Abstract
BACKGROUND
Inhibin B (Inh B) is produced by pre-antral and early antral follicles whereas estradiol (E2) is a product of follicles undergoing antrum formation. This temporal distinction is evident in the patterns of Inh B and E2 release earlier and later during the follicular phase of the menstrual cycle, respectively. However, in previous studies of women with polycystic ovary syndrome (PCOS) and normal controls, release of these granulosa cell (GC) products appears to be simultaneous in response to FSH stimulation. In order to reconcile these disparate findings, we conducted dose–response studies in both PCOS women and normal controls to determine whether GC product responses were due to the amount of FSH administered. In addition, we compared FSH-stimulated responses in PCOS women at various stages of recovery following ovarian suppression with a long-acting GnRH agonist to examine whether Inh B and E2 responses reflected the level of ovarian follicle activity (i.e. circulating E2 levels).
METHODS
Women with PCOS, 18–35 years (n = 23), and normal ovulatory controls, 18–35 years (n = 10) were recruited for study. Dose–responses were assessed over 24 h following intravenous administration of 0 (saline), 37.5, 75 and 150 IU of recombinant human FSH (r-hFSH) in PCOS and normal women. In addition, E2 and Inh B responses to 150 IU of r-hFSH were assessed at baseline and 4, 6 and 8 weeks following suppression of ovarian steroidogenesis by a long-acting GnRH agonist in PCOS women.
RESULTS
In PCOS women and normal controls, serum Inh B and E2 exhibit similar and simultaneous dose-responsiveness to FSH stimulation. During recovery from ovarian suppression, basal and stimulated Inh B release appear to be restored earlier than that of E2 in PCOS women.
CONCLUSIONS
These findings are consistent with the notion that, in PCOS women, the level of ovarian follicle activity largely determines the earlier release of Inh B compared with E2.
Keywords: inhibin B, estradiol, follicle stimulating hormone, polycystic ovary syndrome
Introduction
Within the ovary, inhibin B (Inh B) is produced by granulosa cells (GCs) of pre-antral and early antral follicles whereas estradiol (E2) is a product of aromatase gene expression in GCs of follicles undergoing antrum formation (Fraser et al., 1999; Stocco, 2008). Thus, follicle cohort growth and maturation during the normal menstrual cycle probably account for the rise and peak levels of Inh B during the early portion of the follicular phase compared with the subsequent increment of E2 secretion observed later in the follicular phase. This pattern of early Inh B secretion followed by E2 has also been seen in polycystic ovary syndrome (PCOS) and normal women undergoing ovulation induction with FSH (Anderson et al., 1998; Eldar-Geva et al., 2000). In contrast, our previous studies of PCOS and normal women have demonstrated that Inh B and E2 were released simultaneously and in parallel following provocative FSH stimulation (Wachs et al., 2006). In order to reconcile these disparate findings, we conducted dose–response studies in both PCOS women and normal controls to determine whether GC product responses were due to the amount of FSH administered. In addition, we compared FSH-stimulated responses in PCOS women at various stages of recovery following ovarian suppression with a long-acting GnRH agonist to examine whether Inh B and E2 responses reflected the level of ovarian follicle activity (i.e. circulating E2 levels).
Materials and Methods
Participants
Twenty-three women with PCOS and 10 normal controls with regular menstrual cycles were recruited for study. All PCOS subjects exhibited clinical and biochemical evidence of hyperandrogenism and were either oligomenorrheic or amenorrheic. In the PCOS and normal control groups the mean ages (±SE) were 25.7 ± 1.1 and 28.0 ± 1.6 years, respectively, and were not significantly different. The mean BMI was higher in PCOS subjects compared with normal controls, although the difference was not statistically significant (mean 34.0 ± 1.8 versus 28.7 ± 1.9 kg/m2, median 33.8 versus 26.9, respectively). Each PCOS subject had greater than 12 antral follicles per ovary on transvaginal ultrasound. Late-onset congenital adrenal hyperplasia was excluded by a serum 17-hydroxyprogesterone (17-OHP) level <3 ng/ml. Circulating thyroid stimulating hormone and prolactin levels were normal and not significantly different between groups. None of the subjects in either group had received any hormone medication for at least 3 months before study. The study was approved by the Human Research Protection Program at the University of California, San Diego and written informed consent was obtained from each participant before study.
