Abstract
The worldwide increase in the prevalence and incidence of type 2 diabetes represents a tremendous challenge for the Canadian health care system, especially if we consider that this phenomenon may largely be explained by the epidemic of obesity. However, despite the well-recognized increased morbidity and mortality associated with an elevated body weight, there is now more and more evidence highlighting the importance of intra-abdominal adipose tissue (visceral adipose tissue) as the fat depot conveying the greatest risk of metabolic complications. In this regard, body fat distribution, especially visceral adipose tissue accumulation, has been found to be a key correlate of a cluster of diabetogenic, atherogenic, prothrombotic and inflammatory metabolic abnormalities now often referred to as the metabolic syndrome. This dysmetabolic profile is predictive of a substantially increased risk of coronary artery disease (CAD) even in the absence of hyperglycemia, elevated low-density lipoprotein cholesterol or hypertension. For instance, some features of the metabolic syndrome (hyperinsulinemia, elevated apolipoprotein B and small low-density lipoprotein particles – the so-called atherogenic metabolic triad) have been associated with a more than 20-fold increase in the risk of ischemic heart disease in middle-aged men enrolled in the Quebec Cardiovascular Study. This cluster of metabolic complications has also been found to be predictive of a substantially increased risk of CAD beyond the presence of traditional risk factors. These results emphasize the importance of taking into account in daily clinical practice the presence of metabolic complications associated with abdominal obesity together with traditional risk factors to properly evaluate the cardiovascular risk profile of patients. From a risk assessment standpoint, on the basis of additional work conducted by several groups, there is now evidence that the simultaneous presence of an elevated waist circumference and fasting triglyceride levels (a condition that has been described as hypertriglyceridemic waist) may represent a relevant first-step approach to identify a subgroup of individuals at higher risk of being carriers of the features of the metabolic syndrome. Moreover, a moderate weight loss in initially abdominally obese patients is associated with a selective mobilization of visceral adipose tissue, leading to improvements in the metabolic risk profile predictive of a reduced risk of CAD and type 2 diabetes. In conclusion, hypertriglyceridemic waist as a marker of visceral obesity and related metabolic abnormalities is a useful and practical clinical phenotype to screen persons at risk for CAD and type 2 diabetes.
Keywords: Abdominal obesity, Atherogenic dyslipidemia, Coronary artery disease, Insulin resistance, Metabolic syndrome, Triglycerides
Abstract
La hausse mondiale de prévalence et d’incidence de diabète de type 2 constitue un énorme problème pour le système de santé canadien, notamment lorsqu’on considère que ce phénomène s’explique en grande partie par l’épidémie d’obésité. Cependant, malgré l’augmentation bien connue de la morbidité et de la mortalité associée à un poids corporel élevé, de plus en plus de données probantes soulignent l’importance du tissu adipeux intra-abdominal (tissu adipeux viscéral) à titre de dépôt adipeux entraînant le plus grand risque de complications métaboliques. À cet égard, il est établi que la distribution de la masse grasse, notamment l’accumulation de tissus adipeux, est le corrélat clé d’un groupe d’anomalies diabétogènes, athérogènes, prothrombiques et métaboliques inflammatoires, souvent résumées par le terme « syndrome métabolique ». Ce profil dysmétabolique est prédicteur d’un risque considérablement plus élevé de coronaropathie, même en l’absence d’hyperglycémie, d’un taux élevé de cholestérol à lipoprotéines à basse densité ou d’hypertension. Par exemple, certaines caractéristiques du syndrome métabolique (hyperinsulinémie, apolipoprotéine B élevée et petites particules de lipoprotéines à basse densité, la triade métabolique athérogène) s’associent à plus de vingt fois le risque de cardiopathie ischémique chez les hommes d’âge mûr qui ont participé à l’étude cardiovasculaire de Québec. Il est également établi que ce groupe de complications métaboliques est prédicteur d’un risque considérablement accru de coronaropathie, indépendamment de la présence des facteurs de risque classiques. Ces résultats soulignent l’importance, dans la pratique quotidienne, de tenir compte de la présence de complications métaboliques associées à l’obésité abdominale et des facteurs de risque métaboliques pour bien évaluer le profil de risque cardiovasculaire du patient. Du point de vue de l’évaluation du risque, d’après d’autres travaux menés par plusieurs groupes, des données probantes indiquent désormais que la présence simultanée d’un tour de taille et de taux de triglycérides élevés à jeun (une pathologie décrite par le terme « tour de taille hypertriglycéridémique ») peut constituer une première étape pertinente pour dépister un sous-groupe d’individus plus prédisposés à être porteurs des caractéristiques du syndrome métabolique. De plus, une perte de poids modérée chez des personnes présentant une obésité abdominale est reliée à une mobilisation sélective des tissus adipeux viscéraux, ce qui améliore le profil de risque métabolique et présage une diminution du risque de coronaropathie et de diabète de type 2. En conclusion, le tour de taille hypertriglycéridémique comme marqueur d’obésité viscérale et d’anomalies métaboliques connexes constitue un phénotype clinique utile et pratique pour dépister les personnes vulnérables à une coronaropathie et au diabète de type 2.
A DAY IN THE LIFE...
Another typical lunch for a common prototype of the sedentary Canadian man: as for most of his working days, Mr Albert makes a quick stop in a fast-food restaurant for a double cheeseburger, a large portion of french fries and a very large soft drink. While enjoying an unnecessary but highly palatable energy-dense dessert loaded with saturated fat and refined sugar, this busy, 42-year-old, stressed and sedentary overweight accountant feels an unusual chest pain. The gentleman has been told on numerous occasions that such chest pain should be quickly evaluated. Fortunately, the fast-food establishment is located downtown and, therefore, the man goes straight to the emergency unit of the Quebec Heart Institute, which is 10 min away. Within 20 min of the start of his symptoms, a diagnosis of myocardial infarction (MI) is made. The patient’s electrocardiogram shows a non-ST elevation acute MI in the inferior leads accompanied by increased troponin levels. The patient is immediately sent to the catheterization laboratory, and the angiogram reveals that the culprit lesion is located at the mid right artery, which is successfully dilated. A stent is put into place and symptoms are relieved within 1 h of onset. No other significant lesions are noted in the left coronary tree. The patient is then sent to the magnetic resonance imaging (MRI) facilities, which reveal a transmural infarct that nevertheless leaves the patient with very limited ventricular damage. The news is relatively good under the circumstances: the patient’s ventricular function is not significantly impaired. The interventional cardiologist who treated Mr Albert tells him that he was lucky, that he got an early warning sign, and that he went through the easiest and quickest part of his treatment. For reasons described below, lifetime lifestyle management will now be mandatory for this patient.
