Abstract
Purpose
Concerns about radiotherapy-related neurocognitive sequelae in young children have led to deferral or avoidance of radiation in contemporary treatment for this fragile group of patients. We compared survival and neurocognitive outcome in two groups of infants with medulloblastoma who received adjuvant conventional craniospinal irradiation (csi) or reduced or no radiotherapy during an era of change in the philosophy of infant medulloblastoma treatment.
Patients and Methods
From 1985 to 2007, 29 patients 3 years of age or younger were diagnosed and treated with curative intent in our institution. Children treated before 1994 received adjuvant radiation with chemotherapy; subsequently, radiation was prescribed essentially for disease progression or relapse.
Results
Median age at diagnosis was 24 months (range: 1–36 months); 15 patients (52%) presented with metastatic disease at diagnosis. As part of initial treatment, 8 children received adjuvant radiotherapy with chemotherapy, and 21 children received postoperative chemotherapy only. Five children treated with chemotherapy alone are in prolonged remission. The 5-year event-free and overall survivals were 35.9% ± 9.8% and 50.2% ± 9.6% respectively. Extent of resection, metastatic status, and desmoplastic histology were not found to be significant prognostic factors.
On serial neurocognitive evaluations, patients treated with chemotherapy with or without reduced radiotherapy demonstrated improvement of intellectual function over time. Patients treated with conventional csi exhibited significantly lower intelligence quotient scores and academic performance, with the exception of receptive vocabulary.
Conclusions
Avoidance of conventional csi in treatment of very young children with medulloblastoma appears to be associated with a preserved neurocognitive profile. Neurocognitive evaluation should be integrated into the primary objectives of future infant protocols.
Keywords: Infants, medulloblastoma, neurocognitive outcome
1. INTRODUCTION
Medulloblastoma is the most common malignant brain tumour in children. Of all medulloblastomas, 30% occur in children under 3 years of age 1, and this population is a very challenging group because of their recognized poorer outcome and, most importantly, because of their increased susceptibility to treatment-related toxicities. Infants with brain tumours are particularly vulnerable to significant intellectual impairment after cranial irradiation 2–5. Although craniospinal irradiation (csi) improves survival, it is usually avoided in young children because of significant neurotoxicity and very adverse neurocognitive outcomes. To delay or eliminate the need for radiation at this early stage of brain maturation, a series of contemporary therapeutic studies relying on primary postoperative chemotherapy have been developed for infants and young children 6–10. Preserved intellectual outcome has been documented for survivors treated without radiation, but comprehensive long-term longitudinal data remain limited to date 11,12. Here, we report a single-institution experience in managing infants with medulloblastoma over a 20-year period and provide comprehensive serial neurocognitive data on long-term survivors.
2. PATIENTS AND METHODS
At the Hospital for Sick Children in Toronto, 34 consecutive patients diagnosed with medulloblastoma before the age of 3 years were treated between April 1985 and April 2007. This retrospective review of those 34 cases was approved by the research ethics board of the institution. Clinical, therapeutic, and survival data were collected from patient files. Patient outcomes included serial neurocognitive measures and endocrine status at completion of the study.
Metastatic status according to the Chang staging system 13 and extent of resection were based on the cerebrospinal fluid (csf) analysis (when available), the surgical report, and postsurgical imaging. Extent of resection was defined as follows:
Gross total resection (gtr)—no radiologic evidence of residual tumour
Radical subtotal resection (rstr)—a resection of less than 100%, but of 95% or more
Subtotal resection (str)—a resection of less than 95%, but of more than 50%
Partial resection (pr)—a resection of less than 49%, but of 10% or more
Biopsy—a resection of less than 10%
All tumour samples were reviewed by one neuropathologist (CH) for the purposes of the study.
Clinical management evolved over the study period. Before 1994, all patients treated with curative intent received adjuvant chemotherapy and radiotherapy. After 1994, all infants with medulloblastoma were treated according to “baby brain” protocols and strategies 7,8, those successively being Baby pog (Pediatric Oncology Group) 1 and 2, and bbsfop (Societe francaise d’oncologie pediatrique).
