Abstract
Aortic valve stenosis (AS) is the third-most frequent heart disease after coronary artery disease and arterial hypertension, and it is associated with a high incidence of adverse outcomes. Recent data support the notion that AS is not an isolated disease uniquely limited to the valve. Indeed, AS is frequently associated with abnormalities of the systemic arterial system, and, in particular, with reduced arterial compliance, which may have important consequences for the pathophysiology and clinical outcome of this disease. Moreover, AS may also be associated with left ventricular systolic dysfunction and reduced transvalvular flow rate, which pose important challenges with regards to diagnostic evaluation and clinical decision making in AS patients. Hence, the assessment of AS severity, as well as its therapeutic management, should be conducted with the use of a comprehensive evaluation that includes not only the aortic valve, but also the systemic arterial system and the left ventricle because these three entities are tightly coupled from both a pathophysiological and a hemodynamic standpoint.
Keywords: Arterial stiffness, Aortic stenosis, Doppler echocardiography, Hemodynamics, Hypertension
Abstract
La sténose aortique (SA) est la troisième cardiopathie en importance après la coronaropathie et l’hypertension artérielle, et elle s’associe à une forte incidence de réactions indésirables. Des données récentes étayent la notion selon laquelle la SA n’est pas une maladie isolée limitée à la valvule. En fait, la SA est souvent reliée à des anomalies du système artériel systémique et, notamment, à une diminution de la compliance artérielle, ce qui peut avoir de graves conséquences pour la physiopathologie et l’issue clinique de cette maladie. De plus, la SA peut s’associer à une dysfonction systolique ventriculaire gauche et à une diminution du débit transvalvulaire, ce qui pose d’importants problèmes en matière d’évaluation diagnostique et de prise de décision clinique chez les patients atteints d’une SA. Ainsi, il faut estimer la gravité de la SA et sa prise en charge thérapeutique dans le cadre d’une évaluation complète qui inclut non seulement la valvule sigmoïde, mais également le système artériel systémique et le ventricule gauche, car ces trois entités sont étroitement reliées, tant en matière de physiopathologie que d’hémodynamie.
Calcific aortic valve disease is a slowly progressive disorder with a disease continuum that ranges from mild valve thickening without obstruction of blood flow, termed aortic sclerosis, to severe calcification with impaired leaflet motion, or aortic stenosis (AS). The prevalence of calcific aortic valve disease increases with age: in the population over 65 years of age, aortic sclerosis is detected in approximately 25% of the subjects, whereas severe AS is found in 2% to 4% of the subjects (1,2). Calcific AS has become the most common cardiac disease in developed countries after systemic arterial hypertension and coronary artery disease (CAD) (3–8).
The natural history of AS typically shows a long latent period of progressive valvular obstruction during which the patient remains asymptomatic. Once the patient becomes symptomatic, the outcome without aortic valve replacement (AVR) is extremely poor, with survival rates as low as 50% at two years and 20% at five years (3,9–11). Calcific AS is now the leading indication for AVR in North America and Europe. This disease is directly responsible for approximately 100,000 AVRs and 15,000 deaths per year in North America. Moreover, the prevalence of AS and the number of AVRs are expected to double by 2020 due to aging of the population.
There is widespread agreement that AVR is indicated for symptomatic severe AS (12). However, the benefit of early elective AVR in asymptomatic patients with severe AS still remains highly controversial, even though some investigators have argued that AVR before symptom onset may prevent irreversible left ventricular (LV) dysfunction and decrease the risk of sudden death (12–14). The clinical decision making for AVR is therefore essentially based on the presence of severe valvular stenosis and symptoms. This approach has, however, several limitations. First, symptom onset is often insidious and may not be recognized by the patient or physician, especially in elderly and/or sedentary patients. Second, there is substantial overlap in hemodynamic severity of AS between symptomatic and asymptomatic patients. Hence, some patients develop clear symptoms with a degree of valvular obstruction that traditionally has not been considered ‘severe’, while others remain asymptomatic with apparently severe obstruction. This underlines the fact that beyond stenosis severity, other factors are involved in the development of LV dysfunction, symptoms and adverse outcomes in AS patients.
PRESSURE RECOVERY: A SOURCE OF ERROR IN THE ASSESSMENT OF AS SEVERITY
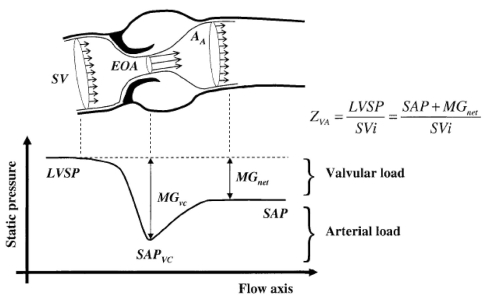
The accurate assessment of the stenosis hemodynamic severity is crucial for clinical decision making in patients with AS (15). The severity of AS is generally determined by measuring the pressure gradient across the valve, or, preferably, because it is less flow-dependent, the effective orifice area (EOA) of the valve (Figure 1). When flow passes through the stenotic valve, there is a contraction and acceleration of the flow, and the area where the flow jet is the smallest is called the vena contracta. The EOA of the valve corresponds to the cross-sectional area of the vena contracta. In the clinical setting, the EOA can be measured either by Doppler echocardiography with the use of the continuity equation, or by left heart catheterization with the Gorlin formula. Unfortunately, there are often discrepancies between Doppler and catheter measurements of EOA. This may result in divergent estimation of AS severity and may bring some uncertainties in clinical decision making. Recent studies have demonstrated that these discrepancies between Doppler and catheter measurements are due, in large part, to the pressure recovery phenomenon (16–18).
Figure 1).
Schematic representation of the flow and static pressure across the left ventricular outflow tract, aortic valve and ascending aorta during systole. AA Aortic cross-sectional area; EOA Effective orifice area (ie, the cross-sectional area of the vena contracta); LVSP Left ventricular systolic pressure; MGnet Transvalvular pressure gradient after pressure recovery (ie, net MG); MGvc Transvalvular pressure gradient at the vena contracta; SAP Systolic aortic pressure; SAPvc Systolic aortic pressure at the vena contracta; SV Stroke volume; SVi Stroke volume index; Zva Vavulo-arterial impedance. Reproduced from (26) with permission
When the blood flow contracts to pass through a stenotic orifice, a portion of the potential energy (ie, blood pressure) is converted into kinetic energy, thus resulting in a pressure drop and acceleration of flow (Figure 1) (19,20). Downstream of the vena contracta, the flow jet re-expands, which causes flow turbulences. As a result of these turbulences, a large part of the kinetic energy is irreversibly lost as heat. Nonetheless, a portion of the kinetic energy is reconverted back to potential energy (pressure). The extent of this pressure recovery essentially depends on the relationship between the size of the valve orifice and the size of the aorta (Figure 1) (19). The smaller the valve orifice relative to the size of the aorta, the more flow turbulence will occur and the less energy will be available to be recovered as pressure.
