Abstract
Calcific aortic stenosis (AS) has been considered a degenerative and unmodifiable process resulting from aging and ‘wear and tear’ of the aortic valve. Over the past decade, studies in the field of epidemiology, molecular biology and lipid metabolism have highlighted similarities between vascular atherosclerosis and calcific AS. In particular, work from the Quebec Heart Institute and from that of others has documented evidence of valvular infiltration by oxidized low-density lipoproteins and the presence of inflammatory cells, along with important tissue remodelling in valves explanted from patients with AS. Recent studies have also emphasized the role of visceral obesity in the development and progression of AS. In addition, visceral obesity, with its attendant metabolic complications, commonly referred to as the metabolic syndrome, has been associated with degenerative changes in bioprosthetic heart valves. The purpose of the present review is to introduce the concept of ‘valvulo-metabolic risk’ and to provide an update on the recent and important discoveries regarding the pathogenesis of heart valve diseases in relation to obesity, and to discuss how these novel mechanisms might translate into clinical practice.
Keywords: Aortic stenosis, Bioprostheses, Dyslipidemia, Heart valve diseases, Metabolic Syndrome, Obesity
Abstract
La sténose aortique (SA) calcifiée a toujours été considérée comme un processus dégénératif et non modifiable causé par le vieillissement et l’usure de la valvule aortique. Depuis dix ans, des études dans les domaines de l’épidémiologie, de la biologie moléculaire et du métabolisme lipidique ont révélé des similarités entre l’athérosclérose vasculaire et la SA calcifiée. Notamment, des travaux de l’Institut de cardiologie de Québec et d’autres ont documenté des indications d’infiltration par des lipoprotéines oxydantes de basse densité et la présence de cellules inflammatoires, ainsi qu’un important remodelage des tissus dans les valvules explantées de personnes atteintes de SA. Des études récentes font également ressortir le rôle de l’obésité viscérale dans l’apparition et l’évolution de la SA. De plus, l’obésité viscérale, avec ses complications métaboliques connexes souvent désignées par le terme syndrome métabolique, s’associe à des modifications dégénératives des valvules cardiaques bioprothétiques. La présente analyse vise à présenter le concept de « risque valvulmétabolique » et une mise à jour des découvertes récentes et importantes au sujet de la pathogenèse des valvulopathies par rapport à l’obésité, ainsi qu’à exposer comment transférer ces nouveaux mécanismes en pratique clinique.
With the decline of rheumatic heart valve diseases in the industrialized world, we have seen an important increase in the incidence of calcific aortic stenosis (AS) (1). In North America and Europe, ‘degenerative’ AS is the number one cause for surgical valve replacements. Although AS has been traditionally associated with aging, recent studies (2) have stressed the importance of other atherosclerotic risk factors in the development of this valve disease. For instance, histological analyses of explanted AS valves have demonstrated similarities between calcific AS and vascular atherosclerosis, such as the presence of oxidized low-density lipoprotein (ox-LDL) and inflammatory cells as well as some receptors involved in the formation of atherosclerotic plaques (3,4). Thus, current evidence supports the notion that AS is, at least in part, related to an atherosclerotic process, where interactions among inflammation, lipids and valvular tissue play a determinant role in the development of valvular calcification.
In recent years, obesity has been recognized as a major public health problem. Obese patients experience more cardiovascular complications and have a shorter life expectancy. Obesity has reached such a high prevalence, particularly in developed countries, that a decline in life expectancy for the American population has been forecasted for the 21st century (5). Although obesity per se, as defined by an increased body mass index, is considered a major independent and modifiable risk factor for cardiovascular disease (CVD), it should be emphasized that there is an important heterogeneity among obese subjects, and that the presence of visceral obesity generally worsens the ‘metabolic portrait’ and thus increases the risk of CVD for any given amount of total body fat (6,7). Visceral obesity associated with metabolic perturbations, commonly referred to as the metabolic syndrome (MS), is associated with an increased risk of type 2 diabetes and CVD (8). While the MS has been associated with cardiovascular events and development of coronary artery disease (CAD), it is only recently that our group has demonstrated for the first time an independent association between the MS and the development of heart valve diseases (9,10). In the present article, the interactions among obesity, metabolism, inflammation and the development of heart valve diseases will be reviewed and the concept of ‘valvulo-metabolic’ risk will be introduced.
AS AN ATHEROSCLEROTIC PROCESS?
