SUMMARY
Background
Long-term success in ventricular assist device (VAD) recipients is limited by thromboembolic events, the prediction of which remains elusive. We evaluated the predictive value of aspirin hyporesponsiveness and markers of coagulation and fibrinolysis.
Methods
We prospectively enrolled patients scheduled to undergo VAD implantation between June 2004 and March 2006. Once before surgery, daily during hospitalization, and weekly after discharge we assessed platelet function, measured prothrombin activation fragment 1.2 (F1.2) and plasminogen activator inhibitor-1 (PAI-1) concentrations, and evaluated aspirin hyporesponsiveness by whole-blood aggregometry and thromboelastography. All patients received 325 mg oral aspirin daily from at least 7 days before VAD implantation. Follow-up continued until heart transplantation, death or closure of the database.
Results
We included 26 patients (median follow-up 315 days, range 9–833 days). In eight (31%) patients, 14 thromboembolic events occurred at a median of 42 (interquartile range 26–131) days. Only six (43%) events based on whole-blood aggregometry and one (7%) based on thromboelastography coincided with aspirin hyporesponsiveness. Within-patient variability was high for both tests (59% and 567%, respectively). Compared with levels before surgery, PAI-1 concentrations were raised for up to 45 days (P <0.0001) and those of F1.2 for up to 3 days (P = 0.0001) after VAD implantation. PAI-1 and F1.2 levels did not rise significantly further before thromboembolic events.
Conclusions
Aspirin hyporesponsiveness was not associated with raised risk of future clinical thromboembolic events after VAD implantation. Impaired fibrinolysis, demonstrated by raised PAI-1 concentrations, might, however, indicate a predisposition to such events early after surgery.
Keywords: aspirin hyporesponsiveness, coagulation, stroke, thromboembolism, ventricular assist device
INTRODUCTION
The use of a ventricular assist device (VAD) in patients with advanced heart failure who are ineligible for transplantation improves survival compared with optimal medical therapy, by about twofold at 1 year and about threefold at 2 years.1 Clinical morbidity with VADs has, however, tempered general enthusiasm; the rate of serious adverse events is a little more than twice that for medical therapy.1 Paramount among risks is that of VAD-associated thromboembolic events, which has remained at 30–50% despite the evolution of device technology and anticoagulation and antiplatelet strategies.2,3 Standard clinical assessment is not useful in predicting thromboembolic risk and new approaches are needed.
Aspirin hyporesponsiveness is the failure of aspirin to adequately inhibit platelet function and activation,4 and is assessed by measurement of platelet aggregation, platelet membrane receptor expression and platelet-release products.5 Mechanisms accounting for aspirin hyporesponsiveness involve a combination of clinical (e.g. nonadherence,6 drug interactions7,8 and smoking9), genetic10–12 and biological effects on platelet function.13,14 This state has been documented in healthy individuals15 and in patients with peripheral vascular disease,16 cerebrovascular disease,17 and cardiac disease,18,19 and has been associated with an increased risk of future vascular atherothrombotic events, such as acute coronary syndromes and cerebrovascular accidents.20 Coagulation and platelet activation indices are also increased in patients with end-stage systolic heart failure and VADs, despite daily aspirin therapy.21,22
The predictive value of aspirin hyporesponsiveness and associated markers of coagulation and impaired fibrinolysis for future clinical thromboembolic events in patients with VADs remains insufficiently understood. Thus, we assessed the prevalence and clinical relevance of aspirin hyporesponsiveness before and after VAD implantation and examined the role of coagulation factor prothrombin activation fragment 1.2 (F1.2), which is a sensitive marker of in vivo thrombin production, and plasminogen activator inhibitor-1 (PAI-1), a marker of impaired fibrinolysis, in the prediction of thromoembolic events.
METHODS
We obtained ethics approval from the Institutional Review Board of the University of Maryland School of Medicine, Baltimore, MD, USA.
Study design and patient population
Consecutive patients scheduled to undergo VAD implantation between June 2004 and March 2006 were prospectively enrolled into this observational study. Inclusion criteria were being a patient at or being transferred to the study hospital for VAD implantation, age 18 years or older and end-stage systolic heart failure. The only exclusion criterion was inability to provide informed consent. Follow-up was planned until patients underwent heart transplantation or died, or until the database closed. Patients were followed up daily while in hospital, and after discharge they attended weekly follow-up visits at the study center clinic.
Implantation protocol for ventricular assist devices
VAD implantation was performed via standard median sternotomy or left thoracotomy, with cardiopulmonary bypass support. Kaolin-based activated clotting time and heparin–protamine titration cartridges (HMS®, Medtronic Inc., Minneapolis, MN) were used to guide heparin dosing to achieve an activated clotting time of more than 480 s and a heparin dose of more than 2.8 IU/ml. Full-dose aprotinin was administered intraoperatively in all patients. Blood flow within pulsatile VADs (Novacor®, Novacor Medical Corp., Oakland, CA; HEARTMATE® and HEARTMATE II®, Thoratec Corp., Pleasanton, CA; and Thoratec®, Thoratec Laboratories Corp., Berkeley, CA) was recorded every 4 h until hospital discharge, at every outpatient follow-up visit and every 4 h during any subsequent inpatient visits. Patients who were implanted with nonpulsatile VADs received either a Jarvik 2000® (Robert Jarvik, New York, NY) or Ventracor® (Ventracor Ltd, Chatswood, New South Wales, Australia) device.
Antiplatelet therapy
All patients were already taking or started to receive 325 mg (coated or uncoated) oral aspirin at least 7 days before VAD implantation. Antiplatelet agents other than aspirin were discontinued at least 3 days before surgery. Aspirin therapy was restarted after surgery as soon as oral treatment was feasible and continued daily thereafter. Daily aspirin administration during the stay in hospital was confirmed by patients’ self-reporting, reports from nursing personnel and reviews of daily pharmacy records. After discharge adherence to daily aspirin was assessed at each weekly visit by verbal self-reporting from patients.
