Context
The Colorectal Cancer Surveillance Panel presents ASCO's 2005 practice guideline update for follow up after primary therapy of stage II or III colorectal cancer. The Panel performed a complete literature review from 1999 through June 2005 and met twice to assemble its recommendations.
Updated 2005 Recommendations
See Table 1 for a summary of the updated 2005 recommendations.
Table 1.
Updated 2005 ASCO recommendations for colorectal cancer surveillance
| Surveillance Tool | Recommendations |
|---|---|
| CT |
|
| Chest x-ray | Yearly chest x-rays are not recommended. |
| Colonoscopy |
|
| Flexible proctosigmoidoscopy | Every 6 months for 5 years for rectal cancer patients who have not been treated with pelvic radiation. |
| History and physical examination and risk assessment |
|
| CEA |
|
| Routine blood tests | CBCs or liver function tests are not recommended. |
| Laboratory-derived prognostic and predictive factors | Until prospective data are available, molecular or cellular markers should not influence the surveillance strategy. |
Abbreviations: CT, computed tomography; CEA, carcinoembryonic antigen.
Imaging Procedures
Computed tomography. Patients who are at higher risk of recurrence, and who could be candidates for curative-intent surgery, should undergo annual computed tomography (CT) of the chest and abdomen for 3 years after primary therapy for colon and rectal cancer. A pelvic CT scan should be considered for surveillance after rectal cancer therapy, especially for patients who have not been treated with radiotherapy. This recommendation represents a major departure from previous ASCO guidelines, in which CT was not recommended.
Chest x-ray. Annual chest x-rays are not recommended. Recommending routine chest CT settled previous disagreements among Panel members about the use of routine chest x-ray.
Endoscopic Surveillance Techniques
Colonoscopy. All patients with colon and rectal cancer should have a colonoscopy for the pre- or perioperative documentation of a cancer- and polyp-free colon. After the surgical treatment of colorectal cancer, the Panel recommends the surveillance guideline presented by the American Gastroenterological Association (AGA): a colonoscopy at 3 years and, if normal, every 5 years thereafter. For colorectal cancer patients with high-risk genetic syndromes, the physician should consider the guideline published by the AGA (available at http://www.gastrojournal.org/article/PIIS0016508502158951/fulltext). The reference for colonoscopy surveillance was updated.
Flexible proctosigmoidoscopy (rectal cancer). For patients who have not received pelvic radiation, flexible sigmoidoscopy of the rectum every 6 months for 5 years is recommended. The recommendation for flexible proctosigmoidoscopy for rectal cancer remains essentially unchanged.
History and Physical Examination and Risk Assessment
Coordinating physician visits should occur every 3 to 6 months for the first 3 years, every 6 months during years 4 and 5, and subsequently at the discretion of the physician. Physician visits should focus on the initial risk assessment followed by the implementation of a surveillance strategy and periodic counseling based on estimated risk and feasibility of surgical interventions such as hepatic resection. These recommendations do not represent a major departure from previous versions of the guideline.
Laboratory Tests
Carcinoembryonic antigen. Postoperative serum carcinoembryonic antigen (CEA) testing should be performed every 3 months in patients with stage II or III disease for at least 3 years after diagnosis, if the patient is a candidate for surgery or systemic therapy (adapted from the 2005 Update of the ASCO Clinical Practice Guideline for the Use of Tumor Markers in Gastrointestinal Cancer). Since fluorouracil-based therapy may falsely elevate CEA values, waiting until adjuvant treatment is finished to initiate surveillance is advised.
Blood tests. Routine blood tests (i.e., CBCs or liver function tests) are not recommended. The 2005 Literature Update is not applicable.
Fecal occult blood test. Periodic fecal occult blood testing is not recommended. The 2005 Literature Update is not applicable.
Laboratory-derived prognostic and predictive factors. Until prospective data are available, use of molecular or cellular markers should not influence the surveillance strategy. This section represents a new category of surveillance for the 2005 update.
Discussion
The most significant change in the updated guideline concerns the use of CT. Prior versions of the ASCO guideline have recommended against CT surveillance. In recent meta-analyses, however, CT scanning or liver imaging was associated with a survival benefit; specifically, the mortality rate for patients who undergo liver imaging is 25% lower than that for patients who do not undergo such imaging. Pelvic CT should be considered for rectal cancer patients with several poor prognostic factors, especially patients who have not been treated with radiation. In addition, although the Panel acknowledges that there is less evidence for surveillance with chest CT, it now recommends the procedure on several grounds. First, the largest proportion of resectable recurrences is found on chest CT. Second, recurrence in the lung is as common as liver relapse in patients with rectal cancer and represents the largest proportion of resected metastases. Finally, recurrence in the lung is less likely to be associated with elevated levels of CEA.