For many years, calcification of heart valves was perceived as a passive mechanism related to aging and a ‘wear and tear’ phenomenon. However, recent data have emphasized the role of a cellular-dependent mechanism involved in the calcification of heart valves (11). Valvular calcification has been ascribed to an ossification process where interactions among cells, matrix, and bone-related proteins play a crucial role. Identification of promoting factors that induce aortic valve remodelling and calcifying processes has revealed potential pathways. In vitro, oxidized lipid products have procalcifying properties, whereas in animal models, hypercholesterolemia has induced aortic valve calcification and stenosis (12,13). In addition, in vitro studies on isolated vascular cells suggest that high-density lipoprotein (HDL) has anti-calcifying properties (14). Immunohistological studies of explanted aortic valves have documented the presence of ox-LDL within AS valves, which are co-localized with calcific nodules and inflammatory infiltrates (Figure 1). Neovascularization as well as tissue remodelling have also been documented to participate in AS development (15). Thus, histological studies and in vitro experiments support the concept that AS is a process akin to atherosclerosis. In support of the latter hypothesis, several investigations have linked atherosclerotic risk factors with calcific AS. For instance, age, diabetes, hypertension, smoking, male sex, c-reactive protein (CRP), lipoprotein (a) and low-density lipoprotein cholesterol (LDL-C) have been documented as risk factors for AS (16,17). Several retrospective studies have demonstrated that statins reduced the progression rate of AS (18,19). However, recent prospective interventional studies have reported conflicting results regarding efficacy of statins in patients with moderate to severe AS (20,21). A recent study has suggested that statins may be efficient in reducing AS progression in the subgroup of patients with hypercholesterolemia (21). However, it remains unclear whether statins are able to slow the stenosis progression in patients having LDL-C levels within normal range. Hence, the development and progression of AS are likely mediated by an atherosclerotic process where heterogeneous mechanisms could be interacting and leading to common pathways of calcification.
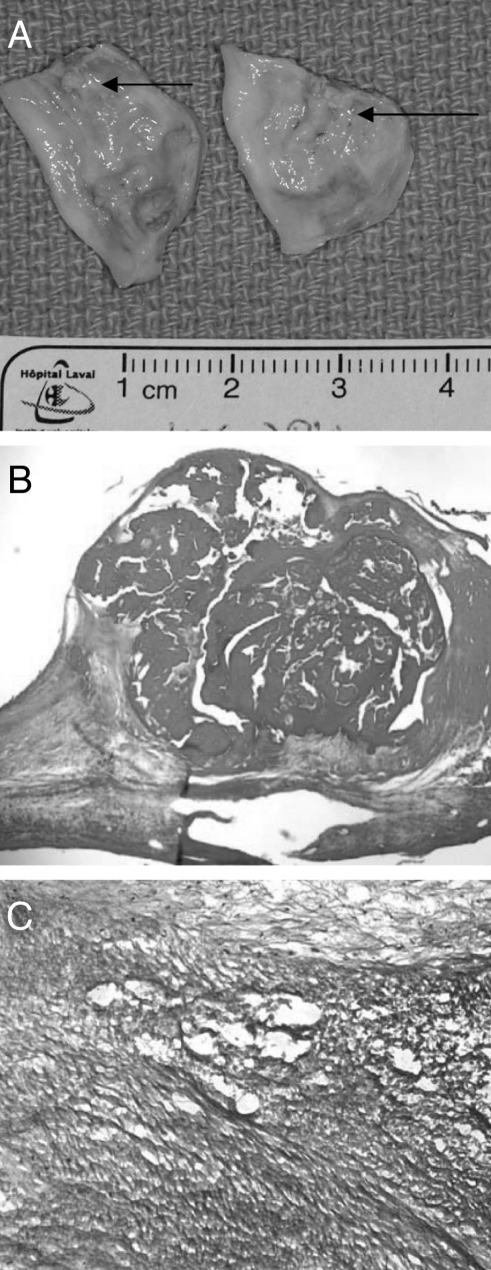
Figure 1).
Macroscopic appearance of explanted leaflets from calcific aortic stenosis showing the presence of yellowish material indicating lipids (arrows) infiltrating the aortic side (A); hematoxylin and eosin staining at low magnification showing the presence of a calcific nodule within the aortic valve (B), which is surrounded by oxidized low-density lipoproteins (immunohistochemistry with high magnification) (C)
VISCERAL OBESITY AND CALCIFIC AS
It is only recently in human life history that obesity has reached such high prevalence. Lack of physical activity and the consumption of energy-dense food have contributed in the development of obesity-related complications such as type 2 diabetes and CVD. Distribution of body fat, particularly abdominal visceral fat accumulation, has been recognized to play a major role in the development of insulin resistance, hypertriglyceridemia, high apolipoprotein B and low HDL cholesterol (HDL-C) levels (22). Prospective observational studies have clearly established that classical risk factors such as age, diabetes, hypertension, smoking and high LDL-C were associated with CAD. However, a substantial proportion of patients with CAD have normal LDL-C levels, suggesting that beyond these factors, other mechanisms could have a significant role on the development of premature atherosclerosis. In the Québec Cardiovascular Study (22), men with features of the MS had a 20-fold increase in their risk of CAD compared with men without such abnormalities, suggesting that metabolic perturbations could act synergistically with classical risk factors.