Assays of platelet function
Blood samples for platelet testing were obtained before VAD implantation (baseline), immediately after surgery when patients had received postoperative protamine, daily at 0600 h during patients’ hospital stay, and weekly after discharge during routine follow-up visits to the clinic.
We used a Whole-Blood Aggregometer® (Chrono-Log Corp., Haverton, PA), which measures collagen values, to assess platelet aggregation, according to previously described methods.23–25 Briefly, platelet aggregation was stimulated with the addition of 1 μg/ml and 5 μg/ml collagen, and platelet aggregability was expressed as the change in electrical impedance. We recorded aggregation curves for 6 min and analyzed the results with Aggrolink software.26 The reported mean coefficient of variability (imprecision value) for whole-blood aggregometry is 9% and, therefore, we used this value as the reference in the current investigation.24 We defined aspirin hyporesponsiveness in this test as at least 50% platelet response according to the following formula (Equation 1):25
| (1) |
We used the Thromboelastograph Coagulation Analyzer 5000 (TEG®, Huron Acquisition Corp., Skokie, IL) as a second measure of hemostasis from the initiation of coagulation to the initial fibrin assembly, the rate of increase in clot rigidity, and the eventual clot lysis.27 In this study, platelet function was defined by the maximum amplitude of the thromboelastography trace. The reported mean coefficient of variability for this assay is 5%,28 and we used this value as a reference. We defined aspirin hyporeponsiveness in this test as at least 50% platelet response to arachidonic acid according to the following normalized formula (Equation 2):25
| (2) |
where MA is maximum amplitude, AA is with arachidonic acid, 0 is without arachidonic acid, and Thr is with standard thrombin-mediated platelet activation.
Assays of coagulation and fibrinolytic activity
Blood samples for testing were obtained at the same times as those for platelet function tests. Citrated blood samples (5 ml) were analyzed by a series of coagulation assays, including tests for platelet count by routine chemistry and measurement of levels of fibrinogen, F1.2 and PAI-1 by enzyme-linked immunosorbent assays (F1.2 by Enzygnost® F1.2 Micro, Dade-Behring, Marberg, Germany, and PAI-1 by Molecular Innovations, Southfield, MI). The coefficients of variability for F1.2 and PAI-1 have been reported as 8%29 and 9%,30 respectively, and we used these values as references for the present investigation. We set a twofold or greater increase in F1.2 levels above a baseline value of up to 0.5 nmol l−1 mg−1 protein as the clinically important threshold, based on prior reports.31 The normal range for PAI-1 concentrations is 5–7 ng/ml and, therefore, we defined a PAI-1 level higher than 7 ng/ml as abnormal.
Detection of thromboembolic events
Before surgery all patients completed a symptom assessment questionnaire and underwent a thorough physical examination, including a neurological assessment, MMSE® mini-mental state examination, and neurocognitive testing with Weschler Adult Intelligence Scale® III, the California Verbal Learning Test® II, the Visual Naming Test, the Controlled Oral Word Association Test, the oral and reading comprehension subsets of the Multilingual Aphasia Examination, Beck Depression Inventory® II, and the NIH Stroke Scale assessment. In addition, all patients underwent head CT.
During the hospital stay after surgery, all patients were followed up according to a structured daily assessment protocol, which continued until discharge. Immediately after surgery and at discharge, all patients were asked to take the MMSE® again. Stringent symptom assessment and complete physical examinations were performed daily for all patients. Patients underwent a head CT scan if they developed any neurological symptoms or there were any indications of a transient ischemic attack or cerebrovascular accident. Neurological consultation was obtained to confirm a diagnosis of any neurological events. Additional appropriate laboratory tests and imaging studies were done if any other clinical thromboembolic events were suspected.
Patients were screened for subclinical stroke by head CT every 3 months for the duration of follow-up. If patients were readmitted to the study hospital for any cause, the same structured daily assessment protocol used during the initial stay was followed. During follow-up clinic visits, patients were systematically assessed for symptoms of thromboembolism, and any other concerns or complications related to their devices were noted and investigated.
We defined thromboembolic events as any new transient or permanent neurological event, including transient ischemic attacks or cerebrovascular accidents, any peripheral arterial occlusion diagnosed clinically and/or supported by laboratory data, arteriography, or CT imaging, and any new thromboembolism to the coronary circulation resulting in a myocardial infarction. Transient ischemic attacks were defined as a transient focal loss of neurological function resulting from brain ischemia lasting less than 24 h, with a head CT scan negative for cerebrovascular accident.32 A cerebrovascular accident was defined as a focal loss of neurological function resulting from brain ischemia lasting longer than 24 h with infarction in the area consistent with the new clinical deficit on brain imaging.32 We defined myocardial infarction according to previously established criteria.33
Statistical analyses
Data are presented as mean (± SD) for continuous demographic and clinical measures, median (interquartile range) for duration measures, and percentage values for categorical variables. Biochemical data are presented as mean and SEM and were logarithmically transformed before statistical analyses if data displayed a positively skewed distribution. Biochemical data were analyzed before VAD placement, and postoperative data were assessed as individual data points per day and as average values from days 0–2, 3–15, 16–30, 31–45, 46–60, and beyond day 60. The coefficient of variability (SD/mean) was used to examine within-patient variability. Biochemical and clinical data for patients with versus without thromboembolic events were compared by t-tests and χ2 tests. If patients had multiple thromboembolic events during follow-up, analyses were conducted on the biomarker values obtained for the first event. We used repeated-measures analysis of variance to examine changes over time. Biochemical data obtained before thromboembolic events were examined by comparing values before and after surgery with those obtained on the day of the event and average levels 2 days before and after the event. Kaplan–Meier analyses were conducted to examine trajectories of event rates during follow-up, with all remaining patients censored at 500 days. Statistical power analysis was based in an a priori anticipated event rate of 30%, and we calculated that enrollment of 26 patients would enable detection of a difference in biochemistry measures of 1.25 SD (effect size V/σ2 = 0.36) at α = 0.05 with a power of 80%, which is equivalent to a difference in whole-blood aggregometry of 38% based on the data distribution in this study. The threshold for statistical significance was set at P = 0.05.
