Abstract
Background
Antiretroviral therapy (ART) affects cardiovascular disease (CVD) risk. In the general population, hsCRP is an established predictor of future coronary events. Little is known about its utility in chronic inflammatory conditions such as HIV-infection. We assessed relationships between hsCRP and metabolic parameters over time in HIV-infected patients on ART.
Methods
Data are from a prospective cohort of HIV-infected adults enrolled June 2005-July 2007. Participants were receiving ART, had HIV-1 RNA <10,000 copies/mL, and no diabetes or CVD. Non-linear mixed effect regression models assessed relationships between body mass index (BMI), lipids, and hsCRP over time adjusting for covariates.
Results
94 individuals had data from ≥ 1 study visit. Median age was 44 years, 27% were female, 57% white, and 54% were on protease inhibitors. Median CD4+ T-cells, HIV-1 RNA, and hsCRP were 502 cells/mm3, 50 copies/mL, and 2.94 mg/L, respectively. Median Framingham score was 3. Multivariate analysis identified associations between increased hsCRP and greater BMI (p=0.001), higher non-HDL-cholesterol (p=0.013) and triglycerides (p=0.017), and lower HDL-cholesterol (p=0.015).
Conclusions
Among HIV-infected adults with low estimated CVD risk and virologic suppression on ART, hsCRP was elevated and independently associated with BMI and lipid changes. Future studies should assess associations between hsCRP and clinical outcomes.
Keywords: HIV, metabolic complications, hsCRP, body mass index, lipids
Introduction
Antiretroviral therapy (ART) for HIV-infection has resulted in dramatic improvements in morbidity and mortality [1]. However, metabolic complications due to ART may increase cardiovascular disease (CVD) risk. Metabolic complications of ART resemble many features of the metabolic syndrome, including abdominal obesity in the setting of lipodystrophy, insulin resistance, and dyslipidemia. Thus, it is not surprising that ART may contribute, at least indirectly, to increased long-term CVD risk [2].
The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study demonstrated an increased risk of myocardial infarction with each year of combination ART exposure. A follow-up analysis showed that this risk was associated with protease inhibitor (PI) therapy, which has long been known to cause metabolic complications, particularly dyslipidemias [3, 4]. Interestingly, the association between PI exposure and myocardial infarction in the DAD study persisted after adjusting for serum lipid profiles, suggesting that any increased CVD risk associated with PIs may, at least in part, be independent of effects on lipids [4].
The critical role of inflammation in CVD among the general population is well recognized [5-7], and is also evidenced by the increased risk of CVD in chronic inflammatory diseases [8-10]. Chronic infection with HIV induces inflammation [11, 12], and the role of this inflammation in CVD has been highlighted by recent data demonstrating an increased risk of CVD events among individuals who interrupt ART despite previously well-suppressed HIV-1 RNA [13, 14]. The potential for dual effects of inflammation resulting from both ART toxicity and HIV replication makes this area of research particularly complex and interesting, and highlights the need to study both traditional and novel markers of inflammation and CVD risk among HIV-infected individuals.
Traditional CVD risk factors, such as those included in the Framingham risk score, may underestimate coronary risk in HIV-infected patients on ART [15]. This suggests the potential need for other surrogate markers of coronary risk. Highly-sensitive c-reactive protein (hsCRP) correlates with risk of an acute coronary event (rather than taking into account other CVD risk factors such as those included in the Framingham score) and outcome in the general population [16], and evaluation of hsCRP in HIV-infected populations has been limited to cross-sectional studies [17, 18]. The implications of elevated hsCRP and its clinical utility in this population are not fully elucidated. Our study is the first to assess longitudinal relationships between hsCRP, body mass index (BMI) and serum lipids over time in individuals with HIV-infection and virologic suppression on ART.
Methods
Study Design
Data are from a prospective clinical cohort study of adults from middle Tennessee with chronic HIV-infection enrolled and followed between June 2005 and July 2007. The cohort was established to assess the contribution of oxidant stress, which involves the generation of highly reactive free radicals in vivo that cause direct tissue damage [19], to the pathogenesis of ART-related toxicities, including metabolic and neurologic complications.
Study Population
Participants were eligible if they were at least 18 years of age, diagnosed with HIV-infection at least 12 months prior to enrollment, currently receiving ART including at least two nucleoside reverse transcriptase inhibitors (NRTIs) for at least 24 consecutive weeks, and had plasma HIV-1 RNA <10,000 copies/mL within 180 days of enrollment. Participants were classified into two study groups: 1) persons with clinical evidence of peripheral neuropathy and/or lipoatrophy, and 2) those with neither symptoms nor clinical evidence of peripheral neuropathy or lipoatrophy. Peripheral neuropathy was defined as vibratory sensation (using a 128 Hz tuning fork applied to the distal interphalangeal joint of the great toe) reduced to less than 5 seconds at the first interphalangeal joint of the great toes, diminished ankle reflexes, and subjective symptoms of numbness, tingling, or pain [20]. Lipoatrophy was defined as subcutaneous fat loss as perceived by both the study participant and study personnel [21]. Lipoatrophy was graded by study personnel using the HIV Outpatient Study (HOPS) Severity Scale: none (score of 0), mild (noticeable on close inspection, score of 1), moderate (readily noticeable by patient or physician, score of 2), and severe (readily noticeable to a casual observer, score of 3) [22].
