Abstract
Matchett, William H. (University of California, San Diego, La Jolla, Calif.) and John A. DeMoss. Factors affecting increased production of tryptophan synthetase by a td mutant of Neurospora crassa. J. Bacteriol. 83:1294–1300. 1962.—Mutant td 201 of Neurospora crassa produces a tryptophan synthetase in significantly larger amount than the parental wild-type strain. Evidence obtained in the present investigation indicates that tryptophan synthetase formation in this mutant is subject to end product regulation by a pool of internal tryptophan. An accelerated rate of formation of enzyme was observed when the level of internal tryptophan fell below the value of 1 μmole per g dry weight of mycelium. It is concluded, therefore, that the high level of enzyme found in this strain results from release of repression. In experiments with the parental wild-type strain, no such release of repression was observed. The significance of these results for the problem concerning the nature of the mutation giving rise to mutant td 201 is discussed.
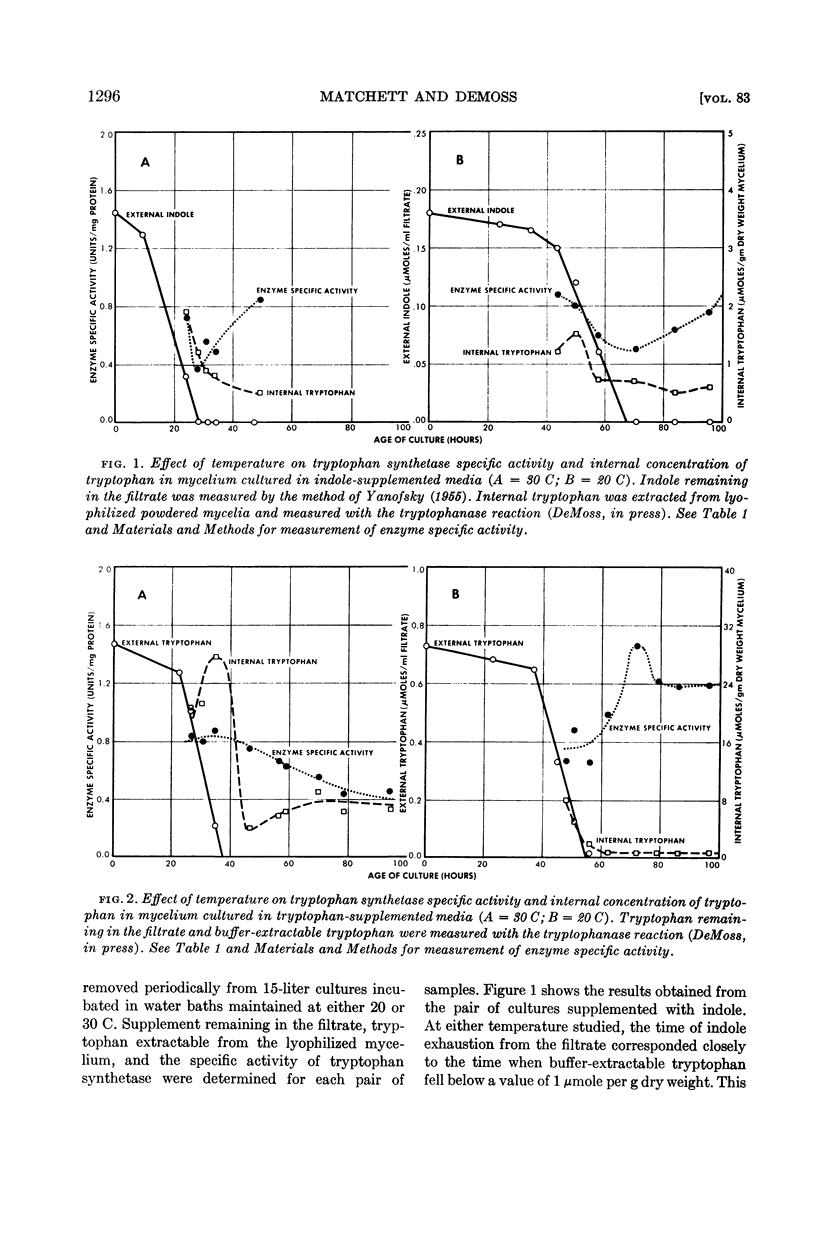
