Abstract
BACKGROUND
Recent studies have shown that both female and male obesity may delay time-to-pregnancy (TTP). Little is known about central adiposity or weight gain and fecundability in women.
METHODS
We examined the association between anthropometric factors and TTP among 1651 Danish women participating in an internet-based prospective cohort study of pregnancy planners (2007–2008). We categorized body mass index (BMI = kg/m2) as underweight (<20), normal weight (20–24), overweight (25–29), obese (30–34) and very obese (≥35). We used discrete-time Cox regression to estimate fecundability ratios (FRs) and 95% confidence intervals (CI), controlling for potential confounders.
RESULTS
We found longer TTPs for overweight (FR = 0.83, 95% CI = 0.70–1.00), obese (FR = 0.75, 95% CI = 0.58–0.97), and very obese (FR = 0.61, 95% CI = 0.42–0.88) women, compared with normal weight women. After further control for waist circumference, FRs for overweight, obese, and very obese women were 0.72 (95% CI = 0.58–0.90), 0.60 (95% CI = 0.42–0.85) and 0.48 (95% CI = 0.31–0.74), respectively. Underweight was associated with reduced fecundability among nulliparous women (FR = 0.82, 95% CI = 0.63–1.06) and increased fecundability among parous women (FR = 1.61, 95% CI = 1.08–2.39). Male BMI was not materially associated with TTP after control for female BMI. Compared with women who maintained a stable weight since age 17 (−5 to 4 kg), women who gained ≥15 kg had longer TTPs (FR = 0.72, 95% CI = 0.59–0.88) after adjustment for BMI at age 17. Associations of waist circumference and waist-to-hip ratio with TTP depended on adjustment for female BMI: null associations were observed before adjustment for BMI and weakly positive associations were observed after adjustment for BMI.
CONCLUSIONS
Our results confirm previous studies showing reduced fertility in overweight and obese women. The association between underweight and fecundability varied by parity.
Keywords: fertility, obesity, body mass index, prospective study, cohort study
Introduction
The prevalence of obesity is increasing rapidly in Denmark and more individuals of childbearing age are becoming overweight and obese (Due et al., 2007). Body mass index (BMI), a measure of absolute body fat, has been associated with delayed fecundability in several studies of women (Zaadstra et al., 1993; Jensen et al., 1999; Bolumar et al., 2000; Diamanti-Kandarakis and Bergiele, 2001; Hassan and Killick, 2004; Gesink Law et al., 2007; Ramlau-Hansen et al., 2007; Nohr et al., 2009). Specifically, fecundability was found in some studies to be lower among women at the extremes of BMI (Zaadstra et al., 1993; Bolumar et al., 2000; Hassan and Killick, 2004; Gesink Law et al., 2007). Underweight may adversely affect fertility through increased FSH levels (Cramer et al., 1994), secondary amenorrhea (Frisch, 1987), and shortened luteal phase (Frisch, 1987), whereas overweight may affect fertility through anovulation (Grodstein et al., 1994; Rich-Edwards et al., 1994, 2002) and biochemical alterations in the pre-ovulatory follicular environment (Robker et al., 2009). In addition, both underweight and overweight are associated with lower follicular-phase estradiol levels (Ziomkiewicz et al., 2008).
Few studies have assessed the influence of central adiposity or body fat distribution on female fertility. Independent of BMI, central adiposity is associated with altered estrogen metabolism (Kirschner et al., 1990), insulin resistance and hyperinsulinemia (Falkner et al., 1999; Moran et al., 1999), oligomenorrhea (De Pergola et al., 2009), and low pH of endocervical mucus (Jenkins et al., 1995), all of which may negatively influence fertility (Nestler 1997a, b; Robker et al., 2009). One study reported an inverse association between waist-to-hip ratio (WHR) and the probability of conception per cycle (Zaadstra et al., 1993), and another study found that a WHR ≥0.80 impaired the pregnancy rate of IVF embryo transfer (Wass et al., 1997).
More recently, male obesity has been linked with subfecundity (Sallmén et al., 2006; Nguyen et al., 2007; Ramlau-Hansen et al., 2007), decreased testosterone and inhibin B levels (Jensen et al., 2004; Aggerholm et al., 2008) and reduced semen quality in some (Jensen et al., 2004; Chavarro et al., 2009) but not all studies (Magnusdottir et al., 2005; Aggerholm et al., 2008).
To clarify the role of body size and body fat distribution on fertility, we examined these associations among women enrolled in an internet-based prospective cohort study of time-to-pregnancy (TTP) in Denmark. We assessed associations of both female and male BMI with fecundability, as well as associations of female weight gain in adulthood, perceived body fat distribution, and central adiposity—measured by waist circumference and WHR—with fecundability.
Materials and Methods
Study population
The ‘Snart-Gravid’ (‘Soon Pregnant’) Study is an internet-based prospective cohort study of pregnancy planners in Denmark. The study methodology has been described in detail elsewhere (Mikkelsen et al., 2008; Rothman et al., 2009). Briefly, participant recruitment began in June 2007 after an advertisement was placed on a popular Danish health-related website (www.netdoktor.dk). Recruitment was enhanced by a coordinated media strategy that included a press release, which received attention from radio, print media, online news sites and television. Enrolment and primary exposure data collection were conducted via self-administered questionnaire on the study website (www.snart-gravid.dk). Contact with participants was maintained by e-mail and the study website.
Before enrolment, potential participants were required to read a consent form and complete an online screening questionnaire to confirm eligibility. Eligible women were aged 18–40 years, residents of Denmark, in a stable relationship with a male partner, who had been attempting to conceive for no more than 12 months, and not using any type of fertility treatment. Women who were planning to discontinue contraception within the next 6 months to attempt pregnancy were asked to provide their e-mail address for later recruitment. Participants were also required to provide their Civil Personal Registration (CPR) number—a unique 10-digit personal identification number assigned to each Danish resident by the Central Office of Civil Registration—and a valid e-mail address.
The baseline questionnaire collected information on demographics, reproductive and medical history, and lifestyle and behavioral factors. Participants were randomized to receive either a short- or a long-form baseline questionnaire, with some questions being asked of only 50% of the cohort. Completion rates and missing data were similar for both versions (Rothman et al., 2009). The follow-up questionnaires assessed changes in various exposures, frequency and timing of intercourse, and whether pregnancy occurred. Women who conceived were asked to complete one questionnaire during early pregnancy to assess changes in exposures, after which active follow-up ceased. Participants were contacted every 2 months by e-mail for 12 months or until conception occurred. Cohort retention after 12 months of follow-up was ∼82%.
Assessment of anthropometric measures
Current height (cm) and body weight (kg) of women and men were self-reported by the female respondent on the baseline questionnaire. In addition, women reported their weight at age 17 years (kg) and were asked ‘When you gain weight, where on your body do you mainly add the weight?’ with the following response categories: ‘don't gain weight’, ‘around the chest and shoulders’, ‘around the waist and stomach’, ‘around the hips and thighs’ or ‘equally all over’. On the long form questionnaire (50% of cohort), women reported their waist circumference (cm) at the level of the umbilicus and hip circumference (cm) at its widest location. These women were asked if they had used a measuring tape to make these measurements (yes versus no). We used BMI [weight (kg) divided by height squared (m2)] to measure overall obesity, WHR to measure relative distribution of fat, and waist circumference to estimate total abdominal fat (Giovannucci et al., 1996). Although WHR is used more widely in the literature, waist circumference provides an estimate of absolute abdominal adiposity, the component most correlated with metabolic abnormalities such as hyperinsulinemia. Because taller women tend to have larger waist circumferences, we created a measure of height-adjusted waist circumference by regressing waist on height and adding the residuals to the average waist size for a woman of average height in our cohort (Giovannucci et al., 1996).
Assessment of pregnancy and cycles at risk
On each follow-up questionnaire, women were asked about the date of their last menstrual period (LMP), whether they were currently pregnant, and whether they had experienced any other pregnancy outcomes since the date of their last questionnaire, including miscarriage, induced abortion or ectopic pregnancy. Women who experienced pregnancy loss were asked how long the pregnancy lasted (in weeks). Our study event of interest was the first report of pregnancy on a follow-up questionnaire, regardless of pregnancy outcome.
Total menstrual cycles at risk were calculated using data from the screening questionnaire (‘For how many months have you been trying to get pregnant?’), the baseline questionnaire (‘What is your usual cycle length?,’ which we defined as ‘the number of days from the first day of one menstrual period to the first day of the next menstrual period’), and the follow-up questionnaire (‘What was the first day of your last menstrual period?’). Women with a range of cycle lengths were asked to provide the midpoint of the range. The following formula was used to calculate total cycles at risk: (months of trying at study entry/cycle length) + (((LMP date from most recent follow-up questionnaire − date of baseline questionnaire completion)/cycle length) + 1), with observed cycles at risk defined as those that were contributed after study entry. We added one cycle to the formula to account for the fact that most women were probably not in the early follicular phase of their cycle when they completed the baseline questionnaire. For women with irregular cycles (24%), we estimated cycle length using their actual LMP data from follow-up questionnaires.