Exclusion criteria included active opportunistic infection diagnosed within 30 days of enrollment and/or on current induction-phase of opportunistic infection therapy, documented lactic acidosis within 12 months of enrollment, diabetes mellitus that was not diet-controlled, documented or reported history of myocardial infarction, active neoplasm, or systemic anti-neoplastic chemotherapy within 12 months of enrollment. The study protocol was approved by the Institutional Review Board at Vanderbilt University School of Medicine, and all participants provided written informed consent.
Data Collection
At enrollment and subsequent follow-up study visits, current ART, BMI, smoking status, and use of lipid-lowering and anti-inflammatory medications were assessed using standardized questionnaires. Serum was obtained at each visit for assays including serum lipids (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides), lactic acid, and hsCRP. The most recent HIV-1 RNA and CD4+ T cell count within three months of the corresponding study visit were obtained from the medical record. Fasting state was assessed and defined as at least an eight hour fast. Heavy smoking was defined as greater than or equal to 20 cigarettes per day.
Statistical Analyses
Baseline characteristics were presented using median and interquartile range (IQR) for continuous variables, and frequency and proportions for categorical variables. Spearman's correlation coefficients and Mann Whitney U tests were used to assess un-adjusted effects of demographic and clinical factors on hsCRP at baseline. Adjusted effects of BMI and serum lipids were assessed using data at all four time points via multivariable general linear models with bootstrap covariance accounting for correlated measures within a patient, with outcome being natural log transformed hsCRP to achieve normality in regression residuals. Demographic factors (sex, race, and age) were included as fixed covariates; other covariates were included as time-dependent factors, including BMI, smoking status (current smoker of any amount, current heavy smoker, and current non-smoker), fasting state, CD4+ T cells, HIV-1 RNA, and the current use of PIs and lipid-lowering medications.
For computing the adjusted effects of BMI and other non-lipid related factors on hsCRP, serum lipid variables (LDL cholesterol, non-HDL cholesterol, HDL cholesterol, total cholesterol and triglycerides) were combined into two lipid components using Factor analysis [23]. The two lipid components were included in the multivariable regression analysis in order to prevent multi-colinearity. Assessment of serum lipid factors on hsCRP was performed separately in a similar multivariable regression where the two lipid components were replaced with each lipid variable. The effect of LDL cholesterol and non-HDL cholesterol were further adjusted for HDL cholesterol as it was of a priori determined clinical relevance. Graphical displays of the relationship of lipid parameters and BMI with reverse log-transformed hsCRP were created based on multivariable regression models using non-linear cubic spines to allow visualization of non-linear association. Data analyses were performed using Stata IC version 10.0 (Stata Corporation, College Station, TX) and R version 2.5.1 [24, 25].
Results
Clinical characteristics of study subjects at baseline and each follow-up visit are shown in Table 1. Ninety-four subjects had data available from at least one (baseline) study visit. The median CD4+ T cells and HIV-1 RNA at baseline were 502 (interquartile range [IQR] 334-738) cells/mm3 and 50 (range 50-9285) copies/mL, respectively, indicating virologic suppression to below the level of assay quantitation in most subjects. Median nadir CD4+ T cells were 190 (90-254) cells/mm3. Median duration of ART was 790 (395-1610) days. Median baseline BMI was 25.6 (IQR 23.3-28.2) kg/m2 and median (IQR) lipid values in mg/dL were: total cholesterol 194 (161-222), LDL cholesterol 98 (77-126), non-HDL cholesterol 139 (116-176), HDL cholesterol 48 (41-53), and triglycerides 173 (117-307). This population had a low baseline CVD risk as assessed by traditional risk factors, with a baseline median Framingham risk score of 3 (IQR 1-6) in both males and females, reflecting a 10-year CVD risk of 5% and 3%, respectively [16]. Seventy-four percent of individuals had low (<10%) 10-year CVD risk by Framingham score, 23% intermediate (10-20%) and 3% high (>20%) risk. However, the median (IQR) hsCRP was 2.94 mg/L (0.83, 5.53), corresponding to high CVD risk (10-20% 10-year risk) by cutoffs based on non-HIV-infected populations [[26]]. Thirty-two (34%) persons had lipoatrophy.
Table 1.
