Abstract
BACKGROUND:
Ischemic mitral regurgitation (MR) often persists after restrictive mitral valve annuloplasty (MVA) and is associated with a poor prognosis. It was hypothesized that the anterior displacement of the posterior aspect of the annulus caused by annuloplasty could induce a change in the direction of MR jet.
METHODS:
The echocardiograms of 21 patients who underwent restrictive MVA for ischemic MR and who had detectable postoperative MR were analyzed before and early after surgery to evaluate the direction of MR jet.
RESULTS:
The MR jet direction was posterior in 15 patients (72%) and central or anterior in six patients (28%) before the operation, compared with four patients (20%) and 17 patients (80%), respectively, after MVA (P<0.001). Overall, the jet direction was modified in 16 of 21 7patients (76%) following MVA. Among the subset of 11 patients with clinically significant persistent MR (vena contracta width greater than 3 mm), the MR jet direction changed in nine patients (82%) compared with their preoperative evaluation. Importantly, the initial clinical interpretation, based on a subjective evaluation, had classified MR severity as nonsignificant in six of 11 patients (55%), likely due to the eccentricity of the jet and its change in direction.
CONCLUSION:
The direction of the persistent MR jet early after annuloplasty is often different from that of preoperative MR jet and may lead to significant misinterpretation of the postoperative echocardiogram.
Keywords: Annuloplasty, Echocardiography, Ischemic mitral regurgitation, Jet direction, Mitral valve
Abstract
HISTORIQUE :
La régurgitation mitrale (RM) ischémique persiste souvent après une annuloplastie restrictive de la valvule mitrale (AVM restrictive) et s’associe à un mauvais pronostic. On a postulé que le déplacement antérieur de l’aspect postérieur de l’anneau causé par l’annuloplastie pourrait modifier l’orientation du flux de RM.
MÉTHODOLOGIE :
Les échocardiogrammes de 21 patients qui ont subi une AVM restrictive en raison d’une RM ischémique et qui présentaient une RM décelable après l’opération ont fait l’objet d’une analyse avant et peu après l’opération pour évaluer l’orientation du flux de RM.
RÉSULTATS :
Avant l’opération, le flux de RM avait une orientation postérieure chez 15 patients (72 %) et une orientation centrale ou antérieure chez six patients (28 %), par rapport à quatre patients (20 %) et à 17 patients (80 %), respectivement, après l’AVM (P<0,001). Dans l’ensemble, l’orientation du flux avait changé chez 16 des 21 patients (76 %) après l’AVM. Dans le sous-groupe de 11 patients présentant une RM persistante significative d’un point de vue clinique (section contractée d’une largeur de plus de 3 mm), l’orientation du flux de RM avait changé chez neuf patients (82 %) par rapport à leur évaluation préopératoire. Qui plus est, d’après l’interprétation clinique initiale, fondée sur une évaluation subjective, la gravité de la RM était classée comme non significative chez six des 11 patients (55 %), probablement à cause de l’excentricité du flux et de son changement d’orientation.
CONCLUSION :
L’orientation du flux de RM persistante peu après l’annuloplastie diffère souvent de ce qu’elle était avant l’opération et peut provoquer une fausse interprétation marquée de l’échocardiogramme postopératoire.
Mitral regurgitation (MR) is a common complication in patients with ischemic heart disease, and it influences long-term prognosis and mortality after revascularization. Although the optimal surgical treatment of ischemic MR is controversial (1–3), restrictive mitral valve annuloplasty (MVA) combined with coronary artery bypass grafting surgery remains the most frequently used approach at the present time (4). However, persistent MR is observed in up to one-third of patients after MVA and can adversely affect outcomes (1,3,4). Recently, Magne et al (5) reported that persistence of MR and clinical outcome could be predicted from preoperative analysis of mitral valve configuration. Patients with persistent MR had markedly lower three-year cardiac event-free survival rates than those with nonpersistent MR (5). The detection of persistent MR after restrictive MVA may thus contribute to improved risk stratification in this high-risk population with ischemic MR. Moreover, the early detection and quantification of persistent MR at the time of operation may help to correct or complete the surgical procedure, and thus improve the postoperative outcome of these patients. The eccentricity of the jet or the change in jet orientation postoperatively may contribute to the underestimation of the presence and severity of persistent MR by perioperative echocardiography (6). The objective of the present study was to evaluate whether MVA could induce changes in the direction of the MR jet after surgery in patients with ischemic MR and thereby possibly affect the interpretation of the postoperative echocardiogram.
