Abstract
Over the past decade, a major shift in the clinical risk factors in the population undergoing a cardiac surgery has been observed. In the general population, an increasing prevalence of obesity has largely contributed to the development of cardiovascular disorders. Obesity is a heterogeneous condition in which body fat distribution largely determines metabolic perturbations. Consequently, individuals characterized by increased abdominal fat deposition and the so-called metabolic syndrome (MetS) have a higher risk of developing coronary artery disease. Recent studies have also emphasized that visceral obesity is a strong risk factor for the development of heart valve diseases. In fact, individuals characterized by visceral obesity and its metabolic consequences, such as the small dense low-density lipoprotein phenotype, have a faster progression rate of aortic stenosis, which is related to increased valvular inflammation. Furthermore, the degenerative process of implanted bioprostheses is increased in subjects with the MetS and/or diabetes, suggesting that a process akin to atherosclerosis could be involved in the failure of bioprostheses. In addition to being an important risk factor for the development of cardiovascular disorders, the MetS is increasing the operative mortality risk following coronary artery bypass graft surgery. Thus, recent evidence supports visceral obesity as a global risk factor that is affecting the development of many heart disorders, and that is also impacting negatively on the results of patients undergoing surgical treatment for cardiovascular diseases. In the present paper, recent concepts surrounding the MetS and its implications in various cardiovascular disorders are reviewed along with the clinical implications.
Keywords: Cardiac surgery, Metabolic syndrome, Valvular heart disease, Visceral obesity
Abstract
Depuis dix ans, on observe un changement majeur des facteurs de risque cliniques de la population qui subit une chirurgie cardiaque. Au sein de la population générale, la prévalence croissante d’obésité a largement contribué à l’apparition de troubles cardiovasculaires. L’obésité est un trouble hétérogène selon lequel la distribution de l’adiposité détermine en grande partie les perturbations métaboliques. Par conséquent, les personnes caractérisées par une augmentation des dépôts de gras abdominal et ce qu’on appelle le syndrome métabolique (SM) courent un plus grand risque de coronaropathie. Des études récentes soulignent également que l’obésité viscérale est un important facteur de risque de maladies valvulaires. En fait, les personnes caractérisées par une obésité viscérale et ses conséquences métaboliques, telles que le phénotype à lipoprotéine de basse densité à la fois petit et dense, présentent un taux d’évolution plus rapide de sténose aortique, relié à un accroissement de l’inflammation valvulaire. En outre, le processus dégénératif des bioprothèses implantées augmente chez les sujets atteints du SM ou du diabète, ce qui laisse supposer qu’un processus semblable à l’athérosclérose pourrait contribuer à l’échec des bioprothèses. En plus d’être un facteur de risque important d’apparition des troubles cardiovasculaires, le SM accroît le risque de mortalité opératoire après un pontage aortocoronarien. Ainsi, les données probantes récentes étayent que l’obésité viscérale est un facteur de risque global qui influe sur l’apparition de nombreux troubles cardiaques, ce qui nuit aux résultats des patients qui subissent un traitement chirurgical en raison d’une maladie cardiovasculaire. Dans le présent article, l’auteur analyse les concepts récents entourant le SM et ses conséquences pour divers troubles cardiovasculaires ainsi que les répercussions cliniques de ces concepts.
Modern lifestyle, epitomized by a substantial decrease in energy expenditure and the consumption of food products with high energy density content, has largely contributed to the very high prevalence of obesity in westernized societies (1). Surprisingly, obesity has also reached epidemic proportions in developing countries and is therefore considered by many as a huge societal problem on a worldwide scale. Increasing evidence indicates that regional body fat distribution is of paramount importance in determining metabolic perturbations and, consequently, the cardiovascular risk (2). In this regard, abdominal fat deposition has consistently been found to be a strong correlate of metabolic perturbations, such as insulin resistance, hypertriglyceridemia and low high-density lipoprotein (HDL) level (3). These metabolic abnormalities in the viscerally obese patient are often associated with hypertension, and have been regrouped under one unifying entity often referred to as the metabolic syndrome (MetS) (4). It should be pointed out that a growing body of evidence indicates that a dysfunction of the lipid partitioning system with the ensuing ectopic fat deposition within the abdomen, and organs such as the liver, pancreas and the heart are believed to be actively involved in the development of metabolic and cardiovascular disorders (5).
