Abstract
Determinative events in vertebrate embryogenesis appear to require the continuous expression of spatial regulators such as the clustered homeobox genes. The mechanisms that govern long-term patterns of gene expression are not well understood. In Drosophila, active and silent states of developmentally regulated loci are maintained by trithorax and Polycomb group. We have examined the developmental role of a mammalian homolog of trx and putative oncogene, Mll. Knockout mice reveal that Mll is required for maintenance of gene expression early in embryogenesis. Downstream targets of Mll including Hoxa7 are activated appropriately in the absence of Mll but require Mll for sustaining their expression. The Mll−/− phenotype manifests later in development and is characterized by branchial arch dysplasia and aberrant segmental boundaries of spinal ganglia and somites. Thus, Mll represents an essential mechanism of transcriptional maintenance in mammalian development, which functions in multiple morphogenetic processes.
During vertebrate development, regions of the embryo become committed to position-specific identities (1, 2). For example, primordial thoracic segments retain their positional identity and form ribs even when ectopically placed into developing cervical regions (3). Moreover, genes that regulate spatial identities such as the evolutionarily conserved homeobox (Hox) genes display committed patterns of expression and are autonomously maintained (4). Studies of Hox gene regulation reveal dual phases of control: an early phase, which establishes the initial anteroposterior pattern of Hox expression, and a late phase, which is required for sustaining this pattern over successive cell generations (5–8). The maintenance of stable patterns of Hox expression may reflect more general mechanisms of gene regulation. In Drosophila, epigenetic mechanisms contribute to the long-term control of gene expression. Trithorax group (trxG) and Polycomb-group (PcG) maintain expression or repression of Antennapediea and bithorax homeotic gene complexes (9, 10) as well as other patterning genes including engrailed (11, 12) and forkhead (13). trxG and PcG proteins are found in association with chromatin and within large multimeric complexes; however, their mechanism of action is not completely understood (14).
Mammalian homologs of trx and PcG genes also have been identified and have been shown to regulate Hox expression (15, 16). Mll (also known as Hrx/All1) resembles trx and was isolated originally as a common target of chromosomal translocations in human acute leukemias (17, 18). Mll and trx encode large nuclear proteins with 9–10 zinc-finger motifs and a highly conserved 200 amino acid SET domain located at their carboxyl-terminal ends. Mll is widely expressed during embryogenesis. Gene-targeting studies in mice demonstrated that Mll is a positive regulator of Hox genes (19). Hox expression is shifted posteriorly in Mll heterozygous (+/−) embryos and completely abolished in Mll homozygous null (−/−) embryos. Shifts in Hox expression also are observed in mice with targeted mutations in PcG (20–23). Collectively, these findings indicate that, at least in part, trxG and PcG functions are conserved in mammals. Curiously, spatial patterns of Hox expression in PcG mutant and Mll mutant embryos once established remain stable over multiple embryonic stages. Whether trxG/PcG acts as maintenance factors in vertebrate development is not known. In this study, we determine that Mll affects gene maintenance rather than activation and explore the timing and biological relevance of maintenance mechanisms in mammalian development.
METHODS
Generation and Genotyping of Mll−/− Embryos.
Knockout mice carrying an insertion of lacZ in the Mll gene were described (19). Mll+/− females were hypofertile but could be effectively bred between 8 and 14 weeks of age with proven Mll+/− males. Vaginal plugs were counted as embryonic day 0.5 (E0.5) of gestation (24, 25). Genomic DNA was prepared from yolk sacs of E9 and younger embryos and from aminotic sacs of older embryos as described (26, 27). Genotyping of embryos was performed by PCR by using a common upstream primer ST2B+ and two downstream primers, ST2B- (wild-type allele) and ST2L- (recombinant allele). Cycle conditions were as follows: 94°C × 5 min; 30 cycles of 94°C × 1 min, 60°C × 1 min, 72°C × 1 min; 72°C × 5 min. This PCR produced an 840-bp wild-type allele and a 400-bp recombinant allele. Primers used include ST2B+ (5′-GAACAGCAGATTCAGCGCCACG-3′), ST2B- (5′-GGACGCTCCAGAAGAAGTTCGATTA-3′), and ST2L- (5′-GAACAAACGGCGGATTGACCGTAATG-3′). Mll-lacZ+/− animals were identified from positive tail biopsies stained overnight in a X-Gal-staining solution (1.65 mg/ml K4Fe(CN)6⋅3H2O/2.1 mg/ml K3Fe(CN)6/2 mM MgCl2/0.1% sodium deoxycholate/0.1% Nonidet-40/0.8 mg/ml X-Gal in PBS).