IMPROVING CORONARY ARTERY DISEASE RISK ASSESSMENT: WE CAN WORK IT OUT...
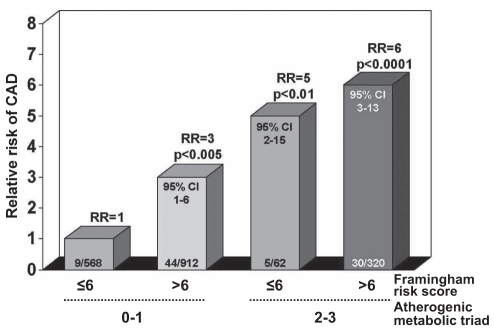
Because the patient described above had never smoked, had a normal resting blood pressure and a normal cholesterol concentration, and was relatively young, his Framingham risk score was low before his MI, therefore predicting a low 10-year risk of coronary artery disease (CAD). Obviously, Framingham did not pick up Mr Albert as being at increased risk for premature CAD. Which factors contributed to the premature development of coronary atherosclerosis in this patient, leading to the clinical event? Being involved in a metabolic study at the Quebec Heart Institute, Mr Albert had previously volunteered to undergo a series of tests and measurements a few weeks before his MI. A 75 g oral glucose tolerance test had been performed, which revealed that he was characterized not only by fasting dysglycemia (6.2 mmol/L) but also by glucose intolerance. A marked hyperinsulinemic state was also found in the fasting state. Apolipoprotein B concentration was measured and found to be markedly elevated, above the 75th percentile for individuals of his age. Plasma C-reactive protein concentration was also increased, with a value of 5 mg/L. Both plasma tumour necrosis factor-alpha and interleukin-6 levels were increased, while the patient was characterized by a low plasma adiponectin concentration. Lipoprotein particle sizes were measured by gradient gel electrophoresis and the patient had both small low-density lipoprotein (LDL) particles and small high-density lipoprotein (HDL) particles. Even in the absence of classical risk factors, Mr Albert was characterized by an insulin-resistant hyperinsulinemic state, by an elevated apolipoprotein B concentration and by small LDL particles. Thus, he presented the features of the atherogenic metabolic triad (hyperinsulinemia, elevated apolipoprotein B and small LDL particles), which were found to be predictive of a 20-fold increased risk of CAD among middle-aged men in the Quebec Cardiovascular Study (1). Would the Framingham risk score have captured such risk? Accordingly, the Framingham risk score was calculated at baseline for all participants of the Quebec Cardiovascular Study. When present, the ‘classical’ risk factors included in the Framingham algorithm predicted the same CAD event rate in the Quebec Cardiovascular Study as in the Framingham study. Thus, when men in the Quebec Cardiovascular Study smoked, had hypertension and had elevated cholesterol levels (even in the absence of the features of the atherogenic metabolic triad), their CAD event rate was increased and essentially comparable with what would have been predicted by Framingham. However, follow-up of men without classical risk factors but with the features of the atherogenic metabolic triad revealed that these men were nevertheless at an increased risk of CAD (Figure 1). Therefore, even in the absence of classical risk factors, men like Mr Albert, who are characterized by insulin resistance (hyperinsulinemia), elevated apolipoprotein B and an increased proportion of small LDL particles are indeed at increased risk of CAD. This is clearly the phenotype that characterized Mr Albert: he had features of the insulin resistance syndrome or the metabolic syndrome. As explained in the next sections, his condition was most probably the consequence of an excess of visceral or intra-abdominal fat.
Figure 1).
Relative risk (RR) of coronary artery disease (CAD) across subgroups of middle-aged men in the Quebec Cardiovascular Study classified on the basis of their Framingham score (≤6 or >6) and with zero to one or two to three features of the atherogenic metabolic triad (hyperinsulinemia, and elevated apolipoprotein B and small low-density lipoprotein particles). Number of cases/controls is indicated within each bar
EVALUATING CAD RISK ON THE BASIS OF ‘TRADITIONAL’ RISK FACTORS
When contacted, Mr Albert’s family doctor confirmed that his patient had no obvious CAD risk factors, apart from a low HDL-cholesterol concentration, whereas his LDL-cholesterol had been considered ‘normal’ (3.0 mmol/L) before taking his statin therapy. The patient did not have diabetes and examination of the overall lipid profile revealed that the patient had elevated triglyceride concentrations (2.8 mmol/L) and an elevated cholesterol/HDL-cholesterol ratio (7.5). Again, the patient was not clinically obese (body mass index [BMI] of 28.5 kg/m2) and had a blood pressure of 120/80 mmHg. However, the primary care physician had noticed that the patient had a positive family history of CAD: the father had an MI at 55 years of age whereas the mother had hypertension, developed angina and had a stroke at the age of 59 years. At the Quebec Heart Institute, a waist circumference measurement was performed which revealed that Mr Albert had a waist circumference of 108 cm. He was therefore clearly abdominally obese. Because his previous lipid profile indicated that he had hypertriglyceridemia, he was therefore diagnosed at the Quebec Heart Institute as having the ‘hypertriglyceridemic waist’ phenotype, which has been shown to be predictive of the likely presence of a cluster of atherogenic and diabetogenic abnormalities often referred to as the metabolic syndrome (2–17).
