Abstract
Acute decompensated heart failure (ADHF) is a common syndrome that precedes over 100,000 hospitalizations in Canada per year (with length of stay in excess of six to eight days), making this the most costly disorder for patients older than 65 years of age. Over 85% of ADHF patients present with shortness of breath and exhibit evidence of volume overload. These findings may be variable in elderly patients, which complicates diagnosis. In fact, even in experienced centres, diagnostic accuracy is less than 80%. Despite advances in the treatment of chronic heart failure, meaningful improvements in outcomes associated with ADHF are very few. The basic assessment and treatments have not changed (early parenteral diuretics, electrocardiographic and oxygen saturation monitoring, supplemental oxygen administration).
The introduction of measurement of natriuretic peptides in those in whom the diagnosis is uncertain may reduced the error rate by over 50%. The use of vasodilator therapy in the absence of cardiogenic shock can lead to earlier amelioration of symptoms, especially in those who do not respond to initial diuretics. Repeated monitoring of vital signs, body weight, electrolytes and creatinine levels is essential to minimize the risk of side effects of treatments. Noninvasive ventilation may reduce the need for endotracheal intubation in patients with severe ADHF and hypoxia at rest. Once the initial phase of heart failure treatment is completed, then the clinician should begin to focus on maximization of chronic heart failure therapy and discharge planning.
Keywords: Acute decompensated heart failure, Treatment options
Abstract
L’insuffisance cardiaque aiguë décompensée (ICAD) est un syndrome courant qui précède plus de 100 000 hospitalisations par année au Canada (d’une durée de plus de six à huit jours), ce qui en fait le trouble le plus coûteux chez les patients de 65 ans et plus. Plus de 85 % des patients atteints d’ICAD consultent en raison d’un essoufflement et présentent des manifestations de surcharge de volume. Ces observations peuvent être variables chez les personnes âgées, ce qui complique le diagnostic. En fait, même dans les centres expérimentés, le traitement a une exactitude inférieure à 80 %. Malgré les progrès dans le traitement de l’insuffisance cardiaque chronique, les améliorations significatives de l’issue de l’ICAD sont très rares. L’évaluation et les traitements de base n’ont pas changé (diurétiques parentéraux rapidement, surveillance par électrocar-diographie et de la saturation d’oxygène, administration d’oxygène d’appoint).
La mesure des peptides natriurétiques chez les patients dont le diagnostic est incertain pourrait réduire le taux d’erreur de plus de 50 %. Le recours à la thérapie vasodilatatrice en l’absence de choc cardiogène peut assurer une amélioration plus rapide des symptômes, notamment chez les personnes qui ne répondent pas aux diurétiques initiaux. La surveillance répétée des signes vitaux, du poids corporel et des taux d’électrolytes et de créatinine est essentielle pour réduire au minimum le risque d’effets indésirables des traitements. La ventilation non effractive peut réduire la nécessité d’intubation trachéale chez les patients atteints d’une grave ICAD et d’hypoxie au repos. Une fois la première phase du traitement de l’insuffisance cardiaque terminée, le clinicien devrait chercher à maximiser le traitement de l’insuffisance cardiaque chronique et la planification du congé.
The goals of treatment of acute decompensated heart failure (ADHF) are to stabilize cardiorespiratory function through improvement of hemodynamics and to improve symptoms of congestion and overall well-being. Because ADHF is characterized by vascular congestion, vasoconstriction, sympathetic overstimulation, often hypertension and occasionally hypoxia, several simple measures should be rapidly and simultaneously instituted.
INITIAL ASSESSMENT AND MANAGEMENT
The fundamentals of the ‘ABCs’ (airway, breathing, circulation) must be followed. These include supplemental oxygen, continuous electrocardiograms and oxygen saturation monitoring in those with shortness of breath at rest, intravenous access, diuretics and vasodilators. The role of parenteral morphine is somewhat controversial because anxiety relief and reduction of sympathetic overload must be balanced against its potential sedative and negative respiratory effects.
Assisted ventilation
Patients who present with systemic oxygen desaturation should undergo intervention before arrival at the hospital. Several studies have supported the role of either continuous positive airway pressure or other forms of noninvasive positive pressure ventilation as a means of providing ventilatory assistance without endotracheal intubation (1,2). The majority of evidence suggests that patients with severe heart failure and oxygen index of less than 250 should be offered this therapy, which can be applied by a paramedic or other health care professional. Importantly, administration of these therapies should not be used instead of vasodilator therapy (see below), but in addition to usual care (3). This is supported by one study (3) that showed noninvasive positive pressure ventilation therapy was associated with worse outcomes when given in preference to vasodilator therapy. None of these slightly different types of assisted ventilation have been shown to be superior to any other and studies of these modalities are ongoing (4–7). These therapies should be considered primarily when the patient is hypoxic with SaO2 of less than 90% (5).