Doppler measurements rely on the maximum velocity or gradient measured across the aortic valve at the level of the vena contracta. On the other hand, catheterization measurements are generally performed a few centimeters downstream of the valve, where the pressure is fully recovered. As a result, the pressure gradient recorded by catheterization, which corresponds to the ‘recovered’ or net pressure gradient, tends to be lower than the Doppler gradient, especially in patients with smaller aortas (ie, aortic diameter at the sinotubular junction less than 30 mm). Consistently, catheter measurements will also yield larger values for EOA, compared with measurements derived from Doppler. In this context, it should be emphasized that the American College of Cardiology/American Heart Association guidelines were first established based on data obtained from catheter measurements (12). The same cut-point values (eg, EOA less than 1.0 cm2 for severe AS) were then extended to echocardiographic data on the assumption that Doppler EOA and catheter EOA were equivalent parameters and indeed, the guidelines make no distinction between catheter and Doppler measurements of EOA. However, these parameters are not, in fact, equivalent, and differences in results of up to 50% may be observed depending on the size of the aorta and the severity of the stenosis (19,21).
These apparent discrepancies can, however, be reconciled by measuring the ‘energy loss coefficient’ (ELCo), which is a new parameter that adjusts the Doppler EOA for the size of the aorta (AA) to better determine the true energy loss across the stenosis (19):
This ELCo is easily measurable by Doppler echocardiography and, as opposed to the Doppler EOA, it takes into account the pressure recovery. Indeed, there is an excellent agreement between catheter EOA and ELCo by Doppler, whereas the Doppler EOA tends to be consistently lower than the catheter EOA (18). Moreover, from a conceptual standpoint, the net or recovered indices, ie, the catheter EOA or the ELCo by Doppler echocardiography, would appear to better reflect the increased workload imposed by the stenosis on the ventricle and, indeed, in multivariate analysis the ELCo is superior to the Doppler EOA in predicting the occurrence of LV dysfunction and of adverse outcomes in these patients (19).
In summary, pressure recovery is a clinically relevant issue in patients with smaller aortas (diameter less than 30 mm) whereby echocardiography tends to overestimate AS severity in these patients relative to American College of Cardiology/American Heart Association guidelines. Indeed, it would appear that the ELCo provides a more relevant estimate of severity in these patients.
AS AND HYPERTENSION: PARTNERS IN CRIME?
As mentioned, AS and hypertension are the two most frequent cardiovascular diseases after CAD in the western world, and 30% to 40% of patients with AS concomitantly have hypertension (22–25). The interaction between valvular and arterial hemodynamics may affect the evaluation of AS severity and the ensuing clinical conduct (15,26–28).
Hypertension may interfere with the assessment of AS severity
Based on clinical experience and the results of recent studies (15,26–31), it would appear that systemic arterial hypertension may significantly interfere with the hemodynamic assessment of AS severity. Indeed, hypertension may induce significant changes in transvalvular flow rate. As well, an increase in blood pressure will tend to markedly decrease (up to 40%) the peak to peak gradient, which is one of the main measures of AS severity used during cardiac catheterization. Hence, confounding results with regards to AS severity may be observed in the same patient depending on blood pressure level.
The practical implication of these findings is that the presence of concomitant hypertension may result in a misclassification of stenosis severity and that to our knowledge there is no easy method to account for this phenomenon. In this context, the following recommendations would therefore appear reasonable:
Blood pressure should be systematically recorded during the echocardiogram in patients evaluated for AS;
Serial evaluations should take into account if the patient’s blood pressure level is within the same range as the previous evaluation; and
Doppler echocardiographic evaluation should be performed when blood pressure control is optimal. Hence, if the blood pressure is elevated at the time of examination, it should ideally be repeated when blood pressure treatment is considered to be optimal.
Concomitance of AS and hypertension: A double load for the left ventricle
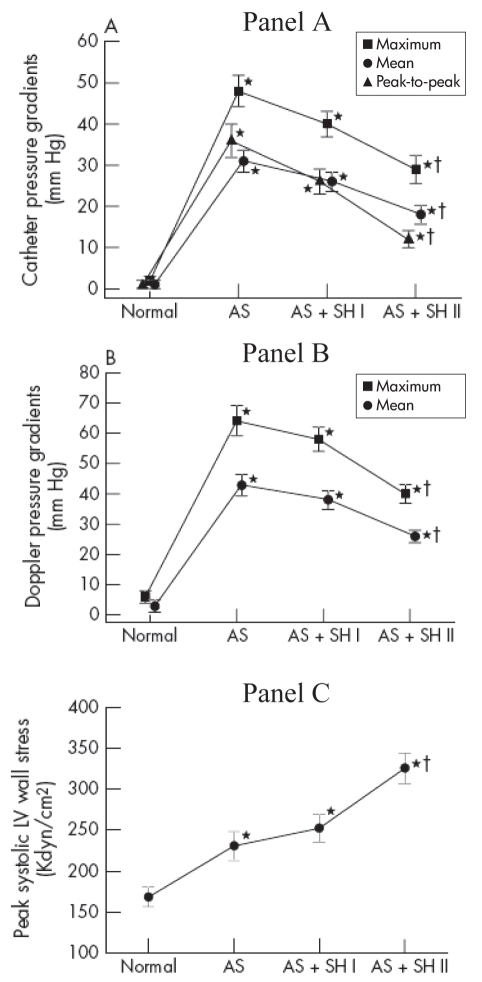
LV pressure overload caused by AS or systemic arterial hypertension generally results in LV concentric hypertrophy, which has been shown to be a strong independent risk factor for morbidity and mortality (32,33). When AS coexists with hypertension, the LV thus faces a double pressure overload and it is possible that both factors add up to adversely affect LV function and patient outcome. The study of Antonini-Canterin et al (24) demonstrated that hypertensive patients with AS develop symptoms at an earlier stage of their disease compared with normotensive ones, suggesting that both factors do indeed add up. As well, we observed in an animal study that despite a significant reduction in transvalvular gradient (related to the concomitant decrease in flow rate), the LV systolic wall stress was markedly increased during hypertension (29) (Figure 2). In this context, a difficult and frequently encountered clinical problem is that of the occurrence of symptoms in hypertensive patients having concomitant AS but whose severity is considered to be only moderate. Hence, it becomes difficult in this situation to delineate the responsibility of each factor in causing symptoms as well as in determining the best treatment and, in particular, if AVR would be beneficial. These patients represent a challenge with regard to management because they fall within the paradigm of a ‘symptomatic AS’ without having the criteria for severity warranting a surgical intervention.