Histology and in Situ Cell Death Assay.
Embryos prepared for histology were fixed in either newly prepared 4% paraformaldehyde in PBS or in 10% formalin and embedded in paraffin. Serial sections (5–7 μm) were stained with hematoxylin and eosin. To examine programmed cell death in situ, tissues were fixed in formalin and embedded in paraffin. The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling method (28) (TUNEL) was used on 5- to 7-μm sections of 2–3 embryos of each genotype and performed according to manufacturer’s methods (TdT TUNEL assay kit, Trevigen, Gaithersburg, MD). Briefly, sections were treated with proteinase K and hydrogen peroxide before incubation in terminal deoxynucleotidyltransferase and biotin-labeled nucleotides. Apoptotic cells then could be detected by staining with a strepavidin-horseradish peroxidase conjugate.
In Situ Hybridization and Immunohistochemistry.
In situ hybridizations on sections were performed with 33P-labeled (Amersham) riboprobes whereas whole mount in situ hybridizations were stained with digoxygenin-labeled riboprobes according to manufacturer’s protocols (Boehringer Mannheim). Details of in situ hybridization protocols are described elsewhere (29). Riboprobes were generated from Hoxa7 (363 bp SmaI), Hoxc8, Pax1, and myf5 DNA templates, and both sense and anti-sense orientations were tested on wild-type embryos. For whole mount neurofilament staining, embryos were fixed in 4% paraformaldehyde in PBS, stained with 2H3 mouse mAb and peroxidase-conjugated rabbit secondary antibody (Sigma), and developed with 4-chloro-1-naphtol (30). En1 and En2 staining were performed at 1:100 dilution of αEnhb-1 rabbit antibody as described (31). Stained trunks were bisected, cleared in glycerol, and mounted on coverslips. Whole embryos were photographed on agarose plates by using an Olympus SZH bifocal dissecting scope (New Hyde Park, NY). Sections and flat-mounted tissue were scanned by the Leaf Micro Lumina camera system (Zeiss) mounted on a Zeiss Axiophot microscope. Images were captured on an Apple Macintosh 7500 by using adobe photoshop 3.0. In situ hybridization experiments were photographed under dark field optics. For all studies described here, three or more embryos of each genotype and embryonic stage were studied with the exception of in situ hybridization of sections. Serial sections of two embryos of each genotype were used for the analysis of Pax1 and myf5 expression.
RESULTS
Mll regulates Hoxa7, Hoxc9, and other Hox genes. Hoxa7 regulation undergoes activation between E7.5 and E8.5 followed by a later stage of tissue-specific maintenance (7). To investigate whether Mll plays a role in gene activation or maintenance, Hoxa7 expression was examined in Mll−/− embryos from E8 and time points thereafter. Before E9, the pattern and level of Hoxa7 expression were similar between wild-type (Fig. 1A) and Mll−/− embryos (Fig. 1B). Expression of Hoxa7 was seen in the neuroectoderm and the presomitic mesoderm of E8.5 embryos. At E9, somitic expression of Hoxa7 in wild-type (not shown) and heterozygous embryos (Fig. 1C) demonstrated establishment of the anterior boundary of Hoxa7. Hoxa7 expression was relatively weak in the neural tubes of wild-type embryos at this stage. In contrast, Mll−/− embryos showed no substantial expression of Hoxa7 in the somites or neural tube at E9, and only faint staining could be detected in the presomitic mesenchyme (Fig. 1D). Hoxc8, which is regulated by early and late response elements (8), also failed to maintain expression beyond E9 (not shown). Gene expression was not globally suppressed in Mll−/− embryos as determined by in situ hybridization of somite lineage markers (see below). Thus, the loss of late Hox expression in the absence of Mll indicates that Mll functions as a maintenance factor.