PATIENTS AND METHODS
Study population
The echocardiograms of 70 consecutive patients with ischemic MR who underwent surgery for restrictive MVA with or without coronary artery bypass grafting surgery at the Quebec Heart Institute (Sainte-Foy, Quebec) between January 2002 and December 2005 were analyzed for the present study. Patients who had concomitant organic mitral valve lesions, significant aortic regurgitation and significant aortic stenosis were excluded from the study.
The initial cohort comprised 51 patients (42 men, nine women; mean age 65±8 years). For the purpose of the present study, only the 21 patients (41%) in this cohort who had MR at predischarge transthoracic echocardiographic examination after MVA were included.
Surgical technique
The mitral valve was accessed intraoperatively through a standard left atriotomy. A complete ring annuloplasty was performed in the majority of patients (81%) using a Carpentier-Edwards Physio ring (Edwards Lifesciences, USA). Ring size was selected by downsizing the measured intertrigonal length by two sizes. Intraoperatively, the MVA was evaluated by transesophageal echocardiography. In the presence of MR 1 or greater, either a smaller sized annuloplasty or a mitral valve replacement was performed.
Echocardiographic measurements
Two-dimensional and Doppler transthoracic echocardiography examinations using commercially available echocardiographic systems (Sonos 5500 or 7500, Philips Medical Systems, Canada) were performed a mean of 5±3 days (range one to 11 days) before surgery and 7±4 days (range one to 12 days) after surgery, at the time of predischarge examination.
Left ventricular geometry and function
Left ventricular (LV) end-diastolic and end-systolic diameters were measured using M mode in the parasternal long-axis view. LV end-diastolic and end-systolic volumes, as well as LV ejection fraction, were determined by the modified biplane Simpson’s method (7,8). To assess the LV shape, LV end-diastolic and end-systolic sphericity indexes were calculated by dividing the LV short-axis dimension by the LV long-axis dimension in the four-chamber view (9). LV stroke volume was calculated by multiplying the LV outflow tract area by the LV outflow tract velocity-time integral measured by pulsed-wave Doppler.
MR assessment
MR was assessed quantitatively using the width of the vena contracta of the regurgitant jet and the ratio of the MR colour flow jet area to the left atrial area. Vena contracta was defined as the narrowest portion of the colour jet that occurred at or just downstream from the regurgitant orifice (10). Vena contracta width was measured in the parasternal long-axis view. The largest diameter during systole was measured in at least three cardiac cycles, and the values were then averaged. MR jet direction was classified as anterior, central or posterior using colour Doppler long-axis and four-chamber views.
Statistical analysis
Results are expressed as mean ± SD or percentages unless otherwise specified. The patients were separated into two groups depending on the severity of the persistent MR. Patients with a vena contracta width of 3 mm or less were classified into the ‘non-significant persistent MR’ group and those with a vena contracta width greater than 3 mm were classified into the ‘signficant persistent MR’ group. Preoperative and operative data of the two groups were compared for statistical significance using Student’s t test, the Mann-Whitney rank sum test, the χ2 test and Fischer’s exact test as appropriate. Preoperative and postoperative echocardiographic data were first compared in the whole cohort using paired Student’s t tests. Afterward, the effects of time (postoperative versus preoperative examination) and group (nonsignificant persistent MR versus signficant persistent MR) were analyzed using two-way analysis of variance for repeated measures.
RESULTS
Patient characteristics and echocardiographic measurements
Of 21 patients, 11 (52%) were classified as having clinically significant persistent MR (vena contracta greater than 3 mm) and 10 (48%) were classified as having clinically nonsignificant persistent MR (vena contracta of 3 mm or less).
There was no significant difference between the clinically nonsignificant and clinically significant MR groups in terms of preoperative demographic and clinical data; myocardial infarction site, operative data, and ring size and type were similar in both groups (Table 1). As well, there were no differences in LV geometry and function, left atrial geometry and preoperative vena contracta width between the two groups. By definition, the postoperative vena contracta width was significantly higher in the group with signficant persistent MR than in the group with nonsignificant persistant MR (5.0±2.0 versus 2.6±0.2; P<0.05) (Table 2).