While these metabolic perturbations associated with visceral obesity have been firmly associated with a substantial risk of developing coronary artery disease (CAD), recent investigations have also established the MetS as an important risk factor for the development of different heart valve diseases as well as an operative risk factor following a coronary artery bypass graft surgery (CABG) (6). Hence, it appears that the MetS, a highly prevalent condition among the population, is a global cardiovascular risk factor with tremendous implications from the development of heart diseases to the treatment of afflicted patients (7). Herein, new developments surrounding the concept of visceral obesity and heart diseases, along with the clinical implications to cardiology and cardiac surgery, are discussed.
AORTIC STENOSIS IN THE LIMELIGHT
Aortic stenosis: An active process
In the past decade, there has been considerable growing interest in the so-called ‘degenerative’ aortic stenosis (AS). This surge of interest is, at least partially, related to the increasing prevalence of this condition in westernized societies, where a progressive, constant aging of the population has occurred (8). Undoubtedly, aging is one crucial factor explaining the increasing prevalence of AS. In fact, severe AS is found in 2% to 4% of the population older than 65 years of age, whereas aortic sclerosis is present in 20% to 30% of the same population (9). For years, calcification of the aortic valve was thought to be a passive process associated with a mechanical ‘wear-and-tear phenomenon’ that, after years of repetitive closure and opening cycles, led to a stiffening of the valve. However, experiments with isolated valve interstitial cells have helped to identify key molecular mechanisms involved in the calcifying process (10). These studies have established that valve interstitial cells, under appropriate conditions, are prone to change their phenotype toward bone-like cells, which produce a calcified matrix. Furthermore, histopathological studies in explanted AS valves have documented the presence of well-differentiated lamellar bone structures as well as osteoblastic markers and growth factors with osteogenic properties (11). Moreover, further examination of explanted AS valves has revealed the presence of oxidized lipids and foam cells (12). Hence, taken together, these observations indicate that calcification of the aortic valve, and thereby the development of AS, is an active process akin to atherosclerosis in which an intricate balance between pro- and anticalcifying mediators are at centre stage in the disease process (13). In this regard, epidemiological studies have also found that traditional cardiovascular risk factors such as age, male sex, hypertension, dyslipidemia and diabetes are associated with the development of AS (14). Thus, rather than being a purely passive degenerative process, AS is now considered to be a complex pathophysiological disorder. This new paradigm about mechanisms participating in the development and progression of AS has thus contributed to open new therapeutic vistas and, incidentally, the hope that a pharmacological approach may be developed to alter the natural course of this disorder (15).
A medical treatment for AS?
Because AS shares risk factors with vascular atherosclerotic disease and to some extent a related histological picture, the next logical step was to expect that similar medical treatments would have benefit on the clinical course of AS. In this regard, retrospective studies have found that statin treatment of subjects with moderate stenosis was associated with a slower disease progression (16,17). However, the first published randomized study (18) evaluating atorvastatin in patients with moderate to severe AS failed to show a difference between placebo and the statin with regard to the calcification of the aortic valve, and the progression rate of the stenosis. On the other hand, a recent open label study (19) has found that rosuvastatin given to patients with moderate AS and hypercholesterolemia was associated with a slower progression rate of valve stenosis compared with patients with AS and without hypercholesterolemia, and not being treated with a statin. Thus, these studies have underlined that statins may be able to slow AS progression in selected individuals with hypercholesterolemia. However, one has to acknowledge that the vast majority of patients with severe AS and referred to surgery for a valve replacement are already receiving lipid-lowering therapy. Therefore, the role and efficacy of statin-based therapy in patients with moderate to severe AS remains contentious for the moment. As another form of medical treatment, angiotensin-converting enzyme (ACE) inhibitors have been examined in the context of AS. Of particular significance, the presence of ACE as well as angiotensin receptor-1 have been documented in explanted AS valves, thus suggesting that ACE inhibitors may have some role in protecting against the evolution of this disorder (20). While one clinical retrospective study has found that ACE inhibitor treatment was associated with a slower progression rate of AS, another study has failed to document a similar association (16,21). Thus, for the time being, the role of ACE inhibitors in AS remains to be clarified.