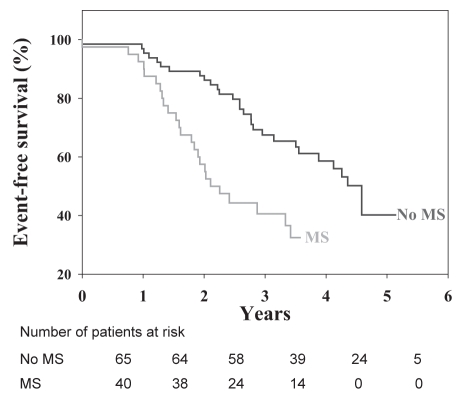
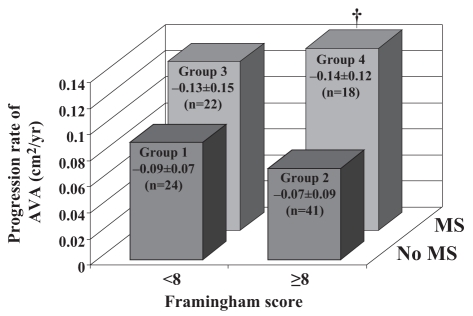
Until recently, the implications of obesity and, particularly, the MS had not been investigated as potential promoting factors in the development of calcific aortic valve disease. In a recent animal study conducted by the team of Jacques Couet and Marie Arsenault (13), the induction of visceral obesity and MS with the use of a high fat, high carbohydrate diet was able to produce aortic valve stenosis in a wild-type mouse strain. In a series of 105 patients with at least moderate AS, we then reported (9) that patients with the features of the MS, identified from the clinical criteria proposed by the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATPIII), had faster stenosis progression than patients without the MS. In addition, patients with the MS had a markedly lower event-free survival (Figure 2). In this study, neither the traditional risk factors nor the Framingham score were significantly associated with AS progression (Figure 3). Patients with the MS were at higher global risk and consequently were treated aggressively with statins and angiotensin-converting enzyme inhibitors. Notwithstanding that patients with the MS receiving lipid-lowering therapy had successfully reached the NCEP-ATPIII recommended target for LDL-C levels, they nevertheless had an average rate of stenosis progression that was twice as fast as patients without the MS. Hence, it is apparent that viscerally obese patients with AS and metabolic abnormalities represent a subgroup of subjects at higher risk for stenosis progression, and for which conventional pharmacological therapy may not be sufficient to prevent hemodynamic progression of the stenosis and the occurrence of cardiovascular events. To identify new therapeutic targets, it is therefore important to identify the causal factors and mechanisms underlying the association between the MS and the progression of AS.
Figure 2).
Kaplan-Meier analysis of event-free survival (absence of death or aortic valve replacement) in patients with or without the metabolic syndrome (MS). Reproduced from Briand et al (9) with permission
Figure 3).
Rate of progression of aortic valve area (AVA) decrease among groups of patients according to the Framingham score (dichotomized at the median value) and the presence or absence of the metabolic syndrome (MS). †Significant difference versus group 2 (P<0.05). Reproduced from Briand et al (9) with permission
PATHOPHYSIOLOGY OF VISCERAL OBESITY AND ITS LINK WITH AS
Visceral obesity and nonesterified fatty acids
It has been shown that for any given amount of total body fat, subjects with increased visceral fat depot are at higher risk of developing metabolic abnormalities and an insulin-resistant state (23). The expanded abdominal fat depot is characterized by the presence of hypertrophied adipocytes with hyperlipolytic activity which generate a large amount of nonesterified fatty acids (NEFA) (24). Increased NEFA delivery to the liver through the portal system has been demonstrated to generate hepatic resistance to insulin along with increased synthesis of triglyceride-rich lipoproteins. Then, the exchange of cholesteryl esters and triglycerides between VLDL and LDL combined with the activity of hepatic lipase leads to the production of small and dense LDL particles (25). A similar mechanism also contributes to produce low HDL-C concentrations. The patients with visceral obesity, therefore, have a high proportion of small and dense circulating LDL particles as well as smaller HDL particles, although their LDL-C level is generally within normal range. Hence, the metabolic perturbations of the MS include, among others, hypertriglyceridemia, insulin resistance, low HDL-C levels, high apolipoprotein B levels, and an increased proportion of small LDL and HDL particles (26).
Alteration of adipokine system
While the NEFA theory behind the metabolic perturbations of visceral obesity has been supported by animal studies and in humans where correlations between visceral fat and NEFA were documented, there are data indicating that portal NEFA are mostly derived from the systemic circulation (23). These findings suggest that other mechanisms may contribute to the dysmetabolic state of the viscerally obese patient. In the past decade, there has emerged a large body of evidence to support the concept that the adipocytes are producing hormones, making the adipose tissue a bona fide endocrine organ, which regulates many metabolic activities (27). Adipokines are adipocyte-derived proteins, which have been characterized in recent years and have been demonstrated to play a prominent role in the development of atherosclerosis. Adiponectin is an adipocyte-produced protein, which is abundant in the plasma and has antiatherosclerotic properties (28,29). Visceral obesity is typically associated with reduced plasma adiponectin levels. Adiponectin has antiatherogenic, antidiabetic and anti-inflammatory properties, and it promotes oxidation of NEFA in peripheral tissues (30).
Proinflammatory state
The expanded abdominal adipose tissue also produces numerous cytokines including the proinflammatory interleukin-6 and tumour necrosis factor-alpha, which contribute to the well documented association between the MS and inflammation (31). Activation of inflammatory pathways could play a significant role in the development of insulin resistance. Moreover, chronic low-grade inflammatory activity has been demonstrated to have an important contributory role in the development and clinical manifestations of atherosclerosis. Circulating levels of CRP, an inflammatory marker produced by the liver and a strong predictor of cardiovascular events, is elevated in patients with the MS and has been suggested to be largely the result of the frequent presence of abdominal obesity among patients with the MS (32,33).
