Abstract
CCL2 is a cytokine prevalent in the prostate cancer tumor microenvironment. Recently, we reported that CCL2 induces the mammalian target of rapamycin (mTOR) pathway to promote prostate cancer PC3 cell survival; however, the mechanism used by CCL2 to maintain mTOR complex-1 (mTORC1) activation requires clarification. This study demonstrates that upon serum starvation, CCL2 functions as a negative regulator of AMP-activated protein kinase (AMPK) by decreasing phosphorylation at its major regulatory site (Thr172) in PC3, DU145, and C4-2B prostate cancer cells. The CCL2-mediated AMPK regulation decreased raptor phosphorylation (Ser792) resulting in hyperactivation of mTORC1. D942, a pharmacological activator of AMPK, stunted CCL2-induced mTORC1 activity, survivin expression, and cell survival without significantly affecting Akt activity. CCL2, however, conferred some resistance to the lethal effect of D942 compared with untreated cells. By using Akt-specific inhibitor X, it was shown that Akt inactivation did not cause an increase in AMPK phosphorylation in CCL2-stimulated cells, suggesting that CCL2-mediated negative regulation of AMPK is independent of Akt. Furthermore, bisindolylmaleimide-V, a specific inhibitor of p70S6K, stunted survivin expression and induced cell death in CCL2-treated PC3. Altogether, these findings suggest that CCL2 hyperactivates mTORC1 through simultaneous regulation of both AMPK and Akt pathways and reveals a new network that promotes prostate cancer: CCL2-AMPK-mTORC1-survivin.
Introduction
AMP-activated protein kinase (AMPK) operates as a cellular energy sensor that is highly specific for AMP and is acutely sensitive to changes in the AMP/ATP ratio. Excessive nutrient depletion or exposure to metabolic stressors rapidly decreases intracellular ATP; the commensurate accumulation of AMP thereby switches on AMPK-mediated energy-generating catabolic processes and turns off energy-consuming processes such as protein synthesis [1–7]. Binding of AMP is thought to enhance AMPK activity both by inducing conformational changes, making AMPK a better substrate for phosphorylation by upstream kinases, including LKB1 (AMPK kinase [AMPKK]), and by preventing subsequent dephosphorylation [1,8]. Conversely, binding of ATP to either the catalytic or the allosteric site antagonizes activation by AMP [8].
Activated AMPK responds by negatively regulating mammalian target of rapamycin (mTOR)-dependent signaling [9]. mTOR operates as a nutrient-sensitive modulator of biogenic and metabolic functions through regulation of key processes, including induction of protein synthesis and inhibition of autophagy [10,11]. mTOR, however, is strictly controlled by interactions with critical binding partners; indeed, mTOR has been confirmed to function as a larger signaling complex (mTORC1), comprising itself and three other subunits: mLST8/GβL, proline-rich Akt substrate 40 (PRAS40), and the regulatory-associated protein of mTOR, raptor [6,11,12]. Raptor interacts directly with mTOR through multiple binding domains and functions as a critical scaffold protein to present regulatory and target substrates (PRAS40, S6K, and eIF4E-binding proteins) to mTORC1 [12,13]. Binding of PRAS40 to the mTORC1 signaling complex attenuates mTOR activity, presumably by inhibition of downstream substrate binding; however, phosphorylation of the Thr246 residue by Akt/PKB relieves PRAS40-mediated mTOR inhibition [13].
It has recently been reported that mTORC1 inhibition, in response to metabolic stress, requires the direct phosphorylation of raptor (Ser792) by AMPK [10]. This inhibition of mTORC1 by AMPK is absolutely essential for activation of the catalytic, ATP-generating process, macroautophagy (herein called autophagy). Importantly, it has been revealed that even basal AMPK activity is sufficient to induce autophagy [5]. In extreme intracellular milieus, cancer cell survival is contingent on acute regulation of energy-consuming processes. Autophagic catabolism of intracellular materials provides the necessary constituents to maintain cellular metabolism. Upon induction of autophagy, unnecessary macromolecules, protein aggregates, and entire organelles are sequestered within double-membrane-bound vesicles (autophagosomes) and delivered to endocytic lysosomes to be degraded, thereby generating these requisite elements. However, autophagy itself is an ATP-consuming process, and consequently, strict regulation is crucial [14–17].
Microtubule-associated protein LC3 is widely used in monitoring autophagy [18]. Upon induction of autophagy, LC3-I is cleaved and conjugated to phosphatidylethanolamine to form LC3-II, a process that is essential for the formation of the autophagosome [19]. Changes in LC3 localization occur as a result of its recruitment to autophagic membranes, and a remarkable increase in punctate LC3 is observable owing to LC3-II colocalization with the autophagosome membranes. Therefore, the amount of LC3-II serves as a good indicator of the number of autophagosomes and of autophagy.
The connection between chemoattractants and inflammation has important implications in tumor progression. It is widely accepted that cytokines elicit a host of responses in the tumor microenvironment, resulting in cell proliferation, differentiation, tumorigenesis, and, recently, in survival [20–23]. CCL2 (monocyte chemoattractant protein-1) is commonly recognized to play a significant role in prostate cancer neoplasia and invasion and is highly expressed in the tumor microenvironment by human bone marrow endothelial cells [24]. We recently reported that treatment of prostate cancer PC3 cells with CCL2 elicits a strong survival advantage by phosphoinositide 3-kinase (PI3K)/Akt-dependent regulation of autophagy through the mTOR pathway while simultaneously upregulating survivin [25,26]. However, the mechanisms through which CCL2 upregulates mTORC1 kinase activity are still poorly understood. Here, we show that CCL2 activates a mechanism that acts in parallel to Akt signaling by inhibiting AMPK/raptor phosphorylation in human prostate cancer PC3 cells. This regulation promotes hyperactivation of mTORC1, sustained survivin expression, and cancer cell survival.