Assessment of covariates
Data on participant's age, partner's age, age at menarche, parity, smoking history, current alcohol intake, physician-diagnosed hypertension, menstrual cycle length, cycle regularity, physical activity and frequency of intercourse were self-reported on the baseline questionnaire. We estimated total metabolic equivalents (METs) per week by summing the METs from moderate physical activity (hours per week multiplied by 3.5) and vigorous exercise (hours per week multiplied by 7.0) (Jacobs et al., 1993).
Validation of anthropometric measures
Using the CPR number, we linked our data with the Danish Medical Birth registry to validate self-reported anthropometric measures (Kristensen et al., 1996; Knudsen and Olsen, 1998). We retrieved data on measured weight and height from the first prenatal visit for 856 cohort members who delivered a live birth resulting from a conception in our study. Self-reported versus registry-supplied means were similar for weight (68.3 versus 68.9 kg) and height (169.0 versus 168.7 cm). The Pearson correlation coefficients for self-reported versus registry-supplied height, weight and BMI were all 0.96. When we compared women with TTP <6 versus ≥6 cycles, correlations were similar for height (0.96 versus 0.98), weight (0.95 versus 0.97) and BMI (0.95 versus 0.96).
Exclusions
After 6 months of recruitment, 2368 women enrolled in the study. Of these, we excluded 340 women who had been trying to conceive for >6 cycles at study entry, 240 women who provided insufficient or implausible information on their LMP date or date of first pregnancy attempt, and 137 women who did not complete a follow-up survey. After these exclusions, 1651 women remained in the cohort and were included in the present analyses. The 241 women who were lost to follow-up in our analysis (mean follow-up of 5.3 months) had lower parity (27.0 versus 36.3%), higher BMI (mean = 25.0 versus 24.0 kg/m2), heavier smoking history (mean = 2.8 versus 1.9 pack-years), and shorter length of time in a steady relationship (mean = 4.5 versus 5.2 years) than the 1410 women who were followed to a study end-point. They were, however, similar in every other factor assessed (e.g. mean age: 28.5 versus 28.7 years; mean partner age: 31.4 versus 31.0 years; mean cycle length: 28.5 versus 28.8 days; ≥5 years of higher education: 21.2 versus 24.7%; and mean alcohol consumption: 3.5 versus 3.4 drinks/week).
Data analysis
We assessed the relation between anthropometric factors and characteristics of the cohort at baseline. We divided female and male BMI (kg/m2) into the following categories: underweight (<20), normal weight (20–24), overweight (25–29), obese (30–34) and very obese (≥35). Weight change since age 17 years was categorized as follows: <−5, −5 to 4, 5–9, 10–14, ≥15 kg. Waist circumference and WHR were categorized into quartiles. We examined the possibility of a non-linear relation or threshold effect of each anthropometric variable on fecundability by using restricted cubic splines (Durrleman and Simon, 1989; Li et al., 2003).
The fecundability ratio (FR) represents the cycle-specific probability of conception among the exposed divided by that among the unexposed. A FR below one indicates reduced fecundability among the exposed relative to the unexposed. We used a discrete-time analogue of the Cox proportional hazards model used to estimate FRs and 95% confidence intervals (CIs) for anthropometric variables in relation to TTP in cycles (Baird et al., 1986). The primary end-point of interest was any pregnancy, regardless of pregnancy outcome. Couples that did not conceive after 12 cycles were censored at 12 cycles, the typical amount of time after which couples seek medical assistance for infertility (Baird et al., 1986; Bonde et al., 2006). Participants who were lost to follow-up (10%), who reported no longer trying to conceive (5%) or who reported the initiation of fertility treatment (9%) were censored at their date of last response.
To handle the problem of left truncation (i.e. women who entered the study after having tried to conceive for one or more cycles), we based risk sets only on observed cycles at risk. For example, if a woman had been trying to conceive for five cycles before entering the study and then reported a pregnancy after 10 cycles of attempt time, she would contribute only five cycles to the analysis starting at cycle 6 when she was first observed in our study and at risk of pregnancy (cycles 6 through 10, not cycles 1 through 5). In contrast, a woman who entered the study without having any attempt time and who reported a pregnancy after 10 cycles of attempt time would contribute the full 10 cycles to the analysis starting at cycle 1. Women contributed cycles at risk until they reached a study end-point—pregnancy, use of fertility treatments, loss to follow-up, or the end of observation (12 cycles), whichever occurred first.
We selected potential confounders from a list of variables that were associated with exposure at baseline and that met criteria for confounding based on a review of the literature and assessment of a causal graph (Greenland and Rothman, 2008). We then controlled for potential confounders that changed the multivariable FR by more than 10% relative to the unadjusted FR (Greenland and Rothman, 2008). On the basis of these criteria, we controlled for female age (<25, 25–29, 30–34, ≥35 years), partner's age (<25, 25–29, 30–34, 35–39, ≥40 years), menstrual cycle length (<25, 25–26, 27–29, 30–31, 32–33, ≥34 days), irregular cycles (yes versus no), frequency of intercourse (<1, 1, 2–3, ≥4 times/week), parity (0, 1, ≥2 births), pack-years of smoking (never smoked, <5, 5–9, ≥10), current alcohol intake (drinks/week) and physical activity (<10, 10–19, 20–39, ≥40 METs/week). To assess the independent contribution of overall and central adiposity, we constructed additional models that simultaneously adjusted for BMI and waist circumference. We used multiple imputation methods to impute missing covariate values (Zhou et al., 2001).
Although most previous studies on body size and fertility have controlled for parity (Zaadstra et al., 1993; Jensen et al., 1999; Bolumar et al., 2000; Hassan and Killick, 2004; Ramlau-Hansen et al., 2007), some epidemiologists argue that adjusting for parity results in over-adjustment since factors that affect the pregnancy under study may have had similar effects on previous pregnancy attempts (Weinberg, 1993). In addition, if parity can be thought of as an effect of fecundability, then control for parity would be another form of over-adjustment. To address this concern, we conducted three sets of analyses: (i) one that controlled for parity in multivariable models, (ii) one that stratified by parity and (iii) one that omitted parity from multivariable models. One rationale for parity stratification is that childbearing is associated with a patterned change in body shape, specifically a decrease in hip and thigh circumferences, and an increase in waist circumference, resulting in a relative decrease in lower-body fat (Lassek and Gaulin, 2006). Moreover, parity stratification allows us to compare our results with previous studies (Bolumar et al., 2000; Gesink Law et al., 2007).
In secondary analyses, we evaluated whether FR results were similar when pregnancy losses were excluded from the outcome definition. In these analyses, women with unsuccessful pregnancy attempts (e.g. conception-free cycles leading up to a spontaneous miscarriage, induced abortion or ectopic pregnancy) were censored at their TTP (Joffe et al., 2005). In addition to parity, we stratified by participant smoking history (ever versus never) and age at baseline (<25, 25–29, ≥30 years), because studies have found stronger effects of BMI among smokers (Bolumar et al., 2000) and younger women (Sneed et al., 2008). We also stratified by cycle regularity (irregular versus regular cycles) and cycles of attempt time before study entry (≤2 versus 3–6), and examined potential effect modification between female BMI and both WHR and weight change since age 17. We assessed departure from the proportional hazards assumption by plotting the log–log survivor functions for each anthropometric variable in categorical form, where parallel log–log survivor curves indicated proportional hazards. SAS statistical software (version 9.1) was used for all analyses (SAS Institute, 2004).
Results
The mean age of cohort participants was 28.6 years (range: 18–40 years). After 6 and 12 cycles of pregnancy attempts, 51.8 and 69.6% of cohort members reported a pregnancy, respectively. Baseline characteristics of the study sample according to female BMI and waist circumference are presented in Table I. Female BMI was positively associated with male partner's BMI, weight change since age 17, waist circumference, WHR, parity, and having a mother who smoked during pregnancy, and inversely associated with age at menarche, cycle irregularity, physical activity, hypertension, diabetes and frequency of intercourse. Waist circumference was similar to BMI in its relation to the characteristics in Table I, with the exception of pack-years of smoking, with which it had a positive association. Pearson correlation coefficients were 0.78 for female BMI versus waist circumference, 0.75 for female BMI versus hip circumference, 0.27 for female BMI versus WHR and 0.30 for female versus male BMI.
Table I.