RESULTS
Patient characteristics
We enrolled 26 patients into the study. Baseline patient characteristics are presented in Table 1. Two (8%) patients were receiving clopidogrel at baseline, which was discontinued at least 3 days before VAD implantation. Pulsatile VADs were implanted into 18 patients and eight received nonpulsative devices (Table 2). No patients dropped out. The database was closed on 30 June 2006, allowing a maximum follow-up of 4 years; median follow-up was 316 days (mean 345 ± 270, range 9–833, interquartile range 70–626 days).
Table 1.
Baseline characteristics of patients.
| Characteristic | Value (n = 26) |
|---|---|
| Demographic characteristics | |
| Mean (SD) age (years) | 52.3 ± 17.1 |
| Male sex | 19 (73%) |
| Race | |
| African American | 11 (42%) |
| White | 15 (58%) |
| Mean (SD) weight (kg) | 85.7 ± 22.3 |
| Clinical characteristics | |
| Mean (SD) LVEF (%) | 15.2 ± 4.8 |
| Mean (SD) duration of disease (months) | 41.4 ± 40.3a |
| Heart failure etiology | |
| Chronic ischemic cardiomyopathy | 10 (39%) |
| Cardiogenic shock (acute MI) | 1 (4%) |
| Nonischemic dilated cardiomyopathy | 14 (54%) |
| Peripartum cardiomyopathy | 1 (4%) |
| Intra-aortic balloon pump support | 7 (27%) |
| Prior cerebrovascular event | 2 (8%) |
| Hypertension | 14 (54%) |
| Hyperlipidemia | 8 (31%) |
| Diabetes mellitus | 8 (31%) |
| Implantable cardioverter-defibrillator | 21 (81%) |
| Smoker (prior or current) | 18 (69%) |
| Therapeutic characteristics | |
| Aspirin | 26 (100%) |
| Clopidogrel | 2 (8%) |
| Diuretic | 25 (96%) |
| ACE inhibitor or ARB | 24 (92%) |
| β-Blocker | 25 (96%) |
| Digoxin | 20 (77%) |
| Spironolactone | 15 (58%) |
| Hydralazine or nitrate | 5 (19%) |
| Statins | 8 (31%) |
| Warfarin | 17 (65%) |
| Intravenous inotropic therapy | 21 (81%) |
| Amiodarone | 6 (23%) |
| Laboratory characteristics | |
| Mean (SEM) hemoglobin (g/l) | 107 ± 11 |
| Mean (SEM) platelets (109/l) | 222.3 ± 108.6 |
| Mean (SEM) creatinine (μmol/l) | 123.8 ± 53.0 |
Range 0–120 months. Abbreviations: ACE, angiotensin-converting-enzyme; ARB, angiotensin-receptor blocker; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Table 2.
Characteristics of ventricular assist devices, requirements, and implantation.
| Characteristic | Number of patients (n = 26) |
|---|---|
| Pump type | |
| Pulsatile | 18 (69%) |
| Nonpulsatile | 8 (31%) |
| Device type | |
| Biventricular assist device | 2 (8%) |
| Left ventricular assist device | |
| Novacor®a | 11 (42%) |
| Jarvik 2000®a | 6 (23%) |
| HEARTMATE®a | 3 (12%) |
| HEARTMATE II®a | 2 (8%) |
| Thoratec®a | 2 (8%) |
| Ventracor®a | 2 (8%) |
| Need for therapy | |
| Bridge to transplantation | 18 (69%) |
| Transplant Ineligible | 8 (31%) |
| Route of implantation | |
| Median sternotomyb | 16 (62%) |
| Left thoracotomyb | 10 (39%) |
Novacor®, Novacor Medical Corp., Oakland, CA; HEARTMATE® and HEARTMATE II®, Thoractec Corp., Pleasanton, CA; and Thoratec®, Thoratec Laboratories Corp., Berkeley, CA; Jarvik 2000®, Robert Jarvik, New York, NY; Ventracor®, Ventracor Ltd, Chatswood, New South Wales, Australia.
With cardiopulmonary bypass support.
Clinical outcomes
VAD insertion was well tolerated for at least the first week after surgery. Clinical outcomes are presented in Table 3. Discharge in a stable condition was achieved in 20 (77%) patients; the remaining 6 (23%) died during the initial hospital stay. Follow-up was complete for 25 (96%) of 26 patients; one patient was lost to follow-up after discharge at 21 days. A total of 14 thromboembolic events were observed in eight (31%) patients during follow-up, at a median of 42 days (interquartile range 26–131). At completion of follow-up 12 (46%) patients had died.
Table 3.