Materials and Methods
Cell Lines
Human prostate cancer cell lines, PC3 and DU145 were obtained from ATCC (Manassas, VA). Cells were maintained in RPMI 1640 (Invitrogen, Carlsbad, CA), supplemented with 10%fetal bovine serum (Invitrogen) and 1% Antibiotic-Antimycotic (Invitrogen). The human prostate C4-2B cells (UroCor, Inc, Oklahoma City, OK) were derived from LNCaP cells through several passages through castrated nude mice and isolated from the tumor that metastasize to bone. C4-2B cells were maintained in T-medium supplemented with 10% fetal bovine serum and 1% Antibiotic-Antimycotic.
WST-1 Cell Viability Assay
Dye conversion of 4-[3-(4-idophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate to formazan (Cell Proliferation Reagent WST-1; Roche, Nutley, NJ) was used to assess cell viability and chemosensitivity of PC3 cells. PC3 cells were grown to 80% confluence then serum-starved for 16 hours in RPMI plus 1% Antibiotic-Antimycotic. Synchronized cells were plated at 104 cells per well into 96-well tissue culture plates (no. 3596; Costar/Corning, Lowell, MA) and allowed to attach for 6 hours. Cells were then treated with increasing concentrations of pharmacological protein kinase inhibitors or activators, in the presence and absence of CCL2 chemokine (Apollo Cytokine Research, Australia), then incubated for 24 to 96 hours. Pharmacological agents used include Akt specific inhibitor X (10-(4′-(N-diethylamino) butyl)-2-chlorophenoxazine, HCl; catalog no. 124020; Calbiochem/EMD Biosciences, San Diego, CA), AMPK inhibitor compound C ((6-[4-(2-piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine; catalog no. 171260; Calbiochem), p70S6K-specific inhibitor bisindolylmaleimide-V (2,3-bis(1H-indol-3-yl)-N-methylmaleimide; catalog no. 203303; Calbiochem), and AMPK activator D942 (5-(3-(4-(2-(4-fluorophenyl)ethoxy)phenyl)propyl) furan-2-carboxylic acid; catalog no. 171256; Calbiochem). WST-1 reagent was added following the manufacturer's instructions, and plates were returned to 37°C for 105 minutes. Dye conversion was ascertained at 440 nm by a VersaMax Microplate Reader and was analyzed using Softmax Pro 3.12 software (Molecular Devices, Sunnyvale, CA). Survival curves were generated using n = 5 sample replicates for each condition ± CCL2 (100 ng/ml). Data for survival curves were normalized to cells untreated with protein kinase activators or inhibitors for both CCL2-stimulated and unstimulated cells. SDs were determined for each set of data points and represented by ±Y error bars.
Western Blot Analysis
Prostate cancer cells (PC3, DU145, C4-2B) were grown to 80% confluence in the appropriate medium. PC3 and DU145 cells were synchronized by starvation in serum-free RPMI 1640 for 16 hours at 37°C. Cells were detached using 0.25 mM EDTA pH 8.0 (Invitrogen), then plated in six-well culture plates at a density of 1.75 x 105 cells/ml. Cells were allowed to attach for 6 hours before treatment with inhibitors (as specified above) and/or CCL2 (100 ng/ml). C4-2B cells were plated in complete medium; 16 hours later, the medium was replaced by serum-free T-medium containing CCL2 (100 ng/ml). After treatment, cells were harvested at increasing time points in cell lysis buffer (no. 9803; Cell Signaling Technology, Danvers, MA). Protein samples were sonicated, followed by centrifugation at 13,000 rpm for 10 minutes. Supernatants were collected, and protein concentrations were determined using the Bradford Assay (Bio-Rad, Hercules, CA). Protein lysates were electrophoresed on 4% to 20% Tris-glycine SDS polyacrylamide gels and transferred onto polyvinylidene fluoride according to Invitrogen instructions. Incubation with antibodies was performed according to Cell Signaling Technology-recommended procedures.
Primary Antibodies
All antibodies against phospho and respective total protein kinases evaluated in these experiments were obtained from Cell Signaling Technology: phospho-p70S6 kinase-Thr389 (catalog no. 9205), p70S6 kinase (catalog no. 9202), phospho-AMPKα-Thr172 monoclonal antibody (mAb; catalog no. 2535), AMPKα (catalog no. 2532), phospho-raptor-Ser792 (catalog no. 2083), raptor mAb (catalog no. 2280), survivin mAb (catalog no. 2808), phospho-Akt-Ser473 mAb (catalog no. 4060), phospho-Akt-Thr308 mAb (catalog no. 4056), Akt (catalog no. 9272), phospho-PRAS40-Thr246 (catalog no. 2640), PRAS40 mAb (catalog no. 2691), LC3B (catalog no. 2775), and β-actin mAb (catalog no. 4967L).