Intravenous loop diuretics
Table 1 summarizes the current treatment options for ADHF. Intravenous loop diuretics have been the mainstay of treatment of congestive heart failure (8). Available therapies include intravenous furosemide, which can be given in doses of 40 mg to 120 mg. Other diuretics include intravenous bumetanide and intravenous torsemide. These two newer diuretics are also more bioavailable than furosemide. Repeat assessment is necessary, including (at minimum) daily electrolytes and renal function assessment. Most physicians believe diuretic therapy is required to promote the approximately 4 L of diuresis associated with a typical hospital admission for ADHF, although this does not occur in reality in many cases (9). More recently, the principles of diuretic therapy have undergone some changes. Recent data show increased mortality, independently associated with increasing doses of diuretic in both acute and chronic heart failure (8,10,11). As a result, there has been interest in limiting the dose of these agents and combining with other modalities, such as vasodilator therapy.
TABLE 1.
Current treatment options for acute decompensated heart failure (ADHF)
| Medication | Route and dose | Indication for use | Comments |
|---|---|---|---|
| Diuretics | |||
| Furosemide | 20 mg to 80 mg oral or IV, according to symptoms | Acute diuresis in ADHF | Should be used in concert with vasoactive therapy. Usually 40 mg for every 1.5 creatinine level to max 160 mg |
| Bumetanide | 0.5 mg to 4.0 mg oral or IV, according to symptoms | Acute diuresis in ADHF | Better absorption than furosemide in edematous states; 1:40 dose conversion with furosemide |
| Torsemide | 10 mg to 40 mg oral or IV | ADHF | |
| Acetazolemide | 0.5 mg oral or IV | Severe alkalosis associated with diuresis | Must closely observe creatinine and electroytes |
| Diuretics – refractory congestion | |||
| Metolazone | 2.5 mg to 10 mg oral | Severe refractory CHF | Potent kaliuretic: closely observe creatinine and electroytes |
| Furosemide | IV infusion 5 mg/h to 20 mg/h | Refractory to bolus diuretic therapy | Prolonged infusion may result in hearing loss and profound electrolyte imbalance |
| Nitroglycerin preparations | |||
| Sublingual 0.4 mg, or buccal isosorbide dinitrate 3 mg every 5 min | Clinical decompensated heart failure, SBP >90 mmHg | For use in severe heart failure, prehospital use, hold for SBP <90 mmHg | |
| IV, 50 mg/250 mL D5W, start at 3–5 mL/h, titrate q 5 min for SBP reduction 20% from baseline | Clinical decompensated heart failure, SBP >90 mmHg | Not formally tested in ADHF, optimal dosage not known. Low doses frequently used, hold for SBP <90 mmHg | |
| Natriuretic peptides | |||
| Nesiritide | Bolus 2 μg/kg, then 0.01 μg/kg/min for 24 h to 48 h | ADHF, SBP >100 mmHg | Hypotension not common but may persist >40 min |
| Narcotics | |||
| Morphine | 3 mg IV bolus | ADHF with distress or restlessness | Avoid overdosing, but usually well tolerated; also causes vasodilation and reduction in heart rate |
| Inotropic drugs – to be used only in ADHF refractory to diuretics and vasodilators | |||
| Dopamine | 1 μg/kg/min to 3 μg/kg/min IV | ‘Renal’ dose | Central venous access, continuous BP monitoring required |
| 3 μg/kg/min to 20 μg/kg/min | To support BP and cardiac output | ||
| Dobutamine | 2 μg/kg/min to 20 μg/kg/min | To support cardiac output | Continuous ECG monitoring needed, increases myocardial oxygen consumption |
| Milrinone | 50 μg/kg bolus over 15 min then 0.25 mg/kg/min to 0.75 mg/kg/min infusion | ADHF refractory to diuretics and vasodilators | Routine administration in ADHF associated with increased side effects |
BP Blood pressure; D5W Dextrose 5% in water; ECG Electrocardiogram; IV Intravenous; SBP Systolic blood pressure
Newer diuretics have also been tested in ADHF. Tolvaptan, a vasopressin antagonist used for the treatment of hyponatremia, has also been shown to enhance diuresis (12). In combination with furosemide and other standard heart failure therapy, tolvaptan has also shown increasing urine output and weight loss in patients with decompensated heart failure, although long-term outcomes were not affected when it was continued after hospital discharge (12,13).
Parenteral treatment for ADHF
Ideally, a successful therapy for acute heart failure should lower blood pressure, attenuate tachycardia, reduce left ventricular filling pressures and alleviate symptoms.