TABLE 1.
Preoperative demographic, clinical and operative data
| Variables | All patients (n=21) | Clinically nonsignificant persistent MR (n=10) | Clinically significant persistent MR (n=11) | P |
|---|---|---|---|---|
| Demographic data | ||||
| Male | 17 (81) | 10 (100) | 7 (64) | NS |
| Age, years | 66.8±9.0 | 69.0±7.0 | 65.0±10.0 | NS |
| BSA, m2 | 1.9±0.2 | 1.9±0.2 | 1.8±0.2 | NS |
| BMI, kg/m2 | 26.5±7.0 | 26.3±3.4 | 26.5±4.4 | NS |
| Risk factors | ||||
| Hypertension | 16 (76) | 8 (80) | 8 (73) | NS |
| Diabetes | 7 (33) | 2 (20) | 5 (45) | NS |
| Dyslipidemia | 19 (90) | 9 (90) | 10 (91) | NS |
| Obesity | 4 (19) | 1 (10) | 3 (27) | NS |
| History of APO | 5 (24) | 4 (40) | 1 (9) | NS |
| Atrial fibrillation | 6 (29) | 4 (40) | 2 (18) | NS |
| Myocardial infarction site | ||||
| Inferior | 17 (81) | 9 (90) | 8 (73) | NS |
| Anterior | 4 (19) | 1 (10) | 3 (27) | NS |
| Revascularization | ||||
| Concomitant CABG | 20 (95) | 10 (100) | 10 (91) | NS |
| Number of grafted vessels | 2.4±1.3 | 2.3±1.3 | 2.5±1.4 | NS |
| Ring size and type | ||||
| Mean ring size, mm | 27.1±1.5 | 27.2±1.5 | 27.0±1.7 | NS |
| 24 mm | 1 (5) | 0 (0) | 1 (9) | NS |
| 26 mm | 9 (43) | 5 (50) | 4 (36) | NS |
| 28 mm | 8 (38) | 4 (40) | 4 (36) | NS |
| 30 mm | 2 (10) | 1 (10) | 1 (9) | NS |
| Carpentier-Edwards Physio* | 17 (81) | 8 (80) | 9 (82) | NS |
| Medtronic Duran† | 2 (10) | 1 (10) | 1 (9) | NS |
| Carpentier-Edwards Classic* | 2 (10) | 1 (10) | 1 (9) | NS |
Data are given as mean ± SD or number of patients (%). NS indicates a non-significant difference (P>0.05) between the mild persistent mitral regurgitation (MR) group and the moderate to severe persistent MR group.
Edwards Lifesciences, USA;
Medtronic Inc, USA. APO Acute pulmonary edema; BMI Body mass index; BSA Body surface area; CABG Coronary artery bypass graft surgery
TABLE 2.
Preoperative and postoperative echocardiographic data
| Variables |
All patients (n=21) |
Clinically nonsignificant persistent MR (n=10) |
Clinically significant persistent MR (n=11) |
|||
|---|---|---|---|---|---|---|
| Preop | Postop | Preop | Postop | Preop | Postop | |
| LV geometry and function | ||||||
| LVED diameter, mm | 61±6 | 59±5 | 59±5 | 57±5* | 63±5 | 61±6* |
| LVES diameter, mm | 49±7 | 48±8 | 46±8 | 45±8 | 51±7 | 51±8 |
| LVED volume, mL | 181±64 | 158±53* | 183±11 | 165±11* | 180±11 | 152±11* |
| LVES volume, mL | 116±48 | 107±42 | 111±8 | 108±8 | 120±8 | 117±8 |
| LVED sphericity index, % | 62±11 | 59±10 | 62±2 | 58±2 | 61±2 | 61±2 |
| LVES sphericity index, % | 54±9 | 55±8 | 52±2 | 53±2 | 56±2 | 56±2 |
| LV ejection fraction, % | 37±9 | 34±8 | 41±2 | 37±2* | 34±2 | 31±2* |
| LV stroke volume, mL | 50±13 | 58±10* | 54±2 | 60±2* | 47±2 | 55±2* |
| LA geometry | ||||||
| LA diameter, mm | 48±4 | 46±5 | 48±1 | 45±1 | 48±1 | 46±1 |
| LA area, cm2 | 26±5 | 25±4 | 25±1 | 24±1 | 28±1 | 25±1 |
| MR | ||||||
| Vena contracta width, mm | 5.9±0.1 | 3.8±0.1* | 5.9±0.2 | 2.6±0.2* | 5.9±0.2 | 5.0±2.0† |
Data are presented as mean ± SD.