ATP Determination Assay
PC3 cells were synchronized by serum-starvation for 16 hours at 37°C. After synchronization, cells were plated in duplicate at a density of 1.75 x 105 cells per milliliter and allowed to attach to plate surfaces for 6 hours, before treatment, or not, with CCL2 (100 ng/ml). At 48 and 72 hours after treatment, cells were lysed as described above and then sonicated, and cell debris was removed by centrifugation. ATP assay was performed using the Invitrogen ATP determination kit (catalog no. A22066) according to the manufacturer's recommended instructions. Briefly, sample lysates were diluted appropriately in cell lysis buffer, and assay was performed at 28°C in triplicate. Luminescent readings were acquired using a SpectraMax M5 microplate reader in combination with SoftMax Pro v5 software (Molecular Devices). Sample concentrations were calculated based on a standard curve of known ATP concentrations (included in assay kit), and background levels were subtracted before final analyses. Cells from duplicate plates were trypsinized and counted using the Invitrogen Countess automated cell counter to normalize ATP concentrations to cell number. ATP concentrations were then expressed as micromolars of ATP per 105 cells.
Statistical Analysis
All average values are presented a means ± SD (SDs). Data were analyzed using GraphPad Prism software (GraphPad Software, Inc, La Jolla, CA) and one-way ANOVA combined with Kruskal-Wallis test or 2-tailed unpaired t-test. P < .05 was considered significant.
Results
CCL2 Signaling Regulates AMPK/Raptor Phosphorylation in PC3 Cells under Serum Deprivation
Several reports have identified CCL2 as a chemokine that impacts cancer progression and metastasis. Recently, we reported that CCL2 induces the mTOR pathway in human prostate cancer PC3 cells, and this activation requires the induction of the PI3K/Akt signaling [25]. Here, we reveal a parallel pathway that leads to mTORC1 activation by CCL2 in PC3 cells. mTORC1 activation was evaluated by the phosphorylation of its target kinase, p70S6K on the Thr389 residue, which is directly phosphorylated by mTORC1, is sensitive to rapamycin in vivo, and is required for the p70S6K kinase activity [27]. CCL2 induced and sustained a higher phosphorylation of p70S6K kinase (Thr389) compared with serum-starved untreated cells (Figure 1A). Although the Akt pathway is hyperactivated in PC3 cells, it was reasoned that to maintain the steady activation of mTOR, another pathway must coexist. This hypothesis was suggested by the observation that insulin-like growth factor 1, a factor known to activate Akt, was able to induce early phosphorylation of p70S6K, but the stimulation disappeared by 24 hours [25]. Other reports have emphasized the role of AMPK as a master sensor of cellular energy status. AMPK, which is activated under conditions of low intracellular ATP resulting from nutrient and serum deficit, acutely inhibits mTORC1 signaling and induces the autophagy machinery [5,28]. Accordingly, we investigated the possible regulation of AMPK by CCL2. When compared with control cells, CCL2 treatment markedly downregulated AMPK phosphorylation on Thr172, an essential phosphorylation site for the activation of AMPK [29,30] (Figure 1B). A densitometric analysis of (phospho-AMPK)/(total AMPK) is shown in the bar graph (Figure 1B) and corroborates the downregulation of AMPK phosphorylation by CCL2. Furthermore, it was recently demonstrated that AMPK directly phosphorylates raptor on Ser792 to inhibit mTORC1 [10]. Consistent with the negative regulation of AMPK, CCL2-treated cells also showed a significantly lower raptor phosphorylation on Ser792 (Figure 1B, bar graph). In contrast, control cells exhibited a sustained up-regulation of phospho-AMPK in time, which also correlated with the increase in phospho-raptor (Ser792) and the inhibition of mTOR activity (lower phospho-p70S6; Figure 1, A and B).
Figure 1.
CCL2 induces mTORC1 activation and downregulates AMPK/raptor phosphorylation in serum-starved PC3 cells. (A and B) Immunoblot analysis depicting the time-dependent effect of CCL2 (100 ng/ml) on (A) phosphorylation of the p70S6 kinase (Thr389 residue), a direct target of mTORC1, and (B) AMPK/raptor phosphorylation and its correlation with survivin up-regulation. Reduction of AMPK and raptor phosphorylation, in response to CCL2 treatment, was quantified by densitometric analysis; the relative change in spot density was compared with total respective proteins and was normalized to actin (shown in respective bar graphs to the right). (C) Quantification of PC3 cellular ATP 48 and 72 hours for control and CCL2-treated cells. Bar graph depicts ATP concentration (µM per 1 x 105 cells), and is representative of n = 3 bioluminescent ATP determination assays.
Because AMPK is acutely sensitive to changes in the AMP/ATP ratio, the effect of CCL2 on intracellular ATP was further investigated. Thus, a quantitative determination of ATP in cell lysates at 48 and 72 hours, for control and CCL2-treated cells, was performed by using an ATP bioluminescence assay. The results shown in Figure 1C demonstrate a decrease in the ATP levels in time, but no significant differences were observed between untreated and CCL2-stimulated cells. Therefore, the down-regulation of AMPK phosphorylation could not be explained by the CCL2-induced metabolic changes that affect the ATP concentration in the cell.