Dysmetabolic state
While visceral obesity is considered a central component of the MS, the possibility cannot be excluded that excess visceral adiposity is partly a marker of a dysmetabolic state. In the so-called ectopic fat theory, the inability of the subcutaneous tissue to adequately handle the energy excess would be associated with the channeling of the extra energy toward skeletal muscle, heart, liver, pancreas and the abdominal fat depot (34). Thus, undesired lipid infiltration in numerous peripheral tissues would explain the development of insulin resistance and the development of metabolic abnormalities.
Link between visceral obesity and AS
The mechanisms described above are not necessarily mutually exclusive and it is possible that several of these mechanisms are concomitantly involved in the development of the metabolic abnormalities typically associated with the MS. How these features of the MS influence the development of valve disease is still a matter of active research, but recent investigations in our laboratory have provided some new insights on the link between ‘cardiometabolic’ risk factors and aortic valve disease.
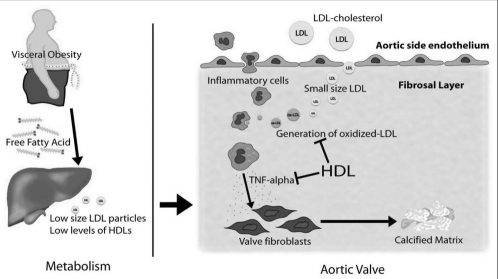
Among the features of the metabolic profile of the viscerally obese patient, the presence of a high quantity of small and dense LDL particles appears to be a hallmark feature (Figure 4). Small, dense LDL particles remain in circulation for a long period of time, are transformed into ox-LDL at higher rates, and have been shown to penetrate the arterial wall with greater facility than their larger buoyant counterparts (35,36). Thus, these particles are extremely atherogenic and may play a central role in the development and/or the progression of AS. In 102 patients undergoing aortic valve replacement, we recently demonstrated that the valvular amount of ox-LDL was correlated with inflammatory activity. Patients with the highest valvular amount of ox-LDL had an increased density of macrophages, T cells, expression of tumour necrosis factor-alpha, as well as higher tissue remodelling activity within their valves. These results underline the central role played by oxidatively modified lipid products at the valvular level. Among the features of the plasma lipid profile, the size of LDL particles was the main determinant of the valvular amount of ox-LDL, whereas there was no association between LDL-C level and the amount of ox-LDL. We proposed, therefore, that among the contributing factors promoting AS development and progression, an increased proportion of small and dense LDL particles could contribute to the strong association that we have previously reported between the MS and AS progression (9).
Figure 4).
Patients with visceral obesity have low high-density lipoprotein (HDL) levels with an increased proportion of circulating small and dense low-density lipoprotein (LDL). The small and dense LDLs have a greater ability to infiltrate the aortic valve and are transformed rapidly to oxidized LDL owing to low HDL levels with a decreased anti-inflammatory activity. Inflammatory cells are attracted within the aortic valve and macrophages are stimulated by oxidized LDL to produce cytokines such as tumour necrosis factor (TNF)alpha, which promotes calcification of valve fibroblasts
Adiponectin is another potential candidate involved in the pathophysiology of AS development and/or progression. Lower circulating levels of adiponectin are closely related to the presence of abdominal fat depot. Thus, through metabolic activities and anti-inflammatory properties, this adipokine could represent an important link between visceral obesity and AS. In our recent study of 124 patients (37), we reported that a lower adiponectin level was a strong and independent predictor of AS progression. Interestingly, a lower adiponectin level was also associated with greater valvular inflammatory activity, supporting the concept that adiponectin may protect the aortic valve from inflammatory and calcifying processes triggered by atherogenic factors.
Among the other contributing factors promoting AS development in the viscerally obese patients, lower HDL levels and/or HDL particle size may also have a prominent role (Figure 4). Indeed, clinical and epidemiological studies have linked a low level of HDL-C to the development of premature CAD (38). Hence, although unproven at the present time, HDL may be one of the key factors underlying the association between the MS and calcific AS. HDLs are a heterogeneous group of particles that have, in addition to their role in the reverse cholesterol transport process, important anti-inflammatory properties (39). It has been demonstrated that HDL protects LDL from oxidative transformation through the paraoxonase-mediated breakdown of oxidized phospholipids, which have a proinflammatory activity (40). Furthermore, recent studies have stressed the role of apolipoprotein A-I as an anti-inflammatory component of HDL, which is independent of HDL-C level (41). Interestingly, the calcification process and transformation of vascular cells to osteoblast-like cells is prevented by HDL, thus supporting the concept that HDL has a broader scope of action that goes beyond the reverse cholesterol transport (14). Patients with the MS have lower HDL-C levels and also smaller HDL particles with decreased anti-inflammatory activity (42). Hence, in light of recent developments in HDL biology, investigations aimed at understanding the relationship between HDL particle phenotype and calcific AS could lead to new and important therapeutic avenues. Indeed, this pathway could represent a potential target in patients with AS and visceral obesity.