Significant difference (P<0.05) between pre- and postoperative data.
Significant difference (P<0.05) versus mild persistent mitral regurgitation (MR) group. LA Left atrial; LV Left ventricular; LVED Left ventricular end-diastolic; LVES Left ventricular end-systolic; Postop Postoperative; Preop Preoperative
MR jet direction
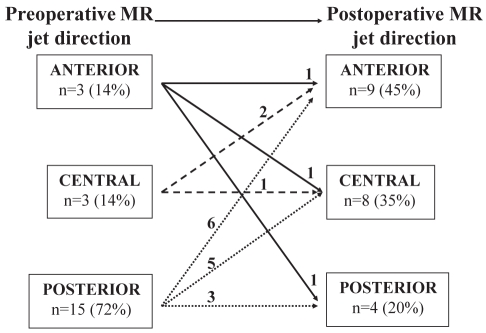
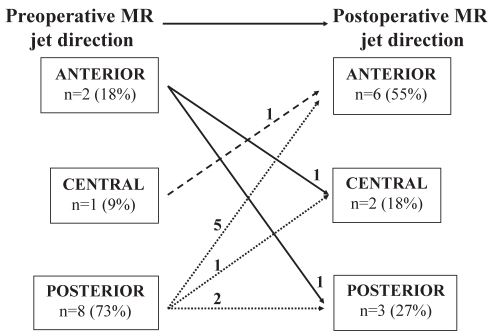
Preoperatively, MR jet direction was posterior in 15 patients and central (n=3) or anterior (n=3) in six patients (72% versus 28%; P=0.002). Postoperative MR jet direction, however, was anterior (n=9) or central (n=8) in 17 patients and posterior in four patients (80% versus 20%; P<0.001). In 16 patients (76%), jet direction was modified postoperatively (Figure 1). In the group of patients with a vena contracta width greater than 3 mm, preoperative MR jet direction was posterior in eight patients and anterior (n=2) or central (n=1) in three patients (73% versus 27%; P=0.09). In this same group, the postoperative MR jet direction was anterior (n=6) or central (n=2) in eight patients and posterior in three patients (73% versus 27%; P=0.09). Moreover, the MR jet direction in nine patients (82%) was different postoperatively than during preoperative evaluation (Figure 2).
Figure 1).
Preoperative mitral regurgitation (MR) jet direction and surgery-induced changes in MR jet direction in the study cohort (n=21)
Figure 2).
Preoperative mitral regurgitation (MR) jet direction and surgery-induced changes in MR jet direction in patients with clinically significant persistent MR (n=11)
Interpretation of postoperative echocardiograms
Clinical interpretation of postoperative echocardiograms is mainly based on subjective evaluation of MR. Hence, the quantification of persistent MR based on jet width was compared with that stated in the clinical report included in the patient’s chart. On this basis, seven of 21 patients (33%) in the entire cohort had an underestimation of their persistent MR severity by at least one grade.
Importantly, in the group of patients with clinically significant persistent MR (vena contracta width greater than 3 mm), MR severity was underestimated and evaluated as nonsignificant in the majority of patients (six of 11 [55%]). In reviewing these studies, it became apparent that the two main reasons explaining this discrepancy were likely the eccentricity of the jet and its change in direction, leading the interpreter to believe that the MR initially seen on the preoperative echocardiogram had largely resolved.