Clinical follow-up of VAD patients.
| Outcome | Number (%) | Median (IQR) time to event (days) |
|---|---|---|
| Discharge from hospital | 20 (77%) | 38 (22–53) |
| TE event | ||
| Number of patients with TE events | 8 (31%) | 26 (10–83) |
| Number of patients with multiple TE events | 4 (15%) | 28 (10–31) |
| Type of event | ||
| All | 14 (100%) | 42 (26–131) |
| CVA | 7 (50%) | 31 (7–101) |
| TIA | 6 (43%) | 120 (29–271) |
| Mesenteric infarction | 1 (8%) | 44a |
| Bleeding event | 21 (81%) | 10 (6–16) |
| Cardiac tamponade | 4 (15%) | 9 (5–11) |
| Heart transplantation | 7 (27%) | 103 (27–120) |
| Mortality | ||
| All causes | 12 (46%) | 81 (46–209) |
| In hospital | 6 (50%) | 51 (35–78) |
| Sepsis | 1 (17%) | 75a |
| Respiratory failure | 1 (17%) | 43a |
| Mulitple-organ failure | 1 (17%) | 56a |
| Major bleeding | 1 (17%) | 86a |
| Mesenteric infarction | 1 (17%) | 45a |
| Failed VAD | 1 (17%) | 9a |
| Postdischarge | 6 (50%) | 185 97–520 |
| Intracranial hemorrhage | 3 (50%) | 447 (138–742) |
| Sepsis | 1 (16.7%) | 49a |
| Trauma | 1 (16.7%) | 232a |
| Complications of heart transplantation | 1 (16.7%) | 113a |
No IQR available because only one event. Abbreviations: CVA, cerebrovascular accident; ICH, intracranial hemorrhage; IQR, interquartile range; TE, thromboembolism; TIA, transient ischemic attack; VAD, ventricular assist device.
Prevalence of aspirin hyporesponsiveness
A total of 1,333 blood samples (mean 51 ± 30 samples per patient, range 9–119) were assayed from 26 patients from baseline and over the duration of follow-up. Aspirin hyporesponsiveness before VAD placement was documented in 14 (54%) of 26 patients (one sample per patient) based on whole-blood aggregometry, in 11 (42%) of 26 based on thromboelastography, and in 9 (35%) of 26 based on both assays. Aspirin administration was restarted within 6 h of surgery in all cases. We identified aspirin hyporesponsiveness in 25 (96%) patients, at some point during follow-up, on whole-blood aggregometry, in 341 of 656 samples tested (mean 52% ± 50%; within-patient range 0–100% of days positive for hyporesponsiveness). Estimated aspirin hyporesponsiveness rates were lower for thromboelastography, with 77% of patients having positive results at some point during follow-up in 10% (± 29%) of all assays (within-patient range 0–36% of days positive for hyporesponsiveness).
Aspirin hyporesponsiveness as a mechanism for thromboembolic events
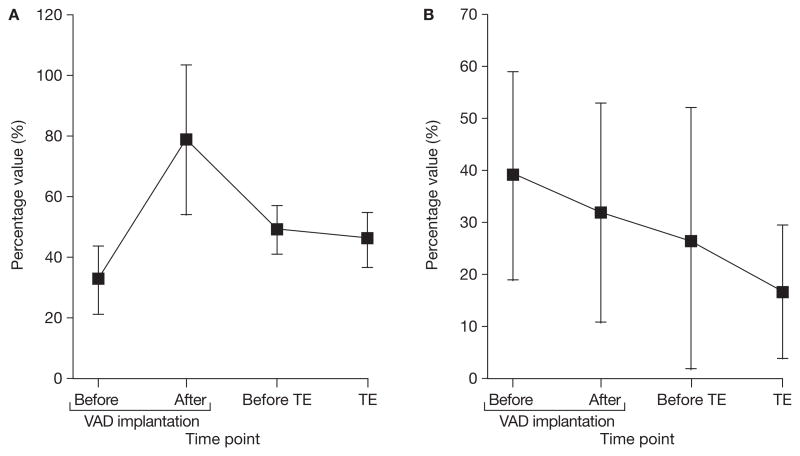
Compared with patients without thromboembolic events (n = 18) patients with thromboembolic events (n = 8) did not have raised platelet aggregation after VAD implantation, when tested by whole-blood aggregometry or thromboelastography, or assessed according to number of days with aspirin hyporesponsiveness (Table 4). In patients with thromboembolism, although the events were preceded by increased values on whole-blood aggregometry and thromboelastography, indicating aspirin hyporesponsiveness, none of these was significant (Figure 1 and Table 5). After implantation, in patients with thromboembolic events, a mean of 55% (± 14%) of whole-blood aggregometry values per patient indicated aspirin hyporesponsiveness (within-patient range 35–82% of days), which was similar to the corresponding values in patients without thromboembolic events (51% ± 22%, within-patient range 0–100%; P = 0.62). Thromboelastography revealed that in patients with thromboembolic events a mean of 5% (± 6%) of values showed aspirin hyporesponsiveness (within-patient range 0–18% of days), whereas in patients without thromboembolic events, 10% (± 10%) of values indicated hyporeponsiveness (within-patient range 0–37% of days; P = 0.23).
Table 4.
Characteristics of patients who did and did not experience thromboembolic events during follow-up.
| Characteristic | Value |
P-value | |
|---|---|---|---|
| No thromboembolic event (n = 18) | Thromboembolic event (n = 8) | ||
| Female sex | 3 (17%) | 4 (50%) | 0.077 |
| Mean (SD) age (years) | 49.9 ± 19.3 | 57.8 ± 9.6 | 0.29 |
| Race African American | 5 (28%) | 6 (75%) | 0.024 |
| Pulsatile device | 11 (61%) | 7 (87%) | 0.18 |
| Device type | |||
| Novacor®a | 5 (28%) | 6 (75%) | 0.024 |
| Other | 13 (72%) | 2 (25%) | |
| History of MI | 8 (44%) | 3 (38%) | 0.74 |
| History of CABG | 8 (44%) | 0 (0%) | 0.023 |
| History of PCI | 1 (6%) | 1 (13%) | 0.54 |
| History of CVA | 1 (6%) | 1 (13%) | 0.54 |
| Hypertension | 10 (56%) | 4 (50%) | 0.79 |
| Mean (±SD) platelet aggregation after VAD implantation | |||
| Mean (SD) whole-blood aggregometry (%) | 57.7 ± 9.3 | 61.5 ± 10.1 | 0.83 |
| Mean (SD) thromboelastography (%) | 13.9 ± 8.3 | 3.6 ± 6.5 | 0.47 |
| Aspirin hyporesponsiveness | |||
| Experienced during follow-up | 17 (94%) | 8 (100%) | 0.50 |
| Mean (SD) percentage of days (%) | 50 ± 22 | 55 ± 13 | 0.62 |
Novacor®, Novacor Medical Corp., Oakland, CA.