CCL2-Dependent Regulation of AMPK Signaling Induces mTORC1 Activation and PC3 Cell Survival
Subsequently, the relevance of AMPK regulation in the mechanism of CCL2-dependent mTORC1 activation and PC3 cell survival was addressed. D942 (Calbiochem [31]), an AMPK activator, was used at concentrations of 10 and 20 µM, and the cell viability was measured at 24-hour increments up to 96 hours in control and CCL2-stimulated cells. CCL2-treated cells showed a higher cell survival compared with control cells. D942 generated a minor effect on cell viability at 10 µM (Figure 2Aa); however, when D942 concentration was increased to 20 µM, a considerable decrease in cell viability was observed in both control and CCL2-treated cells (72 and 96 hours after treatment; Figure 2Ab). Furthermore, CCL2 opposed the effect of D942 in cell viability. In fact, as shown in Figure 2Ac, the ratio of [10 µM D942]/[20 µM D942] WST-1 values, corresponding to 72 and 96 hours, demonstrates a greater reduction in cell viability for control cells compared with CCL2-stimulated cells. Conversely, no significant differences were observed at earlier time points (24–48 hours), which correlates with lower levels of AMPK activation (see Figure 1B). These results indicate that CCL2 promotes cell survival and mitigates the lethal effect of D942 in PC3 cells, suggesting that the negative regulation of AMPK is essential in the CCL2-induced cell survival mechanism. To understand the effect of AMPK activation in PC3 cell signaling mechanisms, cell extracts from control and CCL2-stimulated cells, treated or not with 20 µM D942, were collected 48 hours after treatment and subjected to immunoblot analysis. The activation of AMPK by D942 was confirmed by the observed increase in Thr172 phosphorylation (compare lanes 1 and 2 and lanes 3 and 4, Figure 2B). Furthermore, the increase in AMPK activation induced phosphorylation of raptor on Ser792 (Figure 2B, bar graph), corroborating recent findings that raptor is a direct AMPK substrate [10]. As a result, raptor phosphorylation inhibited mTORC1 activity as was demonstrated by a striking decrease in phospho-p70S6K in response to D942 (Figure 2C). Consequently, the stunted mTORC1 activity abruptly abrogated survivin expression in CCL2-treated cells (compare lanes 3 and 4, Figure 2C). These results were accompanied by an increase in LC3B (compare lanes 1 and 2 and lanes 3 and 4, Figure 2C) correlating with reduced cell survival as previously reported [25]. However, it was necessary to determine if D942 also affected Akt activation. Although the phospho-Akt (Ser473 and Thr308) levels were slightly reduced by the activator, the ratios of (phospho-Akt)/(total Akt) remained equal, as the total Akt levels were also reduced (Figure 2B, bar graph). The unaffected Akt activation by D942 was further corroborated by the analysis of the downstream target PRAS40, which has previously been identified as a raptor binding protein and mTORC1 inhibitor [32,33]. Indeed, as PRAS40 is phosphorylated by Akt on Thr246 [32], an Akt inhibition would also result in PRAS40 inhibition. As shown in Figure 2B, no differences were observed in PRAS40 phosphorylation (compare lanes 1 and 2 or lanes 3 and 4), indicating similar Akt activation. Altogether, our results suggest that the inhibition of mTORC1 activity and the increased cell death by D942 are a consequence of AMPK activation rather than changes in the Akt activity, and therefore, the down-regulation of AMPK activity by CCL2 is essential to control mTORC1 activity and cell survival.
Figure 2.
AMPK activator D942 induces raptor phosphorylation and downregulates mTORC1 signaling promoting cell death in PC3, but CCL2 opposes the lethal effect of D942. Cell viability was evaluated by WST-1 dye conversion at 24-hour increments up to 96 hours. (A) a and b: Time course for PC3 cell survival in response to D942: 10 µM (a) or 20 µM (b), ±CCL2. Bar graphs corresponding to control and CCL2-treated cells, without D942, are also included. Improved PC3 survival in response to CCL2 treatment was determined to be significant compared to control at 72 and 96 hours after stimulation, as evaluated by the Student's t-test; P < .0001 were observed. c: Relative effect of increasing D942 from 10 to 20 µM for control and CCL2-stimulated cells. Data for all graphs are representative of n = 5 assays, and SDs are depicted by ±Y error bars for each data point. (B) Western blot analysis using anti-P-Thr172 AMPK and anti-P-Ser792 raptor phospho-specific antibodies depicts increased AMPK/raptor signaling, in response to D942 treatment (20 µM), 48 hours after treatment. Reduction in raptor phosphorylation compared with total protein was verified by densitometric analysis (shown in respective bar graph). Akt activation was also evaluated in response to D942, and the relative change in phospho and total Akt was quantified and analyzed by densitometric analysis with n = 3 samples (shown in bar graph). Phospho-PRAS40 (Thr246) immunoblots were included as a control for Akt-dependent signaling. (C) Western blot analysis representing the effect of D942 on: p70S6K phosphorylation (Thr389), survivin expression, and the autophagic marker LC3-II. Phospho-specific expression was verified by comparison with total respective proteins.