Procedures
All subjects were admitted to the General Clinical Research Center at the University of California, San Diego on the day of testing. In normal subjects, testing was initiated during the mid-follicular phase of the menstrual cycle, defined as cycle Days 5–9, whereas PCOS women were admitted on a random day. For the FSH-stimulated dose–response studies, recombinant human FSH (r-hFSH) was administered as an intravenous bolus. Seventeen (17) PCOS women and 10 normal subjects each received a dose of 0 (saline), 37.5, 75 and 150 IU of r-hFSH, and the order of doses was randomized by blinded envelope selection. Normal subjects received only one dose per menstrual cycle whereas in PCOS women there was an interval of at least 2 weeks between each dose. Blood samples were drawn through an indwelling intravenous catheter at half-hour intervals for 2 h before and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 20 and 24 h after r-hFSH administration.
Six other PCOS women underwent stimulation testing with 150 IU r-hFSH i.v. before and after ovarian suppression. Blood samples were drawn through an indwelling intravenous catheter at 1 h intervals for 2 h before and at 1, 2, 3, 4, 6, 8, 10, 12 and 24 h after r-hFSH administration. After the 24 h sample, 3.75 mg of long-acting GnRH agonist (depot Lupron kindly provided by TAP Pharmaceuticals) was injected intra-muscularly. Repeat r-hFSH stimulation with the same intravenous dose of 150 IU and with identical blood sampling was performed 4, 6 and 8 weeks later.
The r-hFSH (Gonal-F) was kindly provided by EMD Serono, Inc. None of the PCOS subjects had experienced recent ovulation, as evidenced by serum progesterone (P4) levels <1.5 ng/ml. Samples were allowed to clot and sera were separated by centrifugation and stored at −20°C until assayed. Individual serum samples were analyzed in the same assay in duplicate.
Assays
Serum concentrations of LH and FSH were measured by radio-immunoassay (RIA) with intra- and inter-assay coefficients of variation (CV) of 5.4 and 8.0%, respectively, for LH and 3.0 and 4.6%, respectively, for FSH (Diagnostic Products Corp., Los Angeles, CA, USA). Serum concentrations of E2, androstenedione (A) and testosterone (T) were measured by well-established RIA with intra-assay CV less than 7%. P4, 17-OHP and dehydroepiandrosterone sulfate (DHEAS) were measured by RIA with intra-assay CV less than 7% (Diagnostic Systems Laboratories, Inc., Webster, TX, USA). Serum concentrations of Inh B were measured by ELISA with inter- and intra-assay CV of 6.7 and 4.6% (Diagnostic Systems Laboratories, Inc., Webster, TX, USA). The highly specific two-site ELISA Kit allows for quantitative measurement of dimeric Inh B in human serum. Assay sensitivity for Inh B was 7.0 pg/ml.
Statistical analysis
Baseline hormone values for PCOS and normal women were compared by two-sided, two-sample Wilcoxon Rank Sum tests. In the dose–response studies, E2 and Inh B responses were compared after administration of 0 (saline), 37.5, 75 or 150 IU r-hFSH. Comparisons at each dose were performed using analysis of variance (ANOVA) based on Generalized Estimating Equation (GEE) discrete time models (Liang and Zeger, 1986). These models flexibly estimate the mean response at each dose and time point, and the resulting ANOVA tests the significance of the variance explained by the dose effect. Initial residual plots revealed heteroscedasticity which was stabilized by log transforming the outcome variables. Holm adjustments for the eight comparisons (two outcomes × four doses) were also performed (Holm, 1979). Associations between peak levels of E2 and Inh B as well as time points after each dose of r-hFSH were explored using Spearman's correlation. For recovery after ovarian suppression, E2 and Inh B responses to 150 IU r-hFSH were analyzed and compared at baseline and 4, 6 and 8 weeks after GnRH agonist also using GEE discrete time models with log transformation and Holm adjustments. For all analyses, P-values less than 0.05 were considered statistically significant. Statistical analyses were performed using the R statistical computing software (version 2.6.2, http://www.r-project.org, 2009).