Baseline characteristics of 1651 women according to female BMI and height-adjusted waist circumferencea
| Characteristic | BMI (kg/m2) |
Waist circumference (cm) |
||||||
|---|---|---|---|---|---|---|---|---|
| <20 | 20–24 | 25–29 | ≥30 | <74 | 74–79 | 80–86 | ≥87 | |
| No. of women | 231 | 920 | 300 | 200 | 477 | 331 | 358 | 485 |
| Age, years (mean) | 28.2 | 28.8 | 28.6 | 28.3 | 28.2 | 28.7 | 28.9 | 28.9 |
| Partner's age, years (mean) | 31.0 | 31.0 | 31.4 | 31.1 | 31.0 | 31.1 | 31.0 | 31.2 |
| Age at menarche, years (mean) | 13.4 | 13.1 | 12.7 | 12.5 | 13.3 | 13.0 | 12.9 | 12.7 |
| Irregular cycles, yes (%) | 32.7 | 24.2 | 21.7 | 20.0 | 29.2 | 27.0 | 22.9 | 18.8 |
| Cycle length among regular cyclers, days (mean) | 28.7 | 28.8 | 29.0 | 28.8 | 28.8 | 28.8 | 28.8 | 28.8 |
| Height, cm (mean) | 170.2 | 169.0 | 169.0 | 168.4 | 169.7 | 169.1 | 168.7 | 168.8 |
| BMI, kg/m2 (mean) | 19.0 | 22.3 | 26.9 | 34.5 | 20.9 | 22.3 | 23.8 | 29.0 |
| BMI at age 17, kg/m2 (mean) | 18.5 | 20.3 | 22.7 | 25.8 | 19.9 | 20.1 | 21.1 | 23.3 |
| BMI of male partner, kg/m2 (mean) | 24.1 | 24.9 | 25.8 | 27.5 | 24.3 | 25.3 | 25.0 | 26.4 |
| Weight change since age 17, kg (mean) | 1.4 | 5.7 | 12.1 | 24.8 | 3.1 | 6.3 | 7.9 | 16.2 |
| Waist circumference, cm (mean) | 70.7 | 78.4 | 88.2 | 102.7 | 69.0 | 77.2 | 83.4 | 97.3 |
| WHR (mean) | 0.79 | 0.81 | 0.84 | 0.87 | 0.74 | 0.81 | 0.83 | 0.89 |
| Physical activity, MET h/week (mean) | 25.3 | 26.5 | 22.4 | 18.2 | 26.7 | 26.7 | 25.7 | 20.4 |
| Higher education ≥5 years | 26.5 | 28.8 | 16.3 | 11.7 | 32.7 | 28.8 | 21.3 | 15.1 |
| Parous, ever had live birth (%) | 33.5 | 31.0 | 42.4 | 44.2 | 27.7 | 32.4 | 36.6 | 42.6 |
| Pack-years of ever smoking (mean) | 1.3 | 2.0 | 2.6 | 2.3 | 1.3 | 1.9 | 2.2 | 2.8 |
| Mother smoked during pregnancy, yes (%) | 26.9 | 32.1 | 41.4 | 47.3 | 29.4 | 31.7 | 36.9 | 41.2 |
| Alcohol intake (current), drinks/week (mean) | 3.4 | 3.6 | 3.1 | 2.8 | 4.0 | 3.1 | 3.3 | 3.1 |
| Time in steady relationship, years (mean) | 5.0 | 4.9 | 5.5 | 5.6 | 4.9 | 4.5 | 5.1 | 5.6 |
| Frequency of intercourse, ≥4 times/week (%) | 23.6 | 19.0 | 15.6 | 14.4 | 20.5 | 22.6 | 15.0 | 15.9 |
| Last method of contraception used (%) | ||||||||
| Barrier methods | 26.7 | 26.5 | 32.0 | 31.2 | 24.6 | 26.2 | 30.5 | 31.2 |
| Oral contraceptives | 56.8 | 62.4 | 57.8 | 57.9 | 59.3 | 62.9 | 58.1 | 60.7 |
| Other hormonal contraception | 2.2 | 0.8 | 1.4 | 0.9 | 1.9 | 2.2 | 0.3 | 0.2 |
| Withdrawal/rhythm/charting/other | 14.4 | 10.3 | 8.8 | 10.0 | 14.3 | 8.7 | 11.1 | 7.9 |
| Hypertension, yes (%) | 1.8 | 4.4 | 6.5 | 8.2 | 3.2 | 5.9 | 3.5 | 6.7 |
| Diabetes, yes (%) | 0.0 | 0.2 | 1.0 | 2.8 | 0.0 | 0.0 | 0.4 | 1.7 |
| Attempt time before study entry, cycles (%) | ||||||||
| 0–1 | 54.6 | 54.7 | 56.2 | 50.1 | 54.4 | 53.8 | 54.0 | 55.1 |
| 2–3 | 28.4 | 25.6 | 21.8 | 28.2 | 25.2 | 26.9 | 25.5 | 24.9 |
| 4–6 | 16.9 | 19.7 | 22.0 | 21.7 | 20.3 | 19.2 | 20.5 | 20.0 |
aCharacteristics are presented as means and proportions within categories of each anthropometric variable and are age-standardized to cohort at baseline.
After adjustment for all covariates except waist circumference, FRs associated with underweight, overweight, obese and very obese were 0.94 (95% CI = 0.77–1.15), 0.83 (95% CI = 0.70–1.00), 0.75 (95% CI = 0.58–0.97) and 0.61 (95% CI = 0.42–0.88), respectively, compared with normal weight women (Table II). After further control for waist circumference, the associations of female overweight and obesity with fecundability became stronger (Table II). Deletion of parity from the multivariable model produced similar effect estimates for female BMI: FRs for overweight, obese and very obese women were: 0.75 (95% CI = 0.60–0.95), 0.68 (95% CI = 0.47–0.99) and 0.51 (95% CI = 0.32–0.81), respectively. Results were also similar when we additionally adjusted for weight gain since age 17, with FRs for overweight, obese and very obese women being 0.77 (95% CI = 0.62–0.96), 0.68 (95% CI = 0.47–0.97) and 0.55 (95% CI = 0.35–0.86), respectively. Male partner's BMI was not materially associated with TTP after control for female BMI (Table II). Omission of intercourse frequency from the multivariable model made little difference in the effect estimates for male or female BMI (data not shown).
Table II.
Anthropometric measures at baseline and time to pregnancy
| No. | Cycles | Unadjusted model |
Adjusted modela |
Adjusted modelb |
||||
|---|---|---|---|---|---|---|---|---|
| FR | 95% CI | FR | 95% CI | FR | 95% CI | |||
| BMI, kg/m2 | ||||||||
| <20 | 161 | 843 | 0.95 | 0.78–1.15 | 0.94 | 0.77–1.15 | 1.02 | 0.83–1.27 |
| 20–24 | 666 | 3416 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| 25–29 | 199 | 1178 | 0.83 | 0.70–0.99 | 0.83 | 0.70–1.00 | 0.72 | 0.58–0.90 |
| 30–34 | 85 | 569 | 0.73 | 0.57–0.94 | 0.75 | 0.58–0.97 | 0.60 | 0.42–0.85 |
| ≥35 | 38 | 334 | 0.55 | 0.39–0.78 | 0.61 | 0.42–0.88 | 0.48 | 0.31–0.74 |
| Male partner's BMI, kg/m2 | ||||||||
| <20 | 28 | 168 | 0.85 | 0.56–1.30 | 0.94 | 0.61–1.44 | 0.95 | 0.62–1.46 |
| 20–24 | 611 | 3200 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| 25–29 | 432 | 2423 | 0.93 | 0.81–1.06 | 0.98 | 0.85–1.13 | 0.98 | 0.85–1.13 |
| 30–34 | 60 | 399 | 0.79 | 0.59–1.05 | 0.99 | 0.73–1.35 | 0.97 | 0.72–1.33 |
| ≥35 | 18 | 150 | 0.59 | 0.36–0.98 | 0.72 | 0.43–1.22 | 0.72 | 0.43–1.22 |
| Weight change since age 17 years (kg) | ||||||||
| <−5 | 48 | 242 | 0.91 | 0.65–1.29 | 1.00 | 0.70–1.42 | 1.05 | 0.73–1.52 |
| −5 to 4 | 361 | 1749 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| 5–9 | 330 | 1836 | 0.85 | 0.72–1.00 | 0.91 | 0.77–1.08 | 0.90 | 0.76–1.07 |
| 10–14 | 198 | 1086 | 0.86 | 0.70–1.04 | 0.86 | 0.70–1.05 | 0.86 | 0.70–1.05 |
| ≥15 | 212 | 1427 | 0.68 | 0.57–0.82 | 0.71 | 0.58–0.86 | 0.72 | 0.59–0.88 |
| Waist circumference (cm) | ||||||||
| <74 | 337 | 1941 | 1.00 | (ref.) | 1.00 | (ref) | 1.00 | (ref) |
| 74–79 | 231 | 1231 | 1.04 | 0.83–1.30 | 1.01 | 0.79–1.29 | 1.05 | 0.81–1.36 |
| 80–86 | 259 | 1274 | 1.14 | 0.93–1.39 | 1.10 | 0.89–1.37 | 1.21 | 0.96–1.54 |
| ≥87 | 322 | 1894 | 0.98 | 0.78–1.24 | 1.01 | 0.79–1.29 | 1.44 | 1.03–2.02 |
| WHR | ||||||||
| <0.75 | 274 | 1565 | 1.00 | (ref.) | 1.00 | (ref) | 1.00 | (ref.) |
| 0.75–0.79 | 223 | 1446 | 0.96 | 0.76–1.22 | 0.96 | 0.75–1.22 | 0.98 | 0.77–1.24 |
| 0.80–0.84 | 271 | 1365 | 1.13 | 0.88–1.45 | 1.10 | 0.83–1.45 | 1.15 | 0.88–1.52 |
| ≥0.85 | 381 | 1964 | 1.13 | 0.93–1.37 | 1.15 | 0.89–1.47 | 1.27 | 0.98–1.64 |
| Tendency to gain weight | ||||||||
| Hips/thighs | 307 | 1821 | 0.91 | 0.77–1.09 | 0.86 | 0.72–1.02 | 0.81 | 0.68–0.97 |
| Equally all over | 327 | 1815 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| Waist/stomach | 447 | 2317 | 1.07 | 0.91–1.26 | 1.06 | 0.90–1.24 | 1.00 | 0.85–1.18 |
| Chest/shoulders | 9 | 66 | 0.75 | 0.37–1.54 | 0.65 | 0.31–1.34 | 0.59 | 0.29–1.24 |
| Do not gain weight | 47 | 239 | 1.06 | 0.76–1.50 | 0.98 | 0.69–1.39 | 0.87 | 0.61–1.26 |
FR = fecundability ratio; CI =confidence interval; No. = number of pregnancies.