More recently, in an attempt to improve outcome, high-dose chemotherapy (hdc) protocols were introduced into the management of these children.
2.1 Neuropsychological Assessment
Neurocognitive assessments were conducted for all surviving patients. These assessments included evaluation of intelligence, receptive vocabulary, visual–motor function, memory, and academic achievement. Specifically, the test battery included either the Wechsler Intelligence Scale for Children (3rd edition) or the Wechsler Preschool and Primary Scales of Intelligence–Revised depending on the age of the child and the Peabody Picture Vocabulary Test, the Beery Visual–Motor Integration Test, the Children’s Memory Scale, and the Wide Range Achievement Test (either revised or 3rd edition).
2.2 Statistical Analysis
Progression-free survival (pfs) was determined from the date of diagnosis to the date of disease progression, with censoring on the date of the most recent clinical assessment. Overall survival (os) was assessed from the date of the diagnosis to the date of last follow-up or the date of death. Kaplan–Meier analysis was used to estimate os and pfs, and the log-rank test was used to determine statistical significance. For neurocognitive assessment, individual test scores were converted to standard scores (based on age-related means and standard deviations from test standardization norms) with a mean of 100 and a standard deviation of 15. Analysis of variance was used to examine group differences in neurocognitive functioning for the survivors. All statistical analyses were performed using the SPSS software package (version 15.0: SPSS, Chicago, IL, U.S.A.).
3. RESULTS
3.1 Patient Characteristics at Diagnosis
From April 1985 to April 2007, 34 consecutive patients (19 boys, 15 girls) with a histologically confirmed diagnosis of medulloblastoma were diagnosed and treated at our institution. All were 36 months of age or younger. Median age at diagnosis was 24 months (range: 1–36 months), and 7 patients were less than 12 months old. Median duration of presenting symptoms before diagnosis was 0.8 month (range: 1 week to 6 months).
All patients underwent a surgical procedure, and 5 patients subsequently received postoperative palliation for extensive disease and poor surgical recovery. The patient characteristics, treatment descriptions, and outcomes data that follow are therefore given for the 29 patients who received postoperative therapy with curative intent. Of these 29 patients, 17 underwent gtr or rstr (≥ 95% resection), 10 underwent str, and 2 underwent pr.
In 6 patients (21%), staging investigations (craniospinal axis imaging and csf cytology analysis) were insufficient to provide an accurate metastatic status at diagnosis. Metastasis at diagnosis was found in 15 patients (52%: 4 M1, 4 M2, 6 M3, 1 M2/M3). Histopathologic subtype was desmoplastic in 7 patients (24.1%), anaplastic in 1 patient (3.4%, and classic medulloblastoma in the remaining 21 patients (72.5%).
3.2 Chemotherapy
Chemotherapy with curative intent was prescribed in all 29 patients. Table I details the chemotherapy regimens used. Adjuvant chemotherapy alone was used in 21 patients, with the intent to delay or avoid radiation. This chemotherapy-alone strategy was successfully completed in 5 of the 21 patients (24%), who remain in continuous complete remission 133, 98, 65, 51, and 23 months after diagnosis. Myeloablative hdc followed by stem cell rescue (scr) was given to 8 patients: 6 received this therapy as part of consolidation of initial induction therapy, and 2 received it at the time of relapse or for residual disease. Among these 8 patients, 1 died of toxicity, and 4 remain alive at 81, 51, 36, and 23 months post diagnosis. Median time to progression or relapse for the patients treated with adjuvant chemotherapy alone was 6.7 months (range: 0.4–47.7 months).
TABLE I.