Figure 1.
Changes in platelet aggregation activity in patients experiencing thromboembolic events. Data are presented for eight patients and are expressed as mean (SEM). None of the values was significantly different from those obtained before VAD implantation. (A) Values for collagen measured on whole-blood aggregometry. (B) Values for thrombelastography. Abbreviations: TE, thromboemolic event; VAD, ventricular assist device.
Table 5.
Changes in platelet activation, coagulation, and fibrinolysis markers in relation to ventricular assist device implantation and thromboembolism.
| Test or marker | Mean (SD) platelet activation values |
|||||
|---|---|---|---|---|---|---|
| Before VAD implantation | VAD implantation | 2–4 days after VAD implantation | 2 days before TE | TE | 2 days after TE | |
| WBA (%) | 33.1 ± 10.5 | 78.5 ± 25.3 | 48.8 ± 6.4 | 49.7 ± 8.2 | 46.4 ± 9.1 | 55.3 ± 13.9 |
| TEG (%) | 39.1 ± 19.6 | 31.7 ± 21.2 | 5.0 ± 3.9 | 26.8 ± 26.5 | 16.8 ± 13.0 | 10.4 ± 10.6 |
| PAI-1 (ng/ml) | 6.92 ± 0.68 | 11.68 ± 0.99a | 12.74 ± 1.48a | 10.52 ± 1.35 | 10.61 ± 0.86a | 10.50 ± 0.76a |
| F1.2 nmoll−1 mg−1 | 0.36 ± 0.12 | 0.99 ± 0.27a | 0.41 ± 0.11 | 1.32 ± 0.49 | 0.61 ± 0.19 | 0.41 ± 0.18 |
P <0.005 compared with baseline levels. Abbreviations: F1.2, prothrombin activation fragment 1.2; PAI-1, plasminogen activator inhibitor-1; TE, thromboembolism; TEG, thromboelastography; VAD, ventricular assist device; WBA, whole-blood aggregometry.
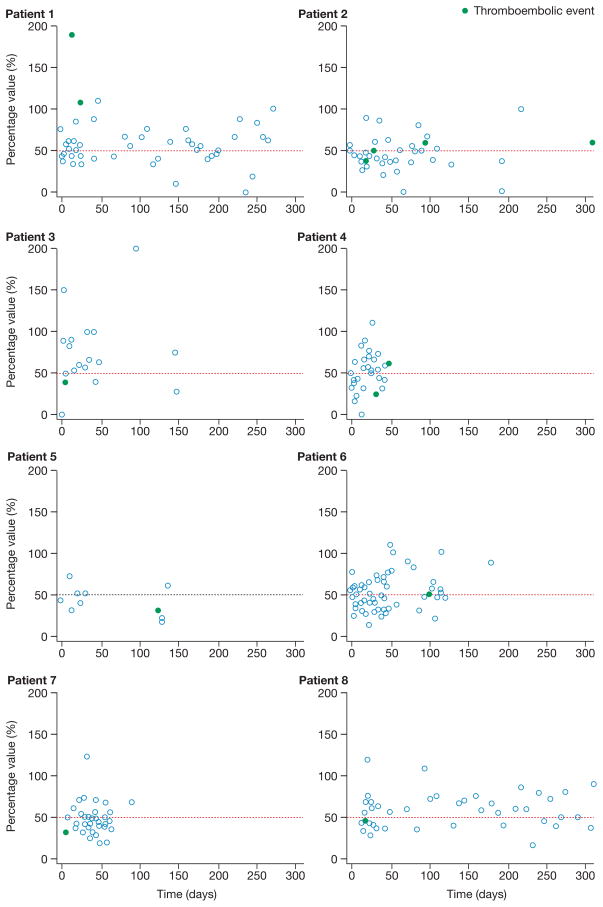
Only 6 (43%) of 14 thromboembolic events coincided with aspirin hyporesponsiveness indicated by whole-blood aggregometry (Figure 2), and only one (7%) when hyporesponsiveness was indicated by thromboelastography. The within-patient variability for whole-blood aggregometry among those who had thromboembolic events was substantial and even greater for thromboelastography (data not shown). The coefficients of variation for the two assays were high at 59% for whole-blood aggregometry and 567% for thromboelastography. Thus, no significant association existed between the presence of aspirin hyporesponsiveness and occurrence of thromboembolic events. Withdrawal of anticoagulation and antiplatelet therapy during episodes of major bleeding was not significantly associated with thromboembolic events.
Figure 2.
Variability of whole-blood aggregometry values in eight patients with thromboembolic events. The horizontal dashed lines indicate the 50% cut-off criterion for aspirin hyporesponsiveness. Whole-blood aggregometry was not recorded for patient 3 at the exact time of their second thromboembolic event (day 232) and, therefore, this event is not represented graphically.