aAdjusted for age, partner's age, cycle regularity, cycle length, partner's BMI (in female BMI analysis only), female BMI (in partner's BMI analysis only), physical activity, smoking, alcohol intake, parity and intercourse frequency.
bAdjusted for factors in ‘a’ as well as waist circumference (in BMI analyses), female BMI (in all analyses except weight change) and BMI at age 17 (in weight change analyses).
Compared with women who maintained a stable weight since age 17 (−5 to 4 kg), women who gained ≥15 kg had longer TTPs (FR = 0.71, 95% CI = 0.58–0.86) (Table II). Additional control for BMI at age 17 had little effect on the strength of this association (Table II), whereas control for current BMI instead of BMI at age 17 attenuated the association: relative to women who maintained a stable weight since age 17 (−5 to 4 kg), FRs for women who gained 5–9, 10–14 and ≥15 kg were: 0.89 (95% CI = 0.75–1.07), 0.88 (95% CI = 0.71–1.09) and 0.82 (95% CI = 0.63–1.05), respectively. Inverse associations between weight gain and fecundability persisted within categories of BMI at baseline (Table III). Given that younger women had less opportunity than older women to gain weight since age 17, we stratified results by age at baseline (<30 versus ≥30 years). Multivariable results with adjustment for BMI at age 17 were similar within each age group: compared with women of stable weight (−5 to 4 kg), FRs for women who gained ≥15 kg were 0.73 (95% CI = 0.56–0.94) for age <30 and 0.70 (95% CI = 0.51–0.96) for age ≥30. With adjustment for current BMI instead of BMI at age 17, these same FRs were 0.82 (95% CI = 0.59–1.14) for age <30 and 0.77 (95% CI = 0.51–1.15) for age ≥30. We also computed a measure of average weight gain per year [(current weight (kg) − weight at age 17 (kg))/(current age − 17 years)]. Multivariable FRs with control for BMI at age 17 were similar to those for total weight gain: compared with women of stable weight (−0.5 to 0.4 kg/year), FRs for women who gained 0.5–0.9, 1.0–1.9 and ≥2 kg per year were 0.88 (95% CI = 0.75–1.04), 0.89 (95% CI = 0.74–1.04) and 0.62 (95% CI = 0.49–0.79), respectively. Adjustment for current BMI instead of BMI at age 17 attenuated the multivariable FR comparing weight gain of ≥2 kg per year with stable weight (FR = 0.72, 95% CI = 0.53–0.99).
Table III.
Weight gain since age 17, waist-to-hip ratio (WHR), and time to pregnancy, by female BMI at baseline
| BMI < 25 |
BMI 25–29 |
BMI ≥ 30 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Cycles | Adjusted modela |
No. | Cycles | Adjusted modela |
No. | Cycles | Adjusted modela |
||||
| FR | 95% CI | FR | 95% CI | FR | 95% CI | |||||||
| Weight change since age 17 years (kg) | ||||||||||||
| <−5 | 43 | 191 | 1.04 | 0.71–1.52 | 3 | 38 | 0.67 | 0.17–2.65 | 1 | 11 | 0.33 | 0.02–4.41 |
| −5 to 4 | 340 | 1608 | 1.00 | (ref.) | 15 | 103 | 1.00 | (ref.) | 4 | 20 | 1.00 | (ref.) |
| 5–9 | 282 | 1540 | 0.90 | 0.75–1.08 | 42 | 261 | 1.01 | 0.50–2.05 | 6 | 21 | 0.69 | 0.13–3.72 |
| 10–14 | 124 | 682 | 0.88 | 0.69–1.11 | 57 | 268 | 1.05 | 0.53–2.11 | 15 | 111 | 0.38 | 0.09–1.72 |
| ≥15 | 38 | 238 | 0.78 | 0.53–1.15 | 63 | 384 | 0.86 | 0.44–1.68 | 97 | 740 | 0.35 | 0.09–1.43 |
| WHR | ||||||||||||
| <0.75 | 241 | 1334 | 1.00 | (ref.) | 24 | 175 | 1.00 | (ref.) | 9 | 56 | 1.00 | (ref.) |
| 0.75–0.79 | 174 | 1065 | 0.99 | 0.76–1.31 | 34 | 262 | 1.01 | 0.48–2.11 | 15 | 119 | 0.92 | 0.22–3.83 |
| 0.80–0.84 | 191 | 879 | 1.18 | 0.87–1.59 | 56 | 303 | 1.07 | 0.46–2.50 | 24 | 183 | 1.38 | 0.34–5.59 |
| ≥0.85 | 221 | 981 | 1.34 | 1.06–1.70 | 85 | 438 | 1.14 | 0.43–3.03 | 75 | 545 | 1.09 | 0.34–3.54 |
FR = fecundability ratio; CI = confidence interval; No. = number of pregnancies.
aAdjusted for age, partner's age, cycle regularity, cycle length, physical activity, smoking, alcohol intake, parity and intercourse frequency.
Neither waist circumference nor WHR was materially associated with TTP in multivariable models that did not control for BMI (Table II). After adjustment for BMI, however, positive associations were observed for waist circumference and WHR with fecundability. The FR comparing waist circumferences ≥87 with <74 cm was 1.44 (95% CI = 1.03–2.02). The FR comparing WHR of ≥0.85 with <0.75 was 1.27 (95% CI = 0.98–1.64). Results for WHR and TTP were strongest among lean women, but there was no evidence of effect modification (Table III). Owing to small numbers of women with narrow waist circumferences in the overweight and obese groups, we could not evaluate effect modification between BMI and waist circumference. Hip circumference indicated a weak inverse association with fecundability, but results were attenuated after adjustment for BMI (data not shown). Among the 28% of women who used a measuring tape for their waist and hip measurements (56% of those who completed the long form questionnaire), results were weaker (FR comparing ≥87 versus <74 cm: 1.29, 95% CI = 0.79–2.12; FR comparing WHR ≥0.85 versus <0.75: 0.94, 95% CI = 0.64–1.39). When parity was omitted from the multivariable model, FRs for waist circumference of 74–79, 80–86 and ≥87 relative to <74 cm were: 1.15 (95% CI = 0.89–1.50), 1.18 (95% CI = 0.93–1.51) and 1.09 (95% CI = 0.83–1.42) without control for female BMI, and 1.15 (95% CI = 0.87–1.51), 1.25 (95% CI = 0.96–1.62) and 1.45 (95% CI = 0.98–2.14) with control for female BMI, whereas FRs for WHR of 0.75–0.79, 0.80–0.84 and ≥0.85 relative to <0.75 were 1.00 (95% CI = 0.87–1.32), 1.25 (95% CI = 1.00–1.56) and 1.23 (95% CI = 0.98–1.54) without control for female BMI, and 1.05 (95% CI = 0.85–1.30), 1.20 (95% CI = 0.96–1.48) and 1.14 (95% CI = 0.91–1.42) with control for female BMI.