Figure 1.
Defective maintenance of Hoxa7 expression in Mll−/− embryos. Whole mount in situ hybridization of E8.5 and E9 control and Mll−/− embryos (lateral views). (A) Hoxa7 expression in the presomitic mesoderm and neural plate of E8.5 wild-type embryos. (B) Hox-7 expression in E8.5 Mll knockout embryos. Arrows indicate the anterior most extent of Hoxa7 expression. (C) Hoxa7 expression in somites 15–18 and presomitic mesoderm in E9 Mll+/− embryos. (D) Absence of Hoxa7 expression in somites of E9 Mll−/− embryos. Faint expression was detected in Mll−/− embryos proximal to the allantois. Arrows point to the anterior boundary of somite 15 (prospective eleventh vertebrae).
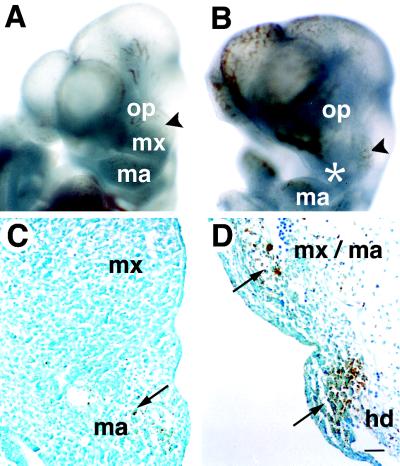
Persistent expression of spatial regulators has been implicated in the control of committed identities of tissues such as neural crest and somites (1, 2). To determine the fate of cranial neural crest in the absence of Mll, we examined branchial arch development and cranial ganglia patterning. Mll knockout mice were embryonic lethal at E10.5 and displayed hypoplasia of all branchial arches and involution of the maxillary process (Fig. 2 A and B). The mesenchyme of arch tissue was hypocellular and showed evidence of apoptosis as confirmed by TUNEL staining (Fig. 2 C and D). Neural crest cells, which contribute to the expansion and outgrowth of arch mesenchyme, also play an important role in the development of other craniofacial structures including cranial ganglia (32).
Figure 2.
Branchial arch abnormalities in Mll knockout embryos. Gross views and TUNEL staining of control and Mll−/− E10.5 embryos. (A) Wild-type embryo (oblique view), demonstrating normal structures of the first branchial arch, maxillary prominence (MX), the mandibular component (MA), and associated trigeminal ganglia (arrowhead). (B) Mll−/− embryo revealing branchial arch hypoplasia and defects in the development of the maxilla (asterisk). (C) First branchial arch of Mll+/+ embryos. Apoptotic cells are indicated by long arrows. (D) First and second branchial arches of Mll−/− embryos; same magnification as (C). Note the severe hypocellularity and apoptosis in the arch mesenchyme but not the surface ectoderm. op; optic vesicle; HD, hyoid or second branchial arch. (Scale bar = 50 μm.)
