003;34:1181–1186. doi: 10.1161/01.STR.0000065431.76788.D9. [DOI] [PubMed] [Google Scholar]
- 68.Prince HE. Biomarkers for diagnosing and monitoring autoimmune diseases. Biomarkers. 2005;10 Suppl. 1:S44–S49. doi: 10.1080/13547500500214194. [DOI] [PubMed] [Google Scholar]
- 69.Zucker S, Hymowitz M, Conner C, et al. Measurement of matrix metalloproteinases and tissue inhibitors of metalloproteinases in blood and tissues. Clinical and experimental applications. Ann. NY Acad. Sci. 1999;878:212–227. doi: 10.1111/j.1749-6632.1999.tb07687.x. [DOI] [PubMed] [Google Scholar]
- 70.El-Menyar AA. Cytokines and myocardial dysfunction: state of the art. J. Card. Fail. 2008;14:61–74. doi: 10.1016/j.cardfail.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 71.Hudson MP, Armstrong PW, Ruzyllo W, et al. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J. Am. Coll. Cardiol. 2006;48:15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 72.Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 73.Anker SD, Coats AJ. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. Int. J. Cardiol. 2002;86:123–130. doi: 10.1016/s0167-5273(02)00470-9. [DOI] [PubMed] [Google Scholar]
- 74.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.