Abstract
Percutaneous devices are critical for health care. Access to tissue, vessels, and internal organs afforded by these devices provides the means to treat and monitor many diseases. Unfortunately, such access is not restricted, and infection may compromise the usefulness of the device and even the life of the patient. New biomaterials offer the possibility of maintaining internal access while limiting microbial access, but understanding of the cutaneous/ biomaterial interface and models to study this area are limited. This review focuses on models useful for studying the morphology and biology of the intersection of skin and percutaneous biomaterials. An organ culture and a mouse model are described that offer promising possibilities for improved understanding of this critical interface.
Keywords: biointegration, cutaneous, epidermis, immunoelectron microscopy, immunohistochemistry, morphology
Introduction
Percutaneous devices are critical for health care. The access provided by intravascular devices, indwelling catheters, glucose sensors, intramedullary prostheses and other devices that penetrate the skin offers the opportunity for improved short- and long-term health. However, this access is not limited to desired effects, and often results in infection, loss of function, and increased risk to the patient. The best estimates of the extent of these problems describe the risk of infection from central venous catheters in the US, which result in as many as 80,000 infections and 20,000 deaths at a cost as high as $2.3 billion annually (Mermel LA, 2000a; Mermel LA, 2000b).
Most early infections of central catheters arise at the interface of the device and the skin (Mermel LA et al., 1991; Raad I et al., 1993). Strategies to address device-related blood stream infections range from simple hand washing to antibiotic-impregnated catheters (Crnich CJ and Maki DG, 2002a; Crnich CJ and Maki DG, 2002b; O'Grady NP et al., 2002). An alternative approach is to design the device to encourage the skin to heal into it. We hypothesize that with this approach one can prevent infection by sealing the interface between the skin and the percutaneous device. To achieve this goal, the interface between the device and the skin must be accessible. This review focuses on models useful for histologic study of the interface between the skin and biomaterials.
Von Recum and Park reviewed percutaneous devices in depth and concluded they “create problems which cannot be overcome at this time [1981]” (von Recum AF and Park JB, 1981). In order to overcome these deficiencies, they suggested that “a surface that allows tissue ingrowth and anchoring” with mechanical properties of the implant material that match those of skin were necessary. They identified critical distinctions, useful for categorizing the types of implants (Table 1).
Table 1.
Types of Percutaneous Implants
| Type | Epithelium | Anchored | Anchor Tissue | Animal |
|---|---|---|---|---|
| Tooth | gingival mucosa/ junctional epithelium |
yes | bone | many |
| Antler | epidermis | yes | bone | cervidae |
| Intramedullary | epidermis | yes | bone | many |
| Urethral | mucosa | no | urethra | many |
| Skin (many)* | epidermis | may be | subcut. tissue | many |
e.g., intravascular, glucose sensor, external ventricular assist)
Von Recum described four types of failure of percutaneous devices (von Recum AF, 1984); marsupialization (the epidermis grows down the side of the implant, creating a pouch), permigration (“the epidermis follows immature connective tissue into the pores of the percutaneous implant and extrudes the implant”), infection, and avulsion (trauma) result in breakdown of the interface of the device with the skin. Von Recum also showed that epidermis and dermis behave similarly in percutaneous implants in dogs, goats and rabbits, and suggested that the choice of an animal model for such studies was not critical (Gangjee T et al., 1985).
The tooth is the best studied percutaneous, biologically anchored implant. Elegant studies demonstrate that even in a setting of high moisture and microbial contamination, percutaneous implants can function effectively (reviewed in Weber HP and Fiorellini JP, 1992). Similarly, percutaneous implants anchored to bone establish a relatively intact cutaneous/ implant interface with formation of a basement membrane zone (Chehroudi B et al., 1991; Kim H et al., 2006). This review will be limited to percutaneous implants that penetrate skin and that are not anchored to bone. These implants are particularly susceptible to microtrauma that disrupts the interface between the skin and biomaterial, leading to inflammation, infection, and loss of the implant.