Results
Baseline hormone concentrations in PCOS and normal women
Baseline circulating hormone levels are shown in Table I. In women with PCOS, mean circulating levels of LH, T, A and 17-OHP were significantly greater than those of normal controls. Serum levels of FSH, DHEAS, E2, Inh B and P4 were similar between groups.
Table I.
Basal circulating steroid hormone and gonadotrophin levels in normal controls and PCOS women
| Controls (n = 10) | PCOS (n = 23) | |
|---|---|---|
| LH (mIU/ml) | 2.75 ± 0.4 | 8.16 ± 1.2* |
| FSH (mIU/ml) | 4.61 ± 0.5 | 5.26 ± 0.3 |
| T (ng/ml) | 0.29 ± 0.03 | 0.61 ± 0.04* |
| A (ng/ml) | 0.87 ± 0.1 | 1.73 ± 0.1* |
| 17-OHP (ng/ml) | 0.67 ± 0.1 | 1.13 ± 0.1* |
| DHEAS (ng/ml) | 1213 ± 307 | 1705 ± 154 |
| E2 (pg/ml) | 67.66 ± 8.9 | 56.64 ± 2.1 |
| P4 (ng/ml) | 0.86 ± 0.1 | 1.11 ± 0.1* |
| Inh B (pg/ml) | 121.27 ± 18.7 | 105.37 ± 11.5 |
To convert to SI units multiply by the following conversion factor: T (3.47); A (3.49); 17-OHP (3.03); DHEAS (0.0027); E2 (3.67); P4 (3.18); Inh B (0.00003125).
*P < 0.05 versus normal controls.
E2 and Inh B dose–responses to r-hFSH in normal women
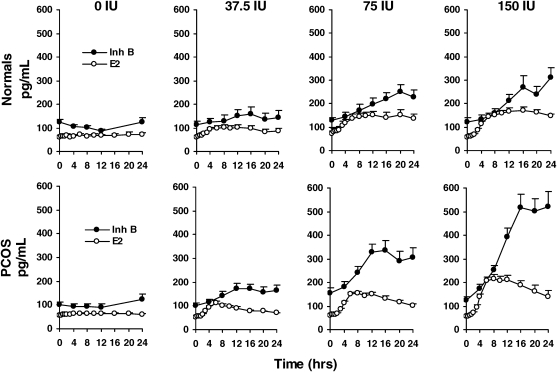
To determine the relative responsiveness of E2 and Inh B to varying doses of r-hFSH in normal and PCOS women, serum hormone levels were assessed following intravenous administration of 0 (saline), 37.5, 75 and 150 IU of r-hFSH (Fig. 1). We used separate discrete time GEE models for each of the two response variables and three active doses. In normal women, the main dose effect on total E2 and Inh B production over 24 h after each dose of r-hFSH was highly significant (P < 0.001) compared with that observed following saline injection based on GEE discrete time models. Mean levels were consistently greater over the 24 h following active doses compared with placebo. Correlation analysis revealed that maximal E2 and Inh B responses to r-hFSH achieved significance at the 150 IU dose (r = 0.69, P < 0.05). With respect to time, maximum E2 responses to r-hFSH were apparent 12–16 h after administration whereas peak Inh B values occurred at 16, 20 and 24 h, respectively (P < 0.001). The correlation between serum E2 and Inh B concentrations was significant only at 24 h after the 150 IU dose (r = 0.77, P < 0.01).
Figure 1.
Mean (±SE) serum E2 and Inh B responses to intravenous administration of FSH, 0 (saline), 37.5, 75 and 150 IU in 17 PCOS and 10 normal women over 24 h.