THE MS AND STRUCTURAL DEGENERATION OF BIOPROSTHESES
Structural valve degeneration (SVD) of bioprostheses (BPs) has been considered as being a purely ‘degenerative’ calcifying process. Calcification of cusp tissue is responsible for approximately 75% of BP failures. While young age at implantation has long been recognized as a strong risk factor for SVD of a BP, it is only recently that traditional atherosclerotic risk factors have been recognized as additional risk factors for reoperation. Indeed, hypercholesterolemia, diabetes and smoking have been associated with increased risk of reoperation (43). Furthermore, one recent study (44) has reported the presence of foam cells in explanted BPs, supporting the hypothesis that, similar to the native aortic valve, calcification of BPs is, at least partially, related to an atherosclerotic-like process. Leaflet tissues of explanted biological valves are often infiltrated by cells expressing bone-regulatory proteins involved in the regulation of the ossifying process, indicating that calcification of biological heart valves is regulated at the molecular level by processes largely similar to what is observed in the bone tissue (45). Triggering factors that promote the initiation of the calcifying process of BPs are, however, poorly identified for the moment.
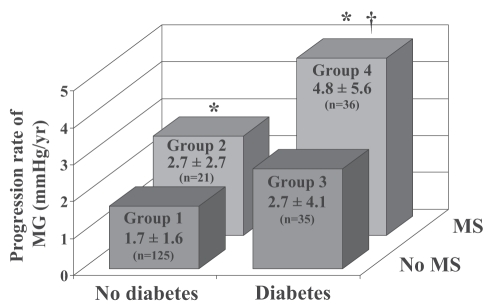
In light of the recent findings obtained in native and bio-prosthetic valves, we recently hypothesized that the MS could, through its proinflammatory and proatherogenic activities, promote the development of SVD in BPs. In 217 patients with an aortic BP and a mean echocardiographic follow-up of 3.1±1.8 years, we reported that the MS was a strong and independent predictor of faster degeneration of BPs (10). Deterioration of hemodynamic function defined as a rate of increase in mean gradient 3 mmHg/year or greater and/or a worsening of regurgitation 1+ or greater occurred in 41% of patients with the MS whereas it was detected in only 25% of patients without the MS (P=0.02). Besides MS, diabetes and renal insufficiency were also associated with faster deterioration of valve hemodynamic function. The combination of diabetes and the MS was associated with a 2.5-fold increase in the progression rate of transprosthetic gradient compared with patients without these two factors (Figure 5). In this cohort, the prevalence of the MS (as defined by the NCEP-ATPIII) was 33%, thus underlining the point that there is a large proportion of the patients with a BP who are potentially at risk to develop hemodynamic failure of their valve. The mechanisms by which the MS participates to the failure of the BPs is still a matter of speculation, but ongoing studies from our group suggest that as for the native aortic valve, lipid oxidative products within the bioprosthetic tissue might participate in the degenerative changes. However, it must be pointed out that, as opposed to the native aortic valve, BPs are processed and chemically fixed tissues. Moreover, BPs are xenogenic tissues able to trigger an immune-mediated response, thereby increasing the inflammatory activity and potentially the calcification of the implanted cusps (46). Thus, multiple mechanisms could act in synergy with atherosclerotic risk factors to promote calcification and degenerative changes of BPs.
Figure 5).
Rate of progression of mean gradient (MG) among groups of patients according to the presence of diabetes and the metabolic syndrome (MS). P<0.05 versus *group 1, †group 2. Reproduced from Briand et al (10) with permission
CLINICAL IMPLICATIONS OF VISCERAL OBESITY ON VALVULAR HEART DISEASES
Although some issues around the MS are controversial, clinical tools developed by official agencies such as the NCEP-ATPIII, the International Diabetes Federation and the World Health Organization have all emphasized the fact that the MS is a strong risk factor for CVD. Recent work has highlighted for the first time that the MS is not only a strong risk factor for CAD, but also for heart valve disease such as calcific AS and calcific degeneration of BPs. While prevention remains the primary goal, clinicians are confronted with a population already with visceral obesity and heart valve disease for whom adequate interventions must be considered to minimize their ‘valvulo-metabolic’ risk.
While statins are the mainstay therapy for CAD, it remains uncertain whether cholesterol-lowering therapy has a major role in the prevention or the progression of AS. Two recent studies, the Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) trial and the Rosuvastatin Affecting Aortic Valve Endothelium (RAAVE) study have provided interesting new insights into the efficacy of statin therapy in patients with AS (20,21). The SALTIRE trial was a double-blind randomized study designed to examine the effect of atorvastin treatment on the progression of moderate to severe AS (average aortic valve area at baseline: 1.0 cm2). Although atorvastatin therapy was associated with a marked reduction in cholesterol levels, it nonetheless failed to slow valvular calcification and stenosis progression. The RAAVE study was an open-label study in which rosuvastatin was administered to patients with moderate AS (baseline aortic valve area comprised between 1.0 cm2 and 1.5 cm2) and hypercholesterolemia (as defined by the NCEP-ATPIII guidelines). Patients with hypercholesterolemia and who were treated with statins had a lower stenosis progression rate compared with patients with normal cholesterol, who were left untreated. This study, therefore, provides the first proof of concept that statins may be effective in reducing the stenosis progression in hypercholesterolemic patients. When analyzed collectively, the results of the SALTIRE and RAAVE prospective clinical studies as well as those of previous experimental studies (47) suggest that statins are likely more effective in early than in end stages of the disease. Hence, statins have a better chance of successfully slowing the stenosis progression when the treatment is instituted early in the course of the disease before the valvular ossification and calcification are too advanced.