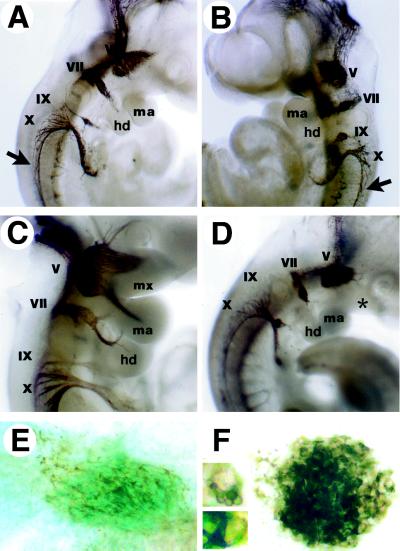
Whole mount immunohistochemical staining for neurofilament revealed that early cranial ganglia development, migration, and differentiation proceeded normally in the absence of Mll. However, slight changes in cranial gangliogenesis became apparent as early as E10 (Fig. 3 A and B). Facial and nodose ganglia of Mll knockout embryos consistently displayed a compact, globoid morphology, which was not representative of any stage of normal development. By E10.5, whereas Mll wild-type and heterozygous embryos showed prominent nerve tracts innervating the arches, Mll−/− cranial ganglia continued to retain this unusual morphology and failed to elaborate nerve fibers toward branchial arch targets (Fig. 3 C and D) Flat-mounted facial ganglia revealed abundant neurofilament but were defective in neurite outgrowth (Fig. 3 E and F). Branchial arch tissues have a chemotropic role in cranial ganglia development (33), and thus defects in the cranial nerves may reflect underlying problems in the branchial arches. In support of this thesis, vagal and spinal accessory nerves, which have targets outside the branchial arches, were relatively spared in the Mll−/− embryos.
Figure 3.
Cranial ganglia development in wild-type and mutant embryos at E10 and E10.5. Neurofilament immunostaining of whole embryos and isolated facial ganglia (lateral views). (A) E10 wild-type embryo demonstrating normal morphology of cranial ganglia. (B) E10 Mll−/− embryo displaying condensed morphology of ganglia and present branchial arch structures. Spinal accessory nerves are indicated by arrows. (C) E10.5 Mll+/− embryo showing cranial innervation of the branchial arches. (D) E10.5 Mll−/− cranial ganglia are condensed and innervation of the branchial arches is absent. Defective positioning of the nodose ganglia was noted in only one of six knockout embryos. Note hypoplastic branchial arch development (∗). (E) Flat mount of Mll+/− facial ganglia; blue staining reflects expression of Mll-lacZ marker in cranial ganglia. (F) Mll−/− facial ganglia. (Inset) Magnified view of cells found within the facial ganglia. V, trigeminal; VII, facial; IX, glossopharyngeal; X, vagal nerves.
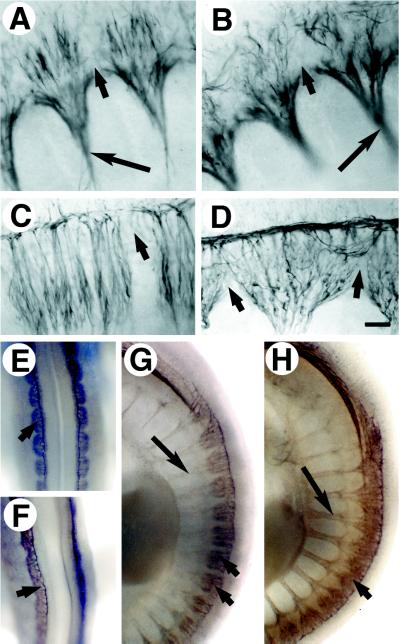
Neural patterning in the trunk also was affected by the loss of Mll. Mll−/− spinal ganglia showed disorganized neurite outgrowth as early as E10 (Fig. 4 A and B) and progressive loss of segmental boundaries through E10.5 (Fig. 4 C and D). Neurite projections from spinal ganglia crossed into neighboring rostral and caudal segments, and spinal ganglia became extensively fused in the Mll−/− E10.5 embryos (Fig. 4E–H). The anterior spinal ganglia were affected more severely than the posterior spinal ganglia suggestive of defect in the later stages of sensory nerve patterning. Motor nerves whose development precedes that of spinal ganglia showed a normal segmented distribution in the absence of Mll (Fig. 4 A and B).