Previous Studies
Winter reported the histologic picture of percutaneous implants in pigs, showing that porous polytetrafluoroethylene (PTFE) (“GoreTex”) and polyhydroxyethylmethacrylate (polyHEMA) allowed the epidermis to grow in contact with the implants, forming “an apparently stable ring of epidermal tissue around the implants” (Winter GD, 1974). Two-hundred forty implants were studied in 5 pigs. The PTFE membrane pore diameter was about 10 μm; intercommunicating pores in the polyHEMA sponge were “irregular,” but averaged about 40 μm. Implants were studied as long as 10 weeks. Materials were fixed in formalin, paraffin-embedded and sections stained with hematoxylin and eosin (H&E) and other tissue stains. Winter postulated that dermal cells were able to grow into the pores, forming a network of mature connective tissue that prevented marsupialization. Von Recum later postulated that permigration occurred when subepidermal connective tissue was “immature,” allowing epidermis to grow through implant pores (rather than around the implant) and effectively marsupialize the implant at its base (von Recum AF, 1984).
Squier and Collins implanted Millipore filters in the back skin of pigs to investigate the relationship between surface pore size, epithelial migration, and connective tissue attachment (Squier CA and Collins P, 1981). Single filters with pore size ranging from 0.025 to 8.0 μm were inserted into 5 pigs for up to 8 weeks. Material was fixed in buffered formalin, and paraffin-embedded sections stained with H&E and other tissue stains. Marsupialization was seen in all specimens, but epidermal migration was inversely related to pore size and appeared to plateau at 3 μm; connective tissue ingrowth correlated with pore size. The authors postulated that connective tissue ingrowth restricted epithelial migration.
Jansen and de Groot reported on dense hydroxylapatite [note: the authors use the terms hydroxylapatite and hydroxyapatite interchangeably] implants in guinea pigs as part of a study of anchored (bone) and nonanchored implants (Jansen JA et al., 1989; Jansen JA and de Groot K, 1988). Formalin-fixed tissue was embedded in methylmethacrylate and stained with Masson and H&E. Numbers of implants or of animals studied was not reported. Implants that were not anchored were lost within 5 weeks by marsupialization. They then studied a percutaneous implant with the use of a two-stage implant procedure. A sintered titanium fiber mesh sheet was implanted in subcutaneous tissue. A percutaneous hydroxyapatite device was implanted and fastened to the subcutaneous portion 3 to 4 months later (Jansen JA et al., 1991). Fifteen devices were implanted into the dorsum of individual rabbits. Implants were studied 1 and 4 months after the second procedure. Materials were fixed in buffered formalin, embedded in methylmethacrylate, sectioned with a diamond blade saw, and stained with methylene blue and basic fuchsin. Four of the fifteen specimens failed mechanically. The remaining 11 implants healed with no gross signs of inflammation or infection. In some (numbers not reported), “direct epithelial tissue-implant contact” was seen, while in others, epidermal downgrowth proceeded to the subcutaneous titanium mesh with accompanying “mild inflammatory response.” Jansen et al. have continued with studies of similar devices in several mammalian animal models, all anchored by sintered titanium fiber mesh, adapted for glucose sensors and continuous ambulatory peritoneal dialysis catheters. They have developed semiquantitative scoring methods for clinical inflammation and infection at the exit site, downgrowth of epidermis (Walboomers XF and Jansen JA, 2005), and connective tissue ingrowth and reaction to the subcutaneous implants (Jansen JA et al., 1994). Although their histologic techniques preclude many histochemical approaches (Gerritsen M et al., 2000), their efforts to quantify clinical and histologic data surpass those in most similar studies and should be adapted by investigators in the field.
Lundgren and Axelsson described a titanium cylinder implanted in the skin of pigs and humans (Lundgren D and Axelsson R, 1989). The upper portion of the cylinder, in contact with the epidermis, was smooth, while the lower portion, in contact with the dermis, was roughened and ridged with holes on the lateral surface of the ridges. Six implants were installed with the use of “a set of specially designed instruments” in the backs of 3 pigs and examined after 3 and 6 months. Clinical signs of infection or inflammation were not seen. Implants were removed with surrounding tissue, fixed in formaldehyde, and skin tissue removed from the implant and examined by “routine histological” techniques. A sinus tract of ~2 mm was seen, along with ingrowth of “tissue” [? connective] into the dermal portion of the implant. Limited details are presented. Five of the devices were implanted into 4 human volunteers for 18 to 28 months. Cyclic shedding of a “collar” of material consisting of epidermal cells and polymorphonuclear leukocytes, associated with varying erythema and edema of surrounding skin, sinus tract formation, and serous exudate were seen, but significant clinical infection was not noted [bacteriologic studies were not done]. The authors confirmed that the extent of epidermal downgrowth could not be determined in the absence of histologic sections of the device in situ.