Vasodilator therapy – nitroglycerin
Several studies suggest that the addition of a vasodilator to diuretic therapy would be most beneficial. Sharon et al (3) and Cotter et al (14) have reported on two such studies in which an aggressive vasodilator regimen was superior to diuretics in short-term outcomes. Fully equipped and community-based paramedic units administered supplemental oxygen and intravenous morphine to patients, and then randomly assigned them to receive either high-dose loop diuretic (80 mg furosemide intravenously repeated every 15 min) and low-dose sublingual vasodilator (4 mg isosorbide dinitrate, single dose) or a low-dose diuretic (40 mg intravenous furosemide; single dose) plus repeated 3 mg aliquots of intravenous isosorbide dinitrate. The end points were needed for endotracheal intubation and improvement in oxygen saturations while in the emergency department. These studies showed significantly improved cardiorespiratory end points in the high-dose vasodilator groups and a trend toward reduced myocardial infarction and in-hospital mortality. These results support the notion that high-dose vasodilator therapy should be given early in severely decompensated heart failure with hypoxia, but mandate the need for advanced care teams to be available as first responders. Hypotension at presentation is uncommon, negating worries about treatment-induced hypotension. In the Acute Decompensated Heart Failure National Registry (ADHERE), in over 100,000 patient presentations to hospital with ADHF, less than 5% of the total population were hypotensive (systolic blood pressure less than 100 mmHg) (9,15). This registry also provided data suggesting that early initiation of intravenous vasodilator therapy was associated with improved outcomes (16).
Traditionally, nitroglycerine (GTN), via sublingual, topical or intravenous administration, has been used most commonly. Buccal, oral or intravenous GTN has been shown to reduce filling pressures in patients with ADHF, although no randomized trial has demonstrated superiority of GTN over placebo in reduction of dyspnea in this population and optimal dosages have not been established (17). Tolerance or tachyphylaxis with GTN is reported to occur in 15% to 30% of patients within 24 h (18). As such, the exact role, dosage and duration of therapy of GTN therapy in ADHF are uncertain, although it is frequently used. While no guidelines exist for GTN therapy in ADHF, many reports suggest either repeated sublingual 3 mg to 4 mg doses of isosorbide dinitrate, or if significant patient distress or hypoxia is present, intravenous GTN titrated to 50 μg/min to 160 μg/min, or systolic blood pressure reduction of approximately 20%, maintained for 12 h to 24 h.
Vasodilator therapy – nesiritide
Human B-type natriuretic peptide (BNP) is released by the ventricles of the heart in response to myocyte wall stress and leads to arterial and venous vasodilation and mild natriuresis. Recombinant BNP, a synthetically manufactured medication identical to native human BNP (nesiritide [Natrecor, Janssen-Ortho Inc, Canada]), has been approved in the United States and now recently in Canada for the treatment of ADHF. Studies testing nesiritide versus placebo in ADHF have shown improvement in both symptoms and hemodynamics (19,20). With these effects, renal blood flow and glomerular filtration rate are not reduced.
In the Vasodilation in the Management of Acute Congestive Heart Failure (VMAC) study (21), 489 ADHF patients were randomly assigned in a double-blind, double-dummy design 2:1:1 structure between nesiritide, GTN and placebo. All patients received standard heart failure therapy in addition to the randomized medication, with a subgroup even receiving oral nitrates. Nesiritide was given as a 2.0 μg/kg intravenous bolus followed by a 0.01 μg/kg/min infusion for at least 24 h while the GTN group was dosed as clinically judged by the attending physician. The primary end point – reduction in pulmonary capillary wedge pressure at 3 h – was significantly reduced compared with placebo for nesiritide, but not for GTN. There was also a significant improvement in global symptom rating and dyspnea score in the nesiritide group but not the GTN group. There was no significant mortality difference. In this study, nesiritide was also superior to GTN in reduction of pulmonary capillary occlusive pressure in the subgroup in which pulmonary catheters were placed. Results of this study suggest that nesiritide is an efficacious therapy for ADHF (22). While one criticism of this study was the low dose of GTN, it is important to note this was a well-blinded study conducted in centres of excellence where, presumably, clinicians expert in the use of GTN were actually caring for the study subjects. This latter finding underscores the lack of dosing guidelines for intravenous GTN for ADHF.
It is noteworthy that an analysis of over 250,000 hospital admissions for ADHF reported in the ADHERE registry, only 27% of patients received intravenous vasoactive therapies during their hospitalization, and the delay averaged 23 h postadmission for those who received this medication on inpatient wards. Retrospective propensity score analysis of the ADHERE registry has shown that administration of systemic vasodilator therapy to patients with ADHF was associated with lower mortality compared with no drug or inotropic medications (23). These results persisted after correction for multiple potential confounding factors, although it must be pointed out this was not a randomized trial. These data add strong argument for the concept of early initiation of vasoactive therapy to patients with ADHF.