Description of postoperative chemotherapy
| Regimen | Patients (n) |
|---|---|
| Conventional chemotherapy | 29 |
| Baby pog 1, 2 | 9 |
| ice | 7 |
| 8-in-1 | 3 |
| bbsfop | 3 |
| moppa | 3 |
| hdc+scr conditioning regimens | 8 |
| ccg 99703 | 3 |
| Busulfan thiotepab | 2 |
| Headstart ii | 2 |
| Etoposide, carboplatin, thiotepab | 1 |
One patient received bbsfop after 1 cycle of mopp and 1 prior to high-dose busulfan thiotepa.
Post conventional chemotherapy.
3.3 Radiation Therapy
Radiation in an adjuvant setting was administered in 8 patients, 7 of whom received csi and a focal boost. In 1 patient who had an incomplete resection at diagnosis, intensity-modulated radiotherapy (5400 cGy) was administered to the original tumour volume after hdc and scr. At a median follow-up of 81 months, 6 of the 8 irradiated patients (75%) remain alive.
In 4 patients, salvage radiotherapy was administered at the time of recurrence or progression. Of these 4 patients, 2 received conventional csi, 1 received stereotactic radiation (5400 cGy) to the tumour bed after hdc and scr, and 1 received a reduced dose of csi (1800 cGy), with a posterior fossa boost, for local relapse 1 year after high-dose chemotherapy. Only 1 of these 4 patients patient is a long-term survivor at 108 months from diagnosis.
3.4 Survival and PFS
Deaths from their disease occurred in 15 patients, from a secondary malignancy (T-cell lymphoma) in 1 patient, and from treatment-related toxicity in 1 patient. At a median follow-up of 90 months (range: 23–194 months), 12 patients remain alive. Progression or relapse was focal in 7 patients (41%), distant in 3 patients (18%), and combined focal and distant in 7 patients (41%).
The 5-year pfs and os were 35.9% ± 9.8% and 50.2% ± 9.6% respectively. None of the following factors were found to be significant for os and event-free survival: age younger than 12 months, extent of resection, and desmoplastic histology. Although nonsignificant, a trend for a better survival was observed in patients presenting with metastatic disease at diagnosis (5-year os of 64.2% ± 13.2%, Table II). The use of adjuvant conventional csi was the only factor associated with a significantly better 5-year pfs (p =0.013) and an os of 72.9% ± 16.5% (p = 0.032).
TABLE II.
Overall survival according to prognostic factors
| Prognostic factor | Patients (n) | 5-Year os (%) | p Value |
|---|---|---|---|
| Age < 12 months at diagnosis | 7 | 28.6±17.1 | 0.54 |
| Resection > 95% | 17 | 58.2±12.1 | 0.50 |
| Metastatic disease | 15 | 64.2±13.2 | 0.055 |
| Desmoplastic histology | 7 | 48.5±11 | 0.69 |
| Use of adjuvant csi | 8 | 72.9±16.5 | 0.032 |
os = overall survival; csi = craniospinal irradiation.
Table III summarizes the characteristics of the surviving patients.
TABLE III.
Characteristics of the survivors
| Pt id | Sex | Age at diagnosis (years) | Pathology | Surgery | Shunt around time of surgery | Metastatic status | Treatment | rt type and dose (cGy) | Age at rt (years) | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 1.7 | Classic | str | + | M3 | ct+rt | csi 3500, boost pf 5000 | 1.9 | 194 |
| 2 | M | 2.5 | Classic | gtr | + | M3 | ct+rt | csi 2880, boost pf 5040 | 2.6 | 173 |
| 3 | F | 0.8 | Classic | gtr | + | M0 | ct alone | – | – | 133 |
| 4 | M | 1.9 | Classic | str | − | M3 | ct+rt | csi 3500 | 2.6 | 126 |
| 5 | F | 0.8 | Desmoplastic | rstr | − | M0 | ct alone | – | – | 98 |
| 6 | M | 2.2 | Classic | str | + | M2 | ct+rta | csi 4140 | 2.8 | 108 |
| 7 | M | 3.0 | Desmoplastic | gtr | + | M3 | ct+rta | csi 3600 | 3.1 | 81 |
| 8 | F | 1.4 | Classic | gtr | + | M1 | hdc+scr+rt | imrt to pf 5400 | 2.0 | 81 |
| 9 | M | 2.6 | Classic | gtr | + | M0 | ct alone | – | – | 65 |
| 10 | M | 2.0 | Anaplastic | gtr | − | M1 | hdc+scr | – | – | 23 |
| 11 | M | 2.0 | Desmoplastic | gtr | − | M1 | hdc+scr | – | – | 51 |
| 12 | F | 2.0 | Classic | pr | − | M2, M3 | hdc+scr+rt | csi 2160, boost pf 5040 | 3.2 | 36 |
Radiotherapy at time of relapse.