Visceral obesity and AS
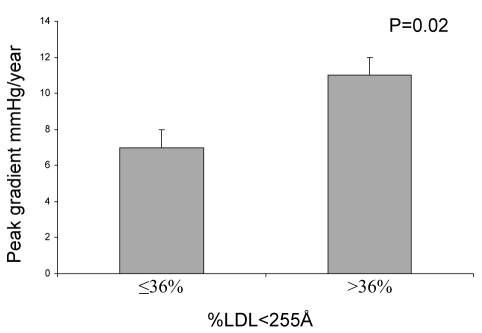
Recently, our group has demonstrated that patients with AS and the MetS had a twofold faster progression rate of their stenosis (22). In this study of 105 patients, the progression rate of AS was neither affected by the traditional risk factors nor the Framingham risk score. Individuals with the MetS had a lower event-free survival, defined as either the need for surgery or death. In this cohort of patients at high risk and therefore treated aggressively with statins, a majority of subjects were within the recommended target for low-density lipoprotein (LDL) cholesterol levels, and nonetheless had a stenosis progression rate which was twice that of patients without the MetS. Then, in a group of 102 patients with severe AS undergoing an aortic valve replacement, we reported (23) that valve content of oxidized-LDL was associated with the relative proportion of circulating small dense LDL and not with the LDL level per se. Moreover, patients having the small dense LDL phenotype had faster progression of stenosis (11±1 mmHg/year versus 7±1 mmHg/year; P=0.02) (Figure 1). Given that statins have only a modest effect on the proportion of small dense LDL, this study may give some new insights as to why statins are of limited efficacy in AS, at least in patients with moderate to severe stenosis. Importantly, a substantial proportion of viscerally obese patients have a LDL level within the normal range but have a high proportion of small dense LDL in the circulation (24). It should be pointed out that small dense LDLs remain in circulation for a longer time, have an increased ability to infiltrate tissues and to become oxidized, thus explaining their detrimental role in the pathophysiology of AS (25,26). From these studies, it is suspected that targeting the size of LDL may be one potential therapeutic strategy to alter the progression of AS. In this regard, other classes of drugs increasing the size of LDL particles, such as fibrates, thiazolidinediones and niacin, could be envisioned to favourably impact the course of AS (27,28). However, further mechanistic and clinical studies are of course necessary before recommending such therapies, but nevertheless are new opportunities for research and development.
Figure 1).
In 102 patients, mean progression of aortic stenosis evaluated with the annualized peak gradient in relation to the proportion of circulating small size low-density lipoprotein (LDL) (percentage of LDL particles less than 255 Å).
DEGENERATIVE PROCESSES IN BIOPROSTHESES
Mechanisms leading to structural valve degeneration
In patients with end-stage valvular disease, valve replacement has been shown to be an efficient procedure that alters the natural course of the disease process and gives a near-normal life expectancy. The development of valve bioprostheses (BPs) has been fostered by the need to have prostheses with a low thromboembolic risk, which would obviate the need for anticoagulation, and thereby would have a clear advantage over mechanical prostheses. Although BPs achieved this goal of low thrombogenicity, studies soon discovered that long-term durability of BPs is impeded by structural valve degeneration (SVD) (29). Over the years, although numerous preimplant treatments of glutaraldehyde-fixed BPs have been developed to reduce ectopic calcification, SVD remains the Achilles’ heel of these biological heart valve substitutes. With conventional stented BPs, the freedom from SVD is 70% to 90% at 10 years and 40% to 70% at 15 years (30). Hence, although BPs offer many advantages, their durability remains a daunting problem precluding their wide-spread use.