For PCOS and normal women, total E2 and Inh B production over 24 h in response to each dose of FSH was significantly higher compared with that observed following saline injection (0 dose). Compared with normal women, total E2 responses in PCOS women were significantly higher after 37.5, 75 and 150 IU of FSH. Total Inh B production in PCOS women was significantly greater than that of normal women after 75 and 150 IU of FSH.
E2 and Inh B dose–responses to r-hFSH in women with PCOS
In women with PCOS, E2 responsiveness to r-hFSH exhibited a significant (P < 0.001) treatment effect at all doses, characterized by progressive rises that achieved peak levels 6–8 h after injection compared with saline administration (Fig. 1). Similarly, the treatment effect on Inh B responses was significant (P < 0.001), with increased responses after all doses of r-hFSH reaching peak levels 12–16 h following FSH stimulation (Fig. 1). We observed a significant (unadjusted P < 0.02) group effect (normal versus PCOS women) on circulating E2 levels following all doses of r-hFSH (the effect of the 75 IU dose failed to reach significance after the Holm adjustment). Likewise, there was a significant (P < 0.001) effect on Inh B levels following 75 and 150 IU of r-hFSH according to GEE discrete time models. Notably, the temporal pattern of Inh B and E2 responses in PCOS women at all doses was remarkably similar through 8 h. After achieving maximal values, circulating E2 concentrations in PCOS women decreased gradually to approximately 40% of maximal levels as previously reported (Coffler et al., 2003). However, in contrast to E2, Inh B levels following r-hFSH injection rose steadily to reach maximal levels that were maintained through the course of study.
A significant correlation between peak E2 and Inh B responses over 24 h was observed at r-hFSH doses of 37 IU (r = 0.72, P < 0.05), 75 IU (r = 0.89, P < 0.0001) and 150 IU (r = 0.87, P < 0.0001). With respect to time, the correlation between serum E2 and Inh B concentrations at 8 and 12 h was significant for all doses. At 16 and 24 h, however, a significant correlation was seen only at the 75 and 150 IU dose.
Effect of ovarian suppression and recovery on E2 and Inh B responses to r-hFSH in PCOS women
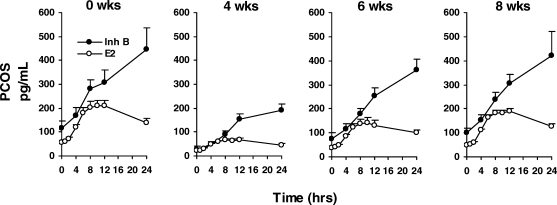
In a subgroup of six women with PCOS, basal levels and stimulated responses of E2 and Inh B to 150 IU r-hFSH were assessed prior to and during recovery from ovarian suppression induced by a long-acting GnRH agonist. Prior to agonist administration, the patterns of E2 and Inh B responses to 150 IU r-hFSH in this subgroup were similar to those observed for the entire group (Fig. 2).
Figure 2.
Mean (±SE) serum E2 and Inh B responses to intravenous administration of 150 IU FSH before and 4, 6 and 8 weeks after ovarian suppression with a single injection of long-acting GnRH agonist (depot Lupron, 3.75 mg, i.m.) in six PCOS women.
Compared with Week 0, total E2 and Inh B release were significantly reduced at Weeks 4 and 6. By Week 8, E2 responsiveness remained significantly reduced whereas Inh B production was no longer different than Week 0.