Results for the self-reported question on ‘tendency to gain weight’ were generally consistent with the overall results for waist circumference and WHR (Table II). Women who reported a tendency to gain weight in their hips and thighs had lower fecundability than women with a tendency to gain weight equally all over (FR = 0.81, 95% CI = 0.68–0.97). In contrast, no difference in fecundability was found among women with a tendency to gain weight in their waist and stomach area (FR = 1.00, 95% CI = 0.85–1.18). The FR was 0.59 for the small number of women who reported a tendency to gain weight in the chest and shoulder area (95% CI = 0.29–1.24).
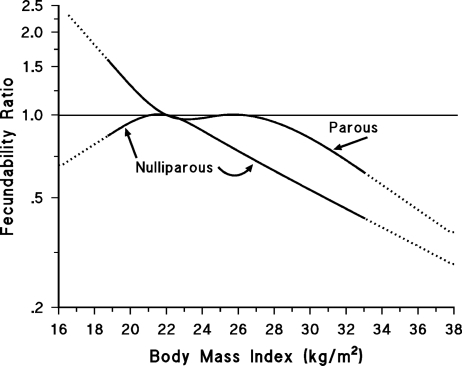
Results for selected anthropometric variables stratified by parity are shown in Table IV. FRs for overweight and obesity were stronger among nulliparous than parous women, and we observed opposing effects of underweight among nulliparous (FR = 0.82, 95% CI = 0.63–1.06) and parous (FR = 1.61, 95% CI = 1.08–2.39) women (Table IV). Figure 1 displays the relation of female BMI to fecundability, by parity status, using restricted cubic splines. Results for weight gain since age 17 were similar across parity status (Table IV) as were results for the ‘tendency to gain weight’ question (data not shown). Waist circumference and WHR results were slightly stronger among parous than nulliparous women, but FRs were generally in the same direction (Table IV).
Table IV.
Anthropometric measures at baseline and time to pregnancy, by parity status at baseline
| Nulliparous |
Parous |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Cycles | Adjusted modela |
Adjusted modelb |
No. | Cycles | Adjusted modela |
Adjusted modelb |
|||||
| FR | 95% CI | FR | 95% CI | FR | 95% CI | FR | 95% CI | |||||
| Female BMI, kg/m2 | ||||||||||||
| <20 | 98 | 637 | 0.76 | 0.60–0.98 | 0.82 | 0.63–1.06 | 63 | 206 | 1.43 | 1.00–2.03 | 1.61 | 1.08–2.39 |
| 20–24 | 441 | 2411 | 1.00 | (ref.) | 1.00 | (ref.) | 225 | 1005 | 1.00 | (ref.) | 1.00 | (ref.) |
| 25–29 | 101 | 742 | 0.73 | 0.57–0.93 | 0.65 | 0.49–0.86 | 98 | 436 | 1.00 | 0.75–1.33 | 0.85 | 0.61–1.17 |
| 30–34 | 36 | 335 | 0.60 | 0.41–0.88 | 0.51 | 0.32–0.82 | 49 | 234 | 0.89 | 0.61–1.31 | 0.68 | 0.43–1.09 |
| ≥35 | 20 | 249 | 0.46 | 0.28–0.76 | 0.39 | 0.22–0.69 | 18 | 85 | 1.00 | 0.56–1.79 | 0.73 | 0.38–1.39 |
| Weight change since age 17 years (kg) | ||||||||||||
| <−5 | 30 | 207 | 0.78 | 0.51–1.19 | 0.83 | 0.53–1.30 | 18 | 35 | 2.88 | 1.37–6.08 | 3.01 | 1.40–6.48 |
| −5 to 4 | 222 | 1212 | 1.00 | (ref.) | 1.00 | (ref.) | 139 | 537 | 1.00 | (ref.) | 1.00 | (ref.) |
| 5–9 | 235 | 1425 | 0.90 | 0.73–1.10 | 0.88 | 0.72–1.08 | 95 | 411 | 0.92 | 0.67–1.25 | 0.91 | 0.67–1.25 |
| 10–14 | 112 | 673 | 0.96 | 0.74–1.25 | 0.96 | 0.74–1.24 | 86 | 413 | 0.77 | 0.56–1.06 | 0.77 | 0.56–1.06 |
| ≥15 | 97 | 857 | 0.64 | 0.49–0.83 | 0.65 | 0.49–0.85 | 115 | 570 | 0.76 | 0.56–1.03 | 0.76 | 0.56–1.04 |
| Waist circumference (cm) | ||||||||||||
| <74 | 239 | 1500 | 1.00 | (ref.) | 1.00 | (ref.) | 98 | 441 | 1.00 | (ref.) | 1.00 | (ref.) |
| 74–79 | 149 | 838 | 1.02 | 0.77–1.34 | 1.03 | 0.78–1.36 | 82 | 393 | 0.99 | 0.60–1.64 | 1.13 | 0.67–1.90 |
| 80–86 | 147 | 806 | 1.18 | 0.91–1.53 | 1.24 | 0.95–1.62 | 112 | 468 | 1.02 | 0.63–1.63 | 1.26 | 0.76–2.07 |
| ≥87 | 161 | 1230 | 0.90 | 0.68–1.18 | 1.32 | 0.89–1.93 | 161 | 664 | 1.14 | 0.69–1.88 | 1.67 | 0.94–2.98 |
| WHR | ||||||||||||
| <0.75 | 197 | 1187 | 1.00 | (ref.) | 1.00 | (ref.) | 77 | 378 | 1.00 | (ref.) | 1.00 | (ref.) |
| 0.75–0.79 | 137 | 1104 | 0.86 | 0.61–1.22 | 0.89 | 0.63–1.25 | 86 | 342 | 1.24 | 0.82–1.89 | 1.29 | 0.85–1.94 |
| 0.80–0.84 | 157 | 786 | 1.14 | 0.81–1.62 | 1.23 | 0.88–1.73 | 114 | 579 | 1.17 | 0.72–1.89 | 1.22 | 0.75–2.00 |
| ≥0.85 | 205 | 1297 | 1.04 | 0.80–1.36 | 1.18 | 0.91–1.54 | 176 | 667 | 1.40 | 0.94–2.09 | 1.53 | 0.98–2.39 |
FR = fecundability ratio; CI = confidence interval; No. = number of pregnancies.
aAdjusted for age, partner's age, cycle regularity, cycle length, partner's BMI (in female BMI analysis only), physical activity, smoking, alcohol intake and intercourse frequency.
bAdjusted for factors in ‘a’ as well as waist circumference (in BMI analyses), female BMI (in all analyses except weight change) and BMI at age 17 (in weight gain analyses).
Figure 1.
Relation between female BMI and fecundability, by parity status at baseline, fitted by restricted cubic splines.
Reference level for fecundability ratio is a BMI of 22 kg/m2. The dotted line segments represent ranges beyond the last knot used in the spline fitting, denoting tail ranges that have less information. The curves are adjusted for age, partner's age, cycle regularity, cycle length, partner's BMI, physical activity, smoking, alcohol intake, intercourse frequency and waist circumference.
Stratification of the female BMI data by age and smoking history showed similar FRs across ever and never smokers, but stronger results for obesity among women aged <25 years, albeit there were few obese women in this stratum (Table V). The inverse associations for overweight and obesity persisted among women with regular cycles (Table V). Overall results were consistent among women who had been trying to conceive for ≤2 cycles and 3–6 cycles at study entry (Table V). Finally, effect estimates were similar when we redefined our outcome variable as pregnancy resulting in birth (data not shown).
Table V.