Markers of coagulation and impaired fibrinolysis
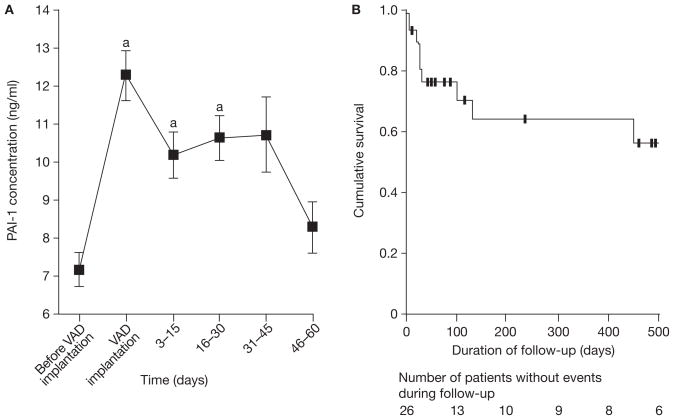
VAD implantation in all patients was associated with significant rises in concentrations of F1.2 (P = 0.0001) and PAI-1 (P <0.0001). F1.2 levels returned to baseline within 3 days of VAD placement, whereas PAI-1 levels remained elevated up to 45 days after implantation (P <0.0001), but had returned to baseline values by day 46 (P = 0.21).
Among the eight patients who experienced thromboloic events, eight (57%) of 14 events occurred in six (75%) patients in the first 45 days after VAD placement (Figure 3). Levels of PAI-1 at the time of thromboembolic event were significantly higher than before surgery (mean change 4.13 ± 0.37 ng/ml, P = 0.0003), whereas those in patients without thromboembolic events to day 45 were significantly less (mean change 2.37 ± 0.48 ng/ml, P for difference = 0.019). PAI-1 levels at the time of the thromboembolic event or in the 2 days preceding the event were not, however, significantly different from levels immediately after surgery (P = 0.87 and P = 0.44; Table 5). In addition, PAI-1 levels before and after surgery were not significantly higher in patients with thromboembolic events than in those without (preoperative 6.9 ± 0.7 ng/ml versus 7.4 ± 0.6 ng/ml, P = 0.64; postoperative 11.7 ± 1.0 ng/ml versus 12.4 ± 0.9 ng/ml, P = 0.39).
Figure 3.
Levels of PAI-1 in relation to VAD placement and incidence of thromboembolic events during follow-up. (A) Mean SEM PAI-1 concentrations. (B) Kaplan–Meier analysis for occurrence of thrombotic events during follow-up. Data were censored for remaining patients at 500 days. aP <0.001 compared with baseline. Abbreviations: PAI-1, plasminogen activator inhibitor-1; VAD, ventricular assist device.
DISCUSSION
We found that biological aspirin hyporesponsiveness is common in patients with end-stage systolic heart failure before and after VAD implantation. Despite meticulous efforts to manage anticoagulation and antiplatelet therapy, thromboembolic events occurred in eight (31%) patients. Thromboembolic events were not, however, associated with aspirin hyporesponsiveness. The fibrinolytic system was adversely affected for several weeks (up to 45 days) after VAD implantation, as indicated by elevated PAI-1 levels, during which period patients seemed to be especially vulnerable to thromboembolic events.
The rates of platelet activation we observed are similar to those noted in previous studies, which have shown persistent platelet activation despite aspirin therapy in patients with end-stage systolic heart failure. Sane et al.21 used a variety of tests, including plasma aggregometry, whole-blood aggregometry, platelet function analysis with the PFA-100® (Dade International, Deerfield, IL), and flow cytometry. According to the criterion of a positive result from four of five tests, persistent platelet activation was detected in 50 (57%) of 88 patients. Despite differing standards in defining aspirin hyporesponsiveness, therefore, this phenomenon seems common in patients with systolic heart failure.
A previous study has specifically evaluated aspirin hyporesponsiveness in patients with VADs.22 This 6-week, prospective, observational study used platelet aggregometry weekly to assess aspirin hyporesponsiveness and demonstrated that 6 (40%) of 15 patients maintained platelet aggregation induced by arachidonic acid, despite 250 mg oral aspirin daily. Our study was notably longer, used two assays to evaluate aspirin hyporesponsiveness, and assessed measures of aspirin hyporesponsiveness daily during hospitalization and weekly after discharge. After VAD implantation substantially more of our patients than before implantation displayed aspirin hyporesponsiveness on whole-blood aggregometry at some point during postoperative follow-up (96% versus 54% in a mean of around approximately half of all assays). The within-patient coefficients of variation were, however, high irrespective of which assay was used. Previous studies in normal individuals have shown substantially lower coefficients of variance for both whole-blood aggregometry and thromboelastography, validating the precision of these tests in assessing aspirin hyporesponsiveness.24,28 Certain factors could account for high variability, such as sampling time and medication administration, but these were kept constant in our study. Furthermore, analysis of values obtained when patients were in hospital compared with those obtained from outpatient visits revealed no significant differences. Given these factors, the absence of any changes in aspirin hyporesponsiveness marker conentrations immediately before the thrombotic event, and the fact that and only 6 (43%) of the 14 and 1 (7%) of the 14 thromboembolic events occurred in the presence of aspirin hyporesponsiveness based on whole-blood aggregometry and thromboelastography, respectively, we believe a relation between aspirin hyporesponsiveness and thromboembolic events is unlikely.
Levels of coagulation markers have been reported elsewhere as being increased in patients after VAD implantation. A study assessing coagulation and fibrinolytic pathways in patients with a HEARTMATE® VAD demonstrated that concentrations of markers of thrombin generation, specifically, thrombin–antithrombin complex and F1.2, rose until 3 days after VAD implantation.34 Previous studies have not, however, investigated whether increased activation of coagulation markers associated with VAD implantation predisposes patients to future thrombombolic events. We found a strong association between elevated PAI-1 levels in the first 45 days after VAD implantation and thromboembolic events during the same period, without significant further elevations immediately before the event. No such association was found with F1.2, and the initial increases in concentrations of this marker were probably due to an acute-phase reaction. The hemostatic environment might, therefore, be the most important factor in thromboembolic risk, and adjustment of antiplatelet therapy based on whole-blood aggregometry and thromboelastography results might be difficult or inadequate to lower risk.