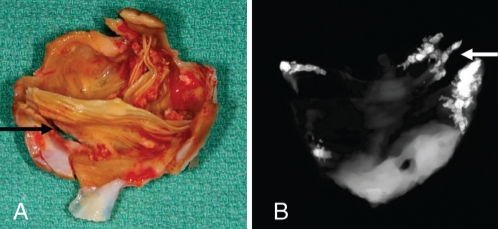
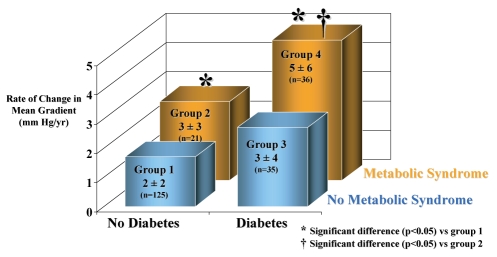
Fixation with glutaraldehyde, a conventional and widely used process to treat BPs by manufacturers, destroys the living cells and induces chemical modification of the collagen matrix (31). This chemical modification of the xenogenic tissues is suspected of being involved in the calcification of BPs, leading to the development of significant transprosthetic gradient and/or regurgitation after years of implantation (29). Regurgitation is related to the development of tears located at the commissures of BPs, which, in turn, is precipitated by calcific degenerative modifications of implanted tissues (Figure 2). Recently, an experimental animal model has emphasized that immune responses against xenoantigens may have a significant contribution to the SVD of implanted BPs (32). While chemical fixation and xenogenic immune response might have a role in SVD of BPs, recent discoveries have shed light on a new and possibly important mechanism relevant to this problem. Histological analysis of explanted BPs has recently demonstrated the presence of oxidized lipids along with inflammatory cells, foam cells and scavenger receptors, thus suggesting that an atherosclerotic-like mechanism might contribute to SVD (6). Furthermore, the inflammatory infiltrate, predominantly composed of macrophage-expressed metalloproteinase-9 in areas of collagen destruction. Moreover, recent studies (33) have noted a clear association between atherosclerotic risk factors and SVD. Using echocardio-graphic criteria of valve dysfunction, we have found in a cohort of 217 patients with a BP and a mean implant time of 3.1±1.8 years that the MetS and diabetes were strong and independent predictors of SVD (34). In this series, patients having both the MetS and diabetes had a 2.5-fold increase in the progression rate of transprosthetic gradient compared with patients without these two factors (Figure 3). It should also be emphasized that 33% of the patients enrolled in this study had the MetS, thus indicating that a large proportion of patients are at high risk of developing short-term BP dysfunction. Taken together, these observations suggest that an active mechanism akin to atherosclerosis, and thus potentially modifiable, is implicated in SVD (35).
Figure 2).
Macroscopic view (A) and x-ray analysis (B) of an explanted bioprosthesis showing that calcifications and tears (arrows) are two interconnected mechanisms actively participating in structural valve degeneration
Figure 3).
In 217 patients with an aortic bioprosthesis, the annualized mean transprosthetic gradient was significantly increased in patients having diabetes and the metabolic syndrome. Reproduced from reference 34 with permission of the American Heart Association
An opportunity to prevent SVD?
Recent studies have contributed to change the paradigm about the mechanisms involved in SVD, and consequently may open new opportunities for preventive measures. In line with this, as opposed to AS, pharmacological treatment for patients with a BP could be started in the immediate postoperative period when no active calcifying process is ongoing, thus potentially increasing the efficacy of such an approach. The rationale behind this is that statins, when given too late in the valvulo-athero-inflammatory process, which is often the case with AS, are of limited value. At this point, it should be emphasized that Antonini-Canterin et al (36) have shown in a cohort of 167 patients a substantial reduction of BP hemodynamic dysfunction (defined as a worsening of valve regurgitation and/or a rate of increase in mean gradient of 3 mmHg/year or greater) in patients using statin therapy. In addition, recent demonstration of an association between the MetS and SVD suggests that lifestyle modification could have a positive effect on SVD (35). Further studies will be necessary to document mechanisms behind these observations and before any clear recommendations can be made with regard to medical treatment to prevent SVD (37). Nevertheless, recent observations have fostered an upsurge of interest from different groups around the world to understand the atheroinflammatory process in BPs and may, in the near future, contribute to design of therapies to prevent SVD.