The objective of the present article is not to review the literature on the risk of cardiovascular disease (CVD) associated with abdominal obesity and the metabolic syndrome, because numerous review papers are already available on the topic (18–22). Rather, we will attempt to convey the notion that with the epidemic proportions reached by obesity and type 2 diabetes, new risk assessment algorithms must be developed to properly evaluate individuals such as Mr Albert who are abdominally obese and who have features of the metabolic syndrome.
Thus, the objectives of this paper are: to provide the evidence that there are individuals on whom risk of CAD is not adequately or optimally assessed by current risk assessment algorithms/engines because these calculators do not incorporate the risk resulting from the presence of visceral obesity and the metabolic syndrome; and to review the literature showing that the simultaneous presence of an elevated waist girth and of hypertriglyceridemia identifies in clinical cardiology a subgroup of patients at high risk of being characterized by the features of the metabolic syndrome.
OBESITY AND CAD RISK: BEYOND BODY WEIGHT
Most physicians recognize that obesity is a significant health problem that has reached an epidemic proportion worldwide (23,24). This situation can be largely attributable to our ‘modern obesogenic environment’ where the daily energy expenditure related to physical activity has considerably decreased, while caloric intake has increased due to the consumption of energy-dense, highly refined food rich in fat and sugar (25). The relationship between body weight and mortality, CAD or diabetes has been investigated in numerous epidemiological studies for decades. When the adiposity of large populations is estimated using a relative index of weight over height such as the BMI (weight in kg divided by height in m2, the most commonly used anthropometric measurement), there is a clear linear or curvilinear relationship between relative weight and total mortality (26) or the presence of comorbidities such as type 2 diabetes and CVD (27,28). Despite this epidemiological evidence, physicians are perplexed by the absence of metabolic abnormalities or of clinical signs of diabetes and/or CVD in some obese patients. Thus, obesity cannot be considered as a homogenous condition. In this regard, 25 years of research have provided evidence that the health hazards of obesity are more closely related to the localization of excess body fat rather than to an elevated body weight per se. Professor Jean Vague, from the University of Marseille, was really the first to propose more than 60 years ago that body fat distribution was a better correlate of the complications of obesity (diabetes, hypertension, CVD) than excess fatness per se (29). Vague defined the male type of fat distribution as ‘android obesity’, mostly characterized by an accumulation of adipose tissue over the trunk, whereas he referred to the common phenotype of women as ‘gynoid obesity’, where adipose tissue accumulates mostly around the hips and thighs, this type of obesity being seldom associated with the common complications of excess fatness (29). However, it took several decades before these remarkable clinical observations became widely studied and accepted by the scientific community and clinicians. For instance, in the mid-1980s, the relationship between obesity, body fat distribution and the risk of developing CVD or type 2 diabetes was examined in men in the Gothenburg Prospective Study followed over a period of more than 13 years (30,31). In this study, within a BMI subgroup, the risk of developing CAD increased with increasing waist-to-hip ratio (WHR; as a crude relative index of the proportion of abdominal fat) (30). Moreover, when the authors also examined the contribution of overall adiposity and of body fat distribution to the 13.5-year incidence of diabetes (31), it was very low among nonobese men characterized by a low WHR (0.5%). However, even among nonobese men, being in the top tertile of WHR (and having an increased relative accumulation of abdominal fat), was associated with a sixfold increased risk of developing diabetes. Finally, the risk of diabetes was increased by 30-fold among men characterized by both overweight/obesity and a high WHR. These results emphasize the critical importance of paying attention to the body fat distribution and to go beyond measuring only BMI to better evaluate the risk associated with overweight and obesity in clinical practice.
These early results are fully consistent with those of Rexrode et al (32), who reported the eight-year incidence of CAD among tertiles of BMI and tertiles of waist circumference (which is a simple anthropometric parameter to evaluate the absolute amount of abdominal obesity) in a cohort of more than 44,000 women free of CAD at baseline (Nurses’ Health Study) (33). They found that within each tertile of BMI, a larger waist circumference was also associated with an increased CAD risk. Moreover, the CAD event rate of obese women not characterized by abdominal obesity was essentially similar to what was recorded among nonobese women with an elevated waist circumference. A longer follow-up of the Nurses’ Health Study (20 years) yielded similar results (34). Finally, in the landmark international cross-sectional, case-control INTERHEART study, involving more than 27,000 individuals stratified on the basis of their BMI values and WHR, subjects in the top quintiles of WHR were characterized by an increased odds ratio of MI irrespective of the BMI category considered (35). These recent results provide robust supportive evidence that the BMI is not an adequate obesity index to identify high-risk overweight and obese patients in clinical practice.
Visceral obesity: The enemy within
Although the waist circumference measurement has been demonstrated to be useful to evaluate the patient beyond the information provided by the BMI because it helps health care professionals identify the subgroup of overweight/obese patients likely to be characterized by a greater accumulation of abdominal fat (the high-risk obesity phenotype), it is important to emphasize that an elevated waist circumference could be the result of an increased amount of subcutaneous fat or of visceral fat. Numerous studies from our group and from other laboratories have clearly shown that it is important to distinguish visceral adipose tissue from abdominal subcutaneous adipose tissue, which is only possible with the use of imaging techniques such as computed tomography or MRI (36–38). Studies that have used these sophisticated techniques have provided evidence that it is the selective accumulation of visceral adipose tissue that is associated with an increased risk of MI, mortality or type 2 diabetes (39–41). In the Health, Aging and Body Composition Study, visceral adipose tissue was an independent predictor of MI in women during the follow-up time of 4.6 years (hazard ratio 1.67; 95% CI 1.28 to 2.17, P<0.001), while there was no association of BMI or total fat mass with MI (39). Furthermore, in a study of men followed over 2.2 years, proper adjustment of the mortality odds ratio associated with visceral fat mass for concomitant variation in subcutaneous fat, waist circumference or for an estimate of liver fat infiltration failed to reduce the odds ratio associated with excess visceral fat accumulation (odds ratio 1.81; 95% CI 1.04 to 3.14, P=0.04) suggesting an independent association between excess visceral adiposity with total and CVD mortality (40). In a prospective study performed in Japanese Americans (41), visceral fat area was also an independent predictor of the development of type 2 diabetes even after adjustment for confounding variables such as BMI or total body fat area.