The mechanisms underlying this dynamic hemostatic milieu are speculative, but several theories are feasible. First, increased platelet turnover after VAD implantation might overcome the inhibitive effect of aspirin on synthesis of thromboxane A2, which would lead to aspirin hyporesponsiveness and platelet aggregation. Second, inflammation associated with VAD implantation could induce increased production of cyclo-oxygenase 2, which is less sensitive to aspirin acetylation than cyclo-oxygenase 1. Third, the sustained inflammatory reaction associated with VAD implantation may be associated with increased levels of interleukins 6 and 8, fibrinogen, von Willebrand factor, and the complement fractions (C3a and C5a) that induce platelet activation. Fourth, persistent thrombin generation after VAD implantation might trigger specific protease-activated receptor cascades, leading to platelet activation aspirin hyporesponsiveness. Finally, production of glycoprotein Ibα, coupled with von Willebrand factor, might be activated by shear stress stimuli created by the VAD and result in platelet activation and aspirin hyporesponsiveness.
One limitation of this study is the small sample size. However, compared with previous VAD trials,1,3,22,35,36 including the REMATCH trial, which included 68 patients,1 a sample size of 26 is small but just as adequate to document the substantial variability of aspirin hyporesponsiveness. Another potential limitation is that we tested for aspirin hyporesponsiveness only once before VAD placement, compared with daily during the hospital stay after implantation and weekly during outpatient follow-ups. Our tests also had high imprecision values; an ideal test would be exceptionally sensitive, yielding high accuracy, precision and reproducibility. We were limited, however, by the currently available tests for aspirin hyporesponsiveness. Therefore, despite finding no association in this study, aspirin hyporesponsiveness might play a part in long-term thromboembolic events in VAD patients.
We conclude that although concentrations of markers of aspirin hyporesponsiveness are elevated in patients with advanced heart failure, they are not predictive of thromboembolic events after VAD placement. We did, however, note substantial variability in concentrations of coagulation and fibrinolysis markers after VAD implantation, suggesting a dynamic hemostatic environment. In particular, PAI-1 levels were raised significantly, compared with baseline, in the first 6 weeks after surgery, which coincided with the majority of thromboembolic events. This finding suggests a period of thrombogenicity and impaired fibrinolysis that might predispose patients to serious adverse events.
KEY POINTS
Biological aspirin hyporesponsiveness is common in patients with end-stage systolic heart failure before and after implantation of ventricular assist devices
Thromboembolic events occur at a high rate—nearly one-third—in patients with ventricular assist devices
No significant association was observed between the presence of aspirin hyporesponsiveness, based on whole-blood aggregometry and thromboelastography assays, and thromboembolic events in patients with ventricular assist devices
Plasminogen activator inhibitor-1 values early after ventricular assist device implantation suggest impaired fibrinolysis predisposes patients to thromboembolic events
Footnotes
Competing interests
F Majeed, WJ Kop, RS Poston and S Kallam declared no competing interests. MR Mehra has declared an association with the following companies: Thoratec and Ventracor. See the article online for full details of these relationships.
References
- 1.Rose EA, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 2.Deng MC, et al. International Society for Heart and Lung Transplantation. Mechanical circulatory support device database of the International Society for Heart and Lung Transplantation: third annual report—2005. J Heart Lung Transplant. 2005;24:1182–1187. doi: 10.1016/j.healun.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Tsukui H, et al. Cerebrovascular accidents in patients with a ventricular assist device. J Thorac Cardiovasc Surg. 2007;134:114–123. doi: 10.1016/j.jtcvs.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 4.Houel R, et al. Resistance to aspirin after external ventricular assist device implantation. J Thorac Cardiovasc Surg. 2003;126:1636–1637. doi: 10.1016/s0022-5223(03)01194-2. [DOI] [PubMed] [Google Scholar]
- 5.Michelson AD. Platelet function testing in cardiovascular diseases. Circulation. 2004;110:e489–e493. doi: 10.1161/01.CIR.0000147228.29325.F9. [DOI] [PubMed] [Google Scholar]
- 6.Cotter G, et al. Lack of aspirin effect: aspirin resistance or resistance to taking aspirin? Am Heart J. 2004;147:293–300. doi: 10.1016/j.ahj.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Catella-Lawson F, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 8.Kurth T, et al. Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal antiinflammatory drugs. Circulation. 2003;108:1191–1195. doi: 10.1161/01.CIR.0000087593.07533.9B. [DOI] [PubMed] [Google Scholar]
- 9.Hung J, et al. Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin. Circulation. 1995;92:2432–2436. doi: 10.1161/01.cir.92.9.2432. [DOI] [PubMed] [Google Scholar]
- 10.Cipollone F, et al. Cyclooxygenase-2 expression and inhibition in atherothrombosis. Arterioscler Thromb Vasc Biol. 2004;24:246–255. doi: 10.1161/01.ATV.0000104005.92603.f2. [DOI] [PubMed] [Google Scholar]
- 11.Undas A, et al. PlA2 polymorphism of β3 integrins is associated with enhanced thrombin generation and impaired antithrombotic action of aspirin at the site of microvascular injury. Circulation. 2001;104:2666–2672. doi: 10.1161/hc4701.099787. [DOI] [PubMed] [Google Scholar]