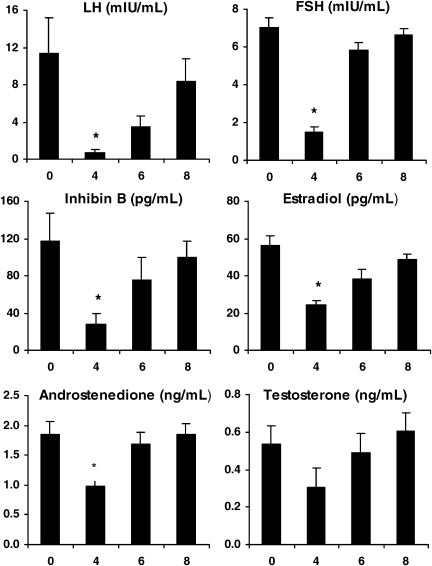
Four weeks following administration of GnRH agonist, marked declines in basal serum FSH and LH were observed as expected (Fig. 3) (Larmore and Klein, 2000). Corresponding decreases in circulating Inh B and E2 levels were also noted. Serum A and T were reduced to approximately 50% of baseline values. In response to r-hFSH stimulation, total circulating E2 and Inh B production was significantly reduced compared with before agonist administration according to GEE discrete time model analysis (Fig. 2, P < 0.001).
Figure 3.
Mean (±SE) basal serum levels of LH, FSH, Inh B, E2, A and T before and 4, 6 and 8 weeks after treatment with a single injection of a long-acting GnRH agonist (depot Lupron, 3.75 mg, i.m.) in six PCOS women; *P < 0.05 versus 0 weeks.
Six weeks after GnRH agonist (2 weeks following the nadir of ovarian hormone suppression), basal serum levels of FSH, LH, Inh B and E2 began to increase but remained below pretreatment concentrations (Fig. 3). Notably, resumption of Inh B secretion was characterized by a 2-fold rise whereas E2 levels increased only 0.5-fold. Serum A and T levels exhibited a rapid return to pretreatment values. Following r-hFSH, peak serum E2 and Inh B responses were greater than those observed at 4 weeks but less than those observed prior to GnRH agonist treatment (Fig. 2, treatment effect P < 0.001).
By 8 weeks, basal secretion of all hormones had completely recovered. Following administration of r-hFSH, total Inh B responses were restored to pretreatment values. In contrast, despite clear increases in E2 levels following r-hFSH stimulation, total E2 responsiveness remained lower than before ovarian suppression (Figs. 2 and 3, treatment effect P < 0.001).
Discussion
The results of this study have demonstrated that in PCOS women and normal controls, responses of serum Inh B and E2 to intravenous FSH stimulation appeared to be similar and simultaneous. However, in PCOS women recovering from GnRH agonist treatment, Inh B release appeared to be restored earlier than that of E2 which is consistent with the presence of small antral follicles following ovarian suppression.
These results confirm and extend our previous findings that acute responses of E2 and Inh B following intravenous administration of FSH in PCOS and normal women occur in parallel with each other (Wachs et al., 2006). In addition, the close correlation between maximal Inh B and E2 responses suggest that both hormones serve to reflect comparable GC responsiveness to FSH. Our dose–response studies failed to discriminate Inh B responses from those of E2 following FSH, which may have been due to intravenous administration resulting in higher increments of FSH than that reported in other studies. When administered as intravenous boluses, FSH doses of 37.5, 75 and 150 IU resulted in peak circulating levels of 10, 15 and 25 mIU/ml, respectively (Coffler et al., 2003). By comparison, i.m. or subcutaneous delivery has been shown to produce correspondingly lower levels of circulating FSH (Anderson et al., 1998; Eldar-Geva et al., 2000). Alternatively, significantly increased GC responsiveness to all doses of FSH stimulation in our PCOS subjects may have masked any possible temporal distinction between Inh B and E2 release. Thus, either the effective dose of FSH or a heightened responsive cohort of follicles or both may have accounted for our findings.