Okada and Ikada implanted 2 types of silicone percutaneous devices with and without immobilized collagen. Four devices (2 types, each with and without immobilized collagen) were implanted into the back of rabbits and integration observed as a function of time for up to 9 weeks (Okada T and Ikada Y, 1995). One animal was used for each of 6 time points. Tissue was excised en block, fixed in buffered formalin, and paraffin-embedded sections stained with H&E were studied. Epidermal downgrowth and clinical signs of infection were reduced by collagen immobilization in both types of implant.
Heaney et al. asked the question, can deep connective tissue or muscle, e.g., murine panniculus carnosus, act as a functional barrier to marsupialization (Heaney TG et al., 1996). They implanted 141 smooth-surfaced, polyethylene devices containing a flange beneath the panniculus carnosus and a stem extending through the skin into 71 mice and examined implants up to 11 weeks after implantation. Implants were designed to maximize marsupialization. Devices were excised en block, embedded in plastic and stained with toluidine blue. Data were presented from 98 specimens. Although the length of the epidermal tongue varied considerably, the distance of the leading epithelial edge from the panniculus carnosus remained constant. Inflammation replaced panniculus carnosus as a function of time, as did extrusion of implants and the number of implants with stems buried beneath the skin surface, supporting increasing implant failure. The authors interpreted their results to support the hypothesis that “deep tissue in the form of muscle or granulation tissue ⋯ can act as a barrier to progressive epithelialization.”
Shin and Akao investigated the interface of dense hydroxyapatite implanted in the dorsal skin of dogs (Shin Y and Akao M, 1997). Implants were examined up to 32 weeks after surgery. Tissue was excised and examined by scanning and transmission electron microscopy and by light microscopy, the last after formalin fixation, paraffin embedding and H&E staining. The skin was found to be “in close contact” with the implant, with limited downgrowth and no clinical signs of infection. “Fibroblasts and collagen fibrils ⋯ were well formed and well oriented perpendicular to the shaft” of the implant. From the images it is not possible to evaluate the basement membrane zone of the epidermis adjacent to the shaft of the implant. Incorporation inferior to that seen with dense hydroxyapatite and signs of infection were seen with 2 forms of porous hydroxyapatite implants. The authors report successful maintenance without signs of infection of implants in the forearm of 3 normal human volunteers and of peritoneal catheters with dense hydroxyapatite percutaneous implants in 7 uremic human patients.
Knabe et al. evaluated the cutaneous/ implant interface of 11 Berlin continuous ambulatory peritoneal dialysis catheters implanted in humans from 1.5 to 30 months (Knabe C et al., 1999). No clinical signs of infection were seen. Formaldehyde-fixed, plastic-embedded sections obtained with a diamond saw microtome were stained with von Kossa Fuchsin or Giemsa after surface polishing and viewed by light microscopy. Epithelial downgrowth, scarring, and inflammation were seen.
In all these studies, morphologic assessment was limited by the mechanical properties of the biomaterials and available techniques for the study of the cutaneous/ biomaterial interface (Gerritsen M et al., 2000). Jansen and coworkers were able to demonstrate hemidesmosomes in implants by electron microscopy (Jansen JA et al., 1991), but high resolution light and electron microscopy and immunohistochemistry was limited. Bacterial studies were not reported.
Current studies
We have adapted many of the suggestions of von Recum (von Recum AF and Park JB, 1981) in studying the interface between biomaterial and skin, beginning with the physical properties of the material (Fukano Y et al., 2006; Isenhath SN et al., 2007; Knowles NG et al., 2005). Our initial approach was to identify biomaterials with physical properties similar to skin in order to study the skin/ biomaterial interface by routine morphologic techniques (Knowles NG et al., 2005). These techniques included microtome sectioning of paraffin embedded material after formalin fixation, cryostat sectioning after freezing in O.C.T., and ultramicrotome sectioning after Polybed 812 embedding of material fixed in half strength Karnovsky for electron microscopy. Two materials, polytetrafluoroethylene and poly (2-hydroxyethyl methacrylate) [poly(HEMA)] were sectioned without signs of physical disruption. These two materials were then embedded into punch biopsies of newborn human foreskin, incubated in organ culture, and studied morphologically, demonstrating the ability to study the cutanous/ biomaterial interface by all 3 methods (Figures 1, 2).