Figure 2).
Changes in catheter (Panel A) and Doppler (Panel B) pressure gradients and in peak systolic LV wall stress (Panel C) in 24 pigs during induction of aortic stenosis (AS) and systemic hypertension (SH) (mild: AS+SH I; severe: AS+SH II). *Significant difference versus normal stage; †Significant difference between severe stenosis + systemic hypertension (AS+SH I or AS+SH II) stages and the severe stenosis (AS) stage. The error bars represent the standard error of the mean. Adapted from (29) with permission
Although significant AS and hypertension may often coexist in the same patient, their combined impact on the occurrence of LV dysfunction, symptoms and adverse outcomes is not well understood. Recently, we performed animal and clinical studies to specifically examine the interaction between AS and hypertension, as well as their impact on LV afterload and function (25,29). Hypertension may be caused by an increase in systemic vascular resistance (ie, systolo-diastolic hypertension), a decrease in systemic arterial compliance (SAC) (ie, systolic hypertension) or both abnormalities. In a retrospective study of 208 patients with AS (25), we found that reduced arterial compliance is frequently observed (41%) and that it independently contributes to the occurrence of LV dysfunction. Moreover, we showed that AS severity may be underestimated in the presence of coexisting hypertension and, inversely, the presence of hypertension may be occulted by coexisting AS (29). This ‘pseudonormalization’ phenomenon probably explains the underestimation of the prevalence of hypertension reported in previous studies. Hence, in our study, 22% of the patients with abnormally low SAC had a systolic arterial pressure below 140 mmHg and would thus have been falsely classified as having normal systemic arterial hemodynamics (25). This pseudonormalization phenomenon is highly insidious because, based on the indices currently used in clinical practice, patients having concomitantly severe AS and severely reduced arterial compliance could be considered as having only moderate AS and no or mild hypertension, when, in fact, they have a markedly increased LV afterload as a result of the double (valvular plus arterial) load and they are thus at higher risk of developing LV dysfunction and symptoms. We thus proposed a new index: valvulo-arterial impedance, calculated by dividing the estimated LV systolic pressure (systolic arterial pressure + mean transvalvular gradient) by the stroke volume index (25) (Figure 1). This index is an estimate of global LV afterload and it represents the cost in mmHg for each systemic millilitre of blood indexed for body surface area pumped by the left ventricle during systole. Providing that blood pressure is measured at the time of the examination, it can be easily calculated from the Doppler echocardiogram and it has been shown to be superior to the standard indices of AS severity in predicting LV dysfunction and patient outcomes. Indeed, a value of the valvulo-arterial impedance greater than 5 mmHg/mL•m2 was independently associated with a fourfold increase in the risk of LV systolic dysfunction in a series of patients with at least moderate AS (25), whereas in a series of patients with severe AS, a value greater than 5.5 mmHg/mL•m2 was associated with a 2.5-fold increase in the risk of overall mortality (34).
These findings are consistent with the concept that calcific AS is not an isolated disease of the valve but rather one manifestation of an atherosclerotic process involving various components of the vascular system including the aorta. Hence, in many patients, the increase in global LV afterload is not only due to the valvular stenotic process but to a decrease in SAC. These findings thus strengthen the need for a more comprehensive evaluation of AS severity going beyond the classical measurements of stenosis severity, and they also emphasize that patients with the combination of AS and systolic hypertension represent both a diagnostic and a therapeutic challenge. Indeed, the presence of symptoms may be logically related to the degree of global afterload and hence, patients with moderate AS may become symptomatic because of the contribution of concomitant hypertension to an increased afterload. In such patients, the logical first step would be to treat their hypertension and then to re-evaluate the situation. Traditionally, vasodilator therapy has been considered contraindicated in patients with severe AS due to the potential hypotensive effect of peripheral vasodilation with fixed valvular obstruction. Recent studies, however, suggest that flow rate can increase in response to a decrease in total afterload, except in patients with very severe disease, suggesting that medical therapy of hypertension may be beneficial in AS patients (15,35). However, caution is needed, especially in patients with severe AS. In these patients, it is preferable to start antihypertensive medications at very low doses and then progressively increase the dosage to a therapeutic level.
Further studies will be necessary to determine if, in symptomatic AS patients with concomitant hypertension, significant improvement in symptomatic status and outcome can be achieved with the intensification of medical treatment alone. Indeed, optimization of blood pressure levels may have its limitations because AS patients often have reduced arterial compliance, which may not be completely normalized by treatment. Likewise, it may well be found that it is worthwhile to operate on some of these patients although their criteria for AS severity do not meet current guidelines for operation. The rationale behind this approach could be that total afterload of these patients is markedly increased and that any significant decrease in either the arterial load or the valvular load may contribute to improve their prognosis and well-being. If the surgical option was contemplated, one would have to ensure that the projected operation would achieve an optimal reduction in the valvular load. To this effect, particular attention should be paid to avoid patient-prosthesis mismatch (36).
LOW-FLOW, LOW-GRADIENT AS: A DIAGNOSTIC AND THERAPEUTIC CHALLENGE
Patients with severe AS and reduced LV ejection fraction represent the most controversial and challenging subset of patients with this disease. This entity is generally characterized by the combination of an aortic valve EOA compatible with severe disease (ie, 1.0 cm2 or less, or 0.6 cm2/m2 or less when indexed for body surface area), a low transvalvular gradient (eg, mean gradient less than 40 mmHg), and a low ejection fraction (40% or less). Indeed, operative mortality for AVR in these patients is high, ranging between 8% and 33% depending on the study (37–46). Moreover, this mode of presentation also represents a diagnostic challenge because at the outset, it is impossible to distinguish between patients having truly severe AS (TS AS) from those having pseudosevere AS (PS AS). In the former, the primary culprit is seen as being the valve disease and the LV dysfunction as being a secondary phenomenon, whereas in the latter, the predominating factor is myocardial disease and AS severity is overestimated due to incomplete opening of the valve in relation with more rigid leaflets due to valve sclerosis and a decrease in the force of opening imposed by the myocardium on the valve. Unfortunately, the resting echocardiogram does not allow one to distinguish between these two conditions. Yet, this distinction is essential because patients with TS AS and poor LV function will generally benefit from AVR, whereas the patients with PS AS may not necessarily benefit.