The actual recommendations for the frequency of history and physical examination with risk assessment do not represent a major departure from previous versions of the guideline. Eighty-five percent of recurrences are diagnosed within the first 3 years after surgical resection of the primary tumor. The Panel has tried to help the oncologist incorporate risk of recurrence into the formulation of the follow-up plan for the individual patient. There was wide agreement that not everyone with stage II and III colorectal cancer should undergo CT scanning. Nevertheless, the Panel could not define a precise percentage risk level (or set of clinical factors) at which CT screening should begin. Currently, other than stage and subsets within a stage, there is no single pathologic feature or statistical model that can be used to build a surveillance strategy. The Mayo Clinic or Adjuvant! calculators http://www.mayoclinic.com/calcs or http://www.adjuvantonline.com) estimate 5-year relapse-free survival both with and without treatment using data available on most pathology reports. Therefore, communication regarding how many of the specific guidelines to follow should occur early on after treatment is finished. Physicians may find ASCO's Colon and Rectal Cancer Follow-Up Flow Sheets (available online at http://www.asco.org/guidelines) useful to print out for documentation and planning. Longer follow-up may be appropriate for locally advanced rectal cancer patients with poor prognostic factors.
Methodology
The literature review for the update focused on three randomized clinical trials and three recent meta-analyses of data from randomized clinical trials. The updated review reflects evidence on both specific methods of surveillance and risk stratification. The individual randomized trials differed in the actual tests that were evaluated and the interval between tests, representing discrepancies that were mentioned in the previous update of this guideline. Each of the meta-analyses involved evaluation of five or six randomized trials in which low-intensity and high-intensity programs of surveillance were compared for patients who underwent curative-intent surgery for adenocarcinoma of the colon and rectum. The Panel did not complete an independent meta-analysis of the data from available randomized clinical trials given the availability of three high-quality and recent meta-analyses identified through the literature search.
Additional Resources
In addition to the full text of the guideline, available online at www.asco.org/guidelines, further online resources include a patient guide, a PowerPoint slide set, and surveillance flow sheets for use in individual patient follow-up.
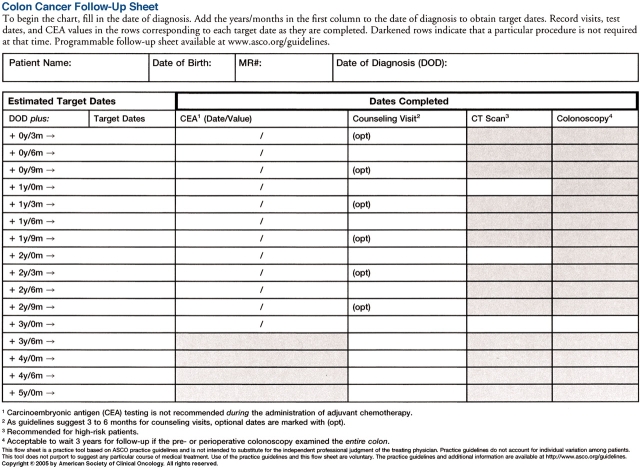
Figure 1.
ASCO's Colon Cancer Follow-up Sheet (available on p. 179)
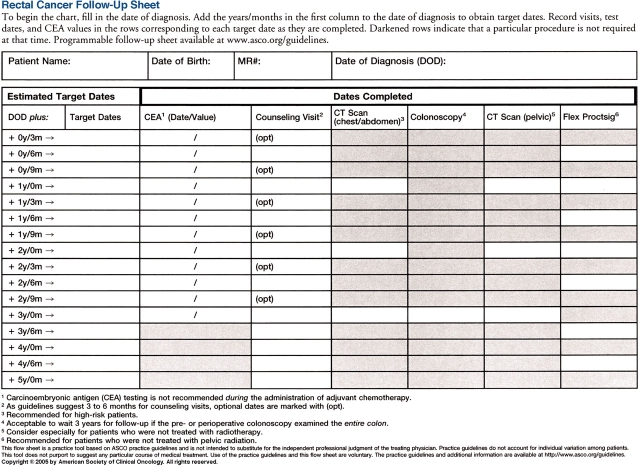
Figure 2.
ASCO's Rectal Cancer Follow-Up Sheet (available on p. 180)
It is important to realize that many management questions have not been comprehensively addressed in randomized trials and guidelines cannot always account for individual variation among patients. A guideline is not intended to supplant physician judgment with respect to particular patients or special clinical situations and cannot be considered inclusive of all proper methods of care or exclusive of other treatments reasonably directed at obtaining the same results. Accordingly, ASCO considers adherence to this guideline to be voluntary, with the ultimate determination regarding its application to be made by the physician in light of each patient's individual circumstances. In addition, the guideline describes administration of therapies in clinical practice; it cannot be assumed to apply to interventions performed in the context of clinical trials, given that clinical studies are designed to test innovative and novel therapies in a disease and setting for which better therapy is needed. Because guideline development involves a review and synthesis of the latest literature, a practice guideline also serves to identify important questions for further research and those settings in which investigational therapy should be considered.
Footnotes
The Colorectal Cancer Surveillance Practice Guideline was developed and written by Christopher E. Desch, Al B. Benson, III, Mark R. Somerfield, Patrick J. Flynn, Carol Krause, Charles L. Loprinzi, Bruce D. Minsky, David G. Pfister, Katherine S. Virgo, and Nicholas J. Petrelli.