Full Activation of mTORC1 and Cell Survival by CCL2 Stimulation Requires Both Positive Regulation of Akt and Negative Regulation of AMPK
The role of AMPK in CCL2 signaling was further investigated by using an ATP competitive inhibitor of AMPK, compound C (dorsomorphin) [34]. PC3 cell survival was analyzed in the presence of 20 µM of compound C. The inhibitor induced rapid cell death in both control and CCL2-stimulated cells (Figure 3A). Immunoblot analysis of cell extracts, treated or not with compound C, evidenced substantial inhibition of raptor phosphorylation on Ser792 (compare lanes 1 and 2 with lanes 3 and 4, Figure 3B). Because raptor is a downstream target of AMPK, this result demonstrated the effective inhibition of AMPK activity by compound C. However, when Akt signaling was analyzed, it was obvious that strong inhibition of Akt phosphorylation was also induced by compound C both in control and CCL2-treated cells (Figure 3B). This Akt inactivation was also reflected by the inhibition of phospho-PRAS40 (Thr246). Consequently, p70S6K phosphorylation was also inhibited (Figure 3C), correlating with down-regulation of survivin expression, up-regulation of autophagy (higher LC3-II levels), and an increase in cell death (Figure 3, A and C). In summary, although reduced raptor phosphorylation was achieved, the overall effect of compound C resulted instead in the inhibition of mTORC1, which could be explained by the inactivation of the Akt pathway. These results and the above findings with the AMPK activator D942 strongly suggest that both the positive regulation of Akt and the negative regulation of AMPK act in parallel and are required for the full mTORC1 activation. Thus, CCL2 hyperactivates mTORC1 via a coordinated regulation of two signaling pathways: Akt and AMPK. Here, induction of PC3 cell death is the consequence of reduced p70S6K activation by high AMPK phosphorylation or low Akt activation.
Figure 3.
AMPK inhibitor, compound C, stunts Akt-dependent signaling, and PC3 cell survival. (A) Cell viability was evaluated by WST-1 dye conversion at 24 and 48 hours. PC3 cell viability graph bars in response to compound C (20 µM) are shown for control and CCL2-stimulated cells. The data are representative of n = 5 assays for each CCL2-stimulated and control cells. SD bars are depicted for each data point. (B) Western blot depicting reduced AMPK activity in response to compound C (20 µM) in cells treated or not with CCL2 as evaluated by raptor phosphorylation (Ser792). The blots also show how compound C hinders Akt activation by inhibiting phosphorylation of key activation sites (Ser473 and Thr308) and thus preventing phosphorylation of a downstream target PRAS40 (Thr246). (C) Immunoblot analysis illustrating the effect of compound C at 24 hours on p70S6K phosphorylation (Thr389), survivin expression, and the autophagic marker LC3-II. (D) CCL2 regulates AMPK signaling through an Akt-independent mechanism. Serum-starved, CCL2-stimulated PC3 cells were treated with increasing concentrations (1.25, 2.5, and 5 µM) of Akt inhibitor Akti-X (Calbiochem). Western blot analysis reveals how Akti-X effectively inhibits Akt activation/phosphorylation (Ser473 and Thr308) resulting in a reduced PRAS40 phosphorylation (Thr246). AMPK phosphorylation (Thr172) and the induction of autophagy (LC3-II) were evaluated in response to Akti-X. Phospho-specific expression was verified by comparison with total respective proteins, and β-actin is included as a loading control.
By using the Akt-specific inhibitor X (Akti-X [25,35]), it was further demonstrated that CCL2-mediated regulation of AMPK is independent of Akt activation. It was hypothesized that if Akt activation induced a negative regulation of AMPK, as previously reported in other cells [36,37], then Akt inhibition would result in the up-regulation of AMPK phosphorylation. Figure 3D shows that Akti-X effectively inhibited Akt phosphorylation at Ser473 and Thr308 (both residues are necessary for full Akt activation). Similarly, phosphorylation of a downstream target PRAS40 was inhibited by Akti-X. However, no upregulation was observed in phospho-AMPK (Thr172), even in lanes 4 and 5 (Figure 3D), where Akt activation was almost completely stunted by Akt inhibitor X. Conversely, LC3-II dose-dependently increased in response to Akti-X, correlating with an increase in both autophagy and cell death, as previously described [25]. These results strongly suggest that AMPK signaling is regulated by CCL2 independently of Akt activation to induce mTORC1 activity and PC3 cell survival.