Female BMI and time to pregnancy by age, smoking history, cycle regularity and attempted cycles before study entry
| Characteristic | BMI (kg/m2) |
||||
|---|---|---|---|---|---|
| <20 | 20–24 | 25–29 | 30–34 | ≥35 | |
| Age at baseline | |||||
| <25 | |||||
| No. | 28 | 89 | 32 | 10 | 4 |
| Cycles | 149 | 420 | 212 | 69 | 74 |
| FR (95% CI)a | 0.89 (0.53–1.48) | 1.00 (reference) | 0.73 (0.46–1.16) | 0.35 (0.16–0.76) | 0.21 (0.06–0.76) |
| FR (95% CI)b | 0.90 (0.50–1.60) | 1.00 (reference) | 0.74 (0.41–1.33) | 0.37 (0.15–0.96) | 0.22 (0.05–0.92) |
| 25–29 | |||||
| No. | 73 | 312 | 87 | 45 | 24 |
| Cycles | 415 | 1571 | 529 | 243 | 173 |
| FR (95% CI)a | 0.85 (0.64–1.15) | 1.00 (reference) | 0.83 (0.63–1.09) | 0.91 (0.62–1.32) | 0.68 (0.42–1.11) |
| FR (95% CI)b | 0.95 (0.69–1.31) | 1.00 (reference) | 0.69 (0.50–0.96) | 0.67 (0.41–1.08) | 0.49 (0.28–0.88) |
| ≥30 | |||||
| No. | 60 | 265 | 80 | 30 | 10 |
| Cycles | 279 | 1425 | 437 | 237 | 87 |
| FR (95% CI)a | 1.09 (0.79–1.52) | 1.00 (reference) | 0.94 (0.70–1.25) | 0.67 (0.43–1.04) | 0.67 (0.33–1.36) |
| FR (95% CI)b | 1.16 (0.82–1.65) | 1.00 (reference) | 0.80 (0.57–1.13) | 0.53 (0.31–0.90) | 0.52 (0.24–1.12) |
| Smoking status | |||||
| Ever | |||||
| No. | 49 | 259 | 89 | 37 | 19 |
| Cycles | 267 | 1480 | 521 | 258 | 168 |
| FR (95% CI)a | 1.08 (0.76–1.55) | 1.00 (reference) | 0.93 (0.70–1.23) | 0.86 (0.58–1.27) | 0.79 (0.44–1.41) |
| FR (95% CI)b | 1.14 (0.77–1.68) | 1.00 (reference) | 0.84 (0.61–1.16) | 0.74 (0.45–1.19) | 0.68 (0.36–1.29) |
| Never | |||||
| No. | 112 | 407 | 110 | 48 | 19 |
| Cycles | 576 | 1935 | 657 | 311 | 166 |
| FR (95% CI)a | 0.89 (0.70–1.14) | 1.00 (reference) | 0.75 (0.59–0.95) | 0.73 (0.52–1.04) | 0.49 (0.29–0.81) |
| FR (95% CI)b | 0.96 (0.74–1.25) | 1.00 (reference) | 0.64 (0.47–0.87) | 0.57 (0.34–0.94) | 0.37 (0.19–0.70) |
| Regular cycles | |||||
| No | |||||
| No. | 49 | 147 | 36 | 11 | 6 |
| Cycles | 264 | 754 | 250 | 102 | 85 |
| FR (95% CI)a | 1.05 (0.71–1.54) | 1.00 (reference) | 0.57 (0.37–0.89) | 0.54 (0.26–1.10) | 0.27 (0.10–0.72) |
| FR (95% CI)b | 1.16 (0.76–1.78) | 1.00 (reference) | 0.49 (0.26–0.91) | 0.43 (0.13–1.35) | 0.22 (0.06–0.81) |
| Yes | |||||
| No. | 112 | 519 | 163 | 74 | 32 |
| Cycles | 579 | 2662 | 928 | 467 | 249 |
| FR (95% CI)* | 0.93 (0.74–1.18) | 1.00 (reference) | 0.87 (0.71–1.07) | 0.85 (0.64–1.12) | 0.70 (0.47–1.06) |
| FR (95% CI)b | 1.01 (0.78–1.30) | 1.00 (reference) | 0.76 (0.60–0.96) | 0.68 (0.48–0.97) | 0.56 (0.35–0.88) |
| Cycle attempts before study entry | |||||
| ≤2 | |||||
| No. | 122 | 502 | 148 | 64 | 28 |
| Cycles | 629 | 2418 | 862 | 409 | 244 |
| FR (95% CI)a | 0.90 (0.72–1.13) | 1.00 (reference) | 0.80 (0.65–0.99) | 0.73 (0.54–0.99) | 0.59 (0.38–0.91) |
| FR (95% CI)b | 0.99 (0.77–1.27) | 1.00 (reference) | 0.67 (0.53–0.85) | 0.55 (0.38–0.79) | 0.43 (0.27–0.70) |
| 3–6 | |||||
| No. | 39 | 164 | 51 | 21 | 10 |
| Cycles | 214 | 998 | 316 | 160 | 90 |
| FR (95% CI)a | 1.24 (0.82–1.87) | 1.00 (reference) | 0.86 (0.60–1.25) | 0.76 (0.44–1.32) | 0.70 (0.34–1.47) |
| FR (95% CI)b | 1.29 (0.82–2.04) | 1.00 (reference) | 0.83 (0.52–1.35) | 0.72 (0.30–1.72) | 0.67 (0.26–1.74) |
FR = fecundability ratio; CI = confidence interval; No. = number of pregnancies.
aWhen applicable, adjusted for age, partner's age, cycle regularity, cycle length, partner's BMI, physical activity, smoking, alcohol intake, intercourse frequency and parity.
bAdjusted for all covariates in ‘a’ as well as waist circumference.
Discussion
In this prospective cohort study of Danish pregnancy planners, we observed longer TTPs among overweight, obese and very obese women, relative to normal weight women. After control for waist circumference, these results became stronger. Although we found little overall relation between underweight and TTP, the association varied by parity status. Among nulliparous women we observed longer TTPs compared with normal weight women, and among parous women we observed shorter TTPs compared with normal weight women. Male BMI was not appreciably associated with fecundability after control for female BMI. There was some suggestion of a weak positive association of waist circumference and WHR with fecundability in women, but only after adjustment for female BMI. Results for perceived body fat distribution were consistent with these anthropometric measures and associations between WHR and fecundability were similar across female BMI categories. In women, the inverse association we observed between weight gain since age 17 and fecundability became weaker after control for current BMI, indicating that current BMI may explain part of this effect.
Our results for female BMI agree with previous studies that have shown reduced fertility in overweight and obese women (Zaadstra et al., 1993; Jensen et al., 1999; Bolumar et al., 2000; Diamanti-Kandarakis and Bergiele, 2001; Hassan and Killick, 2004; Gesink Law et al., 2007; Ramlau-Hansen et al., 2007; Nohr et al., 2009), but not with those that found an inverse association among underweight women (Zaadstra et al., 1993; Bolumar et al., 2000; Hassan and Killick, 2004; Gesink Law et al., 2007). Our finding of effect modification by parity—an inverse association between underweight and fertility among nulliparous women and a positive association among parous women—has not been reported previously. Of note, parous women had considerably higher fecundability rates than nulliparous women in our study. Among women with already proven fertility, it is possible that underweight is not a risk factor for delayed TTP. The stronger inverse association we found between obesity and fertility among younger women is consistent with a study of women undergoing fertility treatment, in which IVF success rates were substantially lower for younger obese women (Sneed et al., 2008). And while no previous studies have investigated weight gain since adolescence and TTP, one study examining weight change between successive pregnancies found that weight loss among overweight women and weight gain among underweight women were associated with increased fecundability (Ramlau-Hansen et al., 2007). Our data were limited in that the precise timing of the weight gain was unknown. Results were similar, however, when we assessed average weight gain per year per woman and when we stratified by age at baseline.
Our results for male BMI are not entirely consistent with the few studies that have examined male fecundability (Sallmén et al., 2006; Nguyen et al., 2007; Ramlau-Hansen et al., 2007). In those studies, Nguyen et al. found an increased risk of infertility (TTP > 12 months) for BMI <20 (OR ∼1.50, 95% CI not reported), BMI 25–29 (OR = 1.20, 95% CI = 1.04–1.38) and BMI 30–34 (OR = 1.36, 95% CI = 1.13–1.63) in men, relative to BMI 20.0–22.4, whereas both Sallmén et al. and Ramlau-Hansen et al. found increased risks of infertility (TTP > 12 months) for overweight (range of ORs: 1.36–1.50) and obese (range of ORs: 1.74–2.00) men only. These studies included more subjects than ours. Like our study, two of the three studies used a measure of male BMI that was self-reported by women (Nguyen et al., 2007; Ramlau-Hansen et al., 2007). Nguyen et al. validated female reports against male reports of BMI in a subset of couples and found high (82%) agreement. It is unlikely that restriction to couples who were infertile (Sallmén et al., 2006) or who conceived (Nguyen et al., 2007; Ramlau-Hansen et al., 2007) explained the differences in study results because all three studies found similar associations for male overweight and obesity. In contrast to our study, however, all three studies were retrospective (Sallmén et al., 2006; Nguyen et al., 2007; Ramlau-Hansen et al., 2007), which may have introduced recall bias (Cooney et al., 2009), and all used infertility (TTP > 12 months) as an outcome definition.
Our observation of nearly null associations for waist circumference and WHR with fecundability before adjustment for BMI but weak positive associations after adjustment for female BMI was unexpected. These results do not agree with the few studies that have examined these exposures in relation to fertility, albeit most of these studies were conducted among women attending fertility clinics. In a prospective study of women attending a donor insemination clinic, a 0.1-unit increase in WHR was associated with a 30% decrease in probability of conception per cycle (FR = 0.71; 95% CI = 0.56–0.89) after adjustment for covariates (Zaadstra et al., 1993). A cross-sectional study found that a WHR ≥0.80, independent of BMI, reduced the pregnancy rate of IVF embryo transfer (Wass et al., 1997). In a third cross-sectional study of women without known fertility problems, women in the lowest quartile of WHR had 29% higher mean mid-cycle 17-β-estradiol levels and 33% higher mean luteal phase progesterone levels than women in the higher quartile of WHR (Jasienska et al., 2004). However, while estradiol correlates with follicle size and oocyte quality (Eissa et al., 1986), quality of cervical mucus (Roumen et al., 1982), and endometrial thickness (Dickey et al., 1993), and low progesterone levels are an indicator of anovulation (van Zonneveld et al., 1994), both estradiol and progesterone have low predictive value for diagnosing ovarian reserve problems and infertility (Broekmans et al., 2006) and are rarely relied on as sole markers of fertility.