- 12.Michelson AD, et al. Platelet GP IIIa PlA polymorphisms display different sensitivities to agonists. Circulation. 2000;101:1013–1018. doi: 10.1161/01.cir.101.9.1013. [DOI] [PubMed] [Google Scholar]
- 13.Larsson PT, et al. Norepinephrine-induced human platelet activation in vivo is only partly counteracted by aspirin. Circulation. 1994;89:1951–1957. doi: 10.1161/01.cir.89.5.1951. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki T, et al. Increased platelet sensitivity to collagen in individuals resistant to low-dose aspirin. Stroke. 2000;31:591–595. doi: 10.1161/01.str.31.3.591. [DOI] [PubMed] [Google Scholar]
- 15.Marshall PW, et al. A comparison of the effects of aspirin on bleeding time measured using the Simplate™ method and closure time measured using the PFA-100™, in healthy volunteers. Br J Clin Pharmacol. 1997;44:151–155. doi: 10.1046/j.1365-2125.1997.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller MR, et al. Variable platelet response to low-dose ASA and the risk of limb deterioration in patients submitted to peripheral arterial angioplasty. Thromb Haemost. 1997;78:1003–1007. [PubMed] [Google Scholar]
- 17.Grundmann K, et al. Aspirin non-responder status in patients with recurrent cerebral ischemic attacks. J Neurol. 2003;250:63–66. doi: 10.1007/s00415-003-0954-y. [DOI] [PubMed] [Google Scholar]
- 18.Gum PA, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001;88:230–235. doi: 10.1016/s0002-9149(01)01631-9. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann N, et al. Functional and biochemical evaluation of platelet aspirin resistance after coronary artery bypass surgery. Circulation. 2003;108:542–547. doi: 10.1161/01.CIR.0000081770.51929.5A. [DOI] [PubMed] [Google Scholar]
- 20.Eikelboom JW, et al. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation. 2002;105:1650–1655. doi: 10.1161/01.cir.0000013777.21160.07. [DOI] [PubMed] [Google Scholar]
- 21.Sane DC, et al. Frequency of aspirin resistance in patients with congestive heart failure treated with antecedent aspirin. Am J Cardiol. 2002;90:893–895. doi: 10.1016/s0002-9149(02)02718-2. [DOI] [PubMed] [Google Scholar]
- 22.Houël R, et al. Platelet activation and aggregation profile in prolonged external ventricular support. J Thorac Cardiovasc Surg. 2004;128:197–202. doi: 10.1016/j.jtcvs.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 23.Dyszkiewicz-Korpanty AM, et al. Approach to the assessment of platelet function: comparison between optical-based platelet-rich plasma and impedance-based whole blood platelet aggregation methods. Clin Appl Thromb Hemost. 2005;11:25–35. doi: 10.1177/107602960501100103. [DOI] [PubMed] [Google Scholar]
- 24.Ivandic BT, et al. Determination of aspirin responsiveness by use of whole blood platelet aggregometry. Clin Chem. 2007;53:614–619. doi: 10.1373/clinchem.2006.081059. [DOI] [PubMed] [Google Scholar]
- 25.Poston RS, et al. Aprotinin shows both hemostatic and antithrombotic effects during off-pump coronary artery bypass grafting. Ann Thorac Surg. 2006;81:104–110. doi: 10.1016/j.athoracsur.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 26.Aggrolink software version 5.2.1. Chrono-Log Corp; Haverton, PA: [Google Scholar]
- 27.Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. doi: 10.1111/j.1365-2257.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 28.Bochsen L, et al. Evaluation of the TEG platelet mapping assay in blood donors. Thromb J. 2007;5:3. doi: 10.1186/1477-9560-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feinberg WM, et al. Markers of thrombin and platelet activity in patients with atrial fibrillation: correlation with stroke among 1531 participants in the stroke prevention in atrial fibrillation III study. Stroke. 1999;30:2547–2553. doi: 10.1161/01.str.30.12.2547. [DOI] [PubMed] [Google Scholar]
- 30.Johansson L, et al. Tissue plasminogen activator, plasminogen activator inhibitor-1, and tissue plasminogen activator/plasminogen activator inhibitor-1 complex as risk factors for the development of a first stroke. Stroke. 2000;31:26–32. doi: 10.1161/01.str.31.1.26. [DOI] [PubMed] [Google Scholar]
- 31.Haude M, et al. Guidance of anticoagulation after intracoronary implantation of Palmaz-Schatz stents by monitoring prothrombin and prothrombin fragment 1 + 2. Am Heart J. 1995;130:228–238. doi: 10.1016/0002-8703(95)90433-6. [DOI] [PubMed] [Google Scholar]
- 32.Lazar RM, et al. Neurological events during long-term mechanical circulatory support for heart failure: the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) experience. Circulation. 2004;109:2423–2427. doi: 10.1161/01.CIR.0000129414.95137.CD. [DOI] [PubMed] [Google Scholar]
- 33.Thygesen K, et al. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction (2007) Universal definition of myocardial infarction. J Am Coll Cardiol. 50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Beppu S, et al. High incidence of left ventricular thrombosis and systemic embolism in patients with left ventricular assist system [Japanese] J Cardiol. 1992;22:713–720. [PubMed] [Google Scholar]
- 35.Fries D, et al. Coagulation monitoring and management of anticoagulation during cardiac assist device support. Ann Thorac Surg. 2003;76:1593–1597. doi: 10.1016/s0003-4975(03)01034-8. [DOI] [PubMed] [Google Scholar]
- 36.Spanier T, et al. Activation of coagulation and fibrinolytic pathways in patients with left ventricular assist devices. J Thorac Cardiovasc Surg. 1996;112:1090–1097. doi: 10.1016/S0022-5223(96)70111-3. [DOI] [PubMed] [Google Scholar]