p70S6 Kinase Phosphorylation at Thr389 Is Strictly Required for the CCL2-Mediated Survivin Up-regulation and PC3 Cell Survival
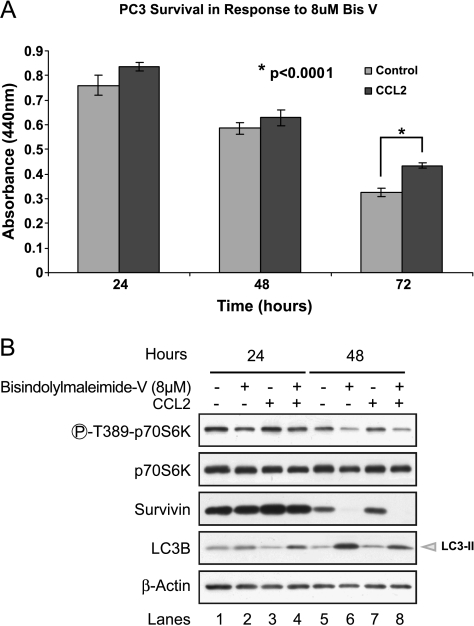
Next, the relevance of p70S6K activation in the CCL2-mediated mechanism of PC3 cell survival was evaluated. The analysis was performed using a cell-permeable compound: bisindolylmaleimide-V (Bis-V), which blocks the activation of p70S6K (IC50 ∼ 8 µM) but has been determined to be inactive for other kinases such as phosphoinositide-dependent protein kinase 1, Akt, protein kinase A, and protein kinase C (IC50 > 50 µM) [38,39]. Previous reports showed that Bis-V inhibition of p70S6K is independent of the upstream activator mTOR and that the residue Thr389 is the critical site for this inhibition [38]. Cell viability assays demonstrated that PC3 both untreated and CCL2-treated cells were in fact sensitive to Bis-V (8 µM; Figure 4A). However, CCL2-treated cells mitigated the effect of p70S6K inhibitor and demonstrated a higher survival at 72 hours after treatment (Figure 4A). Immunoblot analysis of protein extracts revealed an effective blocking of p70S6K phosphorylation by Bis-V (Figure 4B). The inhibitor significantly reduced survivin expression, and consequently, higher levels of LC3-II were observed (Figure 4B, lanes 6 and 8), which correlated with an increase in both autophagy and cell death, as previously reported [25]. Interestingly, the LC3-II increase was higher in control cells than in CCL2-treated cells (compare lanes 6 and 8, Figure 4B), confirming a regulatory role of CCL2 in the control of autophagy. Furthermore, the inhibition of survivin by Bis-V strongly suggests that p70S6K activation by phosphorylation at Thr389 is essential in the mechanism of CCL2-mediated survivin up-regulation and PC3 cell survival. Because p70S6K is regulated by AMPK, this result also links the CCL2-induced cell survival pathway through survivin up-regulation with the control of AMPK activation.
Figure 4.
CCL2-mediated survivin up-regulation and prolonged PC3 cell survival is dependent on p70S6K phosphorylation. (A) Cell viability was evaluated at increasing time points (24, 48, and 72 hours) in response to specific p70S6K inhibitor, Bis-V (8 µM). Data are representative of n = 5 assays. SD bars are depicted for each data point. P value was calculated by the Student's t-test (B) Western blot analysis of cells treated with 8 µM Bis-V in the presence and absence of CCL2. The inhibitor effect was evaluated by the analysis of p70S6K phosphorylation (Thr389), LC3 conversion, and survivin expression. Phospho-specific expression of p70S6K was verified by comparison with the total protein, and β-actin was included as a loading control.
CCL2 Regulates the AMPK Signaling in C4-2B and DU145 Prostate Cancer Cells
The effect of CCL2 in the regulation of AMPK signaling was investigated in C4-2B and DU145 prostate cancer cells upon serum deprivation. Cell lysates from control and CCL2-stimulated cells were collected at different times for the analysis of AMPK activation by Western blot. The results revealed that CCL2 negatively regulated AMPK phosphorylation in both C4-2B and DU145 cells compared with control cells (Figure 5, A and B, bar graphs). These results also correlated with higher phosphorylation of the downstream target raptor (Ser792), suggesting that analogous mechanisms for the negative regulation of AMPK could be activated in other prostate cancer cells to induce mTORC1 signaling and promote survival in cells under nutrient depletion stress.
Figure 5.
CCL2 regulates AMPK signaling in C4-2B and DU145 prostate cancer cells. (A and B) Western blot analysis revealing the inhibition of AMPK/raptor phosphorylation by CCL2 in C4-2B (A) and DU145 (B) prostate cancer cell lines. Reduction in AMPK phosphorylation for both C4-2B and DU145, with respect to total protein, was quantified by densitometric analysis (bar graphs, right). Phosphospecific expression was verified by comparison with total respective proteins, and β-actin was evaluated as a loading control.
Discussion
The cellular response to a shortage of environmental nutrients and resultant loss in energy is a critical pathologic event. Autophagy is an adaptive response to nutrient deprivation and cellular stress to recycle and remove damaged macromolecules and organelles [40]. By mediating catabolism of intracellular contents, autophagy maintains cellular bioenergetics during metabolic stress. Starvation, protein aggregation, and anticancer treatments increase autophagic activity above the basal levels. In solid tumors, cancer cells at the center of a tumor are poorly vascularized, and the induction of autophagy may allow these cells to survive the nutrient depletion stress. However, if the induction of autophagy surpasses the physiological range, it could contribute to cell death, and therefore, strict regulation is crucial in these nutrient-poor settings [15,41–43]. Furthermore, most chemotherapy-resistant cancers exhibit defective apoptosis, and cell death involves the activation of a secondary mechanism [44].
mTOR serves as a major negative regulator of autophagy [14,45]. mTOR functions as a sensor for cellular energy and amino acid levels and acts as a metabolic rheostat-controlling protein synthesis during cellular stress. mTOR links this information with external signals originating from cell surface receptors, and the sensory input is biochemically integrated and coupled to a coordinated response that controls cellular functions. In addition, AMPK is a master regulator of energy balance in the cell, and once activated, it shuts down energy-consuming processes and stimulates the catabolic pathways that generate ATP [8]. The AMPK pathway is linked to mTOR signaling through the ability of AMPK to inhibit mTOR. Thus, the energy demands of protein synthesis can be reduced when cellular energy levels are diminished. We previously reported that CCL2, a chemokine highly prevalent in the tumor microenvironment, induces the mTOR pathway and promotes prostate cancer PC3 cell survival by inhibiting autophagic death through activation of PI3K/Akt signaling and survivin up-regulation [25,26]. In this work, we have examined the possible role of CCL2 in regulating the AMPK activity to control the activation of mTORC1, survivin expression, and cell survival.