Figure 4.
Defective neurite outgrowth and segmentation of Mll−/− spinal ganglia. Immunohistochemical staining for neurofilament was performed on E10 and E10.5 embryos. (A) Flat mount of E10 Mll+/+ spinal ganglia and motor nerves (long arrow). Short arrows indicate boundaries between somites. (B) E10 Mll−/− spinal ganglia and motor nerves from similar axial level as shown in A. Neurites of knockout spinal ganglia are disorganized but did not cross somite boundaries. (C) E10.5 wild-type spinal ganglia. (D) E10.5 Mll−/− spinal ganglia demonstrating aberrant morphology and loss of segmental restriction. (E) Dorsal view of segmented E10.5 Mll+/+ spinal ganglia at the forelimb level. Short arrows indicate spinal ganglia. (F) Fused E10.5 Mll−/− spinal ganglia. (G) Lateral view of segmented spinal ganglia and spinal nerves in E10.5 Mll+/− embryo. (H) E10.5 Mll−/− embryo displays spinal ganglia fusion. Fusion of spinal ganglia was pronounced less posteriorly. Note the segmented pattern of spinal and motor nerves.
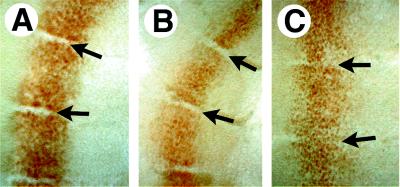
The segmental pattern of spinal ganglia is determined by neighboring somites during trunk neural crest migration and neurite outgrowth (34, 35). To explore somite boundaries, we examined the distribution of Engrailed-1 (En1), a segmentally expressed homeobox gene. Sharp boundaries of En1 expression normally are present in both the newly formed posterior somites and differentiated anterior somites throughout the trunk (Fig. 5A) (31). Although Mll−/− embryos retained a normal segmental distribution of En1 expression posteriorly (Fig. 5B), they failed to maintain sharp boundaries of En1 expression anteriorly (Fig. 5C). This may reflect a loss of segmental boundaries between differentiated somites of the Mll−/− embryos rather than a regulatory defect in En1 because En1 and En2 expression was not altered elsewhere in the embryo (not shown).
Figure 5.
Distribution of EN1-positive cells in E10.5 somites of wild-type and mutant embryos (lateral views). Flat-mounted somites immunostained with EN1/2 antibody. (A) EN1 expression in anterior somites of wild-type embryos; EN1 expression at this stage was in the dermomyotome. Somites shown are at the level of the forelimb. Arrows indicate the somite boundaries. (B) EN1 in posterior somites of Mll−/− embryo. (C) EN1 expression in anterior somites of Mll−/− embryos taken at the level of the forelimb. Note the presence of EN1 positive cells between somites.
Histologic analysis of somitic tissue revealed disrupted somite architecture (Fig. 6 A and B). Extensive cell death was observed in somites of Mll−/− embryos as detected by TUNEL whereas only rare apoptotic cells were observed in wild-type and heterozygous embryos (Fig. 6 C and D). In contrast, Mll−/− spinal ganglia and spinal cord at E10.5 did not show significantly increased cell death.
Figure 6.
Somite architecture and cell death in wild-type and Mll−/− E10.5 embryos. Hematoxylin and eosin staining of lateral sections and TUNEL staining of transverse sections. (A) wild-type somites; note normal morphology of somite epithelium indicated by arrows. (B) Mll−/− somites demonstrating disrupted epithelial boundaries ventrally. (C) Normal cell death in wild-type somites revealed by TUNEL. (D) Cell death in Mll−/− somites; note the relative sparing of spinal ganglia (sg) and neural tube (nt). Arrows indicate areas of apoptosis. (Scale bars = 50 μm.)