Recognition of the importance of assessing abdominal obesity in guidelines
Such a critical role played by abdominal obesity accompanied by an excess of visceral fat justifies the joint recommendations of the National Education Program Adult-Treatment Panel III (NCEP-ATP III), of the National Heart, Lung, and Blood Institute/American Heart Association and of the recent guidelines of the International Diabetes Federation because all these organizations have recognized that abdominal obesity is the most prevalent form of a cluster of atherogenic and diabetogenic metabolic abnormalities that has often been referred to as the metabolic syndrome (42–44). Therefore, numerous organizations now recommend the measurement of waist circumference in addition to the BMI to estimate the amount of abdominal fat. Moreover, they emphasize the importance of taking into account the presence of the prevalent form of the metabolic syndrome (abdominal obesity) as a condition increasing the risk of type 2 diabetes and CVD (42–44). It is, however, important to understand that a distinction must be made between features of the metabolic syndrome and clinical tools that have been proposed by various organizations to find individuals likely to have the metabolic syndrome (42,43,45–47). The NCEP-ATP III and International Diabetes Federation guidelines underlined the central role of abdominal obesity in the development of this syndrome, which also includes an atherogenic dyslipidemia, an insulin resistance state, a proinflammatory state, a prothrombotic state and elevated blood pressure (42). It is now very clear that this form of obesity is associated with a cluster of metabolic abnormalities predictive of an increased risk of type 2 diabetes and CVD. As previously discussed, prospective data from the Quebec Cardiovascular Study have shown that the presence of some features of the metabolic syndrome, frequently found among abdominally obese individuals, was predictive of a substantially increased risk of CAD (1) compared with individuals not having a triad of unconventional risk markers of abdominal obesity and the metabolic syndrome (1). Furthermore, the risk associated with the presence of the triad remained highly significant even after having taken into account the presence of traditional risk factors such as LDL-cholesterol, HDL-cholesterol and triglyceride levels (1). Thus, it appears that paying attention to markers of the metabolic syndrome present in abdominally obese patients could refine CAD risk assessment beyond risk factors traditionally used in clinical practice.
THE HYPERTRIGLYCERIDEMIC WAIST PHENOTYPE
Because many markers of insulin resistance and of the metabolic syndrome cannot currently be widely used in clinical practice due to accessibility, cost and standardization problems (except for apolipoprotein B, which is now standardized [48]), we were interested in developing a simple and inexpensive screening tool that could help general practitioners to identify individuals at risk of developing CAD due to the presence of abdominal obesity and features of the metabolic syndrome.
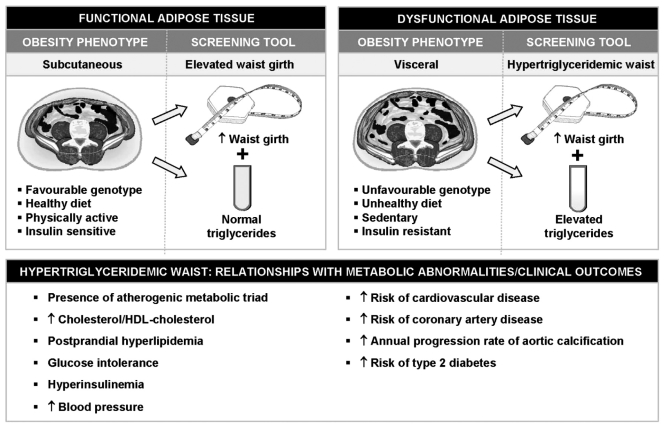
In this regard, we have proposed that the simultaneous presence of an increased waist circumference combined with elevated fasting triglyceride concentrations (hypertriglyceridemic waist) could be used as a first screening phenotype to identify a subgroup of patients likely to be characterized by a cluster of features of the metabolic syndrome such as fasting hyperinsulinemia, elevated apolipoprotein B and in increased proportion of small LDL particles: the atherogenic metabolic triad (Figure 2) (2). The rationale for simultaneously measuring and interpreting waist girth concomitantly with fasting triglyceride concentrations is based on the fact that not all individuals characterized by an elevated waist girth are viscerally obese and at high risk of type 2 diabetes or CVD. We have previously reported that in the presence of an elevated waist circumference, fasting hypertriglyceridemia represented a marker of the patient’s relative inability to manage and store extra energy in the subcutaneous fat depot, which should, under this model, have acted as a protective metabolic sink (49), allowing the rapid clearance and storage of excess dietary triglycerides in the subcutaneous adipose tissue. Thus, abdominal obesity can have two faces: the presence of abdominal obesity observed in isolation (which may often be associated with excess subcutaneous fat); or the presence of abdominal obesity associated with metabolic complications (which may often be found in patients with excess visceral adiposity) (Figure 2). Because the latter condition is associated with an increased risk of type 2 diabetes and CAD, our laboratory has been interested in developing a simple algorithm to find individuals with the metabolic syndrome. We found hyperinsulinemia and elevated apolipoprotein B concentrations to be closely related to visceral adipose tissue accumulation (50). However, the direct and precise measurement of visceral adipose tissue can only be possible with the use of imaging techniques such as computed tomography and MRI, which are expensive and may involve exposure to radiation. However, we have shown that the measurement of waist circumference could serve as a crude surrogate marker of visceral adipose tissue accumulation as well as of insulinemia and apolipoprotein B levels (51). On the other hand, fasting triglyceride levels have been reported to be associated with the presence of small, dense LDL (52). On the basis of these associations between waist girth or triglyceride concentrations and features of the metabolic syndrome, sensitivity and specificity analyses were performed in a sample of Caucasian men aged 28 to 63 years of age, which revealed that cutoffs providing the best discrimination values for presence/absence of features of the atherogenic metabolic triad were 90 cm for waist circumference and 2.0 mmol/L for fasting triglyceride concentrations (2). Of note, these simple clinical criteria were validated and reported before the publication of the NCEP-ATP III guidelines. In our initial study, only 10% of men with a low waist girth (less than 90 cm and triglyceride concentrations less than 2.0 mmol/L were characterized by the atherogenic metabolic triad of abdominal obesity. However, 84% of men with the hypertriglyceridemic waist phenotype (waist girth 90 cm or greater and triglycerides 2.0 mmol/L or greater) were characterized by the atherogenic metabolic triad.