While vasodilator therapy for ADHF with nesiritide has increased in recent years, a reanalysis of previously published data has raised questions regarding the renal safety and mortality effects of this medication (24,25). While reanalysis of the original data did not show evidence of an independent effect of nesiritide on mortality or renal failure (26), observational studies did not detect any signal of adverse impact of nesiritide on mortality outcomes (27–29). However, prospective, randomized studies to date have not been powered to determine this impact.
As a result of this controversy, an independent scientific panel reviewed the available evidence and concluded that use of nesiritide should continue in ADHF patients without hypotension and further investigations pursued (30). As such, the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF), an ongoing, large, 7000-patient randomized trial of nesiritide versus placebo in ADHF will be completed in 2011 to give a definitive answer to these important questions. Until then, careful selection of ADHF patients with volume overload and without hypotension may provide a population that will derive significant symptomatic benefit from intravenous nesiritide.
Other vasodilators
Other therapies, such as intravenous endothelin A and B receptor antagonists, have shown efficacy in reducing filling, systemic and pulmonary pressures, but not symptom improvement, when compared with placebo (31–35). As such, these medications have not been approved for use in ADHF (36).
Intravenous inotropic therapy
The most commonly used agents have been milrinone, dobutamine and dopamine. All agents have increased inotropic activity in common, while there are varying degrees of vasodilation. While these medications improve short-term symptoms and hemodynamics, patient outcomes may be worsened (37–41). The landmark Outcome of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) randomly assigned 949 patients with ADHF to either 48 h intravenous milrinone (0.50 μg/kg/min) or placebo (42). The primary end point was length of hospital stay, which was no different between the two groups. However, there was an increase in episodes of atrial fibrillation, symptomatic hypotension and study drug discontinuation in the milrinone group. As a result of this large, randomized ADHF trial, inotropic therapy is reserved for patients with systemic hypotension or who do not respond to initial therapy and remain highly symptomatic.
If systemic blood pressure is reduced, dobutamine (2.5 μg/kg/min to 10 μg/kg/min infusion) may be preferred due to its more prominent positive inotropic and lesser vasodilatory effect, while dopamine (5 μg/kg/min to 20 μg/kg/min) should be used in patients with low arterial blood pressure due to its vasoconstrictive effects (37–40,43,44). Other vasodilators, such as calcium sensitizers, were received initially with much promise, although subsequent properly controlled studies did not duplicate the initial small randomized studies (45).
Mechanical fluid removal
Many patients with ADHF are diuretic resistant – typically defined as those patients who do not respond clinically to increasing doses of diuretics and remain volume overloaded. While intravenous vasodilator therapy can be very effective in such patients, mechanical means to remove fluid have been developed that do not require traditional dialysis. This method is called ultrafiltration, which is achieved through infusing venous blood through a powered circuit designed to remove sodium and fluid but not large solutes (46,47). Because the circuit is not powered by the systemic blood pressure of the patient, it can be performed in those who cannot receive vasodilators due to hypotension. Early studies have shown ultrafiltration to be superior to intravenous diuretics and to enhance weight loss in hospital. In the Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure (UNLOAD) study (48), 200 patients with ADHF were randomly assigned to ultrafiltration or intravenous diuretics. The intervention was associated with greater in-hospital weight loss (5.0 kg versus 3.1 kg) but no improvement in dyspnea. There was also a surprising reduction of 90-day rate of unscheduled hospital visits or re-hospitalization (14 versus 29) (49). Larger studies are ongoing, which will help to further clarify efficacy and safety of this promising treatment modality.
CURRENT HF TREATMENT GUIDELINES
Although general guidelines for the treatment of ADHF have been published, specific treatment standards do not currently exist. For example, the concept of combined diuretic and vasodilator therapy for the treatment of severe ADHF, particularly in those with symptoms of ADHF at rest, is accepted (49); however, dosing standards for diuretic or vasodilator therapy of ADHF are variable. The resultant lack of clarity has contributed to a high degree of variability in treatment.
Currently, the standard treatment for ADHF includes intravenous loop diuretics, with the addition of vasodilator therapy for those with severe symptoms. The most frequently recommended vasodilators are nitroglycerin (sublingual, oral, intravenous titration), nitroprusside (intravenous titration) and nesiritide (intravenous bolus followed by infusion).