Characteristics of study subjects.*
| Characteristic | Baseline (N=94) | Follow-Up #1 (N=65) | Follow-Up #2 (N=41) | Follow-Up #3 (N=19) |
|---|---|---|---|---|
| Age – yr | 44 (40-50) | -- | -- | -- |
| Sex | ||||
| Male | 69 (73%) | -- | -- | -- |
| Female | 25 (27%) | -- | -- | -- |
| Race | ||||
| White | 54 (57%) | -- | -- | -- |
| Non-White | 40 (43%) | -- | -- | -- |
| CD4+ T cells – cells/mm3 | 502 (334-738) | 524 (360-734) | 426 (337-768) | 396 (288-717) |
| HIV RNA – copies/mL, median (range) | 50 (50-9285) | 50 (50-75135) | 50 (50-5086) | 50 (50-98077) |
| HIV RNA <50 copies/mL | 63 (67%) | 34 (67%) | 21 (66%) | 8 (57%) |
| hsCRP – mg/L | 2.94 (0.83-5.53) | 2.68 (1.22-5.86) | 2.66 (0.90-5.85) | 2.17 (1.46-3.46) |
| hsCRP <1 mg/L | 23 (25%) | 15 (23%) | 12 (29%) | 4 (21%) |
| hsCRP 1-3 mg/L | 24 (26%) | 21 (32%) | 10 (24%) | 9 (47%) |
| hsCRP >3 mg/L | 47 (49%) | 29 (45%) | 19 (46%) | 6 (32%) |
| Framingham score | 3 (1 – 6) | 3 (1 – 5) | 3 (1.5 – 4) | 3.5 (2 – 7) |
| Low 10-yr CVD risk | 70 (74%) | 50 (78%) | 32 (80%) | 12 (67%) |
| Intermediate 10-yr CVD risk | 21 (23%) | 13 (20%) | 8 (20%) | 6 (33%) |
| High 10-yr CVD risk | 3 (3%) | 1 (2%) | 0 (0%) | 0 (0%) |
| BMI – kg/m2 | 25.6 (23.3-28.2) | 25.7 (22.7-28.8) | 26.2 (22.9-30.5) | 27.7 (24.1-31.2) |
| Total cholesterol (mg/dL) | 194 (161-222) | 194 (161-218) | 186 (165-215) | 172 (155-211) |
| LDL cholesterol (mg/dL) | 98 (77-126) | 105 (78-123) | 101 (72-113) | 72 (53-98) |
| non-HDL cholesterol (mg/dL) | 139 (116-176) | 147 (111-173) | 141 (109-164) | 126 (107-160) |
| HDL cholesterol (mg/dL) | 48 (41-53) | 44 (37-50) | 43 (36-56) | 38 (35-58) |
| Triglycerides (mg/dL) | 173 (117-307) | 182 (121-277) | 191 (115-306) | 182 (96-325) |
| Smoking status | ||||
| Smoker | 49 (52%) | -- | -- | -- |
| Heavy smoker+ | 23 (25%) | -- | -- | -- |
| Nonsmoker | 45 (48%) | -- | -- | -- |
| Current lipid-lowering therapy | 16 (17%) | -- | -- | -- |
| Statin | 10 (11%) | -- | -- | -- |
| Fibrate | 5 (5%) | -- | -- | -- |
| Ezetimibe | 1 (1%) | -- | -- | -- |
| Fish oil | 3 (3%) | -- | -- | -- |
| PI-based ART | ||||
| Yes◊ | 51 (54%) | 34 (52%) | 25 (61%) | 11 (58%) |
| No | 43 (46%) | 31 (48%) | 16 (39%) | 8 (42%) |
| NNRTI-based ART | ||||
| Yes | 31 (33%) | 20 (31%) | 15 (37%) | 6 (32%) |
| No | 63 (77%) | 45 (69%) | 26 (66%) | 13 (68%) |
| Lipoatrophy | ||||
| Yes | 32 (34%) | -- | -- | -- |
| Mild | 24 (26%) | -- | -- | -- |
| Moderate | 6 (6%) | -- | -- | -- |
| Severe | 2 (2%) | -- | -- | -- |
| No | 62 (66%) | -- | -- | -- |
Results are n (%) or median (interquartile range) except where noted. Data for time-varying variables used in multivariable models are shown. The other characteristics were included in the models as baseline covariates.
Greater than or equal to 20 cigarettes/day
Atazanavir 22 (24%), lopinavir 26 (27%), nelfinavir 5 (3%)
Note: IQR = interquartile range, hsCRP = highly sensitive c-reactive protein, BMI = body mass index, ART = antiretroviral therapy, PI = protease inhibitor, NNRTI = non-nucleoside reverse transcriptase inhibitor
At baseline, all subjects were receiving at least two NRTIs. Thirty-five percent were on tenofovir, 33% on zidovudine, 22% on abacavir, 9% on didanosine, and 7% on stavudine. Fifty-four percent were on a PI, 33% were on a non-nucleoside reverse transcriptase inhibitor (NNRTI), and 2% were on the fusion inhibitor, enfuvirtide. hsCRP did not differ according to the use of individual antiretroviral agents (data not shown).
Details of PI use are in the footnote for Table 1. Similar proportions of study subjects were on agents from each ART class at each visit (data not shown). One study subject underwent a class change in ART, from an NNRTI-based to a PI-based regimen between study visits 2 and 3. Two other subjects had within-class (NRTI) changes between study visits 2 and 3. The ART regimen of all other study subjects was unchanged during follow-up. Sixty-five subjects had data from 2 follow-up visits, 41 from 3, and 19 from 4 or more visits. Median overall follow-up was 230 (113-362) days. There were a median (IQR) of 122 (99-197) days between enrollment and follow-up visit 2; 122 (112-172) days between follow-up visits 2 and 3; and 119 (111-159) days between follow-up visits 3 and 4. Because this is a clinical cohort, patients were not followed up at standardized time points. Differences in follow-up time were accounted for by repeated measures analysis. As demonstrated in Table 1, parameters were stable over time. There was not a correlation between changes in Framingham risk score and hsCRP over time.
In univariate analyses (Table 2), hsCRP levels correlated with BMI (r=0.33, p=0.021) and were weakly correlated with increasing age (r=0.1, p=0.054), but not with lipids, CD4+ T cells, or HIV-1 RNA. They also did not differ according to race, smoking status, fasting state, PI use, NNRTI use, use of lipid-lowering therapy, or the presence of lipoatrophy. Women had a higher median hsCRP compared to men (4.32 and 2.35 mg/L, respectively), although this difference was not statistically significant.
Table 2.