It should also be considered that a large proportion (approximately 60% to 70%) of patients with calcific AS concomitantly have a history of hypercholesterolemia, diabetes and/or CAD and are thus often already receiving statins. Despite the fact that these patients are taking statins and that their LDL-C plasma level is most often within normal range, there is still an important interindividual variability in the stenosis progression rate and a substantial proportion of these patients show rapid progression. These findings suggest that, beyond LDL-C, there are probably other important therapeutic targets that need to be taken into consideration. Hence, statins may be necessary but not sufficient in a large number of AS patients. In this regard, our recent findings suggest that behavioural or pharmacological interventions targeting visceral adiposity and its associated metabolic abnormalities could contribute to slow the stenosis progression rate in a substantial proportion of the AS population. Given that approximately 40% of the patients with AS have the MS, targeting visceral obesity may be of high clinical relevance.
Thus, patients with mild to moderate AS as well as those with an aortic BP should be screened for the presence of the MS. The criteria developed by the NCEP-ATPIII panel or the International Diabetes Federation include increased waist circumference with specific population cut-off values, hypertriglyceridemia, low HDL-C, elevated blood pressure or treatment for hypertension, and elevated glycemia or treatment with hypoglycemic agents. It should be noted that these criteria have been developed as clinical tools to identify the patients at risk. Hence, measurements of the waist girth along with the lipid blood profile and glycemia will allow identification of patients having the ‘at-risk’ obesity and thus a higher likelihood of rapid progression of AS or BP SVD. Consequently, these patients should have a careful follow-up to ensure early detection of rapid changes in their echocardiographic hemodynamic parameters and/or in their clinical condition. Moreover, the metabolic abnormalities of these patients should be aggressively treated.
Although a genetic predisposition may be involved in the etiology of the MS, acquired risk factors are readily modifiable by appropriate therapy and are, of course, the mainstay of clinical management. Weight loss, even modest, has been shown to reduce insulin resistance and also to decrease circulating levels of CRP and interleukin-6 (48). Lifestyle interventions, combining diet and exercise, have shown efficacy in reducing cardiovascular risk (49). Thus, lifestyle intervention programs should be envisioned in patients with aortic valve disease who also have the MS.
In patients with BPs, one retrospective study has suggested that statin therapy may be effective in reducing SVD (50). Further prospective studies are needed to confirm whether administration of statins after the implantation of a BP could decrease the calcifying and degenerative changes of BPs. Although lipid-modifying agents have been of great help in managing one of the major risk factors, namely LDL-C, it is considered that the major pathways by which the MS influences atherosclerosis are not directly targeted by these pharmacological interventions (8). Therefore, new therapeutic interventions are now developed to correct the proatherogenic and proinflammatory abnormalities typically associated with the MS. The peroxisome proliferator-activated receptor-gamma agonists, thiazolidinediones (TZDs), have been developed as antidiabetic insulin-sensitizing agents, but it is now clear that this class of agents also have anti-inflammatory and antiatherosclerotic activities (51). Results of one clinical trial suggest that TZDs may indeed confer CVD protection in diabetic patients (52). However, it remains to be seen if TZDs could impact the evolution of AS.
Over recent years, the development of a new class of drug, the cannabinoid-1 receptor antagonist rimonabant, has provided evidence that this new pharmacological approach may be relevant for the management of key causal factors involved in the pathogenesis of the MS. In the Rimonabant in Obesity Europe (RIO-Europe) study, a one-year follow-up demonstrated that rimonabant induced a significant weight reduction compared with placebo (53). In this study, the proportion of patients that fulfilled the NCEP ATP III criteria for the MS was reduced by 65% in individuals who completed the treatment. Després et al (54) have recently reported that rimonabant induced a significant increase of adiponectin blood levels (54). Furthermore, a positive correlation was found between changes in adiponectin levels produced by rimonabant and changes in HDL-C concentrations, suggesting that the loss of abdominal fat depot and a specific effect of rimonabant on adiponectin levels were two important factors that could contribute to explain the favourable metabolic effects of the cannabinoid-1 receptor blocker. Thus, rimonabant appears to be a promising drug in the treatment of patients with visceral obesity, and it remains to be determined whether this pharmacological strategy could favourably affect the evolution of patients with AS and obesity at risk.
CONCLUSION
Calcific AS is actually the third leading cause of death among patients with CVD. Its incidence has increased dramatically over the past decade. While surgical treatment remains the mainstay of treatment for severe stenosis in symptomatic patients, no medical therapy has conclusively shown efficacy in altering the progression of AS. Recent studies have linked atherosclerotic risk factors with AS development and SVD of BPs. In particular, the MS has been associated with faster AS progression and hemodynamic degeneration of BPs, suggesting that, beyond traditional risk factors, ‘cardiometabolic’ risk factors may actively participate in the physiopathology of aortic valve disease. These novel insights into ‘valvulo-metabolic risk’ have not only contributed to the elucidation of some of the mechanisms involved in the development and progression of aortic valve disease, but have also opened new therapeutic horizons for the treatment and prevention of this disease. A better understanding of the mechanisms leading to the calcification and remodelling of the valvular tissue in relation to the metabolic features of the viscerally obese patients will help to design tailored therapeutic strategies in these high-risk patients.