Pt = patient; rt = radiotherapy; M = male; str = subtotal resection; ct = chemotherapy; csi = craniospinal irradiation; pf = posterior fossa; gtr = gross total resection; F = female; rstr = radical subtotal resection; hdc+scr = high-dose chemotherapy and stem cell rescue; imrt = intensity-modulated radiation therapy; pr = partial resection.
3.5 Neurocognitive Evaluation
For the 12 surviving patients, neurocognitive outcome was compared between the 6 patients treated with conventional csi (group A) and the 6 patients treated with chemotherapy alone or with chemotherapy associated with reduced-dose or limited-volume radiation (group B). Group A was considered a historical control group.
Age at diagnosis was significantly different between the two groups (2.33 years for group A vs. 1.55 years for group B, p < 0.05). Differences in intelligence, receptive vocabulary, visual–motor skills, academics, and memory were evaluated at a single time (mean of 4.7 years after diagnosis) for all patients. Assessment data for each patient were chosen so that age at testing (6.87 years in group A vs. 6.44 years in group B) and time since diagnosis (4.56 years in group A vs. 4.91 years in group B) did not significantly differ between the two groups (p > 0.10).
In 10 patients, at least two neuropsychological assessments had been administered during follow-up, and we used repeated-measures analyses of variance to examine longitudinal outcomes from the first to the second assessment. For this serial analysis, patients in group A were older (6.20 years at first assessment and 9.65 years at second assessment) than those in group B (3.59 years at first assessment and 7.13 years at second assessment). Further, median time from diagnosis to first and second assessment was 3.93 years and 7.38 years respectively for group A, which is longer than the comparable medians of 2.14 years and 5.68 years for group B. However, given the small sample size, group means for age at testing and for times since diagnosis were not significantly different.
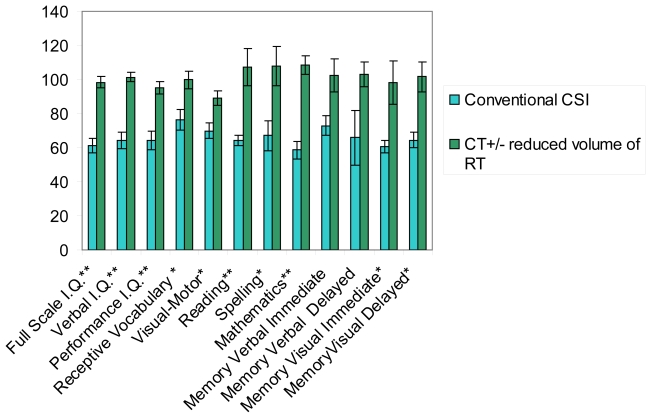
For all measures, Figure 1 sets out the means and standard deviations at 4.7 years of follow-up. Compared with patients treated with chemotherapy with or without reduced radiation, patients treated with conventional csi demonstrated significantly lower standard scores for full-scale intelligence quotient (fiq), visual iq (viq), performance iq (piq), receptive vocabulary, visual–motor skills, reading, spelling, mathematics achievement, and visual memory. The difference for verbal memory did not reach statistical significance. In general, patients treated with conventional csi (group A) demonstrated significant deficits in neurocognitive function; those treated with chemotherapy with or without reduced radiation (group B) exhibited cognitive function within the normal range.
FIGURE 1.