The earlier restoration of basal and stimulated Inh B release relative to that of E2 during recovery from ovarian suppression is not unlike GC responses exhibited by PCOS women undergoing ovulation induction with gonadotrophin therapy. In these studies, ovarian responses were associated with an immediate rise in Inh B unaccompanied by corresponding increments in circulating E2, which instead occurred later with development of a dominant follicle. In normal women undergoing ovulation induction with FSH after suppression with GnRH agonist in the antecedent luteal phase, increases in serum Inh B levels were observed well before rises in circulating E2 (Eldar-Geva et al., 2000). In hypogonadotropic hypogonadal women receiving pulsatile GnRH, significant increases of Inh B were observed within 24 h of GnRH administration whereas increases in E2 were noted only with the appearance of a dominant follicle (Welt et al., 1997). The immediate release of Inh B following FSH was attributed to the preponderance of Inh B generated from GCs of small antral follicles. In non-hyperandrogenic, anovulatory PCOS women treated with FSH during ovulation induction, substantial increases in serum Inh B preceded those of E2 by several days (Anderson et al., 1998). Whether the distinctive separation of Inh B and E2 secretion may have been due to the relatively low dose of FSH (75 IU per day), the non-hyperandrogenic environment, or an apparent absence of enhanced GC sensitivity to FSH is not clear.
During the normal menstrual cycle, the expression of Inh B in the early follicular phase and of E2 later in the same phase is largely follicle stage dependent. In PCOS women, there is an increase in the number of pre-antral and mid-antral follicles; however, this increase is not necessarily accompanied by greater basal secretion of Inh B or E2 relative to that of normal women. It is only with exogenous FSH stimulation that the marked enhanced production of both these hormone becomes apparent (Wachs et al., 2006). In this regard, the PCOS GCs are more sensitive than normal GCs which would lead to greater responses of both Inh B and E2 and at the same time may mask any temporal separation of release. This may account for the finding of early resumption of Inh B release before that of E2 during recovery from ovarian suppression in our PCOS women. At the cellular level, FSH-stimulated E2 production is mediated predominantly through the cAMP/PKA signaling pathway (Hickey et al., 1990). Although the generation of Inh B also involves the same cellular pathway, there appears to be another, as yet, unidentified mechanism(s) that regulates its production (Bernard et al., 2001). Whether differences in signaling pathways may contribute to the temporal disparity of Inh B and E2 release are unknown.
The decline and recovery of circulating gonadotrophin and steroid hormone levels in response to long-acting GnRH agonist were not unexpected and consistent with our previous studies in PCOS women who were treated with a short-acting form of daily GnRH agonist (de Ziegler et al., 1989). In addition, an earlier report by Larmore and Klein showed that premenopausal women treated with a single injection of long-acting GnRH agonist, identical to the preparation used in our study, exhibited a similar temporal decrease and recovery of LH, FSH and E2 as observed here (Larmore and Klein, 2000). Of potential significance was the observation that E2 production in our subjects was not completely suppressed as maximal reduction at 4 weeks was associated with circulating concentrations in the 20 pg/ml range. By comparison, GnRH agonists as well as antagonists have been shown to eliminate ovarian steroidogenesis in women as demonstrated by undetectable levels of serum E2 (Chang et al., 1983). However, the PCOS women in this study were obese and the dose of GnRH agonist administered was not calculated on the basis of weight. Thus, the lack of complete ovarian suppression may have contributed to the increases in Inh B and E2 following FSH administration.
In summary, Inh B and E2 are released in parallel following provocative FSH stimulation in PCOS as well as normal women. In women with PCOS, restoration of baseline and FSH-stimulated Inh B preceded that of E2 during recovery from ovarian suppression. These findings are consistent with the notion that, in PCOS women, the level of ovarian follicle activity largely determines the earlier release of Inh B compared with E2.
Authors’ Roles
M.A.R. participated in data analysis and was the primary author; D.S.W., M.S.C., and P.J.M. participated in study design and execution; M.D. performed statistical analysis and R.J.C. was the primary investigator.
Funding
This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement (U54 HD12303-28) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and in part by NIH grant MO1 RR00827.
Acknowledgements
We are grateful to Mr Jeff Wong for his technical expertise and to the nurses and staff of the University of California, San Diego, General Clinical Research Center for their dedicated care.