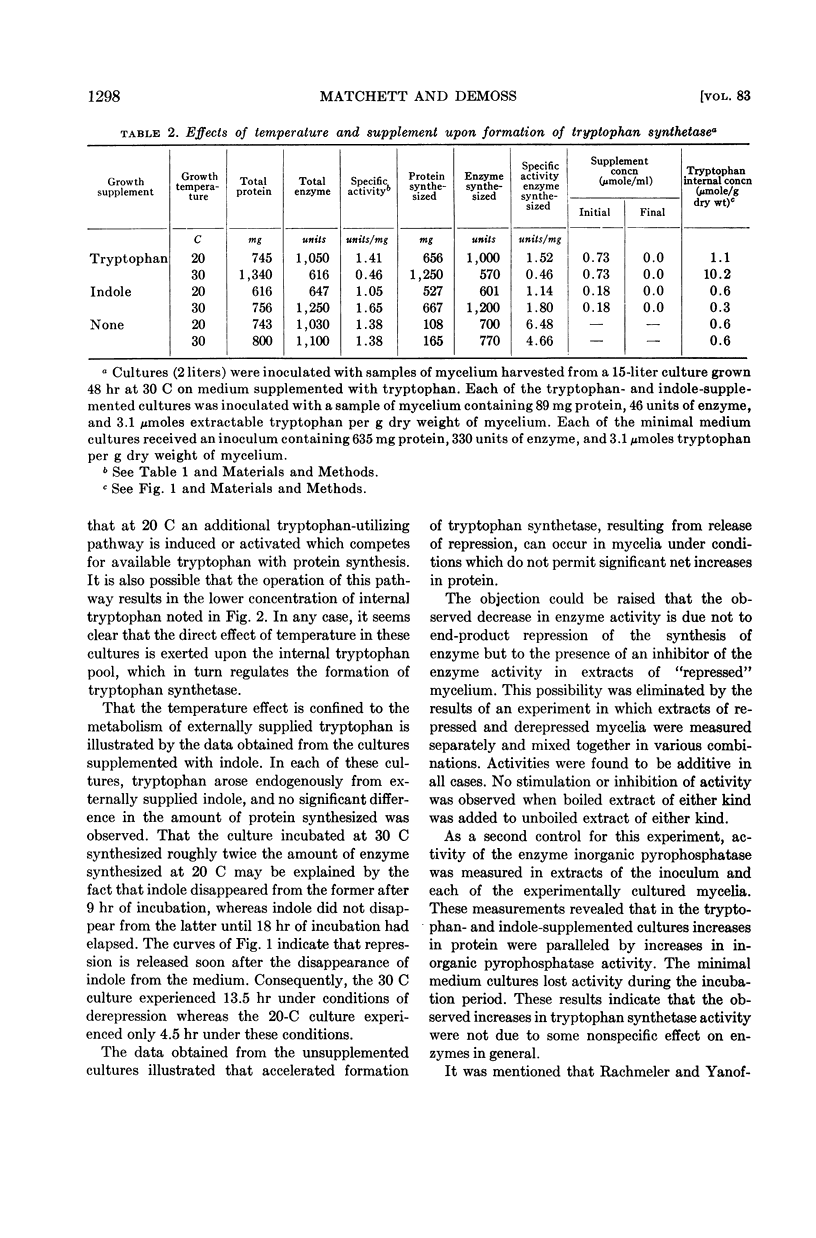
Evidence indicating a marked effect of growth temperature upon metabolism of externally supplied tryptophan is presented.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Demoss J. A., Bonner D. M. STUDIES ON NORMAL AND GENETICALLY ALTERED TRYPTOPHAN SYNTHETASE FROM NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1959 Sep;45(9):1405–1412. doi: 10.1073/pnas.45.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G. Repression and inhibition of indole-synthesizing activity in Neurospora crassa. J Bacteriol. 1961 Aug;82:215–223. doi: 10.1128/jb.82.2.215-223.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G. Some aspects of tryptophan synthetase formation in Neurospora crassa. J Bacteriol. 1961 Jun;81:964–973. doi: 10.1128/jb.81.6.964-973.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- RACHMELER M., YANOFSKY C. Biochemical, immunological, and genetic studies with a new type of tryptophan synthetase mutant of Neurospora crassa. J Bacteriol. 1961 Jun;81:955–963. doi: 10.1128/jb.81.6.955-963.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]