Late effects in survivor patients: neurocognitive outcome at 5 years after diagnosis using the Wechsler Intelligence Scale for Children [full scale intelligence quotient (iq), verbal iq, performance iq], the Receptive Vocabulary test, the Beery Visual–Motor Integration Test, the Wide Range Achievement Test (reading, spelling, mathematics), and the Children Memory Scale (memory verbal and memory visual). * Significantly different at p < 0.05. ** Significantly different at p < 0.01.
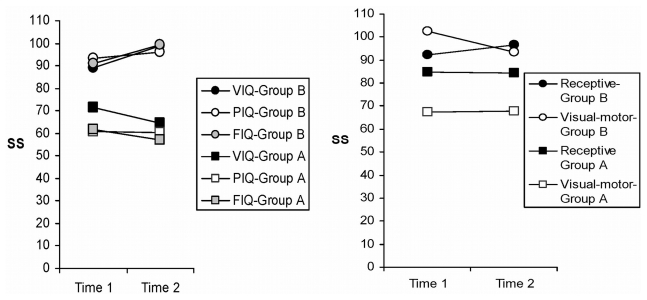
A 2×2 (group × assessment) analysis of variance with repeated measures on the last variables was used to examine changes and stability in fiq, viq, piq, receptive vocabulary, and visual–motor skills over time. Compared with the patients in group B, the patients in group A obtained standard scores that were significantly lower for fiq, viq, piq, and visual–motor functioning across both assessments (p < 0.02, Figure 2). An interaction between group and time of assessment was significant for fiq, with scores increasing from the first to the second assessment for group B, but remaining stable for group A. A notable exception was receptive vocabulary, which was not different between the groups (p > 0.10). Scores fell within the low-average range for group A, which was their highest level of performance.
FIGURE 2.
Longitudinal evaluation at two time points after diagnosis, using the Wechsler Intelligence Scale for Children [verbal intelligence quotient (iq), performance iq (piq), full scale iq (fiq)—left panel] and the Receptive Vocabulary test and the Beery Visual–Motor Integration Test (right panel) in surviving patients. Group A (Time 1: 3.98 years; Time 2: 7.38 years) received conventional craniospinal radiation. Group B (Time 1: 2.14 years; Time 2: 5.68 years) received chemotherapy with or without reduced radiotherapy.
3.6 Endocrine Evaluation
Endocrine status assessment at the last clinical visit was available for 9 of the 12 survivors (in 3 patients, it was still too early after completion of therapy for testing). At a mean follow-up of 108 months post diagnosis (range: 65–194 months), 6 patients had evidence of partial or complete hypopituitarism. Short stature at or below the 10th percentile was found in 5 patients. Of those 5 patients, 2 were on growth hormone (gh) replacement. In 1 case, parents declined gh therapy out of concern that gh might stimulate tumour progression. Precocious puberty developed in 1 patient and was treated with luteinizing hormone–releasing hormone agonist. The 4 patients who did not receive csi are endocrinologically intact. Of those 4 patients, 3 had received chemotherapy alone, and 1 had received high-dose chemotherapy with focal radiation to the tumour bed.
4. DISCUSSION
The increased neurotoxic susceptibility of infants with embryonal tumours of the central nervous system has led to the development, since the late 1980s, of innovative strategies to delay or avoid the use of radiation. Here, we reviewed survival and neurocognitive outcomes in infants treated for medulloblastoma at a single institution using these different strategies.
The study may have some inherent limitations because of its retrospective nature and the prolonged time period, encompassing several eras of therapeutic strategies. For example, because the clinical data were accrued over a long time for children at various ages, different versions of the neurocognitive tests were used for different individuals. Further, the sample size for the neurocognitive comparisons is relatively small (although the patient numbers reflect the clinical reality of medulloblastoma). We acknowledge that, had a larger sample size been available, then the neurocognitive effects may have been more pronounced. Despite the foregoing limitations, our study is unique in that it provides comprehensive longitudinal neurocognitive follow-up not evident in the current literature.