Figure 2).
Use of the hypertriglyceridemic waist as a screening tool to identify individuals likely to be characterized by the cluster of abnormalities of the metabolic syndrome. It is proposed that in the presence of an elevated waist circumference, fasting hypertriglyceridemia could represent a marker of the subject’s relative inability to store energy surplus in subcutaneous adipose tissue, which acts as a ‘metabolic sink’ (functional adipose tissue). Thus, the hypertriglyceridemic waist phenotype could be a simple marker of a ‘dysfunctional adipose tissue’ (visceral obesity) and of its associated metabolic complications resulting from insulin resistance. In other words, ‘hypertriglyceridemic waist’ is a warning red light and its presence should alert physicians that they should pay closer attention to the patient’s risk factor profile
Since the publication of our paper (2) describing for the first time the hypertriglyceridemic waist concept, more than 15 studies worldwide have examined the contribution of this simple screening phenotype in the identification of individuals likely to have features of the metabolic syndrome or reporting associations with type 2 diabetes and CAD (2–17). Although slightly different cutoff values for waist circumference and triglyceride levels have been used across studies, essentially similar conclusions have been reached. This emergent literature on hypertriglyceridemic waist is reviewed in the next sections.
Prevalence of the hypertriglyceridemic waist phenotype
Prevalence data from a representative sample of adults of the province of Quebec aged 18 to 74 years (Quebec Health Survey) have allowed us to quantify the proportion of men characterized by the hypertriglyceridemic waist phenotype in Quebec (3). Results of this study indicated that 19% of adult men of the Quebec Health Survey had an elevated waist circumference (90 cm or greater) and hypertriglyceridemia (2.0 mmol/L or greater) (3). This proportion increased to 29.2% in the subgroup of men aged 40 to 65 years. Despite the use of different cutoffs, the prevalence of this phenotype was similar (19%) in the Tehran Lipid and Glucose Study (6), which involved more than 4000 men. The prevalence of the hypertriglyceridemic waist phenotype was also quantified in a sample of 4448 men and 4735 women from the third National Health and Nutrition Examination Survey (NHANES III) (11). In this study, Kahn and Valdez (11) found that, irrespective of sex, the estimated prevalence of the simultaneous presence of an elevated waist circumference and of increased triglyceride levels was approximately 25%. They also found that the estimated prevalence of this phenotype increased as a function of age and was higher (greater than 40%) in men and women aged 55 to 74 years. Finally, a recent publication from the SUpplémentation en VItamines et Minéraux Anti-oXydants (SU.VI.MAX) study revealed that 12.1% of their sample of middle-aged men were characterized by the hypertriglyceridemic waist phenotype (9). Because the prevalence of obesity, especially abdominal obesity, is rapidly increasing (53), it is expected that the prevalence of hypertriglyceridemic waist will concomitantly rise. Because the prevalence of overweight and obesity in Canada has already surpassed 50% of the population, assessing the hypertriglyceridemic waist phenotype would allow physicians to focus on the subgroup of overweight and obese Canadians (with hypertriglyceridemic waist) at greatest risk for type 2 diabetes and CAD.
Hypertriglyceridemic waist and coronary risk
Additional analyses from our first publication on hypertriglyceridemic waist revealed that only the simultaneous presence of both elevated waist girth and triglyceride concentrations was associated with CAD assessed by angiography (2). In a sample of 287 men who underwent an angiographic procedure for symptoms of CAD, the odds of being diagnosed with significant CAD were increased in men with simultaneous elevations in waist circumference and triglyceride levels compared with men with a waist circumference less than 90 cm and triglyceride concentrations less than 2.0 mmol/L (odds ratio 3.6; 95% CI 1.17 to 10.93, P<0.03). An elevated waist circumference or hypertriglyceridemia in isolation was not associated with a significant increase in CAD. Furthermore, in a small study of heart transplant patients, the odds of CAD were increased fourfold, although it did not reach statistical significance, probably due to the small sample size (n=83) (5). The usefulness of hypertriglyceridemic waist and the presence of the NCEP-ATP III clinical criteria to discriminate the risk of all-cause and cardiovascular mortality as well as to predict the annual progression rate of aortic calcification was examined in a sample of more than 550 women who were followed for a period of 8.5 years (10). The analysis of the survival curves for all-cause or cardiovascular mortality indicated a significant decrease in survival rates among women having an elevated waist girth and high triglyceride concentrations compared with women not meeting this condition. Moreover, relative risks for all-cause mortality (hazard ratio 2.2; 95% CI 1.3 to 3.6, P<0.01) and cardiovascular death (hazard ratio 4.7; 95% CI 2.2 to 9.8, P<0.001) associated with hypertriglyceridemic waist were significant even after adjustment for age, smoking and LDL-cholesterol, and also in a subanalysis where women with diabetes were excluded. The annual progression rate of aortic calcification was significantly greater in women meeting the NCEP-ATP III criteria or the hypertriglyceridemic waist phenotype. However, the highest rates were observed in subgroups with both high waist girth and triglyceride levels irrespective of the presence or absence of NCEP-ATP III, while the presence of NCEP-ATP III alone did not perform as well as the presence of hypertriglyceridemic waist observed in isolation to predict the annual progression rate of aortic calcification. In a recent 7.5-year prospective study of 3430 middle-aged men, the SU.VI.MAX study, using the low waist girth and low triglyceride concentrations as the reference group, also reported that the risk of developing CVD over the follow-up was significantly increased only among men with the hypertriglyceridemic waist phenotype after adjustment for age, active smoking, physical activity, systolic blood pressure, diastolic blood pressure and fasting blood glucose (relative risk 2.13; 95% CI 1.21 to 3.76) (9).