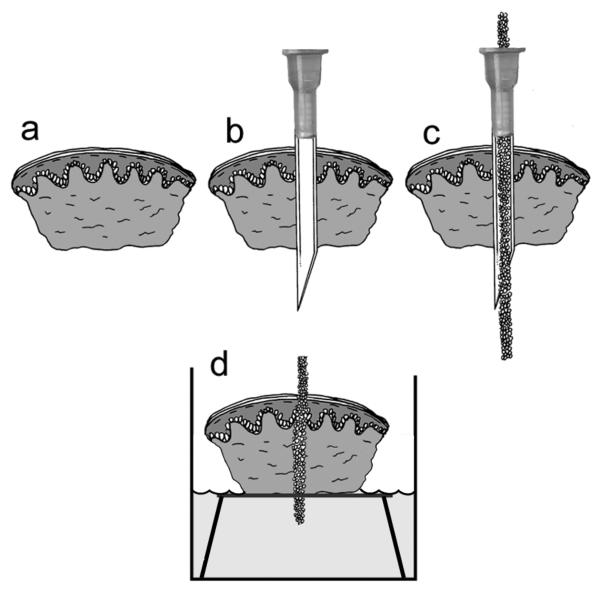
Figure 1.
Organ culture model. A 4mm punch biopsy of newborn foreskin (a) is pierced with an 18-gauge needle (b). Biomaterial is inserted into the barrel of the lumen (c), the needle is withdrawn, and the biopsy containing the implanted biomaterial is cultured at the air-medium interface (d). Modified from Fukano Y, et al., Wound Repair Regen, 14:486, 2006, with permission from the journal.
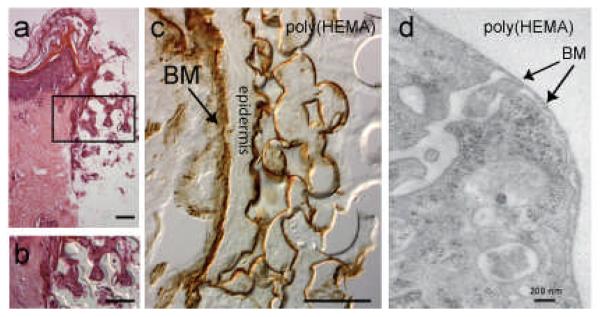
Figure 2.
Morphology of organ culture model after 6 days in culture. (a) H&E stained section of skin/ implant interface. Region within the rectangle is shown at higher magnification in (b). Mag bars = 50 μm. (c) laminin 332 (laminin 5) immunostaining (courtesy of Dr. William Carter, Fred Htuchinson Cancer Research Center, Seattle, WA) showing basement membrane (BM, arrow) along the dermal epidermal junction and within the poly(HEMA) pores. Mag bar = 50 μm. (d) transmission electron micrograph of a keratinocyte within a poly(HEMA) pore showing a basement membrane-like structure (BM) along the surface of the pore. Modified from Fukano Y, et al., Wound Repair Regen, 14:489, 2006, with permission from the journal.
The organ culture model has been optimized to preserve morphologic structure and expression of markers of epidermal functional (Fukano Y et al., 2006). Cutaneous integration into porous poly(HEMA) materials was studied in the organ culture model. Winter showed that porous poly(HEMA) facilitated integration of skin into percutaneous devices. However, the pore size of the material he investigated was variable(Winter GD, 1974). Techniques for the precise control of pore and throat size were employed to engineer porous poly(HEMA) materials with carefully controlled pore and throat diameter and uniform pore shape (Marshall AJ et al., 2004). Surface modification of the material allowed epidermal integration, with the formation of a basement membrane-like structure (demonstrated by electron microscopy) and expression of laminin 332 by (immunohistochemistry) (Figure 2), similar to what is seen in the junctional epithelium of the tooth, one of the two naturally occurring percutaneous devices in nature. Dermal integration was not seen.