Univariate and multivariate analysis of factors potentially associated with hsCRP.
| Median (IQR) hsCRP or Spearman correlation coefficient from baseline dataa | p-value | Adjusted Regression coefficient (95% CI)b | Adjusted p-valuec | |
|---|---|---|---|---|
| Sex | 0.223 | 1.38 (0.76-2.50) | 0.287 | |
| Male | 2.35 (0.75-5.03) | |||
| Female | 4.32 (1.12-8.02) | |||
| Smoking status | 0.412 | 1.16 (0.75-1.80) | 0.509 | |
| Not current smoker | 2.04 (0.66-5.79) | |||
| Current smoker | 3.64 (1.17-5.51) | |||
| BMI | 0.33 | 0.021 | 1.44 (1.16-1.80) | 0.001 |
| Age | 0.10 | 0.054 | 1.35 (1.03-1.80) | 0.032 |
| CD4+ T cells | 0.11 | 0.132 | 1.03 (0.95-1.10) | 0.490 |
| Lipids | ||||
| Total cholesterol | 0.12 | 0.253 | ||
| LDL cholesterol | 0.03 | 0.774 | ||
| Non-HDL cholesterol | 0.14 | 0.199 | ||
| HDL cholesterol | -0.10 | 0.346 | ||
| Triglycerides | 0.20 | 0.056 | ||
| Race/ethnicity | 0.629 | 0.94 (0.58-1.50) | 0.796 | |
| Non-Caucasian | 3.39 (0.64-6.15) | |||
| Caucasian | 2.69 (0.87-5.03) | |||
| Log10 HIV-1 RNA | 0.06 | 0.804 | 1.12 (1.01-1.30) | 0.037 |
| Fasting status | 0.512 | 0.71 (0.48-1.00) | 0.078 | |
| Non-fasting | 3.12 (0.82-6.21) | |||
| Fasting | 2.87 (1.08-5.02) | |||
| Lipid-lowering therapy | 0.386 | 0.72 (0.43-1.20) | 0.221 | |
| No | 2.66 (0.81-5.51) | |||
| Yes | 4.02 (1.69-6.21) | |||
| PI use | 0.235 | 0.88 (0.55-1.40) | 0.614 | |
| No | 3.65 (1.19-6.01) | |||
| Yes | 2.1 (0.76-4.81) |
Spearman's correlation coefficients were used to assess unadjusted effects of each factor on hsCRP, and Mann-Whitney U tests were used for binary factors.
Regression coefficients were obtained by taking the exponential of β-coefficients from the model indicating relative increase (percent) in hsCRP value by one unit increase of factors as listed in the table, in addition to time. Units were chosen for 5 kg/m2 for BMI, 100 cells/mm3 for CD4+ T cells, and 10 years for age.
p-values shown are from multivariable linear mixed effect regression where log hsCRP is the outcome variable, including all variables listed in the table plus time and lipid component variables based on HDL cholesterol, non-HDL cholesterol, and triglycerides simultaneously.
Note: IQR = interquartile range, CI = confidence interval, hsCRP = highly sensitive c-reactive protein, PI = protease inhibitor
In multivariate analyses (Table 2), there was a significant association between increased hsCRP and greater BMI (regression coefficient = 1.44, p=0.001) after adjusting for PI use, CD4+ T cells, HIV-1 RNA, age, race, sex, smoking status, fasting state, use of lipid-lowering therapy and time. Given the significant association of BMI with hsCRP, multivariate analysis of the association of lipid parameters and hsCRP was performed with adjustment for BMI in addition to the above covariates. There were associations between increased hsCRP and higher non-HDL cholesterol (regression coefficient = 1.47, p=0.018) and triglycerides (regression coefficient = 1.31, p=0.019), and lower HDL cholesterol (regression coefficient = 0.81, p=0.050) in these models, as shown in Table 3. The effect of HDL cholesterol was adjusted for non-HDL cholesterol, and the effect of both LDL and non-HDL cholesterol were adjusted for HDL, respectively.
Table 3.
Multivariate analysis of the association of lipid parameters and hsCRP, including BMI in the model.
| Regression coefficient (95% CI)a | p-valueb | |
|---|---|---|
| Total cholesterol (unit=50) | 1.38 (1.03-1.90) | 0.035 |
| LDL (unit=50) | 1.60 (1.05-2.43) | 0.029 |
| Non-HDL (unit=50) | 1.47 (1.07-2.02) | 0.018 |
| HDL (unit=10) | 0.81 (0.66-1.00) | 0.050 |
| Triglycerides (unit=200) | 1.31 (1.05-1.60) | 0.019 |
Regression coefficients were obtained by taking the exponential of β-coefficients from the model, indicating a relative increase (percent) in hsCRP values by one unit increase of the factors listed in the table.
Effect of HDL cholesterol was adjusted for non-HDL cholesterol and the variables listed in Table 1 (age, PI use, CD4+ T cells, HIV-1 RNA, race, sex, smoking status, fasting status, BMI, and use of lipid-lowering therapy) and time. LDL was adjusted for HDL cholesterol and non-HDL cholesterol was adjusted for HDL cholesterol along with the set of covariates in Table 1. Cholesterol and triglycerides were adjusted for only the set of covariates listed in Table 1.
Note: CI = confidence interval, hsCRP = highly sensitive c-reactive protein, BMI = body mass index, LDL = low-density lipoprotein, HDL = high-density lipoprotein
There was a trend toward higher median triglyceride levels at non-fasting visits (170 vs. 136 mg/dL, p=0.31), but there were no statistically significant differences between lipids or hsCRP at non-fasting as compared to fasting visits. We therefore analyzed all lipid data together, adjusting for fasting state in the multivariable model including hsCRP and BMI as covariates. We did not examine the effect of individual lipid-lowering agents or individual PIs due to the small numbers of subjects in each subgroup. Figure 1A-E illustrates the associations of lipid parameters and BMI with hsCRP. These figures are based on data used in the model for Table 3, adjusted for median values of covariates indicated in the footnote of Table 3. We examined whether the effect of BMI or lipids differed according to the presence or absence of ART-related toxicity (either lipoatrophy or neuropathy) by interaction analysis. There was no evidence of effect modification, as all p-values for interaction were >0.3. Therefore, pooled data of individuals with and without ART-related toxicity are presented.