Although it has been reported that hyperglycemia (even in the nondiabetic range) increases CAD (54,55), it is not clear whether there is a direct impact of dysglycemia on CAD risk or whether this is the consequence of the presence of the metabolic syndrome frequently observed among dysglycemic patients. We have recently examined this question and reported that the presence or absence of hypertriglyceridemic waist modulated the CAD risk associated with hyperglycemia in men (7). For instance, in the absence of the hypertriglyceridemic waist phenotype, men characterized by fasting hyperglycemia (in isolation) were not at increased risk of CAD. However, the CAD risk associated with the presence of hypertriglyceridemic waist was significantly increased in both men with normal glucose levels (less than 6.1 mmol/L) or with an impaired fasting glucose concentration (6.1 to 6.9 mmol/L). These results were also confirmed by an investigation conducted in a large sample of men and women from the Hoorn study (56) where the combination of a large waist circumference combined with elevated triglyceride concentrations was associated with CVD among individuals with either normal or abnormal glucose levels (hazard ratio 1.82; 95% CI 1.27 to 2.62 and hazard ratio 2.68; 95% CI 1.89 to 3.81, respectively). Thus, although the relationship between glucose levels and CVD risk is well established, these results suggest that the presence or absence of the hypertriglyceridemic waist phenotype could be of help to the clinician to refine the assessment of CVD risk associated with a dysglycemic or prediabetic state: the presence of abdominal obesity and hypertriglyceridemia should be a warning sign for the physician.
Hypertriglyceridemic waist and diabetes
The hypertriglyceridemic waist phenotype has also been associated with an increased prevalence of type 2 diabetes in adult men and women (3,11). For instance, using data from the NHANES III, it has been reported that the estimated prevalence of diabetes was 25.4% in both men and women with the hypertriglyceridemic waist phenotype and aged 40 to 74 years, while this prevalence was only 8.0% among those without this phenotype (relative risk 3.2; 95% CI 2.4 to 4.0) (11). The odds of diabetes were also markedly increased in men enrolled in the Quebec Health Survey having an elevated waist circumference and an increased triglyceride concentration (3). A 12-fold increase in the prevalent odds ratio of having diabetes (95% CI 5.1 to 27.9, P<0.0001) was observed in men with hypertriglyceridemic waist compared with the reference group of men with both low waist girth and triglyceride levels. It is interesting to note that the prevalent odds ratio was not as high among men characterized by obesity (BMI 30 kg/m2 or greater) as opposed to normal weight individuals (odds ratio 7; 95% CI 3.4 to 13.9, P<0.0001). Furthermore, the metabolic profile of men with hypertriglyceridemic waist was as deteriorated if not more so compared with men with diabetes (3).
Hypertriglyceridemic waist is predictive of metabolic abnormalities
The use of hypertriglyceridemic waist can also be useful in clinical practice to detect individuals likely to have features of the metabolic syndrome such as an elevated cholesterol/HDL-cholesterol ratio, postprandial hyperlipidemia, hyperinsulinemia as well as a dyslipidemic profile typically observed among subjects with abdominal obesity. In this regard, Solati et al (6) have reported that 75% of men of the Tehran Lipid and Glucose Study with hypertriglyceridemic waist had four to six risk factors: increased cholesterol, increased LDL-cholesterol, decreased HDL-cholesterol, elevated systolic blood pressure, elevated diastolic blood pressure and elevated BMI. Other studies have also validated the ability of hypertriglyceridemic waist to identify individuals at high risk of CVD (4,8,11–14). Lamonte et al (4) have published evidence that more than two-thirds of women with elevated waist girth and triglyceride concentrations had the simultaneous presence of hyperinsulinemia, and increased apolipoprotein B and LDL-cholesterol levels. Furthermore, a clear relationship between the cholesterol/HDL-cholesterol ratio, a well-known strong predictor of CAD (57,58), and the presence of hypertriglyceridemic waist has been reported in several study populations (3,7,11,14). The prevalence of subjects with a cholesterol/HDL-cholesterol ratio of at least 6.0 almost reached 50%, whereas the prevalence was only 3% among men with both low waist girth and normal triglyceride concentrations (3). A more deteriorated plasma glucose-insulin homeostasis has also been found among carriers of the hypertriglyceridemic waist phenotype as opposed to individuals not having this phenotype (3,4,11,13,14). Finally, a postprandial study (8) has revealed that men with the hypertriglyceridemic waist phenotype showed the most substantial increase in triglyceride concentrations during the postprandial state compared with control subjects. The latter study has also indicated that the presence of this phenotype was more useful to identify a hyperlipidemic state during the postprandial phase than the presence of an elevated waist circumference or of hypertriglyceridemia measured in isolation.
Hypertriglyceridemic waist: ‘Maxwell’s Silver Hammer’?
Because the metabolic syndrome increases the risk of type 2 diabetes and CVD, numerous organizations have proposed screening approaches to identify patients with the features of the metabolic syndrome. However, different variables and cutoffs have been proposed and, therefore, clinicians are sometimes confused and perplexed. Because of the evidence that waist circumference and triglycerides may be as sufficient as other, more tedious approaches such as the NCEP-ATP III criteria, hypertriglyceridemic waist may represent the ‘Maxwell’s Silver Hammer’, the simplest and most effective tool for the initial screening of the metabolic syndrome in clinical practice. However, additional prospective studies will have to be performed in order to validate cut-off values of fasting triglyceride concentrations and waist circumference in various ethnic populations, in both sexes and across different age groups.