In patients with PS AS, the depression of myocardial contractility is caused by a coexisting cardiomyopathy, the most frequent being ischemic cardiomyopathy due to severe CAD. The management of these patients should therefore be mainly focused on the treatment of the cardiomyopathy. In patients with TS AS, heart failure may be caused by excessive LV workload associated with severe AS and/or by a coexisting cardiomyopathy. It should be mentioned that severe AS and cardiomyopathy due to obstructive CAD are often present concomitantly (47–51). This underlines the heterogeneity and complexity of this condition and that although the dichotomization of patients into two categories is convenient, the classification of the individual patient may not always be as easy as it may appear (42–46,52–54).
Distinguishing between TS AS and PA AS: Role of dobutamine echocardiography
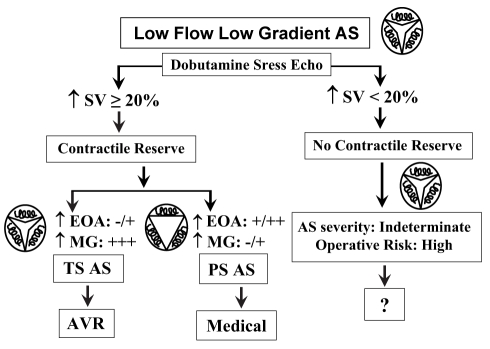
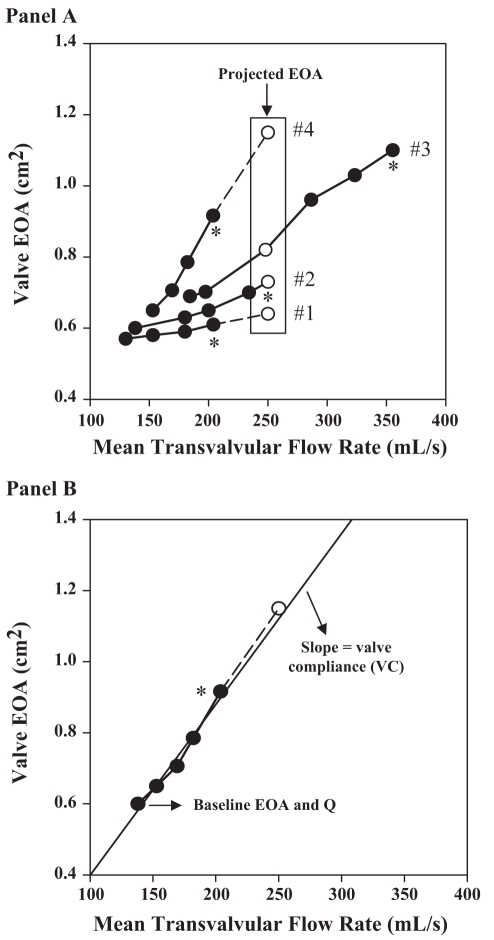
The evaluation of the changes in valve EOA and gradient during a gradual infusion of a low dose of dobutamine may be helpful in differentiating TS AS from PS AS (42–45,52–58). Typically, the valve EOA increases significantly with increasing flow in PS AS because the valve is semiflexible, whereas one expects no or minimal increase in EOA and marked increase in gradient when flow is increased in TS AS because the valve is rigid (Figure 3). Several criteria have been proposed in the literature to differentiate TS AS from PS AS, including: a peak stress mean gradient greater than 30 mmHg, a peak stress EOA 1.0 cm2 or less or less than 1.2 cm2, depending on the study, and an absolute increase in EOA of less than 0.3 cm2 during dobutamine stress echocardiography (DSE) (42,44,52,57,58). However, the changes in gradient and EOA during stress largely depend on the magnitude of flow augmentation achieved during DSE, which may vary considerably from one patient to another (52,53,59,60). This variability of flow response to DSE may be due to multiple factors including the degree of impairment of LV contractile reserve, the chronotropic response to DSE, the use of medication (ie, beta-blocker therapy) and the AS severity itself (53,61,62). The EOA and gradient are therefore measured at flow conditions that differ dramatically from one patient to another, and the utilization of these indices, which are not normalized with respect to flow increase, may be misleading (Figure 4A). To overcome this important limitation, our group proposed a new parameter: the projected valve EOA (EOAproj) at a normal transvalvular flow rate (53). To do this projection, we selected a standardized value of flow rate of 250 mL/s on the basis of data reported in previous studies of patients with AS and normal LV function (59,63). For each patient, EOA is plotted against transvalvular flow (Q) at each dobutamine stage, and valve compliance (VC) is derived as the slope of the regression line fitted to the EOA versus Q plot (Figure 4B); EOAproj is calculated as:
where EOArest and Qrest are the EOA and Q at rest (Figure 4). The diagnostic accuracy of this new index was tested in the context of the Truly or Pseudo Severe Aortic Stenosis (TOPAS) multicentre prospective study of low-flow AS (53). When compared with surgical findings, the percentage of correct classification was 83% when using a value of EOAproj 1.0 cm2 or less to separate TS AS from PS AS, and 91% when using a value of indexed EOAproj 0.55 cm2/m2 or less. The performance of the EOAproj was superior to that (percentages of correct classification: 61% to 74%) of the other echocardiographic indices usually utilized for this purpose. These results show that EOAproj can correct for important interindividual variability in the flow response to DSE and thus allow an assessment of AS severity under similar flow conditions (53). Thus, this new index has the potential to improve the diagnostic accuracy of DSE to distinguish TS AS from PS AS in patients with low-flow, low-gradient AS.
Figure 3).
Usefulness of dobutamine stress echocardiography for the evaluation of low-flow aortic stenosis (AS). AVR Aortic valve replacement; EOA Valve effective orifice area; MG Mean transvalvular gradient; PS AS Pseudosevere AS; SV Stroke volume; TS AS True severe AS
Figure 4).