Figure 1.



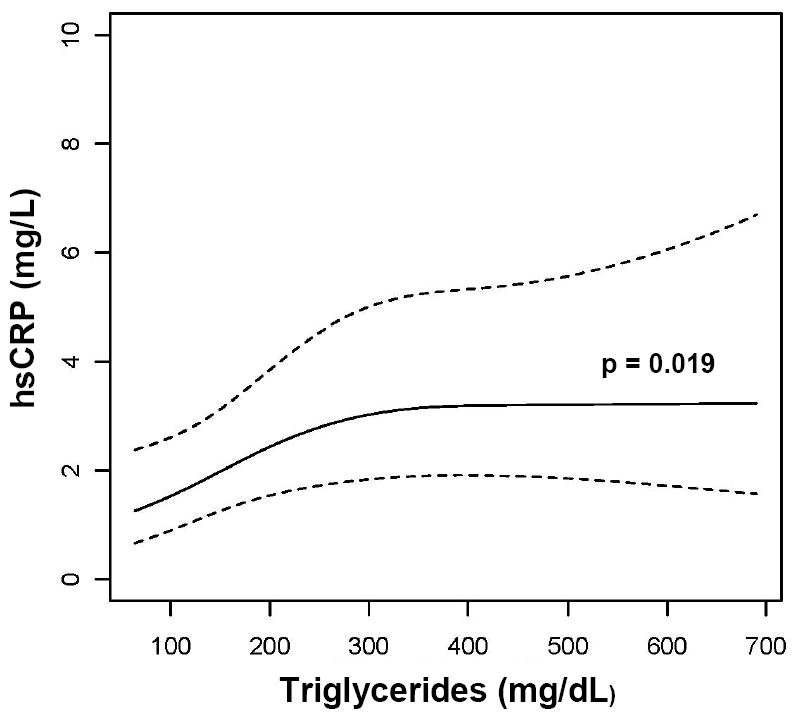
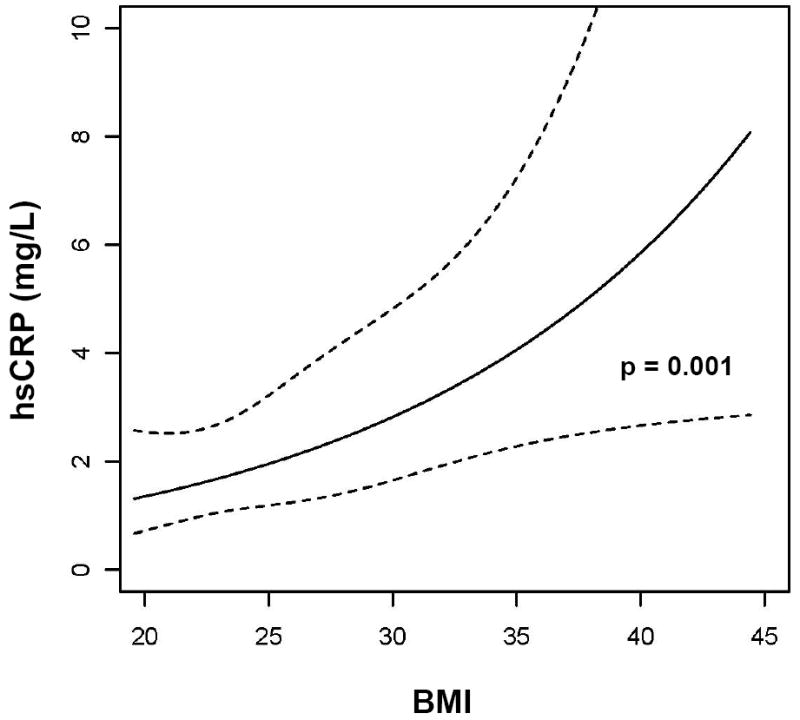
Association of (A) LDL cholesterol, (B) Non-HDL cholesterol, (C) HDL cholesterol, (D) triglycerides, and (E) BMI with hsCRP. Data are reverse-log-transformed from multivariable repeated measures analysis adjusting for sex, race, age, smoking status, fasting state, CD4+ T cells, HIV-1 RNA, and use of PIs and lipid-lowering therapy. Note: hsCRP = highly sensitive c-reactive protein, LDL = low-density lipoprotein, HDL = high-density lipoprotein, BMI = body mass index, PI = protease inhibitor, R = regression coefficient
Discussion
The most important finding of our study is that hsCRP was elevated in individuals with low Framingham risk score and correlated with changes in serum lipids and BMI over time. Forty-nine percent of the individuals in our study had an hsCRP >3 mg/L, consistent with a high risk of future acute coronary events, despite a low overall CVD risk by Framingham risk score. Changes in hsCRP were not associated with changes in Framingham risk score. These data highlight the potential disparity in traditional CVD risk factors and risk of future acute coronary syndrome in HIV-infected patients on ART. To our knowledge, these associations have not been described in a longitudinal cohort of HIV-infected persons.