PREVENTION OF ABDOMINAL OBESITY, METABOLIC SYNDROME AND CAD: THE LONG AND WINDING ROAD…
Let us now return to our patient. Mr Albert left the hospital with a beta-blocker, acetylsalicylic acid, an angiotensin converting enzyme inhibitor, a statin and clopidogrel. Do we provide him with the optimal pharmacotherapy for his CAD risk? Studies that have examined the residual risk of statin-treated patients have noticed that the residual risk remains elevated when statin-treated patients with the metabolic syndrome were compared with patients without the metabolic syndrome (59–61). What can we do to further reduce the residual risk? We currently have no randomized trial that has shown that losing abdominal fat could be beneficial to CAD risk beyond the management of classical risk factors. Studies are underway to address this issue. But another aspect of Mr Albert’s profile deserves attention: with his hypertriglyceridemic waist phenotype and with his dysglycemia, this patient is at very high risk of developing type 2 diabetes. Here is a tremendous window of opportunity for the physician, because intervention studies focusing on reshaping nutritional and physical activity habits have clearly shown that such lifestyle modification programs associated with about a 5% weight loss could reduce the risk of converting to diabetes by almost 60% (62,63). The challenge now will be to implement these lifestyle modification programs in clinical practice. It will be a ‘long and winding road’, but we are learning from the science of changing lifestyles that certain conditions must be met to obtain success. It is not with general recommendations such as ‘you should eat better and you should exercise’ that we will convince Canadians to take care of their cardiovascular health. Regular consultations with a team of health care professionals (dieticians, kinesiologists, psychologists with expertise in lifestyle modifications, etc) reimbursed by our health care system should be seriously considered.
LET IT BE…
For Mr Albert, it is the beginning of a long journey. Let us hope that he will have the chance of getting the support of a multidisciplinary team who will help him reshape his nutritional and physical activity habits for the sake of his cardiovascular health and quality of life. This should not be considered as a further financial burden to our health care system but rather an investment which may eventually save billions of dollars of public expenditures while giving back to millions of Canadians better cardiovascular health and quality of life.
Acknowledgments
The work of the authors has been supported by research grants from the Canadian Institutes of Health Research, the Canadian Diabetes Association, the Heart and Stroke Foundation of Canada and by the Foundation of the Quebec Heart Institute. Dr Després is Scientific Director of the International Chair on Cardiometabolic Risk, which is supported by an unrestricted grant from Sanofi Aventis awarded to Université Laval.
REFERENCES
- 1.Lamarche B, Tchernof A, Mauriège P, et al. Fasting insulin and apolipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. JAMA. 1998;279:1955–61. doi: 10.1001/jama.279.24.1955. [DOI] [PubMed] [Google Scholar]
- 2.Lemieux I, Pascot A, Couillard C, et al. Hypertriglyceridemic waist. A marker of the atherogenic metabolic triad (hyperinsulinemia, hyperapolipoprotein B, small, dense LDL) in men? Circulation. 2000;102:179–84. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Lemieux I, Alméras N, Mauriège P, et al. Prevalence of “hypertriglyceridemic waist” in men who participated in the Quebec Health Survey: Association with atherogenic and diabetogenic metabolic risk factors. Can J Cardiol. 2002;18:725–32. [PubMed] [Google Scholar]
- 4.LaMonte MJ, Ainsworth BE, DuBose KD, et al. The hypertriglyceridemic waist phenotype among women. Atherosclerosis. 2003;171:123–30. doi: 10.1016/j.atherosclerosis.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Sénéchal M, Lemieux I, Beucler I, et al. Features of the metabolic syndrome of “hypertriglyceridemic waist” and transplant coronary artery disease. J Heart Lung Transplant. 2005;24:819–26. doi: 10.1016/j.healun.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Solati M, Ghanbarian A, Rahmani M, Sarbazi N, Allahverdian S, Azizi F. Cardiovascular risk factors in males with hypertriglycemic waist (Tehran Lipid and Glucose Study) Int J Obes Relat Metab Disord. 2004;28:706–9. doi: 10.1038/sj.ijo.0802582. [DOI] [PubMed] [Google Scholar]
- 7.St-Pierre J, Lemieux I, Vohl MC, et al. Contribution of abdominal obesity and hypertriglyceridemia to impaired fasting glucose and coronary artery disease. Am J Cardiol. 2002;90:15–8. doi: 10.1016/s0002-9149(02)02378-0. [DOI] [PubMed] [Google Scholar]
- 8.Blackburn P, Lamarche B, Couillard C, et al. Postprandial hyperlipidemia: Another correlate of the “hypertriglyceridemic waist” phenotype in men. Atherosclerosis. 2003;171:327–36. doi: 10.1016/j.atherosclerosis.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Czernichow S, Bruckert E, Bertrais S, et al. Hypertriglyceridemic waist and 7.5-year prospective risk of cardiovascular disease in asymptomatic middle-aged men. Int J Obes (Lond) 2006. [DOI] [PubMed]
- 10.Tanko LB, Bagger YZ, Qin G, et al. Enlarged waist combined with elevated triglycerides is a strong predictor of accelerated atherogenesis and related cardiovascular mortality in postmenopausal women. Circulation. 2005;111:1883–90. doi: 10.1161/01.CIR.0000161801.65408.8D. [DOI] [PubMed] [Google Scholar]
- 11.Kahn HS, Valdez R. Metabolic risks identified by the combination of enlarged waist and elevated triacylglycerol concentration. Am J Clin Nutr. 2003;78:928–34. doi: 10.1093/ajcn/78.5.928. [DOI] [PubMed] [Google Scholar]
- 12.Bell D, McAuley KA, Mann J, Murphy E, Williams S. The hypertriglyceridaemic waist in New Zealand Maori. Asia Pac J Clin Nutr. 2004;13:74–7. [PubMed] [Google Scholar]
- 13.Gazi IF, Filippatos TD, Tsimihodimos V, et al. The hypertriglyceridemic waist phenotype is a predictor of elevated levels of small, dense LDL cholesterol. Lipids. 2006;41:647–54. doi: 10.1007/s11745-006-5015-8. [DOI] [PubMed] [Google Scholar]
- 14.Hiura Y, Acklin F, Newman J, et al. Hypertriglyceridemic waist as a screening tool for CVD risk in indigenous Australian women. Ethn Dis. 2003;13:80–4. [PubMed] [Google Scholar]
- 15.Esmaillzadeh A, Mirmiran P, Azizi F.Clustering of metabolic abnormalities in adolescents with the hypertriglyceridemic waist phenotype Am J Clin Nutr 20068336–46. quiz 183–4. [DOI] [PubMed] [Google Scholar]
- 16.Esmaillzadeh A, Mirmiran P, Azadbakht L, Azizi F. Prevalence of the hypertriglyceridemic waist phenotype in Iranian adolescents. Am J Prev Med. 2006;30:52–8. doi: 10.1016/j.amepre.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 17.Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain intake and the prevalence of hypertriglyceridemic waist phenotype in Tehranian adults. Am J Clin Nutr. 2005;81:55–63. doi: 10.1093/ajcn/81.1.55. [DOI] [PubMed] [Google Scholar]