The MetS and cardiac surgery
Although important progress has been made with percutaneous interventions and the medical treatment of CAD, a substantial number of patients still require CABG. It is estimated that worldwide, approximately 800,000 CABG procedures are performed each year (38). During the past decade, a major shift in the risk factor profile has been documented in the surgical population. For instance, the proportion of obese and diabetic patients undergoing CABG at our institution has increased by 18% over the past decade. In addition, as one would suspect, the proportion of patients undergoing CABG and having the MetS has reached a high level. In one recent study (39) of 5304 patients undergoing CABG at Laval Hospital, we have found that 43% of the subjects were characterized as having the MetS, which conferred a threefold risk for in-hospital mortality. Also important, patients with features of the MetS had an increased risk of developing postoperative complications such as atrial fibrillation and renal failure (40). Considering the high prevalence of the MetS among patients with heart disease, these observations have important clinical impact. Although not yet proven, it is suspected that a low-grade inflammatory state encountered in patients with the MetS may promote a detrimental and amplified inflammatory reaction to cardiopulmonary bypass, which would trigger a different set of complications.
The fatty heart: A biological tug-of-war
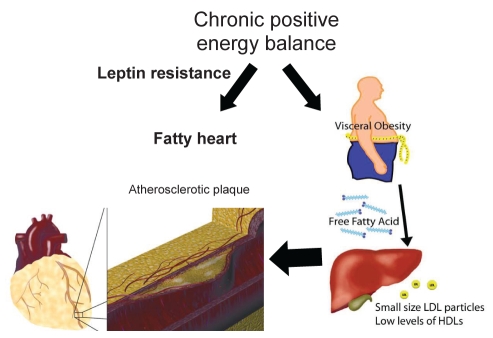
Biological systems have evolved to accomplish specific functions and to prevent detrimental reaction at the cellular and organ levels. In this regard, the adipose tissue, an energy repository, is also an extraordinarily complex endocrine organ that controls energy flux within the body. In normal conditions, when an individual is in positive energy balance, which is a frequent occurrence at present with the modern lifestyle, an exquisite and intricate process ensures a safe and efficient partitioning of lipids to the subcutaneous fat, a metabolic sink (3,41). However, when these regulatory systems of lipid partitioning are overwhelmed, ectopic fat deposition will occur with damaging consequences (42). For instance, visceral ectopic fat deposition will occur in individuals in chronic positive energy balance and an inability to adequately handle lipid partitioning. Importantly, dysregulation of lipid partitioning will not only lead to increased abdominal fat deposition, but it will also promote fat deposition in different organs such the liver, pancreas and heart (43). In fact, recent studies have identified that the amount of epicardial fat is significantly correlated with visceral fat (44). Incidentally, the epicardial fat shares the same embryological origin with the mesenteric and omental adipose tissue (45).
Normally distributed over the course of coronary arteries and the anterior surface of the right ventricle, epicardial fat can cover the entire surface of the heart of obese individuals. In the recent years, the question has arisen as to whether the epicardial fat is of some pathophysiological relevance. Animal models in rodents have conclusively shown that the Zucker diabetic fatty rats, which have a loss-of-function of tissue receptors for leptin, develop dilated cardiomyopathy and lipid accumulation in cardiomyocytes (46). It has been suggested that lipid-induced apoptosis is one potential mechanism explaining this observation. Furthermore, basic studies suggest that leptin resistance may be one crucial mechanism explaining lipid overload of normally protected organs, such as the heart (43). In humans, the use of magnetic resonance imaging and spectroscopic techniques have shown that intracellular accumulation of lipids in cardiomyocytes is inversely related to systolic function (47). During CABG, surgeons have for a long time noticed that intramyocardial arteries are in pristine condition, which is in stark contrast to the epicardial course of the same arteries. It has been suggested that particular hemodynamic conditions of intramyocardial coronary arteries may protect the vessels from lipid accumulation. However, one can not exclude the possibility that epicardial fat, by producing cytokines, may affect vascular wall biology and plaque development (Figure 4). In support of the latter assertion, recent studies indicate that when compared with the subcutaneous fat, epicardial fat produces more proinflammatory cytokines and less adiponectin (48). Although still speculative, epicardial fat may make a significant contribution to the development of heart pathology. Thus, recent discoveries in animal models and humans have fuelled interest in the field of lipid partitioning and its relationship with heart disease. Understanding this biological tug-of-war between protective and promoting factors influencing ectopic fat deposition may prove of crucial importance in the near future to treat heart disease.