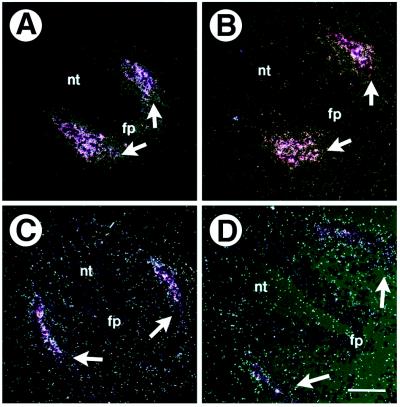
Somites differentiate into sclerotome and dermomyotome; the sclerotome of differentiated somites has been shown to provide segmental signals to migrating trunk neural crest and axons of spinal ganglia (2). Somite maturation as assessed by expression of the sclerotome and dermomyotome lineage markers, Pax1 (Fig. 7 A and B) and myf5 (Fig. 7 C and D), respectively, was not appreciably altered in the Mll−/− embryos. These results argue that during differentiation, Mll is essential for the maintenance of segmental boundaries in somites and spinal ganglia.
Figure 7.
Differentiation of somites in wild-type and Mll−/− E10.5 embryos. In situ hybridization of Pax-1 (A and B) and myf-5 (C and D) performed on transverse sections. (A) Pax-1 expression in the sclerotome of wild-type embryo. White arrows show areas of expression. (B) Pax-1 expression in Mll−/− embryo. (C) Myf-5 expression in the dermomyotome of Mll+/+ embryo. (D) Myf-5 expression in Mll−/− embryo. nt, neural tube; fp, floor plate. (Scale bars = 50 μm.)
DISCUSSION
In this study, we show that Mll acts as a maintenance factor necessary for correct development of multiple tissues during embryogenesis. Mll is ubiquitously expressed and is required for successful skeletal (19), hematopoietic (27), neural, and craniofacial development. Maintenance mechanisms have been proposed to impart stable gene expression important for retaining cell identity and positional cues during cell proliferation, migration, and differentiation. The complex phenotype of the Mll−/− embryos could reflect the lack of sustained expression of spatial regulators. For example, the effects of Mll deficiency on branchial arch development and cranial nerve outgrowth are reminiscent of defects described in Hox knockout models (36). Progressive aberrations in the segmentation of somites and spinal ganglia also were found in Mll knockout embryos. Defective maintenance of genes involved in specifying the rostrocaudal polarity of somites might explain the later onset of segmentation defects in the Mll−/− embryos. The anteroposterior fate of Mll−/− somites also may be transformed but could not be accurately assessed because of early embryonic lethality. Segmentation defects in the somites might reflect the anterior transformation of mesoderm in the Mll−/− embryos. Interestingly, the anterior most mesoderm does not normally form discrete somites (6). The distribution of Mll−/−-derived cells in a chimeric mouse model would help to define their capacity to acquire different anteroposterior fates.
We have identified a critical interval between E8.5 and E9 after which gene expression becomes dependent on Mll. This stage in development may reflect a dynamic period of chromatin reorganization, which leads to either silencing or persistent expression of developmentally regulated loci. In the Mll knockout embryos, this balance may be influenced by loss of positive signals and/or by unopposed PcG proteins resulting in the silencing of Hox expression. The observed shifts in Hox patterns in Mll(+/−) and PcG mutant mice also may reflect defects incurred in this early period. During this critical window, patterns of Hox expression appear to stabilize set by the relative levels of trxG/PcG proteins. Interactions among trx and PcG proteins are thought to transmit active and silent chromatin states to successive cell generations thereby creating stable patterns of gene expression (37). Motifs within Mll such as the AT-hook and SET domain have been implicated in chromatin regulation (38, 39). Recently described interactions of the Mll SET domain with components of the SWI/SNF complex argue that Mll is involved in recruiting chromatin remodeling machinery and opening repressed loci (40, 41). The Mll knockout mice provide a model system for studying maintenance mechanisms involved in patterning the vertebrate body plan.