Concept of the projected effective orifice area (EOAproj) in four different patients (Panel A) and calculation of the EOAproj at a flow rate of 250 mL/s in an individual patient (#4) (Panel B). Open circles represent the projected EOA. *Peak valve EOA obtained during dobutamine stress echocardiography. Reproduced from (54) with permission
Contractile reserve: An important predictor of operative risk
The assessment of LV contractile reserve is an essential aspect of risk stratification in low-flow AS because patients with no evidence of contractile reserve have a high risk of operative mortality following AVR independently of the degree of valve stenosis. In a multicentre French study (45), the presence of contractile reserve on DSE, defined by a relative increase in stroke volume of 20% or greater, was associated with a low operative risk (operative mortality: 6%), whereas operative mortality was very high (33%) in the absence of contractile reserve (Figure 3). Nonetheless, in the subset of patients with no contractile reserve who survived the operation, the postoperative improvement in the LV ejection fraction (17±11% versus 19±10%), as well as the two-year survival rate (90±5% versus 92±7%) was as good as in the subset of patients with contractile reserve (45). These findings suggest that the assessment of contractile reserve by DSE is useful to estimate the risk for operative mortality. Nonetheless, the absence of LV contractile reserve should not preclude consideration of AVR in symptomatic subjects with low-flow AS.
Usefulness of natriuretic peptides
Beyond echocardiographic parameters, other indices may be useful for the assessment of LV functional impairment and for risk stratification in low-flow AS. In the multicentre prospective study of low-flow AS (TOPAS study), the cumulative one-year survival of patients with B-type natriuretic peptide (BNP) 550 pg/mL or greater was only 47±9%, compared with 97±3% with BNP less than 550 pg/mL (P<0.0001) (46). In the subset of patients undergoing AVR, postoperative one-year survival was also markedly lower in patients with BNP 550 pg/mL or greater (53±13% versus 92±7%, P=0.02) and operative mortality was twice as high (19% versus 8%). This simple blood biomarker could therefore be highly useful to improve risk stratification in patients with low-flow AS. However, more data are required before BNP levels can be used for therapeutic recommendations.
Management of patients with PS AS
Current wisdom suggests that patients with PS AS are unlikely to benefit from valve replacement (12,54) and should therefore be treated medically. However, there are no prospective data showing that medical treatment is better than surgical treatment in this situation. In fact, recent studies show that the two-year mortality rate in patients with PS AS treated medically is very high (50% to 63%) and much worse than that initially reported by deFillipi et al (52). Hence, it cannot be excluded that AVR would not be beneficial in patients with a theoretically ‘moderate’ AS but severe LV dysfunction. Indeed, it is well known that a failing ventricle is much more sensitive to a moderate increase in afterload than a normal ventricle (64,65). Although there are no direct data regarding this issue in the context of low-flow AS, an analogy can, however, be made with recent studies showing that moderate patient-prosthesis mismatch, which is a postoperative equivalent to moderate native AS in terms of LV afterload, has no significant impact on mortality in patients with preserved LV systolic function, but a major impact on mortality in patients with poor ventricular function (66–68). Hence, it cannot be excluded that relief of valvular obstruction, even if only moderate by conventional criteria, may have beneficial effects on morbidity and mortality in patients with PS AS.
New perspectives in the treatment of low-flow AS
The improvement in the clinical outcome of patients with low-flow AS will, in large part, come from a better assessment of stenosis severity and a better risk stratification as described above. Furthermore, the optimization of the operative strategies should contribute to reduce the operative mortality in the subset of patients treated surgically. To this effect, several studies recently demonstrated a strong interaction between prosthesis-patient mismatch and depressed LV function with regard to occurrence of heart failure as well as to early and late mortality after AVR (66–69). As outlined above, these findings are consistent with the fact that an increased hemodynamic burden is less well tolerated by a poorly functioning ventricle than by a normal ventricle. In light of these recent data (66–68), every effort should be made to avoid prosthesis-patient mismatch in the patients with low-flow AS. The clinical implications of these findings are important given that prosthesis-patient mismatch is frequent (20% to 30%) after AVR and, as opposed to other risk factors, it can largely be avoided or its severity can be reduced with the use of a prospective strategy at the time of operation (36,70–74).
PARADOXICAL LOW-FLOW AS DESPITE NORMAL EJECTION FRACTION: A NEW DISEASE PATTERN
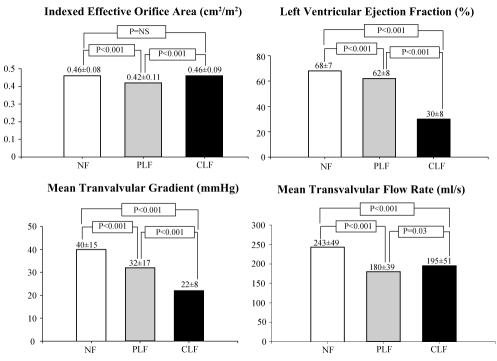
The classical form of low-flow, low-gradient AS is characterized by the combination of low ejection fraction (40% or less), a low cardiac output and transvalvular flow rate, and a low transvalvular gradient despite the presence of a severely reduced valve EOA. We recently reported that an important proportion of the patients with severe AS on the basis of the aortic valve EOA paradoxically have a low transvalvular flow rate and a low gradient despite the presence of a preserved LV ejection fraction (50% or greater) (34). We retrospectively studied the clinical and Doppler-echocardiographic data of 512 consecutive patients with severe AS (indexed EOA 0.6 cm2/m2 or less) and preserved LV ejection fraction (50% or greater). Of these patients, 331 (65%) had normal LV flow output defined as a stroke volume index greater than 35 mL/m2 and 181 (35%) had paradoxically low-flow output (PLF group) defined as stroke volume index 35 mL/m2 or less. When compared with normal flow patients, PLF patients were more likely to be female (P<0.05), had a lower transvalvular gradient (32±17 mmHg versus 40±15 mmHg; P<0.001), a more pronounced LV concentric remodelling, a lower LV diastolic volume index (52±12 mL/m2 versus 59±13 mL/m2; P<0.001), a lower LV ejection fraction (62±8% versus 68±7%; P<0.001), a higher level of LV global afterload reflected by a higher valvulo-arterial impedance (5.3±1.3 mmHg•mL−1•m−2 versus 4.1±0.7 mmHg•mL−1•m−2; P<0.001), and a lower overall three-year survival (76% versus 86%, P=0.006) (Figure 5). Interestingly, the PLF patients had similar transvalvular flow rate (180±039 mL/s versus 195±051 mL/s) compared with the patients included in the TOPAS study (53), ie, the patients with the classical form of low-flow AS with reduced LV EF (CLF patients) (Figure 5). The valve EOA was significantly lower (0.42±0.11 cm2/m2 versus 0.46±0.08 cm2/m2) and the transvalvular gradient was higher (32±17 mmHg versus 22±8 mmHg) in the PLF group than in the CLF group, but there was a considerable overlap between the groups. In fact, these two subsets of patients (PLF and CLF) have very similar baseline characteristics except for LV ejection fraction that is higher in the PLF group (62±8%) than in the CLF group (30±8%) (Figure 5). Finally, it is interesting to note that probably because of underestimation of symptoms, only 50% of PLF patients are referred for operation. Yet, the prognosis of the medically treated patients is much worse than those treated surgically. These data show that patients with severe AS on the basis of valve EOA may nonetheless have low transvalvular flow and low gradient despite normal LV ejection fraction. This PLF pattern generally reflects an advanced stage of the disease and is associated with a poorer prognosis, particularly if treated medically (34). Such findings have important implications because the condition is highly prevalent (35% of the AS population) and may often be misdiagnosed, leading to an erroneous choice of treatment.