A joint statement from the Centers for Disease Control and American Heart Association [26] recommends categorizing patients according to break points for hsCRP <1, 1-3 and >3 mg/L to define low-, average- and high-risk, respectively, of future acute coronary events. Measuring hsCRP in individuals judged at intermediate risk by global risk assessment for primary prevention of coronary disease was a Class IIa recommendation. hsCRP is a promising marker that may provide prognostic information not possible from serum lipids alone. In patients with acute coronary syndrome, a significant difference in the risk of recurrent myocardial infarction after statin therapy was observed among those with hsCRP less than versus greater than 2 mg/L. This effect was present at all levels of LDL cholesterol achieved, suggesting that attenuating inflammation in addition to addressing LDL cholesterol may be important [29]. It should be noted that this study involved individuals at high risk of CVD since they already had a prior acute coronary event, unlike our study which involves individuals of overall low CVD risk by traditional risk assessment.
In the DAD study, the rate of myocardial infarction increased with duration of combination ART [4]. However, the Strategies for Management of Antiretroviral Therapy (SMART) study demonstrated that ART interruption increased CVD risk compared to continuous therapy [14]. In addition, recently presented data from a prospective cohort demonstrated a trend toward decreased CVD risk in those remaining on ART more than 95% of the time over an 8-year period, and no apparent association between particular ART and CVD risk [30]. Such conflicting reports suggest that chronic ART exposure, uncontrolled HIV replication, or both, likely contribute to CVD in HIV-infected patients. hsCRP is a minimally invasive method for assessing risk of future acute coronary events in an HIV-negative population with intermediate risk as determined by traditional risk assessment. Ultimately, its use in HIV may allow practitioners to tailor ART and primary prevention strategies in at-risk individuals.
In the SMART study [31], hsCRP was higher in those on abacavir compared to other NRTIs, suggesting that this agent may have pro-inflammatory properties. This was a pertinent finding after the DAD study found an increased risk of myocardial infarction in those receiving or having recently received abacavir [32]. However, the HEAT study found a longitudinal reduction in hsCRP with an abacavir-containing regimen, similar to that with tenofovir [33]. There was an increased risk of myocardial infarction with didanosine in the DAD study, although no significant change in hsCRP was seen with its use in the SMART study. Although the number of individuals in our study on abacavir [20 (21%)] and didanosine [9 (9%)] was small, there was no difference in hsCRP according to the use of either agent.
A cross-sectional study [17] of 245 subjects on and off ART found that hsCRP positively correlated with LDL cholesterol and triglycerides and negatively with HDL cholesterol. No correlation was seen with HIV-1 RNA or CD4+ T cells. When adjusting for smoking, LDL cholesterol, and HDL cholesterol, ART use was associated with higher hsCRP, suggesting an enhanced cardiovascular risk on these medications. Those on PI-based regimens or a concomitant PI and NNRTI had a nonsignificant trend toward a higher hsCRP. However, we did not find a difference in hsCRP in subjects on PI vs. non-PI-based ART. This suggests, at least in our small study population, that this inflammatory marker does not appear to underlie the effect of PIs on cardiovascular risk.
A potential limitation of our study is that subjects were recruited based on the presence of other ART-related toxicities that may influence hsCRP – lipoatrophy and peripheral neuropathy. Lipodystrophy is associated with features of metabolic syndrome, and elevated hsCRP has been reported in that setting [34, 35]. hsCRP was associated with greater visceral and subcutaneous adipose tissue in a cross-sectional study of HIV-positive and -negative individuals [35]. There is no evidence that peripheral neuropathy is associated with hsCRP, nor did we find an association; thus, we did not adjust for peripheral neuropathy in multivariable models. We did not have an HIV-negative control population. Since our study was designed to follow individuals with and without ART-associated toxicities, selection bias could affect generalizability of results. Interaction analysis did not reveal evidence of effect modification of ART-related toxicities. However, our study may have limited statistical power to detect an interaction and further study with a larger sample size may be warranted. Finally, a small proportion of our study population had an intermediate 10-year CVD risk by Framingham score, where hsCRP may be most useful.
We did not utilize other indirect measures of CVD risk, such as carotid intima-media thickness (IMT) or flow-mediated dilation. Previously presented data demonstrated that subjects with HIV-infection had higher hsCRP and carotid IMT than uninfected persons, although these were not correlated [18]. We excluded persons receiving therapy for diabetes mellitus, but did not assess insulin resistance, a factor that may influence inflammation and CVD risk.
Statins may have important anti-inflammatory in addition to lipid-lowering effects, reducing hsCRP compared to placebo in HIV-negative individuals [36, 37]. We did not find a significant effect of lipid-lowering therapy on hsCRP. However, only 16% of subjects in our study were on lipid-lowering therapy, with 11% on a statin. Lipid-lowering therapy was included as a covariate in our multivariate model; it did not affect the independent relationships between hsCRP and BMI or lipids. LDL cholesterol was missing for some of the study subjects. Thus, non-HDL cholesterol was used in the primary analyses. Data suggest that non-HDL cholesterol may be a reasonable measure of CVD risk [38].
We found that higher BMI was strongly correlated with elevated hsCRP. Due to changes in body habitus resulting from ART, it would not be surprising if BMI were less predictive of CVD risk in HIV-infected persons. Data regarding the prognostic use of BMI in HIV-infected patients are limited. However, our results suggest that BMI may be quite useful in this population at predicting CVD risk. Visceral fat accumulation in lipodystrophy likely plays a key role, and visceral abdominal fat by computed tomography scan was a significant predictor of elevated hsCRP in HIV-infected women [39]. A limitation of our study is that lipoatrophy assessments were subjective. Studies using single-slice computed tomography scan or other objective measures for visceral adiposity are needed to extend our observations.