Acknowledgments
We thank Raphael Kopan for critical review of this manuscript. We thank P. Gruss for Hoxa7 and Pax1; A. Schumacher for Hoxc8; J. Miner for myf5; A. Joyner for αEnhb-1 antibody; and Developmental Studies Hybridoma Bank for 2H3 neurofilament antibody. We thank Mary Pichler for expert secretarial assistance. This work was supported in part by a grant from The Parker Hughes Trust, B.D.Y. was supported by a training grant from the National Institutes of Health, and R.D.H. was supported by a physician fellowship from the Howard Hughes Medical Institute.
ABBREVIATIONS
- trxG
trithorax-group
- PcG
Polycomb-group
- En1
Engrailed-1
- Hox
homeobox
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling
- E0.5–E10.5
embryonic day 0.5–10.5
References
- 1.Noden D M. J Neurobiol. 1993;24:248–261. doi: 10.1002/neu.480240210. [DOI] [PubMed] [Google Scholar]
- 2.Tam P P L, Trainor P A. Anat Embryol. 1994;189:275–305. doi: 10.1007/BF00190586. [DOI] [PubMed] [Google Scholar]
- 3.Kieny M, Mauger A, Sengel P. Dev Biol. 1972;28:142–161. doi: 10.1016/0012-1606(72)90133-9. [DOI] [PubMed] [Google Scholar]
- 4.Beddington R S P, Püschel A W, Rashbass P. In: Postimplantation Development in the Mouse. Chadwick D J, Marsh J, editors. New York: Wiley; 1992. pp. 61–77. [Google Scholar]
- 5.Deschamps J, Wijgerde M. Dev Biol. 1993;156:473–480. doi: 10.1006/dbio.1993.1093. [DOI] [PubMed] [Google Scholar]
- 6.Gaunt S J, Strachan L. Dev Dyn. 1994;199:229–240. doi: 10.1002/aja.1001990307. [DOI] [PubMed] [Google Scholar]
- 7.Püschel A W, Balling R, Gruss P. Development (Cambridge, UK) 1991;112:279–287. doi: 10.1242/dev.112.1.279. [DOI] [PubMed] [Google Scholar]
- 8.Bieberich C J, Utset M F, Awgulewitsch A, Ruddle F H. Proc Natl Acad Sci USA. 1990;87:8462–8466. doi: 10.1073/pnas.87.21.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis E B. Nature (London) 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 10.Sedkov Y, Tillib S, Mizrokhi L, Mazo A. Development (Cambridge, UK) 1994;120:1907–1917. doi: 10.1242/dev.120.7.1907. [DOI] [PubMed] [Google Scholar]
- 11.Chinwalla V, Jane E P, Harte P J. EMBO J. 1995;14:2056–2065. doi: 10.1002/j.1460-2075.1995.tb07197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moazed D, O’Farrell P H. Development (Cambridge, UK) 1992;116:805–810. doi: 10.1242/dev.116.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzin B, Tillib S, Sedkov Y, Mizrokhi L, Mazo A. Genes Dev. 1994;8:2478–2490. doi: 10.1101/gad.8.20.2478. [DOI] [PubMed] [Google Scholar]
- 14.Pirrotta V. Cell. 1998;93:333–336. doi: 10.1016/s0092-8674(00)81162-9. [DOI] [PubMed] [Google Scholar]
- 15.Gould A. Curr Opin Genet Dev. 1997;7:488–494. doi: 10.1016/s0959-437x(97)80075-5. [DOI] [PubMed] [Google Scholar]
- 16.Schumacher A, Magnuson T. Trends Genet. 1997;13:167–170. [PubMed] [Google Scholar]