BMI is strongly correlated with percent body fat (r = 0.84) in women aged 20–39 years (Flegal et al., 2009). Anthropometric and covariate data were self-reported by the female partner at baseline before the occurrence of pregnancy or any censoring event. Because not all women entered the study when they were first attempting to conceive, both differential (bias in either direction) and non-differential misclassification (bias towards null unless exposure has multiple categories) of anthropometric variables was possible. Although we did not update anthropometric measures over time and longer TTPs could have been associated with changes in eating patterns (e.g. stress-induced eating or medical advice to lose weight), it is unlikely that anthropometric measures would have changed appreciably over the short follow-up period. In a subset of cohort participants, self-reported height and weight showed excellent agreement with measures provided by the Danish Medical Birth Registry. Finally, it is reassuring that our results did not differ by number of cycle attempts before study entry, suggesting that reporting of anthropometric measures was not strongly influenced by TTP.
The association between high BMI and reduced fecundability in women has biological plausibility. Obesity may affect fertility through anovulation (Grodstein et al., 1994; Rich-Edwards et al., 1994, 2002) and alterations in the pre-ovulatory follicular environment (Robker et al., 2009). Obese women have been shown to have abnormally high levels of fats and inflammation in the fluid surrounding their oocytes, which can negatively influence the oocyte's development (Robker et al., 2009). Increasing BMI is associated with increasing follicular fluid levels of insulin, lactate, triglycerides, and C-reactive protein, and decreasing levels of sex hormone binding globulin (Robker et al., 2009). Differences in insulin-regulated genes in granulosa cells have also been found between obese and normal weight women (Robker et al., 2009).
Because Denmark has a high prevalence of internet access (www.oecd.org/denmark), our study was accessible to nearly all Danish women thinking about pregnancy. Some studies indicate a decrease in fecundability in Denmark over time (Juul et al., 1999; Jensen et al., 2002, 2008) whereas others have seen no clear time trends in fecundability (Jensen et al., 2005), although the data are scarce and methods have been inconsistent across studies (Sallmén et al., 2005). The proportion of couples in the Snart-Gravid study that conceived after 1 year was somewhat lower than that found in other prospective studies (Zinaman et al., 1996; Gnoth et al., 2003), and those interested in our study may have had lower fertility on average than the general population. Because we were only able to capture clinically-recognized pregnancies and up to 22% of early pregnancies are lost before clinical detection (Wilcox et al., 1988), our findings only relate to time to clinically-recognized pregnancy. Moreover, this was a study of pregnancy planners and although rates of unintended pregnancy are lower in Denmark compared with other developed countries (Jones et al., 1988), and were estimated to be as low as 25% in some regions of Denmark (Juul et al., 1999), a non-negligible proportion of pregnancies may have been unplanned. If pregnancy intention was related both to the exposures studied here and to fertility, our results may not be generalizable to women with unplanned pregnancies. Nonetheless, our study should have high internal validity because cohort retention was high and compares favorably with what has been reported for other large volunteer cohort studies (Olsen et al., 2001; Russell et al., 2001). The small proportion of women lost to follow-up tended to be heavier and have lower parity, which suggests that the effect of selective losses, if any, would have been to underestimate the associations of BMI with fecundability.
In summary, we found that women who were overweight, obese and very obese, as well as women who gained excess weight since age 17 years, had reduced fertility. Underweight was associated with reduced fertility among nulliparous women only. Results for the relation of waist circumference and WHR to fecundability depended on adjustment for BMI and were either close to null or weakly positive, findings that are at odds with the few other studies on this topic. Overall, our findings suggest that the increasing prevalence of obesity in Denmark may have deleterious effects on female fertility.
Funding
The study was funded by the NIH/NICHD (R21-050264) and the Danish Medical Research Council (271-07-0338).
Acknowledgements
We acknowledge Tina Christensen for her support with data collection and media contact, Krista Huybrechts for her assistance with data quality control, Donna Day Baird for her feedback on questionnaire development, and Thomas Jensen for his assistance with website design.
References
- Aggerholm AS, Thulstrup AM, Toft G, Ramlau-Hansen CH, Bonde JP. Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil Steril. 2008;90:619–626. doi: 10.1016/j.fertnstert.2007.07.1292. [DOI] [PubMed] [Google Scholar]
- Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol. 1986;124:470–480. doi: 10.1093/oxfordjournals.aje.a114417. [DOI] [PubMed] [Google Scholar]
- Bolumar F, Olsen J, Rebagliato M, Saez-Lloret I, Bisanti L. Body mass index and delayed conception: a European Multicenter Study on Infertility and Subfecundity. Am J Epidemiol. 2000;151:1072–1079. doi: 10.1093/oxfordjournals.aje.a010150. [DOI] [PubMed] [Google Scholar]
- Bonde JP, Joffe M, Sallmén M, Kristensen P, Olsen J, Roeleveld N, Wilcox A. Validity issues relating to time-to-pregnancy studies of fertility. Epidemiology. 2006;17:347–349. doi: 10.1097/01.ede.0000210239.80406.46. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2009 doi: 10.1016/j.fertnstert.2009.01.100. March 2 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology. 2009;20:56–59. doi: 10.1097/EDE.0b013e31818ef47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer DW, Barbieri RL, Xu H, Reichardt JKV. Determinants of basal follicle stimulating hormone levels in premenopausal women. J Clin Endocrinol Metab. 1994;79:1105–1109. doi: 10.1210/jcem.79.4.7962282. [DOI] [PubMed] [Google Scholar]
- De Pergola G, Tartagni M, d'Angelo F, Centoducati C, Guida P, Giorgino R. Abdominal fat accumulation, and not insulin resistance, is associated to oligomenorrhea in non-hyperandrogenic overweight/obese women. J Endocrinol Invest. 2009;32:98–101. doi: 10.1007/BF03345694. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bergiele A. The influence of obesity on hyperandrogenism and infertility in the female. Obes Rev. 2001;2:231–238. doi: 10.1046/j.1467-789x.2001.00041.x. [DOI] [PubMed] [Google Scholar]
- Dickey RP, Olar TT, Taylor SN, Curole DN, Matulich EM. Relationship of endometrial thickness and pattern to fecundity in ovulation induction cycles: effect of clomiphene citrate alone and with human menopausal gonadotropin. Fertil Steril. 1993;59:756–760. doi: 10.1016/s0015-0282(16)55855-5. [DOI] [PubMed] [Google Scholar]
- Due P, Heitmann BL, Sorensen TIA. Prevalence of obesity in Denmark. Obes Rev. 2007;8:187–189. doi: 10.1111/j.1467-789X.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- Eissa MK, Obhrai MS, Docker MF, Lynch SS, Sawers RS, Newton JR. Follicular growth and endocrine profiles in spontaneous and induced conception cycles. Fertil Steril. 1986;45:191–195. doi: 10.1016/s0015-0282(16)49153-3. [DOI] [PubMed] [Google Scholar]
- Falkner B, Sherif K, Sumner A, Kushner H. Hyperinsulinism and sex hormones in young adult African Americans. Metabolism. 1999;48:107–112. doi: 10.1016/s0026-0495(99)90018-5. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Harris TB, Everhart JE, Schenker N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch RE. Body fat, menarche, fitness and fertility. Hum Reprod. 1987;2:521–533. doi: 10.1093/oxfordjournals.humrep.a136582. [DOI] [PubMed] [Google Scholar]
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22:414–420. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes Control. 1996;7:253–263. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- Gnoth C, Godehardt D, Godehardt E, Frank-Herrmann P, Freundl G. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Hum Reprod. 2003;18:1959–1966. doi: 10.1093/humrep/deg366. [DOI] [PubMed] [Google Scholar]
- Greenland S, Rothman KJ. Introduction to stratified analysis: selecting confounders for control. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. New York: Lippincott Williams & Wilkins; 2008. pp. 261–263. [Google Scholar]
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5:247–250. doi: 10.1097/00001648-199403000-00016. [DOI] [PubMed] [Google Scholar]