Figure 5).
Comparison of resting Doppler-echocardiographic data in patients with normal flow (NF, n=331), paradoxical low flow (PLF, n=181) and classical low flow (CLF, n=62)
Acknowledgments
Dr Pibarot holds the Canada Research Chair in Valvular Heart Diseases, Canadian Institutes of Health Research (Ottawa, Ontario, Canada). The work presented in this review article was supported by research grants from the Canadian Institutes of Health Research (MOP 57745, MOP-79342, MOP 10929), the Heart and Stroke Foundation of Canada (Montreal, Quebec), and the Québec Heart Institute Foundation (Quebec, Quebec).
REFERENCES
- 1.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: An echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–5. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 2.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–26. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 3.Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J. 1988;9:57–64. doi: 10.1093/eurheartj/9.suppl_e.57. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald E. On the natural history of severe aortic stenosis. J Am Coll Cardiol. 1990;15:1018–20. doi: 10.1016/0735-1097(90)90235-h. [DOI] [PubMed] [Google Scholar]
- 5.Davies MJ, Treasure T, Parker DJ. Demographic characteristics of patients undergoing aortic valve replacement for stenosis: Relation to valve morphology. Heart. 1996;75:174–8. doi: 10.1136/hrt.75.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanian R, Olson LJ, Edwards WD. Surgical pathology of pure aortic stenosis: A study of 374 cases. Mayo Clin Proc. 1984;59:683–90. doi: 10.1016/s0025-6196(12)62057-6. [DOI] [PubMed] [Google Scholar]
- 7.Peterson MD, Roach RM, Edwards JE. Types of aortic stenosis in surgically removed valves. Arch Pathol Lab Med. 1985;109:829–32. [PubMed] [Google Scholar]
- 8.Otto CM. Aortic stenosis In: Valvular Heart Disease. Philadelphia: WB Saunders Company; 1999. pp. 179–217. [Google Scholar]
- 9.Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105–10. doi: 10.1161/01.cir.66.5.1105. [DOI] [PubMed] [Google Scholar]
- 10.Ross J, Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38:61–7. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 11.Lester SJ, Heilbron B, Gin K, Dodek A, Jue J. The natural history and rate of progression of aortic stenosis. Chest. 1998;113:1109–14. doi: 10.1378/chest.113.4.1109. [DOI] [PubMed] [Google Scholar]
- 12.Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): Developed in collaboration with the Society of Cardiovascular Anesthesiologists: Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 13.Shavelle D, Otto CM. Aortic valve diseases: Aortic stenosis. In: Crawford MH, Dimarco JP, editors. Cardiology. Vol. 9. London: Mosby; 2000. pp. 1–9.pp. 10 [Google Scholar]
- 14.Rosenhek R, Maurer G, Baumgartner H. Should early elective surgery be performed in patients with severe but asymptomatic aortic stenosis? Eur Heart J. 2002;23:1417. doi: 10.1053/euhj.2002.3163. [DOI] [PubMed] [Google Scholar]
- 15.Otto CM. Valvular aortic stenosis: Disease severity and timing of intervention. J Am Coll Cardiol. 2006;47:2141–51. doi: 10.1016/j.jacc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Niederberger J, Schima H, Maurer G, Baumgartner H. Importance of pressure recovery for the assessment of aortic stenosis by Doppler ultrasound. Role of aortic size, aortic valve area, and direction of the stenotic jet in vitro. Circulation. 1996;94:1934–40. doi: 10.1161/01.cir.94.8.1934. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner H, Steffenelli T, Niederberger J, Schima H, Maurer G. “Overestimation” of catheter gradients by Doppler ultrasound in patients with aortic stenosis: A predictable manifestation of pressure recovery. J Am Coll Cardiol. 1999;33:1655–61. doi: 10.1016/s0735-1097(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 18.Garcia D, Dumesnil JG, Durand LG, Kadem L, Pibarot P. Discrepancies between catheter and Doppler estimates of valve effective orifice area can be predicted from the pressure recovery phenomenon: Practical implications with regard to quantification of aortic stenosis severity. J Am Coll Cardiol. 2003;41:435–42. doi: 10.1016/s0735-1097(02)02764-x. [DOI] [PubMed] [Google Scholar]
- 19.Garcia D, Pibarot P, Dumesnil JG, Sakr F, Durand LG. Assessment of aortic valve stenosis severity: A new index based on the energy loss concept. Circulation. 2000;101:765–71. doi: 10.1161/01.cir.101.7.765. [DOI] [PubMed] [Google Scholar]
- 20.Weyman AE, Scherrer-Crosbie M. Aortic stenosis: Physics and physiology – what do the numbers really mean? Rev Cardiovasc Med. 2005;6:23–32. [PubMed] [Google Scholar]
- 21.Garcia D, Kadem L. What do you mean by aortic valve area: Geometric orifice area, effective orifice area, or gorlin area? J Heart Valve Dis. 2006;15:601–8. [PubMed] [Google Scholar]
- 22.Ikram H, Marshall DE, Moore SM, Bones PJ. Hypertension in valvar aortic stenosis. N Z Med J. 1979;89:204–7. [PubMed] [Google Scholar]
- 23.Pate GA. Association between aortic stenosis and hypertension. J Heart Valve Dis. 2002;11:612–4. [PubMed] [Google Scholar]
- 24.Antonini-Canterin F, Huang G, Cervesato E, et al. Symptomatic aortic stenosis: Does systemic hypertension play an additional role? Hypertension. 2003;41:1268–72. doi: 10.1161/01.HYP.0000070029.30058.59. [DOI] [PubMed] [Google Scholar]
- 25.Briand M, Dumesnil JG, Kadem L, et al. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and functions in aortic stenosis: Implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–8. doi: 10.1016/j.jacc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 26.Chambers J. Can high blood pressure mask severe aortic stenosis? J Heart Valve Dis. 1998;7:277–8. [PubMed] [Google Scholar]