References
- Anderson RA, Groome NP, Baird DT. Inhibin A and inhibin B in women with polycystic ovarian syndrome during treatment with FSH to induce mono-ovulation. Clin Endocrinol (Oxf) 1998;48:577–584. doi: 10.1046/j.1365-2265.1998.00442.x. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Chapman SC, Woodruff TK. Mechanisms of Inhibin Signal Trandsuction. Recent Prog Horm Res. 2001;56:417–450. doi: 10.1210/rp.56.1.417. [DOI] [PubMed] [Google Scholar]
- Chang RJ, Laufer LR, Meldrum DR, DeFazio J, Lu JK, Vale WW, Rivier JE, Judd HL. Steroid secretion in polycystic ovarian disease after ovarian suppression by a long-acting gonadotropin-releasing hormone agonist. J Clin Endocrinol Metab. 1983;56:897–903. doi: 10.1210/jcem-56-5-897. [DOI] [PubMed] [Google Scholar]
- Coffler MS, Patel K, Dahan MH, Malcom PJ, Kawashima T, Deutsch R, Chang RJ. Evidence for abnormal granulosa cell responsiveness to follicle-stimulating hormone in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:1742–1747. doi: 10.1210/jc.2002-021280. [DOI] [PubMed] [Google Scholar]
- de Ziegler D, Steingold K, Cedars M, Lu JK, Meldrum DR, Judd HL, Chang RJ. Recovery of hormone secretion after chronic gonadotropin-releasing hormone agonist administration in women with polycystic ovarian disease. J Clin Endocrinol Metab. 1989;68:1111–1117. doi: 10.1210/jcem-68-6-1111. [DOI] [PubMed] [Google Scholar]
- Eldar-Geva T, Robertson DM, Cahir N, Groome N, Gabbe MP, Maclachlan V, Healy DL. Relationship between serum inhibin A and B and ovarian follicle development after a daily fixed dose administration of recombinant follicle-stimulating hormone. J Clin Endocrinol Metab. 2000;85:607–613. doi: 10.1210/jcem.85.2.6383. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Groome NP, McNeilly AS. Follicle-stimulating hormone-inhibin B interactions during the follicular phase of the primate menstrual cycle revealed by gonadotropin-releasing hormone antagonist and antiestrogen treatment. J Clin Endocrinol Metab. 1999;84:1365–1369. doi: 10.1210/jcem.84.4.5586. [DOI] [PubMed] [Google Scholar]
- Hickey GJ, Krasnow JS, Beattiee WG, Richard JS. Aromatase cytochrome P450 in rat ovarian granulosa cells before and after luteinization: adenosine 3′,5′-monophosphate-dependent and independent regulation. Cloning and sequencing of rat aromatase cDNA and 5′genomic DNA. Mol Endocrinol. 1990;4:3–12. doi: 10.1210/mend-4-1-3. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Larmore KA, Klein KO. Estradiol suppression and recovery during leuprolide acetate treatment in women as determined weekly by an ultrasensitive recombinant cell bioassay. Gynecol Endocrinol. 2000;14:405–410. doi: 10.3109/09513590009167711. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; R: A language and environment for statistical computing. 2009:ISBN 3-900051-07-0;URL http://www.R-project.org . [Google Scholar]
- Stocco C. Aromatase expression in the ovary: hormonal and molecular regulation. Steroids. 2008;73:473–487. doi: 10.1016/j.steroids.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs DS, Coffler MS, Malcom PJ, Chang RJ. Comparison of follicle-stimulating-hormone-stimulated dimeric inhibin and estradiol responses as indicators of granulosa cell function in polycystic ovary syndrome and normal women. J Clin Endocrinol Metab. 2006;91:2920–2925. doi: 10.1210/jc.2006-0442. [DOI] [PubMed] [Google Scholar]
- Welt CK, Martin KA, Taylor AE, Lambert-Messerlian GM, Crowley WF, Jr, Smith JA, Schoenfeld DA, Hall JE. Frequency modulation of follicle-stimulating hormone during the luteal-follicular transition: evidence for FSH control of inhibin B in normal women. J Clin Endocrinol Metab. 1997;82:2645–2652. doi: 10.1210/jcem.82.8.4138. [DOI] [PubMed] [Google Scholar]