Figure 4).
Patients in chronic positive energy balance and deficient lipid partitioning, which may be at least in part dependent on leptin resistance, will develop ectopic fat accumulation. Abdominal fat promotes the formation of small, dense low-density lipoprotein (LDL) particles as well as lower level of high-density lipoproteins (HDLs), which in turn have detrimental roles in the development of atherosclerotic plaque. Accumulation of ectopic epicardial fat could influence atherosclerosis development through the production of cytokines diffusing to the plaque by the vasa vasorum and neo-vascularization process
CONCLUSION
Modern lifestyle has undoubtedly created an unprecedented condition where, for the first time in human history, the worldwide number of overfed, overweight individuals will soon surpass the number of undernourished (49). In the past decades, investigations from various groups have underlined that obesity is a heterogeneous condition, in which body fat distribution is in large measure responsible for metabolic perturbations, and therefore associated with different cardiovascular risk. Visceral obesity, the most common form of insulin resistance, is associated with a considerable risk of developing CAD. Recently, visceral obesity has also been linked to the development of different heart valve diseases, including AS and the SVD of BPs, and moreover it has been associated with an increased risk of developing postoperative complications following CABG surgery. Thus, the MetS appears to be a major cardiovascular risk that goes way beyond the traditional risk factors, affecting the development of different heart disorders and also the surgical treatment of afflicted individuals. Hence, decreasing the number of viscerally obese patients would definitely have a positive impact on the health care system. Meanwhile, more research will possibly help to uncover specific mechanisms implicated in the different manifestations of the MetS in the field of cardiology, and may then translate into treatment targets in the years to come.
Acknowledgments
The work of the author is supported by the Canadian Institute of Health Research (CIHR), Ottawa, Canada, grant number MOP 79342, and MOP 86666 and the Quebec Heart Institute Foundation. Dr Mathieu is a research scholar from the Fonds de Recherches en Santé du Québec, Montreal, Canada. No conflicts of interest.
REFERENCES
- 1.Zimmet P. Diabesity – the biggest epidemic in human history. MedGenMed. 2007;9:39. [Google Scholar]
- 2.Brochu M, Starling RD, Tchernof A, Matthews DE, Garcia-Rubi E, Poehlman ET. Visceral adipose tissue is an independent correlate of glucose disposal in older obese postmenopausal women. J Clin Endocrinol Metab. 2000;85:2378–84. doi: 10.1210/jcem.85.7.6685. [DOI] [PubMed] [Google Scholar]
- 3.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 4.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: A global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 5.Unger RH. Longevity, lipotoxicity and leptin: The adipocyte defense against feasting and famine. Biochimie. 2005;87:57–64. doi: 10.1016/j.biochi.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Mathieu P, Despres JP, Pibarot P. The ‘valvulo-metabolic’ risk in calcific aortic valve disease. Can J Cardiol. 2007;23(Suppl B):32B–9B. doi: 10.1016/s0828-282x(07)71008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathieu P, Pibarot P, Larose E, Poirier P, Marette A, Després JP. Visceral obesity and the heart. Int J Biochem Cell Biol. 2008;40:821–36. doi: 10.1016/j.biocel.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien KD. Epidemiology and genetics of calcific aortic valve disease. J Investig Med. 2007;55:284–91. doi: 10.2310/6650.2007.00010. [DOI] [PubMed] [Google Scholar]
- 9.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: An echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–5. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 10.Mathieu P, Voisine P, Pepin A, Shetty R, Savard N, Dagenais F. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis. 2005;14:353–7. [PubMed] [Google Scholar]