Acknowledgments
The authors thank Brigitte Dionne, Stephanie Dionne and Martine Fleury for their technical assistance. Some of the work presented in this review paper was supported by the Canadian Institute of Health Research (CIHR), Ottawa, Canada, grant number MOP 79342 and the Quebec Heart Institute Foundation. Dr Mathieu is a research scholar from the Fonds de Recherches en Santé du Québec, Montreal, Quebec. Dr Pibarot holds the Canada Research Chair in Valvular Heart Diseases, CIHR, Ottawa, Ontario. Dr Després is the scientific director of the International Chair on Cardiometabolic Risk at University Laval, which is supported by an unrestricted grant of Sanofi-Aventis to Université Laval.
REFERENCES
- 1.Rajamannan NM. Calcific aortic stenosis: A disease ready for prime time. Circulation. 2006;114:2007–9. doi: 10.1161/CIRCULATIONAHA.106.657759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossebø AB, Pedersen TR. Hyperlipidaemia and aortic valve disease. Curr Opin Lipidol. 2004;15:447–51. doi: 10.1097/01.mol.0000137229.00020.fe. [DOI] [PubMed] [Google Scholar]
- 3.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19:1218–22. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 4.Otto CM, Kuusisto J, Reichenbach DD, et al. Characterization of the early lesion of degenerative valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–53. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 5.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. New Engl J Med. 2005;352:1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 6.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 7.St-Pierre A, Cantin B, Mauriège P, et al. Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. CMAJ. 2005;172:1301–5. doi: 10.1503/cmaj.1040834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Després JP. Inflammation and cardiovascular disease: Is abdominal obesity the missing link? Int J Obesity. 2003;27:S22–4. doi: 10.1038/sj.ijo.0802495. [DOI] [PubMed] [Google Scholar]
- 9.Briand M, Lemieux I, Dumesnil JG, et al. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J Am Coll Cardiol. 2006;47:2229–36. doi: 10.1016/j.jacc.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 10.Briand M, Pibarot P, Després JP, et al. Metabolic syndrome is associated with faster degeneration of bioprosthetic valves. Circulation. 2006;114(Suppl I):I512–I517. doi: 10.1161/CIRCULATIONAHA.105.000422. [DOI] [PubMed] [Google Scholar]
- 11.Mathieu P, Voisine P, Pépin A, et al. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis. 2005;14:353–7. [PubMed] [Google Scholar]
- 12.Parhami F, Morrow AD, Balucan J, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–7. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 13.Drolet MC, Lachance D, Plante E, et al. A high fat/high carbohydrate diet induces aortic valve disease in c57BL/6J mice. J Am Coll Cardiol. 2006;47:850–5. doi: 10.1016/j.jacc.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 14.Parhami F, Basseri B, Hwang J, et al. High-density lipoprotein regulates calcification of vascular cells. Circ Res. 2002;91:570–6. doi: 10.1161/01.res.0000036607.05037.da. [DOI] [PubMed] [Google Scholar]
- 15.Charest A, Pépin A, Shetty R, et al. Distribution of SPARC during neovascularisation of degenerative aortic stenosis. Heart. 2006;92:1844–9. doi: 10.1136/hrt.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajamannan NM, Gersh B, Bonow RO. Calcific aortic stenosis: From bench to the bedside-emerging clinical and cellular concepts. Heart. 2003;89:801–5. doi: 10.1136/heart.89.7.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. J Am Coll Cardiol. 1997;29:630–4. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 18.Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–5. doi: 10.1161/01.CIR.0000140723.15274.53. [DOI] [PubMed] [Google Scholar]
- 19.Bellamy MF, Pellika PA, Klarich KW, et al. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. 2002;40:1723–30. doi: 10.1016/s0735-1097(02)02496-8. [DOI] [PubMed] [Google Scholar]
- 20.Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–97. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 21.Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–61. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamarche B, Tchernof A, Mauriège P, et al. Fasting insulin and apoliporotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. JAMA. 1998;279:1955–61. doi: 10.1001/jama.279.24.1955. [DOI] [PubMed] [Google Scholar]
- 23.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–6. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 24.Mittelman SD, Van Citters GW, Kirkman EL, et al. Extreme insulin resistance of the central adipose depot in vivo. Diabetes. 2002;51:755–61. doi: 10.2337/diabetes.51.3.755. [DOI] [PubMed] [Google Scholar]
- 25.Carmena R, Duriez P, Fruchart JC. Atherogenic lipoprotein particles in atherosclerosis. Circulation. 2004;109:III2–III7. doi: 10.1161/01.CIR.0000131511.50734.44. [DOI] [PubMed] [Google Scholar]
- 26.Lamarche B, Lemieux I, Després JP. The small, dense LDL phenotype and the risk of coronary heart disease: Epidemiology, pathophysiology and therapeutic aspects. Diabetes Metab. 1999;25:199–211. [PubMed] [Google Scholar]
- 27.Staiger H, Häring HU. Adipocytokines: Fat-derived humoral mediators of metabolic homeostasis. Exp Clin Endocrinol Diabetes. 2005;113:67–79. doi: 10.1055/s-2004-830555. [DOI] [PubMed] [Google Scholar]