- Hassan MAM, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril. 2004;81:384–392. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- Jasienska G, Ziomkiewicz A, Ellison PT, Lipson SF, Thune I. Large breasts and narrow waists indicate high reproductive potential in women. Proc Biol Sci. 2004;271:1213–1217. doi: 10.1098/rspb.2004.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins JM, Brook PF, Sargeant S, Cooke ID. Endocervical mucus pH is inversely related to serum androgen levels and waist to hip ratio. Fertil Steril. 1995;63:1005–1008. doi: 10.1016/s0015-0282(16)57538-4. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10:422–428. doi: 10.1097/00001648-199907000-00011. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Carlsen E, Jorgensen N, Berthelsen JG, Keiding N, Christensen K, Petersen JH, Knudsen LB, Skakkebaek NE. Poor semen quality may contribute to recent decline in fertility rates. Hum Reprod. 2002;17:1437–1440. doi: 10.1093/humrep/17.6.1437. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Andersson AM, Jorgensen N, Andersen AG, Carlsen E, Petersen JH, Skakkebaek NE. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril. 2004;82:863–870. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Joffe M, Scheike T, Skytthe A, Gaist D, Christensen K. Time trends in waiting time to pregnancy among Danish twins. Hum Reprod. 2005;20:955–964. doi: 10.1093/humrep/deh723. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Sobotka T, Hansen MA, Pedersen AT, Lutz W, Skakkebaek NE. Declining trends in conception rates in recent birth cohorts of native Danish women: a possible role of deteriorating male reproductive health. Int J Androl. 2008;31:81–92. doi: 10.1111/j.1365-2605.2007.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe M, Key J, Best N, Keiding N, Scheike T, Jensen TK. Studying time to pregnancy by use of a retrospective design. Am J Epidemiol. 2005;162:115–124. doi: 10.1093/aje/kwi172. [DOI] [PubMed] [Google Scholar]
- Jones EF, Forrest JD, Henshaw SK, Silverman J, Torres A. Unintended pregnancy, contraceptive practice and family planning services in developed countries. Fam Plann Perspect. 1988;20:53–67. [Google Scholar]
- Juul S, Karmaus W, Olsen J. Regional differences in waiting time to pregnancy: pregnancy-based surveys from Denmark, France, Germany, Italy and Sweden. The European Infertility and Subfecundity Study Group. Hum Reprod. 1999;14:1250–1254. doi: 10.1093/humrep/14.5.1250. [DOI] [PubMed] [Google Scholar]
- Kirschner MA, Samojlik E, Drejka M, Szmal E, Schneider G, Ertel N. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab. 1990;70:473. doi: 10.1210/jcem-70-2-473. [DOI] [PubMed] [Google Scholar]
- Knudsen LB, Olsen J. The Danish medical birth registry. Dan Med Bull. 1998;45:320–323. [PubMed] [Google Scholar]
- Kristensen J, Langhoff-Roos J, Skovgaard LT, Kristensen FB. Validation of the Danish Birth Registration. J Clin Epidemiol. 1996;49:893–897. doi: 10.1016/0895-4356(96)00018-2. [DOI] [PubMed] [Google Scholar]
- Lassek WD, Gaulin SJC. Changes in body fat distribution in relation to parity in American women: a covert form of maternal depletion. Am J Phys Anthropol. 2006;131:295–302. doi: 10.1002/ajpa.20394. [DOI] [PubMed] [Google Scholar]
- Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. The SAS LGTPHCURV8 Macro. Boston, MA: Channing Laboratory; 2003. [Google Scholar]
- Magnusdottir EV, Thorsteinsson T, Thorsteinsdottir S, Heimisdottir M, Olafsdottir K. Persistent organochlorines, sedentary occupation, obesity and human male subfertility. Hum Reprod. 2005;20:208–215. doi: 10.1093/humrep/deh569. [DOI] [PubMed] [Google Scholar]
- Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sorensen HT. Cohort Profile: The Danish Web-based Pregnancy Planning Study–'Snart-Gravid. Int J Epidemiol. 2008 doi: 10.1093/ije/dyn191. [EPub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C, Hernandez E, Ruiz JE, Fonseca ME, Bermudez JA, Zarate A. Upper body obesity and hyperinsulinemia are associated with anovulation. Gynecol Obstet Invest. 1999;47:1–5. doi: 10.1159/000010052. [DOI] [PubMed] [Google Scholar]
- Nestler JE. Insulin regulation of human ovarian androgens. Hum Reprod. 1997a;12:53–62. doi: 10.1093/humrep/12.suppl_1.53. [DOI] [PubMed] [Google Scholar]
- Nestler JE. Role of hyperinsulinemia in the pathogenesis of the polycystic ovary syndrome, and its clinical implications. Semin Reprod Endocrinol. 1997b;15:111–122. doi: 10.1055/s-2007-1016294. [DOI] [PubMed] [Google Scholar]
- Nguyen RH, Wilcox AJ, Skjaerven R, Baird DD. Men's body mass index and infertility. Hum Reprod. 2007;22:2488–2493. doi: 10.1093/humrep/dem139. [DOI] [PubMed] [Google Scholar]
- Nohr EA, Vaeth M, Rasmussen S, Ramlau-Hansen CH, Olsen J. Waiting time to pregnancy according to maternal birthweight and prepregnancy BMI. Hum Reprod. 2009;24:226–232. doi: 10.1093/humrep/den357. [DOI] [PubMed] [Google Scholar]
- Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, Taxbol D, Hansen KD, Juhl M, Schow TB, et al. The Danish National Birth Cohort–its background, structure and aim. Scand J Public Health. 2001;29:300–307. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22:1634–1637. doi: 10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, Manson JE. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171:171–177. doi: 10.1016/0002-9378(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Spiegelman D, Garland M, Hertzmark E, Hunter DJ, Colditz GA, Willett WC, Wand H, Manson JE. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002;13:184–190. doi: 10.1097/00001648-200203000-00013. [DOI] [PubMed] [Google Scholar]
- Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, Lane M, Norman RJ. Obese Women Exhibit Differences in Ovarian Metabolites, Hormones, and Gene Expression Compared to Moderate Weight Women. J Clin Endocrinol Metab. 2009;94:533–540. doi: 10.1210/jc.2008-2648. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Mikkelsen EM, Riis A, Sørensen HT, Wise LA, Hatch EE. Randomized trial of questionnaire length. Epidemiology. 2009;20:154. doi: 10.1097/EDE.0b013e31818f2e96. [DOI] [PubMed] [Google Scholar]
- Roumen FJ, Doesburg WH, Rolland R. Hormonal patterns in infertile women with a deficient postcoital test. Fertil Steril. 1982;38:42–47. doi: 10.1016/s0015-0282(16)46394-6. [DOI] [PubMed] [Google Scholar]
- Russell C, Palmer JR, Adams-Campbell LL, Rosenberg L. Follow-up of a large cohort of Black women. Am J Epidemiol. 2001;154:845–853. doi: 10.1093/aje/154.9.845. [DOI] [PubMed] [Google Scholar]
- Sallmén M, Weinberg CR, Baird DD, Lindbohm ML, Wilcox AJ. Has human fertility declined over time?: why we may never know. Epidemiology. 2005;16:494–499. doi: 10.1097/01.ede.0000165391.65690.e1. [DOI] [PubMed] [Google Scholar]
- Sallmén M, Sandler DP, Hoppin JA, Blair A, Baird DD. Reduced fertility among overweight and obese men. Epidemiology. 2006;17:520–523. doi: 10.1097/01.ede.0000229953.76862.e5. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. Cary, NC: SAS Institute; 2004. SAS/STAT User's Guide. Version 9.1. [Google Scholar]
- Sneed ML, Uhler ML, Grotjan HE, Rapisarda JJ, Lederer KJ, Beltsos AN. Body mass index: impact on IVF success appears age-related. Hum Reprod. 2008;23:1835–1839. doi: 10.1093/humrep/den188. [DOI] [PubMed] [Google Scholar]
- van Zonneveld P, te Velde ER, Koppeschaar HP. Low luteal phase serum progesterone levels in regularly cycling women are predictive of subtle ovulation disorders. Gynecol Endocrinol. 1994;8:169–174. doi: 10.3109/09513599409072451. [DOI] [PubMed] [Google Scholar]
- Wass P, Waldenstrom U, Rossner S, Hellberg D. An android body fat distribution in females impairs the pregnancy rate of in-vitro fertilization-embryo transfer. Hum Reprod. 1997;12:2057–2060. doi: 10.1093/humrep/12.9.2057. [DOI] [PubMed] [Google Scholar]
- Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol. 1993;137:1–8. doi: 10.1093/oxfordjournals.aje.a116591. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Zaadstra BM, Seidell JC, Van Noord PA, te Velde ER, Habbema JD, Vrieswijk B, Karbaat J. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. BMJ. 1993;306:484–487. doi: 10.1136/bmj.306.6876.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20:1541–1549. doi: 10.1002/sim.689. [DOI] [PubMed] [Google Scholar]
- Zinaman MJ, Clegg ED, Brown CC, O'Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65:503–509. [PubMed] [Google Scholar]
- Ziomkiewicz A, Ellison PT, Lipson SF, Thune I, Jasienska G. Body fat, energy balance and estradiol levels: a study based on hormonal profiles from complete menstrual cycles. Hum Reprod. 2008;23:2555–2563. doi: 10.1093/humrep/den213. [DOI] [PubMed] [Google Scholar]