A potential confounding issue regarding the use of hsCRP in persons with HIV-infection at assessing risk of an acute coronary event is the effect of chronic infection such as HIV on hsCRP. Individuals with AIDS or non-suppressed HIV RNA had much higher hsCRP than those with virologic suppression [40]. Therefore, uncontrolled viral replication may confound interpretation of hsCRP at assessing coronary risk. High hsCRP in patients with HIV-infection predicts HIV-related mortality and disease progression [40, 41]. Although there was a trend toward higher hsCRP with higher viral load in our well-suppressed population, this association did not reach statistical significance and did not substantially affect the relationship between hsCRP and BMI and serum lipids.
In summary, our study is the first to show in HIV-infected subjects with well-suppressed HIV-1 RNA on ART and low CVD risk by Framingham score that hsCRP is longitudinally correlated with BMI and serum lipid changes. This data is important because it suggests that inflammation is relevant to the pathophysiology of metabolic disturbances in chronically HIV-infected persons on treatment with minimal plasma HIV-1 replication, confirms that CVD risk by traditional risk factors and risk of acute coronary syndrome by hsCRP are often discordant in HIV-infected persons, and that non-invasive inflammatory markers may be of utility. hsCRP is a particularly useful marker because it allows risk stratification and provides prognostic information. Larger studies are needed to assess the associations between hsCRP and clinical outcomes in this population.
Acknowledgments
The authors wish to thank the many study volunteers, participants, and staff of the Comprehensive Care Center and the Vanderbilt AIDS Clinical Trials Center (ACTC), who made this study possible. The authors would like to posthumously acknowledge JD Morrow, MD, who served as a co-author and mentor and was a pioneer in oxidant stress pharmacology until his untimely death on July 8, 2008. Dr. Morrow was instrumental in the design and development of this study. This work was facilitated by the infrastructure and resources provided by the Vanderbilt-Meharry Center for AIDS Research (CFAR), an NIH funded program (P30 AI 54999), the Vanderbilt University School of Medicine Emphasis Program, and NIH Molecular Basis of Infectious Diseases Training Program T32 AI07474-13. Funding was provided by NIH/NCCAM Career Development Award K23 AT002508 and Vanderbilt CTSA grant 1 UL 1 RR024975 from NCRR/NIH.
Funding was provided by NIH/NCCAM Career Development Award K23 AT002508 (TH) and Vanderbilt CTSA grant 1 UL 1 RR024975 from NCRR/NIH. This work has been facilitated by the infrastructure and resources provided by the Vanderbilt-Meharry Center for AIDS Research (CFAR), an NIH funded program #P30 AI 54999.
Footnotes
A portion of these data were presented at the XVII International AIDS Conference, Mexico City, Mexico, August 7, 2008
Conflict of interest disclosure: MS Boger-no conflict, A Shintani-no conflict, LA Redhage-no conflict, V Mitchell-no conflict, DW Haas-no conflict, JD Morrow-no conflict, and T Hulgan-no conflict.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Waters L, Nelson M. Long-term complications of antiretroviral therapy: lipoatrophy. Int J Clin Pract. 2007 Jun;61(6):999–1014. doi: 10.1111/j.1742-1241.2007.01385.x. [DOI] [PubMed] [Google Scholar]
- 3.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003 Nov 20;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 4.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007 Apr 26;356(17):1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 5.Vaccarino V, Johnson BD, Sheps DS, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007 Nov 20;50(21):2044–50. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 6.Kinlay S, Schwartz GG, Olsson AG, et al. Inflammation, statin therapy, and risk of stroke after an acute coronary syndrome in the MIRACL study. Arterioscler Thromb Vasc Biol. 2008 Jan;28(1):142–7. doi: 10.1161/ATVBAHA.107.151787. [DOI] [PubMed] [Google Scholar]
- 7.Wang TJ, Nam BH, Wilson PW, et al. Association of C-reactive protein with carotid atherosclerosis in men and women: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2002 Oct 1;22(10):1662–7. doi: 10.1161/01.atv.0000034543.78801.69. [DOI] [PubMed] [Google Scholar]
- 8.Urowitz MB, Gladman D, Ibanez D, et al. Accumulation of coronary artery disease risk factors over three years: data from an international inception cohort. Arthritis Rheum. 2008 Feb 15;59(2):176–80. doi: 10.1002/art.23353. [DOI] [PubMed] [Google Scholar]
- 9.Hahn BH, Grossman J, Chen W, McMahon M. The pathogenesis of atherosclerosis in autoimmune rheumatic diseases: roles of inflammation and dyslipidemia. J Autoimmun. 2007 Mar-May;28(23):69–75. doi: 10.1016/j.jaut.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Dessein PH, Joffe BI, Veller MG, et al. Traditional and nontraditional cardiovascular risk factors are associated with atherosclerosis in rheumatoid arthritis. J Rheumatol. 2005 Mar;32(3):435–42. [PubMed] [Google Scholar]
- 11.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008 Jan;214(2):231–41. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 12.Coll B, Parra S, Alonso-Villaverde C, et al. The role of immunity and inflammation in the progression of atherosclerosis in patients with HIV infection. Stroke. 2007 Sep;38(9):2477–84. doi: 10.1161/STROKEAHA.106.479030. [DOI] [PubMed] [Google Scholar]