First, it was demonstrated that CCL2 hyperactivates mTORC1 in prostate cancer PC3 cells and sustains its activation over time as monitored by phosphorylation of p70S6K (Figure 1A). mTORC1 stimulation was accompanied by a negative regulation of AMPK phosphorylation on the crucial residue (Thr172) [46] (Figure 1B). Furthermore, raptor, an essential component of mTORC1 and a direct target of AMPK [10], was also negatively regulated by CCL2. The AMPK regulation by CCL2 also correlates with the up-regulation of survivin expression that was shown to enhance survival through inhibition of autophagic cell death (Figure 1B) [25].
The significance of AMPK regulation by CCL2 to sustain the mTORC1 activation and PC3 cell survival was demonstrated by using the specific AMPK activator D942. Indeed, D942 induced the AMPK and raptor phosphorylation (Figure 2B), resulting in a significant decrease of p70S6K activation by mTORC1 (Figure 2C). Consequently, the CCL2-induced survivin up-regulation was stunted, correlating with an increase in autophagosome formation (higher LC3-II reflects higher autophagy [19,25]) and rapid induction of cell death (Figure 2, A, a and b, and C). These findings also corroborate our previous data showing that CCL2 modulates the amount and localization of LC3 and that survivin short hairpin RNA induces changes in LC3 punctate pattern that could not be reversed by CCL2 [25]. Furthermore, CCL2-treated cells exhibited a higher resistance to AMPK activator when compared with control cells (Figure 2A, a, b, and c), indicating a crucial role of AMPK signaling in the survival mechanism induced by this cytokine. Concurrently, D942 showed insignificant alteration of Akt activation (Figure 2B, bar graph), suggesting that the changes inmTORC1 and cell survival are a direct consequence of AMPK activation.
Conversely, the ATP competitive inhibitor of AMPK, compound C, induced a strong inhibition of Akt/PRAS40 phosphorylation, and consequently, mTORC1 was also inhibited (Figure 3, B and C). Although the inhibitor resulted in lower raptor phosphorylation (Figure 3B), the mTORC1 complex remained inactive, which could be explained by the inhibition of Akt signaling. Inhibition of autophagy by using compound C has been reported in other mammalian cells such as HT-29 and HeLa cells [5]. However, in PC3 cells, compound C induced autophagic death, as evidenced by the increase in autophagosome formation (higher LC3-II) and lower survivin expression (Figure 3, A and C). In addition, reports in other cells suggest that Akt negatively regulates AMPK phosphorylation [36,37]. However, it was found that CCL2-mediated AMPK regulation is independent of Akt signaling. In fact, the Akt-specific inhibitor X (Akti-X) effectively blocked Akt/PRAS40 phosphorylation and stimulated autophagy (increase in LC3-II); conversely, AMPK was not activated by Akti-X (Figure 3D). In summary, these results suggest that AMPK functions in parallel to Akt signaling; nevertheless, both pathways are indispensable for the induction of mTORC1 signaling and promotion of cell survival. Independent inactivation of any of these two CCL2-induced signals will blunt mTORC1 activity and induce cell death. Moreover, the presented findings also suggest an important physiological role of raptor phosphorylation by AMPK in the control of autophagy. Importantly, this study also shows that under serum deprivation, CCL2 induces a negative regulation of AMPK in other prostate cancer cells: C4-2B and DU145 (Figure 5, A and B). In these cells, survivin is also upregulated by CCL2 [25], suggesting that CCL2 induces an analogous survival mechanism.
Further significance of p70S6K phosphorylation at Thr389 came from the findings that the p70S6K-specific inhibitor, Bis-V, also stunted survivin expression and induced autophagy and cell death (Figure 4, A and B). Because CCL2 is a negative regulator of AMPK, and p70S6K phosphorylation is inhibited by AMPK, these results imply that this is a major mechanism used by CCL2 to control survivin expression and induce prostate cancer cell survival. Figure 6 summarizes this mechanism: CCL2 induces a negative regulation of AMPK and a positive regulation of Akt, and both signaling pathways act in parallel and are necessary to sustain mTORC1 activation and p70S6K phosphorylation to induce survivin up-regulation and promote cell survival.
Figure 6.
Proposed mechanism of mTORC1 regulation by CCL2 in human prostate cancer PC3 cells. CCL2 induces negative regulation of AMPK and positive regulation of Akt; both signaling pathways act in parallel and are necessary to sustain mTORC1 activation and p70S6K phosphorylation over time, leading to survivin up-regulation and prolonged cell survival.
Growing evidence suggests that signaling abnormalities within nutrient signaling pathways can lead to cancer. For example, the AMPK kinase, LKB1, is a tumor suppressor protein that activates AMPK when the AMP/ATP ratio increases in starved cells [47,48]. Mutations in the AMPK kinase, LKB1, are associated with several types of related cancers [47,49,50]. Although it is not clear how CCL2 exerts AMPK regulation, the findings presented here suggest that CCL2 does not induce metabolic changes that affect the ATP concentration in the cell (Figure 1C). Nevertheless, the fact that CCL2 regulates AMPK signaling pathway to sustain mTORC1 activation, survivin expression, and survival in prostate cancer cells suggests that CCL2 and AMPK may serve as therapeutic targets for the treatment of prostate cancer.