- 17.Tkachuk D C, Kohler S, Cleary M L. Cell. 1992;71:691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- 18.Gu Y, Nakamura T, Alder H, Prasad R, Canaani O, Cimino G, Croce C M, Canaani E. Cell. 1992;71:701–708. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- 19.Yu B D, Hess J L, Horning S E, Brown G A J, Korsmeyer S J. Nature (London) 1995;378:505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- 20.van der Lugt N M T, Alkema M, Berns A, Deschamps J. Mech Dev. 1996;58:153–164. doi: 10.1016/s0925-4773(96)00570-9. [DOI] [PubMed] [Google Scholar]
- 21.Akasaka T, Kanno M, Balling R, Mieza M A, Taniguchi M, Koseki H. Development (Cambridge, UK) 1996;122:1513–1522. doi: 10.1242/dev.122.5.1513. [DOI] [PubMed] [Google Scholar]
- 22.Coré N, Bel S, Gaunt S J, Aurrand-Lions M, Pearce J, Fisher A, Djabali M. Development (Cambridge, UK) 1997;121:2847–2852. doi: 10.1242/dev.124.3.721. [DOI] [PubMed] [Google Scholar]
- 23.Takihara Y, Tomotsune D, Shirai M, Katoh-Fukui Y, Nishii K, Motaleb M A, Nomura M, Tsuchiya R, Fujita Y, Shibata Y, et al. Development (Cambridge, UK) 1997;124:3673–3682. doi: 10.1242/dev.124.19.3673. [DOI] [PubMed] [Google Scholar]
- 24.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 25.Kaufman M H. The Atlas of Mouse Development. San Diego: Academic; 1992. [Google Scholar]
- 26.Laird P W, Zijderveld A, Linders K, Rudnicki M A, Jaenisch R, Berns A. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hess J L, Yu B D, Li B, Hanson R, Korsmeyer S J. Blood. 1997;90:1799–1806. [PubMed] [Google Scholar]
- 28.Gavrieli Y, Sherman Y, Ben-Sasson S A. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson D G, Neito M A. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- 30.Swiatek P J, Gridley T. Genes Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- 31.Davis C A, Holmyard D P, Millen K J, Joyner A L. Development (Cambridge, UK) 1991;111:287–298. doi: 10.1242/dev.111.2.287. [DOI] [PubMed] [Google Scholar]
- 32.Serbedzija G N, Bronner-Fraser M, Fraser S E. Development (Cambridge, UK) 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- 33.Davies, A. M. (1988) Development (Cambridge, U.K.) 103, Suppl., 175–183. [DOI] [PubMed]
- 34.Kalcheim C, Teillet M-A. Development (Cambridge, UK) 1989;106:85–93. doi: 10.1242/dev.106.1.85. [DOI] [PubMed] [Google Scholar]
- 35.Keynes R, Cook G, Davies J, Lumsden A, Norris W, Stern C. J Physiol (Paris) 1990;84:27–32. [PubMed] [Google Scholar]
- 36.Gavalas A, Studer M, Lumsden A, Rijli F M, Krumlauf R, Chambon P. Development (Cambridge, UK) 1998;125:1123–1136. doi: 10.1242/dev.125.6.1123. [DOI] [PubMed] [Google Scholar]
- 37.Paro R. Curr Opin Cell Biol. 1993;6:373–379. doi: 10.1016/0955-0674(93)90084-4. [DOI] [PubMed] [Google Scholar]
- 38.Girard F, Bello B, Laemmli U K, Gehring W J. EMBO J. 1998;17:2079–2085. doi: 10.1093/emboj/17.7.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laible G. EMBO J. 1997;16:3219–3232. doi: 10.1093/emboj/16.11.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roszenblatt-Rosen O, Rozovskaia T, Burakov D, Sedkov Y, Tillib S, Blechman J, Nakamura T, Croce C M, Mazo A, Canaani E. Proc Natl Acad Sci USA. 1998;95:4152–4157. doi: 10.1073/pnas.95.8.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kingston R E, Bunker C A, Imbalzano A N. Genes Dev. 1996;10:905–920. doi: 10.1101/gad.10.8.905. [DOI] [PubMed] [Google Scholar]