- 27.Bermejo J. The effect of hypertension on aortic valve stenosis. Heart. 2005;91:280–2. doi: 10.1136/hrt.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pibarot P, Dumesnil JG. Assessment of aortic stenosis severity: Check the valve but don’t forget the arteries. Heart. 2007;93:780–2. doi: 10.1136/hrt.2006.111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadem L, Dumesnil JG, Rieu R, Durand LG, Garcia D, Pibarot P. Impact of systemic hypertension on the assessment of aortic stenosis. Heart. 2005;91:354–61. doi: 10.1136/hrt.2003.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadem L, Garcia D, Durand LG, Rieu R, Dumesnil JG, Pibarot P. Value and limitations of the peak-to-peak gradient for the evaluation of aortic stenosis. J Heart Valve Dis. 2006;15:609–16. [PubMed] [Google Scholar]
- 31.Little SH, Chan KL, Burwash IG. Impact of blood pressure on the Doppler echocardiographic assessment of aortic stenosis severity. Heart. 2007;93:848–55. doi: 10.1136/hrt.2006.098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orsinelli DA, Aurigemma GP, Battista S, Krendel S, Gaasch WH. Left ventricular hypertrophy and mortality after aortic valve replacement for aortic stenosis. A high risk subgroup identified by preoperative relative wall thickness. J Am Coll Cardiol. 1993;22:1679–83. doi: 10.1016/0735-1097(93)90595-r. [DOI] [PubMed] [Google Scholar]
- 33.Aurigemma GP, Silver KH, McLaughlin M, Mauser J, Gaasch WH. Impact of chamber geometry and gender on left ventricular systolic function in patients over 60 years of age with aortic stenosis. Am J Cardiol. 1994;74:794–8. doi: 10.1016/0002-9149(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 34.Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–64. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien KD, Zhao XQ, Shavelle DM, et al. Hemodynamic effects of the angiotensin-converting enzyme inhibitor, ramipril, in patients with mild to moderate aortic stenosis and preserved left ventricular function. J Investig Med. 2004;52:185–91. doi: 10.1136/jim-52-03-33. [DOI] [PubMed] [Google Scholar]
- 36.Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: Definition, clinical impact, and prevention. Heart. 2006;92:1022–9. doi: 10.1136/hrt.2005.067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brogan WC, Grayburn PA, Lange RA, Hillis LD. Prognosis after valve replacement in patients with severe aortic stenosis and a low transvalvular pressure gradient. J Am Coll Cardiol. 1993;21:1657–60. doi: 10.1016/0735-1097(93)90383-c. [DOI] [PubMed] [Google Scholar]
- 38.Blitz LR, Gorman M, Herrmann HC. Results of aortic valve replacement for aortic stenosis with relatively low transvalvular pressure gradients. Am J Cardiol. 1998;81:358–62. doi: 10.1016/s0002-9149(97)00905-3. [DOI] [PubMed] [Google Scholar]
- 39.Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction: Prognostic indicators. Circulation. 1997;95:2395–400. doi: 10.1161/01.cir.95.10.2395. [DOI] [PubMed] [Google Scholar]
- 40.Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction. Result of aortic valve replacement in 52 patients. Circulation. 2000;101:1940–6. doi: 10.1161/01.cir.101.16.1940. [DOI] [PubMed] [Google Scholar]
- 41.Smith RL, Larsen D, Crawford MH, Shively BK. Echocardiographic predictors of survival in low gradient aortic stenosis. Am J Cardiol. 2000;86:804–7. doi: 10.1016/s0002-9149(00)01089-4. [DOI] [PubMed] [Google Scholar]
- 42.Schwammenthal E, Vered Z, Moshkowitz Y, et al. Dobutamine echocardiography in patients with aortic stenosis and left ventricular dysfunction: Predicting outcome as a function of management strategy. Chest. 2001;119:1766–77. doi: 10.1378/chest.119.6.1766. [DOI] [PubMed] [Google Scholar]
- 43.Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Range JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients. J Am Coll Cardiol. 2001;37:2101–7. doi: 10.1016/s0735-1097(01)01339-0. [DOI] [PubMed] [Google Scholar]
- 44.Monin JL, Quere JP, Monchi M, et al. Low-gradient aortic stenosis: Operative risk stratification and predictors for long-term outcome: A multicenter study using dobutamine stress hemodynamics. Circulation. 2003;108:319–24. doi: 10.1161/01.CIR.0000079171.43055.46. [DOI] [PubMed] [Google Scholar]
- 45.Quere JP, Monin JL, Levy F, et al. Influence of preoperative left ventricular contractile reserve on postoperative ejection fraction in low-gradient aortic stenosis. Circulation. 2006;113:1738–44. doi: 10.1161/CIRCULATIONAHA.105.568824. [DOI] [PubMed] [Google Scholar]
- 46.Bergler-Klein J, Mundigler G, Pibarot P, et al. B-type natriuretic peptide in low-flow, low-gradient aortic stenosis: Relationship to hemodynamics and clinical outcome: results from the Multicenter Truly or Pseudo-Severe Aortic Stenosis (TOPAS) study. Circulation. 2007;115:2848–55. doi: 10.1161/CIRCULATIONAHA.106.654210. [DOI] [PubMed] [Google Scholar]
- 47.Galante A, Pietroiusti A, Vellini M, et al. C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J Am Coll Cardiol. 2001;38:1078–82. doi: 10.1016/s0735-1097(01)01484-x. [DOI] [PubMed] [Google Scholar]
- 48.Pohle K, Maffert R, Ropers D, et al. Progression of aortic valve calcification: association with coronary atherosclerosis and cardiovascular risk factors. Circulation. 2001;104:1927–32. doi: 10.1161/hc4101.097527. [DOI] [PubMed] [Google Scholar]
- 49.Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001;104:2205–9. doi: 10.1161/hc4301.098249. [DOI] [PubMed] [Google Scholar]
- 50.Nassimiha D, Aronow WS, Ahn C, Goldman ME. Association of coronary risk factors with progression of valvular aortic stenosis in older persons. Am J Cardiol. 2001;87:1313–4. doi: 10.1016/s0002-9149(01)01531-4. [DOI] [PubMed] [Google Scholar]
- 51.Wierzbicki A, Shetty C. Aortic stenosis: An atherosclerotic disease? J Heart Valve Dis. 1999;8:416–23. [PubMed] [Google Scholar]