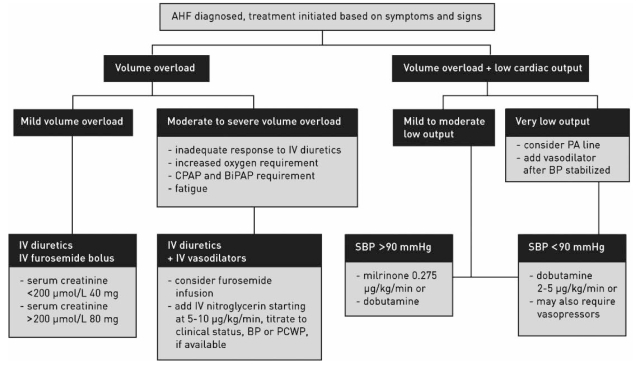
The Heart Failure Society of America has recently delineated a comprehensive set of guidelines for patients with HF (50). They note that most hospitalized patients have considerable volume overload, that congestive symptoms predominate over those of low cardiac output, and that cardiogenic shock presentation occurs in a small minority of patients. The Task Force on Acute Heart Failure of the European Society of Cardiology have issued the only guidelines specifically devoted to the diagnosis and treatment of acute HF (51). The Canadian Cardiovascular Society recently reported on the recommendations from a recent consensus conference on HF (52). These guidelines note the need for personalized care for each patient, based on symptoms, clinical presentation and severity of disease. They are particularly useful because visual algorithms are supplied to assist in initial management. Furthermore, they suggest rapid clinical assessment to categorize patient presentation emphasizing clinical perfusion (‘warm’ or ‘cold’) and volume overload (‘wet’ or ‘dry’). Patients who are ‘warm and wet’ (approximately 70% of acute HF patients), are typically candidates for combined early diuretic and vasodilator therapy. Figure 1 illustrates a proposed algorithm for management of ADHF patients. Table 2 shows a general overview comparison of the Heart Failure Society of America, the European Society of Cardiology and the Canadian Cardiovascular Society guidelines for the management of ADHF.
Figure 1).
Treatment algorithm for acute heart failure (AHF) suggested by the Canadian Cardiovascular Society (53). BiPAP Bilevel positive airway pressure; BP Blood pressure; CPAP Continuous positive airway pressure; IV Intravenous; PA Pulmonary artery; PCWP Pulmonary capillary wedge pressure; SBP Systolic blood pressure. Reprinted with permission of the publisher
TABLE 2.
Comparison of published practice guidelines of the Heart Failure Society of America (HFSA), the European Society of Cardiology (ESC) and the Canadian Cardiovascular Society (CCS) for the management of acute decompensated heart failure (ADHF)
| HFSA, 2006 (50) | ESC, 2005 (51) | CCS, 2007 (52) | |
|---|---|---|---|
| Timing of diagnosis and treatment | Not mentioned | As soon as possible after arrival at ED | Within 2 h of presentation to ED. Response determined within 2 h. Disposition within 8 h |
| Primary diagnostic tools | Clinical history, physical examination, chest x-ray, ECG, routine biochemical studies | Clinical history, physical examination, chest x-ray, ECG, routine biochemical studies | Clinical history, physical examination, chest x-ray, ECG, routine biochemical studies |
| Secondary diagnostic tools | Echocardiography, BNP or NT-proBNP when there is clinical uncertainty about the diagnosis | ECG, chest x-ray, plasma BNP/NT-proBNP and other laboratory tests, and echocardiography | If available, BNP/NT-proBNP if clinical uncertainty about diagnosis. Echochardiogram if available |
| Primary treatment goal | Symptom relief (especially congestion and low output symptoms) | Symptom relief and stabilization of hemodynamic status | Symptom relief and stabilization of hemodynamic status |
| Initial treatment | Loop diuretics (furosemide, bumetanide, torsemide) at adequate dose to achieve optimal volume status. Monitoring of vital signs, urine output, electrolytes, renal function and weight required |
Loop diuretics when symptoms/fluid retention present. Monitoring of vital signs, urine output, electrolytes, renal function and weight required | IV diuretic (furosemide) for volume overload. Monitoring of vital signs, urine output, electrolytes, renal function and weight required |
| Vasodilators* | In patients with acute pulmonary edema or hypertension, IV vasodilators (nitroglycerin, nitroprusside, nesiritide) in combination with diuretics | First line therapy if HF is associated with organ hypoperfusion in absence of hypotension* | If inadequate response to diuretics, administration of combined IV diuretics and vasodilator therapy (IV nitroglycerin infusion started at 5 to 10 μmol/L) is recommended* |
| Role of inotropes | For relief of symptoms, to improve end organ function in patients with evidence of fluid overload not responsive to IV diuretics or vasodilators or poor perfusion | When peripheral hypoperfusion is present, as evidenced by hypotension and decreased renal function | In patients with evidence of low cardiac output and systolic BP <90 mmHg |
| Role of ACE inhibitors | Not mentioned | Not recommended in early stabilization of ADHF. Note a role for ACE inhibitors once stabilized over 12 h to 24 h | Not recommended in early stabilization of ADHF. Note a role for ACE inhibitors once stabilized over 12 h to 24 h |