The 5-year os rate of 50.2% ± 9.6% in this study is in keeping with previous reports 2,7,10,14–17, with most relapses occurring within the first year after diagnosis (median: 7 months).
Desmoplastic medulloblastoma histology was found in 24% of the patients, a frequency lower than that found in three recent multicentric trials, which demonstrated frequency ranges between 33% and 46% in patients with infant medulloblastoma 8,9,18. This variation in frequency highlights the importance of uniformity in diagnostic criteria for desmoplasia 19, because desmoplastic histology appears to be associated with better outcome and has been proposed as a major element for risk stratification in future infant medulloblastoma protocols 20,21. The reason for the lack of prognostic correlation with desmoplastic histology in our series is unclear, but is likely influenced by the heterogeneity in treatment received by our group of patients.
At diagnosis, 15 patients (52%) had metastatic disease. The actual frequency may be even higher in light of the 20% of patients whose records lacked a complete staging evaluation. The high incidence of disease dissemination is consistent with several other institutional and cooperative group reports 2,22,23. Although nonsignificant, a trend to better outcomes in patients with metastatic disease was observed (p = 0.055). This contra-intuitive trend can likely be explained by differences in treatment. The use of adjuvant radiotherapy was significantly associated with a better survival rate (p = 0.032), and of the metastatic patients, 66% received radiation as compared with 14% of the M0 patients. Similar trends were reported by Walter et al. 2 in a population with infant medulloblastoma treated with chemotherapy and deferred radiotherapy.
We also did not find a significant correlation between extent of surgical resection and survival, most likely because of the small sample size in this series. However, in most recent infant studies, the association of extent of resection with metastatic status at diagnosis has allowed for the identification of risk groups—such as older children—with significant differences in outcome8,9,18,24.
In our study, half the patients who received high-dose chemotherapy with scr are long-term survivors. Longer follow-up is necessary, because this finding relates to the more recent cohort. However, these results appear encouraging and should be confirmed in larger prospective clinical trials that include a systematic neurocognitive evaluation of long-term survivors18,25,26.
Although there is growing evidence that omission or delay of radiation in infants allows for preservation of intellectual outcome, neurocognitive evaluations remain critical to appraising the benefits of the new strategies 4,8,9,27. Among the available evaluations, the best approach remains to be determined, not only for assessing the neurocognitive effects of first-line therapy, but also for evaluating the consequences of additional salvage therapy in patients whose initial treatment fails. For instance, in a group of patients treated with salvage therapy (including high-dose chemotherapy and focal radiation), Grill et al. 8 reported a mean iq of 72 as compared with 91 in the group of survivors who received conventional chemotherapy only. Similarly, the German ghop group (Gesellschaft fur Padiatrische Onkologie und Hamatologie) reported neurocognitive data on infants treated with methotrexate-based chemotherapy (high-dose systemic, and intraventricular). Although the intellectual outcomes in their series appear to be improved compared with a historical control group treated with radiation, the ghop data also suggested significant decline in intellectual performance for patients who received salvage radiation after methotrexate therapy 9.
With few exceptions, most infant series in the literature are limited in their evaluation of intellectual functioning. Deficits in other neurocognitive domains have been documented in older children with brain tumours treated with cranial radiation. Few attempts have been made to examine broader areas of function in children during infancy or early childhood 12. Our study contributes to this field by assessing, in a longitudinal fashion, multiple areas of neurocognitive functioning in long-term survivors. We present further evidence that strategies avoiding csi in infants are associated with preserved neurocognitive outcome. At a mean interval of 5 years after diagnosis, patients who received csi showed, as expected, evidence of poor neurocognitive outcome across most measures. In this subgroup, standard iq scores were at least 2 standard deviations below the associated normative means. Standard scores for patients treated with chemotherapy alone or with a reduced radiation field were significantly higher, falling within the average range.