The organ culture model has been extended to an animal model (Isenhath SN et al., 2007). Porous poly(HEMA) strips were implanted through an incision in the skin, beneath the panniculus carnosus, and out through a second skin incision of C57BL/6 mice (Figure 3).
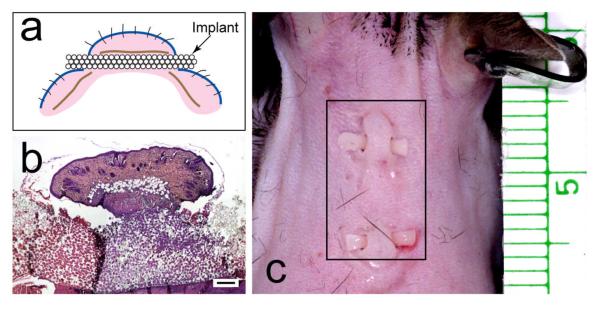
Figure 3.
Mouse implant model. (a) Cartoon of the mouse model. Blue line, epidermis; brown line, panniculus carnosus; pink, dermis. (b) low-power, H&E-stained light micrograph of a sagittal section of implant with skin above and below. Mag bar = 250 μm. (c) Photograph of implants in dorsal skin of mouse. Plate (b) from Isenhath SN, et al., J Biomed Mater Res A 83A:919, 2007, with permission from the journal.
We chose a mouse model for of the ease of study, plethora of reagents, and remarkable utility of mouse models (Bockamp E et al., 2002; Grose R and Werner S, 2004; Reid RR et al., 2004). In vivo studies extend the model to neuroinflammatory and dermal interactions with implanted biomaterials. Material can be implanted with minimal trauma, with retention of implants and histologic demonstration of epidermal and dermal incorporation (Figure 4). Implants have been retained successfully for as long as 35 days (Fukano et al., unpublished).
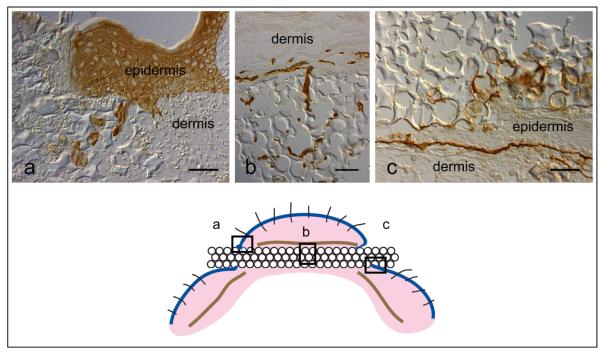
Figure 4.
Mouse implant immunomorphology. 40 μ pore diameter porous poly(HEMA) rod harvested 7 days after implant into a mouse. (a) Pan-keratin stain (Dako Corp, Carpinteria, CA), (b) Vessels [Platelet/ Endothelial cell adhesion molecule-1 (PECAM-1), Research Diagnostics, Concord, MA], (c) Laminin 332 (laminin 5) stain (courtesy of Dr. William Carter, Fred Hutchinson Cancer Research Center, Seattle, WA). The boxes in the cartoon below indicate the areas illustrated. Mag bar = 50 μm.
Von Recum recognized the adverse role of microtrauma on failure of percutaneous devices (von Recum AF and Park JB, 1981). It may be that unanchored percutaneous devices are doomed to failure. However, the models described afford easy access to tissue and advance the opportunity for study of the skin/ implant interface in order to test the hypothesis that new biomaterials will allow stable integration of percutaneous devices. Identifying biomaterials with physical properties similar to those of skin facilitates study of the biology of the interface in ways not demonstrated previously. Molecular interactions with biomaterials can be defined with the use of high resolution light microscopy using histochemical and immunochemical techniques (Figures 2, 3, 4). Conventional transmission electron microscopy expands these studies to the subcellular level (Figure 2). Addition of ultrathin cryomicrotomy offers the possibility of increasing the molecules that can be identified ultrastructurally while maintaining morphology (Tokuyasu KT, 1989). Current studies include modification of the animal model to improve the cutaneous/ implant interface while minimizing trauma, quantification of percutaneous integration, and assessment of bacterial infection and biofilm formation.
Acknowledgements
Supported by NSF EEC 9529161, NIH DK 59221, USPHS NIBIB 1R01 EB004422-03, the Warren G. Magnuson Research Grant, the George F. Odland Endowed Research Fund, and the Marvin and Judy Young Research Fund. Special thanks to Marcia Usui for figures.