DISCUSSION
The main finding of the present study is that restrictive MVA induces a change in the direction of the jet in the vast majority of patients having persistent MR early after the operation. The diagnosis of persistent MR is important, because it is associated with a high risk of postoperative adverse outcomes. However, the eccentricity and the change in direction of the MR jet associated with the surgery may interfere with the early detection of persistent MR after the operation. There are very few data in the literature about the direction of MR jet in patients with ischemic MR and about the changes in jet direction following MVA. Nonetheless, previous data are consistent with the data of the present study. In our series, the MR jet direction was mainly posterior before surgery (72%) but rarely posterior after annuloplasty (20%). Consistently, Agricola et al (11) reported that the preoperative jet direction was posterior in the vast majority (83%) of their patients with ischemic MR. On the other hand, Zhu et al (6) reported that in a subset of patients (n=6) with persistent MR after surgery, postoperative MR jet direction was most frequently anterior (n=2) or central (n=4). The detection and evaluation of the severity of persistent MR may be difficult because of the eccentricity of the jet. Moreover, our results show that surgery induced changes in the MR jet direction in 76% of the entire cohort and in 82% of the subset of patients with mild to moderate MR (ie, clinically significant MR). Not being aware that the MR jet direction can change following MVA may lead to an underdetection of persistent MR and/or to an underestimation of the severity. The failure to detect and adequately assess persistent MR after MVA may, in turn, negatively affect patient postoperative outcomes, given that persistent MR is associated with poor functional and clinical results (5). Indeed, our comparison of the interpretations of the postoperative echocardiograms shows that subjective evaluations may lead to frequent underestimation of MR severity in this situation. These findings underline the necessity of using proper quantification methods in the presence of an eccentric jet. Quantification of regurgitation using Doppler colour flow imaging is possible in ischemic MR but inappropriate in eccentric jets, where quantitative Doppler study should be recommended (12). Moreover, eccentric MR can also be quantified by measuring vena contracta width, and Lesniak-Sobelga et al (13) have shown excellent correlation between vena contracta width and effective regurgitant orifice area, regurgitant volume and regurgitant fraction.
Several echocardiographic studies have provided new insights into the mechanisms of ischemic MR (6,11). The basic mechanism of ischemic MR is leaflet tethering by the outward displacement of papillary muscles due to LV remodelling. The persistence of MR after MVA can be explained by the postoperative persistence of tethering of both leaflets and/or by worsening of posterior leaflet tethering potentially caused by ring prosthesis implantation, which anteriorly displaces the posterior leaflet and reduces its mobility (5,6,11). Zhu et al (6) have demonstrated that surgical annuloplasty hoists the posterior annulus anteriorly and increases posterior leaflet tethering. Interestingly, in such cases, the anterior leaflet angle was similar before and after surgery, whereas the posterior leaflet angle was significantly increased postoperatively in patients with persistent MR compared with those with nonpersistent MR (111°±13° versus 83°±7°; P<0.05). Thus, it is conceivable that in the subset of patients with insufficient coaptation length, the anterior displacement of the posterior annulus and the augmentation of the posterior leaflet angle may induce a change in the MR jet direction from the posterior region of the atrium pre-operatively to the central or anterior region postoperatively (Figure 3). We recently reported that a preoperative posterior leaflet angle of 45° or greater was the best predictor of postoperative persistence of MR compared with other indexes of valve and LV geometry (ie, tenting area, coaptation distance, LV diameter and sphericity index) as well as with preoperative MR severity (5). The reason why posterior leaflet angle was superior in predicting persistence of MR and outcomes can be explained by the fact that an extreme posterior leaflet angle is usually present in all patients with persistent MR, whatever the pattern of tethering (symmetrical versus asymmetrical tethering).
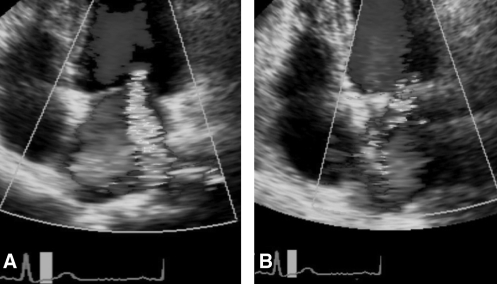
Figure 3).