- 11.Charest A, Pepin A, Shetty R, et al. Distribution of SPARC during neovascularisation of degenerative aortic stenosis. Heart. 2006;92:1844–9. doi: 10.1136/hrt.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–53. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 13.Helske S, Kupari M, Lindstedt KA, Kovanen PT. Aortic valve stenosis: An active atheroinflammatory process. Curr Opin Lipidol. 2007;18:483–91. doi: 10.1097/MOL.0b013e3282a66099. [DOI] [PubMed] [Google Scholar]
- 14.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–4. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 15.Moura LM, Zamorano J, Perez-Oteyza C, Rocha-Gonclves F, Rajamannan NM. The role of statins in aortic stenosis. Myth or reality? Rev Port Cardiol. 2007;26:51–62. [PubMed] [Google Scholar]
- 16.Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–5. doi: 10.1161/01.CIR.0000140723.15274.53. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy MF, Pellikka PA, Klarich KW, Tajik AJ, Enriquez-Sarano M. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. 2002;40:1723–30. doi: 10.1016/s0735-1097(02)02496-8. [DOI] [PubMed] [Google Scholar]
- 18.Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–97. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 19.Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–61. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien KD, Shavelle DM, Caulfield MT, et al. Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation. 2002;106:2224–30. doi: 10.1161/01.cir.0000035655.45453.d2. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien KD, Probstfield JL, Caulfield MT, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med. 2005;165:858–62. doi: 10.1001/archinte.165.8.858. [DOI] [PubMed] [Google Scholar]
- 22.Briand M, Lemieux I, Dumesnil JG, et al. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J Am Coll Cardiol. 2006;47:2229–36. doi: 10.1016/j.jacc.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 23.Mohty D, Pibarot P, Despres JP, et al. Association between plasma LDL particle size, valvular accumulation of oxidized LDL, and inflammation in patients with aortic stenosis. Arterioscler Thromb Vasc Biol. 2008;28:187–93. doi: 10.1161/ATVBAHA.107.154989. [DOI] [PubMed] [Google Scholar]
- 24.Lamarche B, Lemieux I, Despres JP. The small, dense LDL phenotype and the risk of coronary heart disease: Epidemiology, pathophysiology and therapeutic aspects. Diabetes Metab. 1999;25:199–211. [PubMed] [Google Scholar]
- 25.Bjornheden T, Babyi A, Bondjers G, Wiklund O. Accumulation of lipoprotein fractions and subfractions in the arterial wall, determined in an in vitro perfusion system. Atherosclerosis. 1996;123:43–56. doi: 10.1016/0021-9150(95)05770-6. [DOI] [PubMed] [Google Scholar]
- 26.Tribble DL, Holl LG, Wood PD, Krauss RM. Variations in oxidative susceptibility among six low density lipoprotein subfractions of differing density and particle size. Atherosclerosis. 1992;93:189–99. doi: 10.1016/0021-9150(92)90255-f. [DOI] [PubMed] [Google Scholar]
- 27.Lemieux I, Laperriere L, Dzavik V, Tremblay G, Bourgeois J, Despres JP. A 16-week fenofibrate treatment increases LDL particle size in type IIA dyslipidemic patients. Atherosclerosis. 2002;162:363–71. doi: 10.1016/s0021-9150(01)00711-0. [DOI] [PubMed] [Google Scholar]
- 28.Yoshino G, Hirano T, Kazumi T. Treatment of small dense LDL. J Atheroscler Thromb. 2002;9:266–75. doi: 10.5551/jat.9.266. [DOI] [PubMed] [Google Scholar]
- 29.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: Progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–80. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 30.Ruel M, Kulik A, Rubens FD, et al. Late incidence and determinants of reoperation in patients with prosthetic heart valves. Eur J Cardiothorac Surg. 2004;25:364–70. doi: 10.1016/j.ejcts.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Tomazic BB, Brown WE, Schoen FJ. Physicochemical properties of calcific deposits isolated from porcine bioprosthetic heart valves removed from patients following 2–13 years function. J Biomed Mater Res. 1994;28:35–47. doi: 10.1002/jbm.820280106. [DOI] [PubMed] [Google Scholar]