- 28.Chandran M, Phillips SA, Ciaraldi T, et al. Adiponectin: More than just another fat cell hormone? Diabetes Care. 2003;26:2442–50. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 29.Arita Y, Kihara S, Ouchi N, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–63. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 30.Côté M, Mauriège P, Bergeron J, et al. Adiponectemia in visceral obesity; impact on glucose tolerance and plasma lipoprotein and lipid levels in men. J Clin Endocrinol Metab. 2005;90:1434–9. doi: 10.1210/jc.2004-1711. [DOI] [PubMed] [Google Scholar]
- 31.Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis. 2000;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 32.Yudkin JS, Stehouwer CD, Emeis JJ, et al. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM. Cardiology Patient Page. C-reactive protein: A simple test to help predict risk of heart attack and stroke. Circulation. 2003;108:e81–5. doi: 10.1161/01.CIR.0000093381.57779.67. [DOI] [PubMed] [Google Scholar]
- 34.Unger RH. Longevity, lipotoxicity and leptin: The adipocyte defense against feasting and famine. Biochimie. 2005;87:57–64. doi: 10.1016/j.biochi.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Bjornheden T, Babyi A, Bondjers G, et al. Accumulation of lipoprotein fractions and subfractions in the arterial wall, determined in an in vitro perfusion system. Atherosclerosis. 1996;123:43–56. doi: 10.1016/0021-9150(95)05770-6. [DOI] [PubMed] [Google Scholar]
- 36.Tribble DL, Holl LG, Wood PH, et al. Variations in oxidative susceptibility among six low density lipoprotein subfractions of differing density and particle size. Atherosclerosis. 1992;93:189–99. doi: 10.1016/0021-9150(92)90255-f. [DOI] [PubMed] [Google Scholar]
- 37.Mohty D, Cartier A, Pibarot P, et al. Hypoadiponectemia is associated with aortic valve inflammation and faster disease progression in patients with aortic stenosis Circulation 2006114II657 (Abst). [DOI] [PubMed] [Google Scholar]
- 38.Gordon DH, Rifkind BM. High-density lipoprotein: The clinical implications of recent studies. N Engl J Med. 1989;321:1311–6. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 39.Von Eckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Current Opin Clin Nutr Met Care. 2005;8:147–52. doi: 10.1097/00075197-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Getz GS, Reardon CA. Paraoxonase, a cardioprotective enzyme: Continuing issues. Curr Opin Lipidol. 2004;114:529–41. doi: 10.1097/00041433-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Hyka N, dayer JM, Modoux, et al. Apolipoprotein A-I inhibits the production of interleukin-1 beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–90. doi: 10.1182/blood.v97.8.2381. [DOI] [PubMed] [Google Scholar]
- 42.Hansel B, Giral P, Nobecourt E, et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004;89:4963–71. doi: 10.1210/jc.2004-0305. [DOI] [PubMed] [Google Scholar]
- 43.Nollert G, MiKsh J, Kreuzer E, et al. Risk factors for atherosclerosis and the degeneration of pericardial valves after aortic valve replacement. J Thorac Cardiovasc Surg. 2003;126:965–8. doi: 10.1016/s0022-5223(02)73619-2. [DOI] [PubMed] [Google Scholar]
- 44.Bottio T, Thiene G, Pettenazzo E, et al. Hancock II bioprosthesis: A glance at the miscroscope in mid-long-term explants. J Thorac Cardiovasc Surg. 2003;126:99–105. doi: 10.1016/s0022-5223(03)00131-4. [DOI] [PubMed] [Google Scholar]
- 45.Shetty R, Pepin A, Charest A, et al. Expression of bone-regulatory proteins in human valve allografts. Heart. 2006;92:1303–8. doi: 10.1136/hrt.2005.075903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manji RA, Zhu LF, Nijjar N, et al. Glutharaldehyde-fixed bioprosthetic heart valves conduits calcify and fail from xenograft rejection. Circulation. 2006;114:318–27. doi: 10.1161/CIRCULATIONAHA.105.549311. [DOI] [PubMed] [Google Scholar]
- 47.Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathways. Circulation. 2005;112(Suppl I):I229–34. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts CK, Barnard J. Effects of exercise and diet on chronic disease. J Applied Physiol. 2005;98:3–30. doi: 10.1152/japplphysiol.00852.2004. [DOI] [PubMed] [Google Scholar]
- 49.Bonow RO, Eckel RH. Diet, obesity, and cardiovascular risk. N Engl J Med. 2003;348:2057–8. doi: 10.1056/NEJMp030053. [DOI] [PubMed] [Google Scholar]
- 50.Antonini Canterini F, Zuppirali A, Popescu BA, et al. Effect of statins on the progression of bioprosthetic aortic valve degeneration. Am J Cardiol. 2003;92:1479–82. doi: 10.1016/j.amjcard.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 51.Puddu P, Puddu GM, Muscari A. Peroxisome proliferators-activated receptors: Are they involved in atherosclerosis progression? Int J Cardiol. 2003;90:133–40. doi: 10.1016/s0167-5273(02)00565-x. [DOI] [PubMed] [Google Scholar]
- 52.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 53.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S, RIO-Europe Study Group Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–97. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 54.Després JP, Golay A, Sjöström L, Rimonabant in Obesity-Lipids Study Group Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–34. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]