- 13.Tebas P, Henry WK, Matining R, et al. Metabolic and immune activation effects of treatment interruption in chronic HIV-1 infection: implications for cardiovascular risk. PLoS ONE. 2008;3(4):e2021. doi: 10.1371/journal.pone.0002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 15.Law MG, Friis-Moller N, El-Sadr WM, et al. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV Med. 2006 May;7(4):218–30. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 16.Sabatine MS, Morrow DA, Jablonski KA, et al. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007 Mar 27;115(12):1528–36. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 17.Masia M, Bernal E, Padilla S, et al. The role of C-reactive protein as a marker for cardiovascular risk associated with antiretroviral therapy in HIV-infected patients. Atherosclerosis. 2007 Nov;195(1):167–71. doi: 10.1016/j.atherosclerosis.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Huse P, F A, Younes N, Martin J, Deeks S, Waters D. C-reactive protein levels in patients with HIV: a marker of cardiovascular risk of chronic infection?. Presented at the Conference on Retroviruses and Opportunistic Infections; 2008. Abstract 864. [Google Scholar]
- 19.Yin H, Havrilla CM, Gao L, Morrow JD, Porter NA. Mechanisms for the formation of isoprostane endoperoxides from arachidonic acid. “Dioxetane” intermediate versus beta-fragmentation of peroxyl radicals. J Biol Chem. 2003 May 9;278(19):16720–5. doi: 10.1074/jbc.M300604200. [DOI] [PubMed] [Google Scholar]
- 20.McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005 Sep;4(9):543–55. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 21.Nolan D, Mallal S. The role of nucleoside reverse transcriptase inhibitors in the fat redistribution syndrome. J HIV Ther. 2004 May;9(2):34–40. [PubMed] [Google Scholar]
- 22.Carr A, Emery S, Law M, Puls R, Lundgren JD, Powderly WG. An objective case definition of lipodystrophy in HIV-infected adults: a case-control study. Lancet. 2003 Mar 1;361(9359):726–35. doi: 10.1016/s0140-6736(03)12656-6. [DOI] [PubMed] [Google Scholar]
- 23.Asanuma Y, Chung CP, Oeser A, et al. Serum osteoprotegerin is increased and independently associated with coronary-artery atherosclerosis in patients with rheumatoid arthritis. Atherosclerosis. 2007 Dec;195(2):e135–41. doi: 10.1016/j.atherosclerosis.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.StatCorp. Stata Statistical Software: Release 10. College Station: TSL; 2007. [Google Scholar]
- 25.www.r-project.org
- 26.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003 Jan 28;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 27.Giraldez RR, Giugliano RP, Mohanavelu S, et al. Baseline low-density lipoprotein cholesterol is an important predictor of the benefit of intensive lipid-lowering therapy: a PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) analysis. J Am Coll Cardiol. 2008 Sep 9;52(11):914–20. doi: 10.1016/j.jacc.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 28.Wenger NK, Lewis SJ, Welty FK, Herrington DM, Bittner V. Beneficial effects of aggressive low-density lipoprotein cholesterol lowering in women with stable coronary heart disease in the Treating to New Targets (TNT) study. Heart. 2008 Apr;94(4):434–9. doi: 10.1136/hrt.2007.122325. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Morrow DA, Rose LM, Rifai N, Cannon CP, Braunwald E. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: an analysis of the PROVE-IT TIMI-22 trial. J Am Coll Cardiol. 2005 May 17;45(10):1644–8. doi: 10.1016/j.jacc.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 30.Lichtenstein K, A C, Buchacz K, Moorman A, Wood K, Brooks J. Analysis of cardiovascular risk factors in the HIV outpatient study cohort. Presented at Conference on Retroviruses and Opportunistic Infections; 2008. Abstract 735. [Google Scholar]
- 31.Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. Aids. 2008 Sep 12;22(14):F17–24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabin CA, Worm SW, Weber R, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008 Apr 26;371(9622):1417–26. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McComsey GA, S K, Patel P, et al. Similar reductions in markers of inflammation and endothelial activation after initiation of abacavir/lamivudine (ABC/3TC) or tenofovir/emtricitabine (TDF/FTC) in the HEAT study [Poster 732]. Presented at: 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 34.Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care. 2007 Jan;30(1):113–9. doi: 10.2337/dc06-1075. [DOI] [PubMed] [Google Scholar]
- 35.Reingold J, Wanke C, Kotler D, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008 Jun 1;48(2):142–8. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Harst P, Asselbergs FW, Hillege HL, et al. Effect of withdrawal of pravastatin therapy on C-reactive protein and low-density lipoprotein cholesterol. Am J Cardiol. 2007 Nov 15;100(10):1548–51. doi: 10.1016/j.amjcard.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 37.Liang YJ, Shyu KG, Wang BW, Lai LP. Simvastatin Inhibits C-Reactive Protein-Induced Pro-Inflammatory Changes in Endothelial Cells by Decreasing Mevalonate Pathway Products. Cardiology. 2007 Dec 4;110(3):182–90. doi: 10.1159/000111928. [DOI] [PubMed] [Google Scholar]
- 38.Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005 Nov 29;112(22):3375–83. doi: 10.1161/CIRCULATIONAHA.104.532499. [DOI] [PubMed] [Google Scholar]
- 39.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005 May 1;39(1):44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]
- 40.Lau B, Sharrett AR, Kingsley LA, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006 Jan 9;166(1):64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 41.Drain PK, Kupka R, Msamanga GI, Urassa W, Mugusi F, Fawzi WW. C-reactive protein independently predicts HIV-related outcomes among women and children in a resource-poor setting. Aids. 2007 Oct 1;21(15):2067–75. doi: 10.1097/QAD.0b013e32826fb6c7. [DOI] [PMC free article] [PubMed] [Google Scholar]