| Invasive monitoring | invasive hemodynamic monitoring is not recommended unless the patient is refractory to initial therapy, or unclear hemodynamics with clinical deterioration | Arterial line as needed – when patients are not responding in predictable ways to traditional treatments | Arterial line ± pulmonary artery catheterization when there is evidence of very low cardiac output/compromised tissue perfusion |
*At the time of publication of the ESC and CCS guidelines, nesiritide was not yet approved for use in the European Union and Canada, and was not recommended in those documents. Subsequently, nesiritide was approved for use in Canada in late 2007. ACE Angiotensin-converting enzyme; BNP B-type natriuretic peptide; BP Blood pressure; ED Emergency department; ECG Eletrocardiogram; HF Heart failure; IV Intravenous; NT-proBNP N-terminal prohormone BNP
CONCLUSIONS
ADHF is responsible for a large health care burden. In terms of guidelines and standards of care, we are very far behind the more mature chronic heart failure setting, as evidenced by significant heterogeneity between major cardiovascular society recommendations for diagnosis and treatment of ADHF. Recently significant advances been made in the treatment of this complex condition and it is because of this increase in attention that the landscape of potential treatments for ADHF will undoubtedly increase as we observe the results of ongoing clinical trials. Presently, state-of-the-art therapy rests in the rapid diagnosis of ADHF, early and aggressive combination diuretic/vasodilator therapy, and avoidance of potentially deleterious inotropic agents unless clearly required to support blood pressure.
Footnotes
DISCLOSURE: Dr Howlett is Chair, Canadian Cardiovascular Society Heart Failure Guidelines Primary Panel. Dr Howlett reports that he has received consulting fees from Ortho Biotech, who currently market nesiritide in Canada. He is also a paid member of the ASCEND-HF Steering Committee.
REFERENCES
- 1.Murray S. Bi-level positive airway pressure (BiPAP) and acute cardiogenic pulmonary oedema (ACPO) in the emergency department. Aust Crit Care. 2002;15:51–63. doi: 10.1016/s1036-7314(02)80006-6. [DOI] [PubMed] [Google Scholar]
- 2.Rasanen J, Heikkila J, Downs J, Nikki P, Vaisanen I, Viitanen A. Continuous positive airway pressure by face mask in acute cardiogenic pulmonary edema. Am J Cardiol. 1985;55:296–300. doi: 10.1016/0002-9149(85)90364-9. [DOI] [PubMed] [Google Scholar]
- 3.Sharon A, Shpirer I, Kaluski E, et al. High-dose intravenous isosorbide-dinitrate is safer and better than Bi-PAP ventilation combined with conventional treatment for severe pulmonary edema. J Am Coll Cardiol. 2000;36:832–7. doi: 10.1016/s0735-1097(00)00785-3. [DOI] [PubMed] [Google Scholar]
- 4.Rusterholtz T, Bollaert PE, Feissel M, et al. Continuous positive airway pressure vs. proportional assist ventilation for noninvasive ventilation in acute cardiogenic pulmonary edema. Intensive Care Med. 2008;34:840–6. doi: 10.1007/s00134-008-0998-7. [DOI] [PubMed] [Google Scholar]
- 5.Ursella S, Mazzone M, Portale G, Conti G, Antonelli M, Gentiloni Silveri N. The use of non-invasive ventilation in the treatment of acute cardiogenic pulmonary edema. Eur Rev Med Pharmacol Sci. 2007;11:193–205. [PubMed] [Google Scholar]
- 6.Nadar S, Prasad N, Taylor RS, Lip GY. Positive pressure ventilation in the management of acute and chronic cardiac failure: A systematic review and meta-analysis. Int J Cardiol. 2005;99:171–85. doi: 10.1016/j.ijcard.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 7.Kelly CA, Newby DE, McDonagh TA, et al. Randomised controlled trial of continuous positive airway pressure and standard oxygen therapy in acute pulmonary oedema. Effects on plasma brain natriuretic peptide concentrations. Eur Heart J. 2002;23:1379–86. doi: 10.1053/euhj.2001.3156. [DOI] [PubMed] [Google Scholar]
- 8.Moser M. Diuretics in the prevention and treatment of congestive heart failure. Cardiovasc Drugs Ther. 1997;11(Suppl 1):273–7. doi: 10.1023/a:1007791814100. [DOI] [PubMed] [Google Scholar]
- 9.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, ADHERE Scientific Advisory Committee and Investigators Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: A report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database J Am Coll Cardiol 20064776–84.(Erratum in 2006;47:1502). [DOI] [PubMed] [Google Scholar]