References
- 1.Bockamp E, Maringer M, Spangenberg C, Fees S, Fraser S, Eshkind L, et al. Of mice and models: improved animal models for biomedical research. Physiol Genomics. 2002;11:115–32. doi: 10.1152/physiolgenomics.00067.2002. [DOI] [PubMed] [Google Scholar]
- 2.Chehroudi B, Gould TR, Brunette DM. A light and electron microscopic study of the effects of surface topography on the behavior of cells attached to titanium-coated percutaneous implants. J. Biomed. Mater. Res. 1991;25:387–405. doi: 10.1002/jbm.820250310. [DOI] [PubMed] [Google Scholar]
- 3.Crnich CJ, Maki DG. The promise of novel technology for the prevention of intravascular device-related bloodstream infection. I. Pathogenesis and short-term devices. Clin. Infect. Dis. 2002a;34:1232–42. doi: 10.1086/339863. [DOI] [PubMed] [Google Scholar]
- 4.Crnich CJ, Maki DG. The promise of novel technology for the prevention of intravascular device-related bloodstream infection. II. Long-term devices. Clin. Infect. Dis. 2002b;34:1362–68. doi: 10.1086/340105. [DOI] [PubMed] [Google Scholar]
- 5.Fukano Y, Knowles NG, Usui ML, Underwood RA, Hauch KD, Marshall AJ, et al. Characterization of an in vitro model for evaluating the interface between skin and percutaneous biomaterials. Wound. Repair Regen. 2006;14:484–91. doi: 10.1111/j.1743-6109.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- 6.Gangjee T, Colaizzo R, von Recum AF. Species-related differences in percutaneous wound healing. Ann. Biomed. Eng. 1985;13:451–67. doi: 10.1007/BF02407772. [DOI] [PubMed] [Google Scholar]
- 7.Gerritsen M, Lutterman JA, Jansen JA. The influence of impaired wound healing on the tissue reaction to percutaneous devices using titanium fiber mesh anchorage. J. Biomed. Mater. Res. 2000;52:135–41. doi: 10.1002/1097-4636(200010)52:1<135::aid-jbm17>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Grose R, Werner S. Wound-healing studies in transgenic and knockout mice. Mol. Biotechnol. 2004;28:147–66. doi: 10.1385/MB:28:2:147. [DOI] [PubMed] [Google Scholar]
- 9.Heaney TG, Doherty PJ, Williams DF. Marsupialization of percutaneous implants in presence of deep connective tissue. J. Biomed. Mater. Res. 1996;32:593–601. doi: 10.1002/(SICI)1097-4636(199612)32:4<593::AID-JBM12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Isenhath SN, Fukano Y, Usui ML, Underwood RA, Irvin CA, Marshall AJ, et al. A mouse model to evaluate the interface between skin and a percutaneous device. J. Biomed. Mater. Res. A. 2007;83:915–22. doi: 10.1002/jbm.a.31391. [DOI] [PubMed] [Google Scholar]
- 11.Jansen JA, de Groot K. Guinea pig and rabbit model for the histological evaluation of permanent percutaneous implants. Biomaterials. 1988;9:268–72. doi: 10.1016/0142-9612(88)90096-8. [DOI] [PubMed] [Google Scholar]
- 12.Jansen JA, Dhert WJ, van der Waerden JP, von Recum AF. Semi-quantitative and qualitative histologic analysis method for the evaluation of implant biocompatibility. J. Invest Surg. 1994;7:123–34. doi: 10.3109/08941939409015356. [DOI] [PubMed] [Google Scholar]
- 13.Jansen JA, van der Waerden JP, de Groot K. Epithelial reaction to percutaneous implant materials: in vitro and in vivo experiments. J. Invest Surg. 1989;2:29–49. doi: 10.3109/08941938909016502. [DOI] [PubMed] [Google Scholar]
- 14.Jansen JA, van der Waerden JP, de Groot K. Development of a new percutaneous access device for implantation in soft tissues. J. Biomed. Mater. Res. 1991;25:1535–45. doi: 10.1002/jbm.820251210. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Murakami H, Chehroudi B, Textor M, Brunette DM. Effects of surface topography on the connective tissue attachment to subcutaneous implants. Int. J. Oral Maxillofac. Implants. 2006;21:354–65. [PubMed] [Google Scholar]