Footnotes
K.J. Pienta is supported by a National Institutes of Health grant CA093900, an American Cancer Society Clinical Research Professorship, a National Institutes of Health SPORE in prostate cancer grant P50 CA69568, Cancer Center support grant P30 CA 46592, the Southwest Oncology Group CA32102, and the Prostate Cancer Foundation.
References
- 1.Birnbaum MJ. Activating AMP-activated protein kinase without AMP. Mol Cell. 2005;19:289–290. doi: 10.1016/j.molcel.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Meijer AJ, Codogno P. AMP-activated protein kinase and autophagy. Autophagy. 2007;3:238–240. doi: 10.4161/auto.3710. [DOI] [PubMed] [Google Scholar]
- 4.Meijer AJ, Dubbelhuis PF. Amino acid signalling and the integration of metabolism. Biochem Biophys Res Commun. 2004;313:397–403. doi: 10.1016/j.bbrc.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, Meijer AJ. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281:34870–34879. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- 6.Harris TE, Lawrence JC., Jr TOR signaling. Sci STKE. 2003;2003:re15. doi: 10.1126/stke.2122003re15. [DOI] [PubMed] [Google Scholar]
- 7.Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ. 2008;15:1572–1581. doi: 10.1038/cdd.2008.84. [DOI] [PubMed] [Google Scholar]
- 8.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 9.Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda) 2006;21:48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- 10.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 12.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Harris TE, Roth RA, Lawrence JC., Jr PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- 14.Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12(Suppl 2):1509–1518. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 15.Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Reggiori F, Klionsky DJ. Autophagosomes: biogenesis from scratch? Curr Opin Cell Biol. 2005;17:415–422. doi: 10.1016/j.ceb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Viana R, Aguado C, Esteban I, Moreno D, Viollet B, Knecht E, Sanz P. Role of AMP-activated protein kinase in autophagy and proteasome function. Biochem Biophys Res Commun. 2008;369:964–968. doi: 10.1016/j.bbrc.2008.02.126. [DOI] [PubMed] [Google Scholar]
- 18.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 19.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bar-Shavit Z. The osteoclast: a multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell. J Cell Biochem. 2007;102:1130–1139. doi: 10.1002/jcb.21553. [DOI] [PubMed] [Google Scholar]
- 21.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 22.Pienta KJ, Loberg R. The “emigration, migration, and immigration” of prostate cancer. Clin Prostate Cancer. 2005;4:24–30. doi: 10.3816/cgc.2005.n.008. [DOI] [PubMed] [Google Scholar]
- 23.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 24.Loberg RD, Day LL, Harwood J, Ying C, St John LN, Giles R, Neeley CK, Pienta KJ. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8:578–586. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roca H, Varsos Z, Pienta KJ. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3-kinase/AKT-dependent survivin up-regulation. J Biol Chem. 2008;283:25057–25073. doi: 10.1074/jbc.M801073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roca H, Varsos ZS, Mizutani K, Pienta KJ. CCL2, survivin and autophagy: new links with implications in human cancer. Autophagy. 2008;4:969–971. doi: 10.4161/auto.6822. [DOI] [PubMed] [Google Scholar]
- 27.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Hardie DG. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 31.Kosaka T, Okuyama R, Sun W, Ogata T, Harada J, Araki K, Izumi M, Yoshida T, Okuno A, Fujiwara T, et al. Identification of molecular target of AMP-activated protein kinase activator by affinity purification and mass spectrometry. Anal Chem. 2005;77:2050–2055. doi: 10.1021/ac0484631. [DOI] [PubMed] [Google Scholar]
- 32.Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, Roth RA. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem. 2003;278:10189–10194. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 33.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thimmaiah KN, Easton JB, Germain GS, Morton CL, Kamath S, Buolamwini JK, Houghton PJ. Identification of N10-substituted phenoxazines as potent and specific inhibitors of Akt signaling. J Biol Chem. 2005;280:31924–31935. doi: 10.1074/jbc.M507057200. [DOI] [PubMed] [Google Scholar]
- 36.Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 37.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 38.Marmy-Conus N, Hannan KM, Pearson RB. Ro 31-6045, the inactive analogue of the protein kinase C inhibitor Ro 31-8220, blocks in vivo activation of p70(s6k)/p85(s6k): implications for the analysis of S6K signalling. FEBS Lett. 2002;519:135–140. doi: 10.1016/s0014-5793(02)02738-2. [DOI] [PubMed] [Google Scholar]
- 39.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 40.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 41.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine B. Cell biology: autophagy and cancer. Nature. 2007;446:745–747. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- 43.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 44.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 45.Klionsky DJ, Meijer AJ, Codogno P. Autophagy and p70S6 kinase. Autophagy. 2005;1:59–60. doi: 10.4161/auto.1.1.1536. discussion 60-1. [DOI] [PubMed] [Google Scholar]
- 46.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Marignani PA. LKB1, the multitasking tumour suppressor kinase. J Clin Pathol. 2005;58:15–19. doi: 10.1136/jcp.2003.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei C, Amos CI, Stephens LC, Campos I, Deng JM, Behringer RR, Rashid A, Frazier ML. Mutation of Lkb1 and p53 genes exert a cooperative effect on tumorigenesis. Cancer Res. 2005;65:11297–11303. doi: 10.1158/0008-5472.CAN-05-0716. [DOI] [PubMed] [Google Scholar]