- 52.deFilippi CR, Willett DL, Brickner E, et al. Usefulness of dobutamine echocardiography in distinguishing severe from nonsevere valvular aortic stenosis in patients with depressed left ventricular function and low transvalvular gradients. Am J Cardiol. 1995;75:191–4. doi: 10.1016/s0002-9149(00)80078-8. [DOI] [PubMed] [Google Scholar]
- 53.Blais C, Burwash IG, Mundigler G, et al. The projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low flow aortic stenosis: The multicenter TOPAS (Truly or Pseudo Severe Aortic Stenosis) study. Circulation. 2006;113:711–21. doi: 10.1161/CIRCULATIONAHA.105.557678. [DOI] [PubMed] [Google Scholar]
- 54.Bermejo J, Yotti R. Low-gradient aortic valve stenosis. Value and limitations of dobutamine stress testing. Heart. 2007;93:298–302. doi: 10.1136/hrt.2005.066860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh JK, Seward JB, Tajik AJ. Valvular heart disease. In: Weinberg RW, Simmons LA, Madrigal R, editors. The Echo Manual Philadelphia. New York: Lippincott-Raven; 1999. pp. 103–32. [Google Scholar]
- 56.Takeda S, Rimington HM, Chambers JB.The use of the pressure drop-flow slope as a measure of severity in patients with aortic stenosis J Am Coll Cardiol 199831246A (Abst) [Google Scholar]
- 57.Nishimura RA, Grantham JA, Connolly HM, Schaff HV, Higano ST, Holmes DR., Jr Low-output, low-gradient aortic stenosis in patients with depressed left ventricular systolic function: The clinical utility of the dobutamine challenge in the catheterization laboratory. Circulation. 2002;106:809–13. doi: 10.1161/01.cir.0000025611.21140.34. [DOI] [PubMed] [Google Scholar]
- 58.Zuppiroli A, Mori F, Olivotto I, Castelli G, Favilli S, Dolara A. Therapeutic implications of contractile reserve elicited by dobutamine echocardiography in symptomatic, low-gradient aortic stenosis. Ital Heart J. 2003;4:264–70. [PubMed] [Google Scholar]
- 59.Blais C, Pibarot P, Dumesnil JG, Garcia D, Chen D, Durand LG. Comparison of valve resistance with effective orifice area regarding flow dependence. Am J Cardiol. 2001;1:45–52. doi: 10.1016/s0002-9149(01)01584-3. [DOI] [PubMed] [Google Scholar]
- 60.Burwash IG, Hay KM, Chan KL. Hemodynamic stability of valve area, valve resistance and stroke work loss in aortic stenosis: A comparative analysis. J Am Soc Echocardiogr. 2002;15:814–22. doi: 10.1067/mje.2002.120287. [DOI] [PubMed] [Google Scholar]
- 61.Otto CM, Pearlman AS, Kraft CD, Miyake-Hull CY, Burwash IG, Gardner CJ. Physiologic changes with maximal exercise in asymptomatic valvular aortic stenosis assessed by Doppler echocardiography. J Am Coll Cardiol. 1992;20:1160–7. doi: 10.1016/0735-1097(92)90373-u. [DOI] [PubMed] [Google Scholar]
- 62.Kadem L, Pibarot P, Dumesnil JG, et al. Independent contribution of the left ventricular ejection time to the mean gradient in aortic stenosis. J Heart Valve Dis. 2002;11:615–23. [PubMed] [Google Scholar]
- 63.Bermejo J, Rojo-Alvarez JL, Antoranz JC, et al. Estimation of the end of ejection in aortic stenosis. An unreported source of error in the invasive assessment of severity. Circulation. 2004;110:1114–20. doi: 10.1161/01.CIR.0000139846.66047.62. [DOI] [PubMed] [Google Scholar]
- 64.Khot UN, Novaro GM, Popovic ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med. 2003;348:1756–63. doi: 10.1056/NEJMoa022021. [DOI] [PubMed] [Google Scholar]
- 65.Zile MR, Gaasch WH. Heart failure in aortic stenosis – improving diagnosis and treatment. N Engl J Med. 2003;348:1735–6. doi: 10.1056/NEJMp030035. [DOI] [PubMed] [Google Scholar]
- 66.Blais C, Dumesnil JG, Baillot R, Simard S, Doyle D, Pibarot P. Impact of prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation. 2003;108:983–8. doi: 10.1161/01.CIR.0000085167.67105.32. [DOI] [PubMed] [Google Scholar]
- 67.Kulik A, Burwash IG, Kapila V, Mesana TG, Ruel M. Long-term outcomes after valve replacement for low-gradient aortic stenosis: Impact of prosthesis-patient mismatch. Circulation. 2006;114:I5553–8. doi: 10.1161/CIRCULATIONAHA.105.001180. [DOI] [PubMed] [Google Scholar]
- 68.Ruel M, Al-Faleh H, Kulik A, Chan KL, Mesana TG, Burwash IG. Prosthesis-patient mismatch after aortic valve replacement predominantly affects patients with preexisting left ventricular dysfunction: Effect on survival, freedom from heart failure, and left ventricular mass regression. J Thorac Cardiovasc Surg. 2006;131:1036–44. doi: 10.1016/j.jtcvs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 69.Dumesnil JG, Pibarot P. Prosthesis-patient mismatch and clinical outcomes: The evidence continues to accumulate. J Thorac Cardiovasc Surg. 2006;131:952–5. doi: 10.1016/j.jtcvs.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 70.Pibarot P, Dumesnil JG. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol. 2000;36:1131–41. doi: 10.1016/s0735-1097(00)00859-7. [DOI] [PubMed] [Google Scholar]
- 71.Pibarot P, Dumesnil JG, Cartier PC, Métras J, Lemieux M. Patient-prosthesis mismatch can be predicted at the time of operation. Ann Thorac Surg. 2001;71:S265–8. doi: 10.1016/s0003-4975(01)02509-7. [DOI] [PubMed] [Google Scholar]
- 72.Castro LJ, Arcidi JMJ, Fisher AL, Gaudiani VA. Routine enlargement of the small aortic root: A preventive strategy to minimize mismatch. Ann Thorac Surg. 2002;74:31–6. doi: 10.1016/s0003-4975(02)03680-9. [DOI] [PubMed] [Google Scholar]
- 73.Bleiziffer S, Eichinger WB, Hettich I, et al. Prediction of valve prosthesis-patient mismatch prior to aortic valve replacement: Which is the best method. Heart. 2007;93:615–20. doi: 10.1136/hrt.2006.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pibarot P, Dumesnil JG. Prevention of valve prosthesis-patient mismatch before aortic valve replacement: does it matter and is it feasible? Heart. 2007;93:549–51. doi: 10.1136/hrt.2006.107672. [DOI] [PMC free article] [PubMed] [Google Scholar]