- 10.Hasselblad V, Gattis Stough W, Shah MR, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: Results of the ESCAPE trial. Eur J Heart Fail. 2007;9:1064–9. doi: 10.1016/j.ejheart.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 2003;42:705–8. doi: 10.1016/s0735-1097(03)00765-4. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Konstam MA, Burnett JC, Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST Clinical Status Trials. JAMA. 2007;297:1332–43. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 13.Konstam MA, Gheorghiade M, Burnett JC, Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA. 2007;297:1319–31. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 14.Cotter G, Metzkor E, Kaluski E, et al. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet. 1998;351:389–93. doi: 10.1016/S0140-6736(97)08417-1. [DOI] [PubMed] [Google Scholar]
- 15.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Peacock WFt, Fonarow GC, Emerman CL, Mills RM, Wynne J. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE. Cardiology. 2007;107:44–51. doi: 10.1159/000093612. [DOI] [PubMed] [Google Scholar]
- 17.Verma SP, Silke B, Reynolds GW, Richmond A, Taylor SH. Nitrate therapy for left ventricular failure complicating acute myocardial infarction: a haemodynamic comparison of intravenous, buccal, and transdermal delivery systems. J Cardiovasc Pharmacol. 1989;14:756–62. doi: 10.1097/00005344-198911000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Fung HL, Bauer JA. Mechanisms of nitrate tolerance. Cardiovasc Drugs Ther. 1994;8:489–99. doi: 10.1007/BF00877927. [DOI] [PubMed] [Google Scholar]
- 19.Abraham WT, Cheng ML, Smoluk G. Clinical and hemodynamic effects of nesiritide (B-type natriuretic peptide) in patients with decompensated heart failure receiving beta blockers. Congest Heart Fail. 2005;11:59–64. doi: 10.1111/j.1527-5299.2005.03792.x. [DOI] [PubMed] [Google Scholar]
- 20.Colucci WS, Elkayam U, Horton DP, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343:246–53. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 21.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: A randomized controlled trial. JAMA. 2002;287:1531–40. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 22.Elkayam U, Akhter MW, Singh H, Khan S, Usman A. Comparison of effects on left ventricular filling pressure of intravenous nesiritide and high-dose nitroglycerin in patients with decompensated heart failure. Am J Cardiol. 2004;93:237–40. doi: 10.1016/j.amjcard.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 23.Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: An analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) J Am Coll Cardiol. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 24.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: A pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–5. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 25.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–91. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 26.Butler J, Emerman C, Peacock WF, Mathur VS, Young JB. The efficacy and safety of B-type natriuretic peptide (nesiritide) in patients with renal insufficiency and acutely decompensated congestive heart failure. Nephrol Dial Transplant. 2004;19:391–9. doi: 10.1093/ndt/gfg558. [DOI] [PubMed] [Google Scholar]
- 27.Arnold LM, Crouch MA, Carroll NV, Oinonen MJ. Outcomes associated with vasoactive therapy in patients with acute decompensated heart failure. Pharmacotherapy. 2006;26:1078–85. doi: 10.1592/phco.26.8.1078. [DOI] [PubMed] [Google Scholar]
- 28.Emerman CL. Safety and efficacy of nesiritide for the treatment of decompensated heart failure. Rev Cardiovasc Med. 2002;3(Suppl 4):S28–34. [PubMed] [Google Scholar]
- 29.Costanzo MR, Johannes RS, Pine M, et al. The safety of intravenous diuretics alone versus diuretics plus parenteral vasoactive therapies in hospitalized patients with acutely decompensated heart failure: A propensity score and instrumental variable analysis using the Acutely Decompensated Heart Failure National Registry (ADHERE) database. Am Heart J. 2007;154:267–77. doi: 10.1016/j.ahj.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Scios. Panel of Cardiology Experts Provides Recommendations to Scios Regarding NATRECOR 2005. < http://www.sciosinc.com/sciosinc/pr_1118721302.html> and < www.natrecor.com/> (Version current at May 20, 2008)
- 31.Torre-Amione G, Young JB, Colucci WS, et al. Hemodynamic and clinical effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2003;42:140–7. doi: 10.1016/s0735-1097(03)00556-4. [DOI] [PubMed] [Google Scholar]