- 32.Manji RA, Zhu LF, Nijjar NK, et al. Glutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation. 2006;114:318–27. doi: 10.1161/CIRCULATIONAHA.105.549311. [DOI] [PubMed] [Google Scholar]
- 33.Farivar RS, Cohn LH. Hypercholesterolemia is a risk factor for bioprosthetic valve calcification and explantation. J Thorac Cardiovasc Surg. 2003;126:969–75. doi: 10.1016/s0022-5223(03)00708-6. [DOI] [PubMed] [Google Scholar]
- 34.Briand M, Pibarot P, Despres JP, et al. Metabolic syndrome is associated with faster degeneration of bioprosthetic valves. Circulation. 2006;114(1 Suppl):I512–7. doi: 10.1161/CIRCULATIONAHA.105.000422. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien KD. Do bioprosthetic aortic valves deteriorate more rapidly in patients with the metabolic syndrome? Nat Clin Pract Cardiovasc Med. 2007;4:192–3. doi: 10.1038/ncpcardio0826. [DOI] [PubMed] [Google Scholar]
- 36.Antonini-Canterin F, Zuppiroli A, Popescu BA, et al. Effect of statins on the progression of bioprosthetic aortic valve degeneration. Am J Cardiol. 2003;92:1479–82. doi: 10.1016/j.amjcard.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 37.Antonini-Canterin F, Popescu BA, Zuppiroli A, Nicolosi GL. Are statins effective in preventing bioprosthetic aortic valve failure? A need for a prospective, randomized trial. Ital Heart J. 2004;5:85–8. [PubMed] [Google Scholar]
- 38.Nalysnyk L, Fahrbach K, Reynolds MW, Zhao SZ, Ross S. Adverse events in coronary artery bypass graft (CABG) trials: A systematic review and analysis. Heart. 2003;89:767–72. doi: 10.1136/heart.89.7.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Echahidi N, Pibarot P, Despres JP, et al. Metabolic syndrome increases operative mortality in patients undergoing coronary artery bypass grafting surgery. J Am Coll Cardiol. 2007;50:843–51. doi: 10.1016/j.jacc.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 40.Echahidi N, Mohty D, Pibarot P, et al. Obesity and metabolic syndrome are independent risk factors for atrial fibrillation after coronary artery bypass graft surgery. Circulation. 2007;116(11 Suppl):I213–I219. doi: 10.1161/CIRCULATIONAHA.106.681304. [DOI] [PubMed] [Google Scholar]
- 41.Unger RH. Lipid overload and overflow: Metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab. 2003;14:398–403. doi: 10.1016/j.tem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–36. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 43.Unger RH. Hyperleptinemia: Protecting the heart from lipid overload. Hypertension. 2005;45:1031–4. doi: 10.1161/01.HYP.0000165683.09053.02. [DOI] [PubMed] [Google Scholar]
- 44.Iacobellis G, Sharma AM. Adiposity of the heart. Ann Intern Med. 2006;145:554–5. doi: 10.7326/0003-4819-145-7-200610030-00021. [DOI] [PubMed] [Google Scholar]
- 45.Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: Structure, foetal development and biochemical properties. Comp Biochem Physiol B. 1989;94:225–32. doi: 10.1016/0305-0491(89)90337-4. [DOI] [PubMed] [Google Scholar]
- 46.Wang MY, Unger RH. Role of PP2C in cardiac lipid accumulation in obese rodents and its prevention by troglitazone. Am J Physiol Endocrinol Metab. 2005;288:E216–21. doi: 10.1152/ajpendo.00004.2004. [DOI] [PubMed] [Google Scholar]
- 47.McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–24. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- 48.Baker AR, Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanumihardjo SA, Anderson C, Kaufer-Horwitz M, et al. Poverty, obesity, and malnutrition: An international perspective recognizing the paradox. J Am Diet Assoc. 2007;107:1966–72. doi: 10.1016/j.jada.2007.08.007. [DOI] [PubMed] [Google Scholar]