- 16.Knabe C, Grosse-Siestrup C, Gross U. Histologic evaluation of a natural permanent percutaneous structure and clinical percutaneous devices. Biomaterials. 1999;20:503–10. doi: 10.1016/s0142-9612(98)00195-1. [DOI] [PubMed] [Google Scholar]
- 17.Knowles NG, Miyashita Y, Usui ML, Marshall AJ, Pirrone A, Hauch KD, et al. A model for studying epithelial attachment and morphology at the interface between skin and percutaneous devices. J. Biomed. Mater. Res. A. 2005;74:482–88. doi: 10.1002/jbm.a.30384. [DOI] [PubMed] [Google Scholar]
- 18.Lundgren D, Axelsson R. Soft-tissue-anchored percutaneous device for long-term intracorporeal access. J. Invest Surg. 1989;2:17–27. doi: 10.3109/08941938909016501. [DOI] [PubMed] [Google Scholar]
- 19.Marshall AJ, Irvin CA, Barker T, Sage EH, Hauch KD, Ratner BD. Biomaterials with tightly controlled pore size that promote vascular in-growth. Polym. Preprints. 2004;45:100–101. [Google Scholar]
- 20.Mermel LA. Correction: Cather-related bloodstream infections. Ann. Intern. Med. 2000a;133:395. [Google Scholar]
- 21.Mermel LA. Prevention of intravascular catheter-related infections. Ann. Intern. Med. 2000b;132:391–402. doi: 10.7326/0003-4819-132-5-200003070-00009. [DOI] [PubMed] [Google Scholar]
- 22.Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery Swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am. J. Med. 1991;91:197S–205S. doi: 10.1016/0002-9343(91)90369-9. [DOI] [PubMed] [Google Scholar]
- 23.O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm. Rep. 2002;51:1–29. [PubMed] [Google Scholar]
- 24.Okada T, Ikada Y. Surface modification of silicone for percutaneous implantation. J. Biomater. Sci. Polym. Ed. 1995;7:171–80. doi: 10.1163/156856295x00689. [DOI] [PubMed] [Google Scholar]
- 25.Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J. Infect. Dis. 1993;168:400–407. doi: 10.1093/infdis/168.2.400. [DOI] [PubMed] [Google Scholar]
- 26.Reid RR, Said HK, Mogford JE, Mustoe TA. The future of wound healing: pursuing surgical models in transgenic and knockout mice. J. Am. Coll. Surg. 2004;199:578–85. doi: 10.1016/j.jamcollsurg.2004.05.262. [DOI] [PubMed] [Google Scholar]
- 27.Shin Y, Akao M. Tissue reactions to various percutaneous materials with different surface properties and structures. Artif. Organs. 1997;21:995–1001. doi: 10.1111/j.1525-1594.1997.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 28.Squier CA, Collins P. The relationship between soft tissue attachment, epithelial downgrowth and surface porosity. J. Periodontal Res. 1981;16:434–40. doi: 10.1111/j.1600-0765.1981.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 29.Tokuyasu KT. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem. J. 1989;21:163–71. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]
- 30.von Recum AF. Applications and failure modes of percutaneous devices: a review. J. Biomed. Mater. Res. 1984;18:323–36. doi: 10.1002/jbm.820180403. [DOI] [PubMed] [Google Scholar]
- 31.von Recum AF, Park JB. Permanent percutaneous devices. Crit Rev. Bioeng. 1981;5:37–77. [PubMed] [Google Scholar]
- 32.Walboomers XF, Jansen JA. Effect of microtextured surfaces on the performance of percutaneous devices. J. Biomed. Mater. Res. A. 2005;74:381–87. doi: 10.1002/jbm.a.30337. [DOI] [PubMed] [Google Scholar]
- 33.Weber HP, Fiorellini JP. The biology and morphology of the implant-tissue interface. Alpha. Omegan. 1992;85:61–64. [PubMed] [Google Scholar]
- 34.Winter GD. Transcutaneous implants: reactions of the skin-implant interface. J. Biomed. Mater. Res. 1974;8:99–113. doi: 10.1002/jbm.820080311. [DOI] [PubMed] [Google Scholar]