- 32.Kalra PR, Moon JC, Coats AJ. Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure? Int J Cardiol. 2002;85:195–7. doi: 10.1016/s0167-5273(02)00182-1. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor CM, Gattis WA, Adams KF, Jr, et al. Tezosentan in patients with acute heart failure and acute coronary syndromes: Results of the Randomized Intravenous TeZosentan Study (RITZ-4) J Am Coll Cardiol. 2003;41:1452–7. doi: 10.1016/s0735-1097(03)00194-3. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Villa F, Cuppoletti A, Rossel V, Vallejos I, Roig E. Initial experience with bosentan therapy in patients considered ineligible for heart transplantation because of severe pulmonary hypertension. Clin Transplant. 2006;20:239–44. doi: 10.1111/j.1399-0012.2005.00475.x. [DOI] [PubMed] [Google Scholar]
- 35.Packer M, McMurray J, Massie BM, et al. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: Results of a pilot study. J Card Fail. 2005;11:12–20. doi: 10.1016/j.cardfail.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Spieker LE, Luscher TF. Endothelin receptor antagonists in heart failure – a refutation of a bold conjecture? Eur J Heart Fail. 2003;5:415–7. doi: 10.1016/s1388-9842(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 37.Bollano E, Tang MS, Hjalmarson A, Waagstein F, Andersson B. Different responses to dobutamine in the presence of carvedilol or metoprolol in patients with chronic heart failure. Heart. 2003;89:621–4. doi: 10.1136/heart.89.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burger AJ, Aronson D, Horton DP, Burger MR. Comparison of the effects of dobutamine and nesiritide (B-type natriuretic peptide) on ventricular ectopy in acutely decompensated ischemic versus nonischemic cardiomyopathy. Am J Cardiol. 2003;91:1370–2. doi: 10.1016/s0002-9149(03)00335-7. [DOI] [PubMed] [Google Scholar]
- 39.Silver MA, Horton DP, Ghali JK, Elkayam U. Effect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failure. J Am Coll Cardiol. 2002;39:798–803. doi: 10.1016/s0735-1097(01)01818-6. [DOI] [PubMed] [Google Scholar]
- 40.Follath F, Cleland JG, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): A randomised double-blind trial. Lancet. 2002;360:196–202. doi: 10.1016/s0140-6736(02)09455-2. [DOI] [PubMed] [Google Scholar]
- 41.Lewis DA, Gurram NR, Abraham WT, Akers WS. Effect of nesiritide versus milrinone in the treatment of acute decompensated heart failure. Am J Health Syst Pharm. 2003;60(Suppl 4):S16–20. doi: 10.1093/ajhp/60.suppl_4.S16. [DOI] [PubMed] [Google Scholar]
- 42.Cuffe MS, Califf RM, Adams KF, Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA. 2002;287:1541–7. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 43.Yamani MH, Haji SA, Starling RC, et al. Comparison of dobutamine-based and milrinone-based therapy for advanced decompensated congestive heart failure: Hemodynamic efficacy, clinical outcome, and economic impact. Am Heart J. 2001;142:998–1002. doi: 10.1067/mhj.2001.119610. [DOI] [PubMed] [Google Scholar]
- 44.Ungar A, Fumagalli S, Marini M, et al. Renal, but not systemic, hemodynamic effects of dopamine are influenced by the severity of congestive heart failure. Crit Care Med. 2004;32:1125–9. doi: 10.1097/01.ccm.0000124871.58281.d1. [DOI] [PubMed] [Google Scholar]
- 45.Cleland JG, Freemantle N, Coletta AP, Clark AL. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur J Heart Fail. 2006;8:105–10. doi: 10.1016/j.ejheart.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Sackner-Bernstein JD. Management of diuretic-refractory, volume-overloaded patients with acutely decompensated heart failure. Curr Cardiol Rep. 2005;7:204–10. doi: 10.1007/s11886-005-0078-3. [DOI] [PubMed] [Google Scholar]
- 47.Costanzo MR, Saltzberg M, O’Sullivan J, Sobotka P. Early ultrafiltration in patients with decompensated heart failure and diuretic resistance. J Am Coll Cardiol. 2005;46:2047–51. doi: 10.1016/j.jacc.2005.05.099. [DOI] [PubMed] [Google Scholar]
- 48.Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–83. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 49.Elkayam U, Janmohamed M, Habib M, Hatamizadeh P. Vasodilators in the management of acute heart failure. Crit Care Med. 2008;36(1 Suppl):S95–105. doi: 10.1097/01.CCM.0000297161.41559.93. [DOI] [PubMed] [Google Scholar]
- 50.Heart Failure Society Of America HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12:e1–2. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Nieminen MS, Bohm M, Cowie MR, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- 52.Arnold JM, Howlett JG, Dorian P, et al. Canadian Cardiovascular Society Consensus Conference recommendations on heart failure update 2007: Prevention, management during intercurrent illness or acute decompensation, and use of biomarkers. Can J Cardiol. 2007;23:21–45. doi: 10.1016/s0828-282x(07)70211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnold JM, Liu P, Demers C, et al. Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: Diagnosis and management. Can J Cardiol. 2006;22:23–45. doi: 10.1016/s0828-282x(06)70237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]