- 18.Després JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 19.Després JP, Lemieux I, Prud'homme D. Treatment of obesity: Need to focus on high risk abdominally obese patients. BMJ. 2001;322:716–20. doi: 10.1136/bmj.322.7288.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM. Does a diagnosis of metabolic syndrome have value in clinical practice? Am J Clin Nutr. 2006;83:1248–51. doi: 10.1093/ajcn/83.6.1248. [DOI] [PubMed] [Google Scholar]
- 22.Sattar N. The metabolic syndrome: Should current criteria influence clinical practice? Curr Opin Lipidol. 2006;17:404–11. doi: 10.1097/01.mol.0000236366.48593.07. [DOI] [PubMed] [Google Scholar]
- 23.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 24.Katzmarzyk PT. The Canadian obesity epidemic, 1985–1998. CMAJ. 2002;166:1039–40. [PMC free article] [PubMed] [Google Scholar]
- 25.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–4. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 26.Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–85. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 27.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA. 1995;273:461–5. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 28.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–6. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 29.Vague J. Sexual differentiation, a factor affecting the forms of obesity. Presse Méd. 1947;30:339–40. [PubMed] [Google Scholar]
- 30.Larsson B, Svardsudd K, Welin L, et al. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow-up of participants in the study of men born in 1913. Br Med J. 1984;288:1401–4. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohlson LO, Larsson B, Svardsudd K, et al. The influence of body fat distribution on the incidence of diabetes mellitus: 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34:1055–8. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- 32.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–8. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 33.Pouliot MC, Després JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: Best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–8. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 34.Li TY, Rana JS, Manson JE, et al. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113:499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 36.Ross R, Aru J, Freeman J, Hudson R, Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab. 2002;282:E657–63. doi: 10.1152/ajpendo.00469.2001. [DOI] [PubMed] [Google Scholar]
- 37.Ross R, Freeman J, Hudson R, Janssen I. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. J Clin Endocrinol Metab. 2002;87:5044–51. doi: 10.1210/jc.2002-020570. [DOI] [PubMed] [Google Scholar]
- 38.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: Race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–40. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]
- 39.Nicklas BJ, Penninx BW, Cesari M, et al. Association of visceral adipose tissue with incident myocardial infarction in older men and women: The Health, Aging and Body Composition Study. Am J Epidemiol. 2004;160:741–9. doi: 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 40.Kuk JL, Katzmarzyk PT, Nichaman MZ, et al. Visceral fat is an independent predictor of all-cause mortality in men. Obes Res. 2006;14:336–41. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 41.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: A prospective study among Japanese Americans. Diabetes Care. 2000;23:465–71. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 42.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 43.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome – a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 44.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 45.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 46.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–3. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 47.Einhorn D, Reaven GM, Cobin RH, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9:237–52. [PubMed] [Google Scholar]
- 48.Marcovina S, Packard CJ. Measurement and meaning of apolipoprotein AI and apolipoprotein B plasma levels. J Intern Med. 2006;259:437–46. doi: 10.1111/j.1365-2796.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 49.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 50.Lemieux S, Prud'homme D, Tremblay A, Bouchard C, Després JP. Anthropometric correlates of changes in visceral adipose tissue over 7 years in women. Int J Obes Relat Metab Disord. 1996;20:618–24. [PubMed] [Google Scholar]
- 51.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Després JP. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64:685–93. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- 52.McNamara JR, Jenner JL, Li Z, Wilson PW, Schaefer EJ. Change in LDL particle size is associated with change in plasma triglyceride concentration. Arterioscler Thromb. 1992;12:1284–90. doi: 10.1161/01.atv.12.11.1284. [DOI] [PubMed] [Google Scholar]
- 53.Ford ES, Mokdad AH, Giles WH. Trends in waist circumference among U.S. adults. Obes Res. 2003;11:1223–31. doi: 10.1038/oby.2003.168. [DOI] [PubMed] [Google Scholar]
- 54.Gerstein HC, Yusuf S. Dysglycaemia and risk of cardiovascular disease. Lancet. 1996;347:949–50. doi: 10.1016/s0140-6736(96)91420-8. [DOI] [PubMed] [Google Scholar]
- 55.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–40. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 56.Bos G, Dekker JM, Heine RJ, Hoom study Non-HDL cholesterol contributes to the “hypertriglyceridemic waist” as a cardiovascular risk factor: the Hoorn study. Diabetes Care. 2004;27:283–4. doi: 10.2337/diacare.27.1.283. [DOI] [PubMed] [Google Scholar]
- 57.Kinosian B, Glick H, Garland G. Cholesterol and coronary heart disease: Predicting risks by levels and ratios. Ann Intern Med. 1994;121:641–7. doi: 10.7326/0003-4819-121-9-199411010-00002. [DOI] [PubMed] [Google Scholar]
- 58.Lemieux I, Lamarche B, Couillard C, et al. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men. The Quebec Cardiovascular Study. Arch Intern Med. 2001;161:2685–92. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 59.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: A randomised placebo-controlled trial. Lancet. 2003;361:2005–16. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 60.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–9. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 61.Deedwania P, Barter P, Carmena R, et al. Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: Analysis of the Treating to New Targets study. Lancet. 2006;368:919–28. doi: 10.1016/S0140-6736(06)69292-1. [DOI] [PubMed] [Google Scholar]
- 62.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]