Example of an echocardiographic four-chamber view of a surgery-induced change in mitral regurgitation (MR) jet direction. A Preoperative posterior MR jet. B Postoperative anterior MR jet
Clinical implications
The persistence of mild to moderate MR after MVA is associated with a dismal prognosis in patients with ischemic MR (5). The results of the present study show that the jet direction often changes after MVA, which could lead to underestimation of or failure to detect the presence of persistent MR after operation. These results have several clinical implications. First, they provide a strong impetus for the development of meticulous intraoperative and early postoperative echocardiographic examinations in several views to search for the presence of any residual MR jet(s). Importantly, the early detection of persistent MR by intraoperative transesophageal echocardiography may influence postoperative outcomes. If persistent MR is detected at the time of intraoperative examination, the surgeon may indeed optimize the MVA and/or perform another concomitant procedure to avoid or reduce the severity of the residual MR. Accurate identification and quantification of persistent MR at the predischarge transthoracic echocardiographic examination may also be useful for risk stratification of patients (5). Patients having mild to moderate persistent MR at this early postoperative examination require a closer follow-up and may need more aggressive treatment.
CONCLUSIONS
The results of the present study show that MR jet direction often changes after restrictive MVA, which may predispose patients to misdiagnosis of persistent MR after the procedure. These results emphasize the importance of meticulous perioperative transesophageal and early postoperative transthoracic echocardiography to correctly identify and thereby improve the clinical management of these high-risk patients with persistent MR.
REFERENCES
- 1.Gillinov AM, Wierup PN, Blackstone EH, et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2001;122:1125–41. doi: 10.1067/mtc.2001.116557. [DOI] [PubMed] [Google Scholar]
- 2.Al-Radi OO, Austin PC, Tu JV, David TE, Yau TM. Mitral repair versus replacement for ischemic mitral regurgitation. Ann Thorac Surg. 2005;79:1260–7. doi: 10.1016/j.athoracsur.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 3.Wu AH, Aaronson KD, Bolling SF, Pagani FD, Welch K, Koelling TM. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol. 2005;45:381–7. doi: 10.1016/j.jacc.2004.09.073. [DOI] [PubMed] [Google Scholar]
- 4.Bolling SF, Pagani FD, Deeb GM, Bach DS. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg. 1998;115:381–6. doi: 10.1016/S0022-5223(98)70282-X. [DOI] [PubMed] [Google Scholar]
- 5.Magne J, Pibarot P, Dagenais F, Hachicha Z, Dumesnil JG, Sénéchal M. Preoperative posterior leaflet angle accurately predicts outcome after restrictive mitral valve annuloplasty for ischemic mitral regurgitation. Circulation. 2007;115:782–91. doi: 10.1161/CIRCULATIONAHA.106.649236. [DOI] [PubMed] [Google Scholar]
- 6.Zhu F, Otsuji Y, Yotsumoto G, et al. Mechanism of persistent ischemic mitral regurgitation after annuloplasty: Importance of augmented posterior mitral leaflet tethering. Circulation. 2005;112(9 Suppl):I396–401. doi: 10.1161/CIRCULATIONAHA.104.524561. [DOI] [PubMed] [Google Scholar]
- 7.St John Sutton M, Otterstat JE, Plappert T, et al. Quantitation of left ventricular volumes and ejection fraction in post-infarction patients from biplane and single plane two-dimensional echocardiograms. A prospective longitudinal study of 371 patients. Eur Heart J. 1998;19:808–16. doi: 10.1053/euhj.1997.0852. [DOI] [PubMed] [Google Scholar]
- 8.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 9.Kono T, Sabbah HN, Rosman H, Alam M, Jafri S, Goldstein S. Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. J Am Coll Cardiol. 1992;20:1594–8. doi: 10.1016/0735-1097(92)90455-v. [DOI] [PubMed] [Google Scholar]
- 10.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. American Society of Echocardiography Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 11.Agricola E, Oppizzi M, Maisano F, et al. Echocardiographic classification of chronic ischemic mitral regurgitation caused by restricted motion according to tethering pattern. Eur J Echocardiogr. 2004;5:326–34. doi: 10.1016/j.euje.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Enriquez-Sarano M, Tajik AJ, Bailey KR, Seward JB.Color flow imaging compared with quantitative Doppler assessment of severity of mitral regurgitation: Influence of eccentricity of jet and mechanism of regurgitation J Am Coll Cardiol 1993211211–9. (Erratum in 1993;22:342). [DOI] [PubMed] [Google Scholar]
- 13.Lesniak-Sobelga A, Olszowska M, Pienazek P, Podolec P, Tracz W. Vena contracta width as a simple method of assessing mitral valve regurgitation. Comparison with Doppler quantitative methods. J Heart Valve Dis. 2004;13:608–14. [PubMed] [Google Scholar]