A significant observation in our series was that patients treated with less-intensive therapy demonstrated improvement in intellectual functioning over time. Serial assessments are important not only for monitoring functional declines in highly vulnerable patients (such as those treated with cranial radiation), but also for documenting improvement in patients treated with less-intensive therapy. This improvement may reflect functional normalization as the medical condition of these children improved following illness and as they experienced increased learning opportunities that stimulated their behavioural and cognitive development.
Several studies have reported continuous neurocognitive decline after cranial radiation 2,27–30. In our cohort of survivors, although patients treated with conventional csi demonstrated no functional declines across the two assessments, their scores were already at the lowest level by the time of the first assessment, which was performed 3.93 years after diagnosis. Hence, room for further decline was limited. Despite the pervasive neurocognitive deficits in patients treated with adjuvant csi at a young age, some areas of neurocognitive function were preserved. Notably, these patients demonstrated low-average function on a measure of receptive vocabulary. Indices of receptive vocabulary have been found to be relatively robust to the effects of cranial radiation in older children 31. Thus, by examining multiple domains of neurocognitive function, we were able to identify an isolated area of relative strength in patients who typically present with other pervasive deficits. It is therefore important to ensure that all patients have access to comprehensive neuropsychological assessment, because identification of areas of relative strength is important for programming and developing compensatory strategies.
6. CONCLUSIONS
This institutional review provides comprehensive longitudinal outcome and neurocognitive data on a population of infants successfully treated for medulloblastoma. Our study provides further indications that alternative therapeutic approaches, such as adjuvant chemotherapy and reduced or no radiation, are associated with encouraging intellectual performances. Efforts are required to better integrate neuropsychological evaluation into the objectives of future infant medulloblastoma protocols.
7. REFERENCES
- 1.Walker DA, Perilongo G, Punt JAG, Taylor RE, editors. Brain and Spinal Tumors of Childhood. London, U.K.: Arnold; 2004. [Google Scholar]
- 2.Walter AW, Mulhern RK, Gajjar A, et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children’s Research Hospital. J Clin Oncol. 1999;17:3720–8. doi: 10.1200/JCO.1999.17.12.3720. [DOI] [PubMed] [Google Scholar]
- 3.Jakacki RI, Feldman H, Jamison C, Boaz JC, Luerssen TG, Timmerman R. A pilot study of preirradiation chemotherapy and 1800 cGy craniospinal irradiation in young children with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;60:531–6. doi: 10.1016/j.ijrobp.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 4.Copeland DR, deMoor C, Moore BD, 3rd, Ater JL. Neurocognitive development of children after a cerebellar tumor in infancy: a longitudinal study. J Clin Oncol. 1999;17:3476–86. doi: 10.1200/JCO.1999.17.11.3476. [DOI] [PubMed] [Google Scholar]
- 5.Kellie SJ. Chemotherapy of central nervous system tumours in infants. Childs Nerv Syst. 1999;15:592–612. doi: 10.1007/s003810050548. [DOI] [PubMed] [Google Scholar]
- 6.van Eys J, Cangir A, Coody D, Smith B. mopp regimen as primary chemotherapy for brain tumors in infants. J Neurooncol. 1985;3:237–43. doi: 10.1007/BF00165184. [DOI] [PubMed] [Google Scholar]
- 7.Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–31. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- 8.Grill J, Sainte-Rose C, Jouvet A, et al. Treatment of medulloblastoma with postoperative chemotherapy alone: an sfop prospective trial in young children. Lancet Oncol. 2005;6:573–80. doi: 10.1016/S1470-2045(05)70252-7. [DOI] [PubMed] [Google Scholar]
- 9.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–86. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 10.Geyer JR, Sposto R, Jennings M, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol. 2005;23:7621–31. doi: 10.1200/JCO.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 11.Moore BD, 3rd, Ater JL, Copeland DR. Improved neuropsychological outcome in children with brain tumors diagnosed during infancy and treated without cranial irradiation. J Child Neurol. 1992;7:281–90. doi: 10.1177/088307389200700308. [DOI] [PubMed] [Google Scholar]
- 12.Sands SA, van Gorp WG, Finlay JL. Pilot neuropsychological findings from a treatment regimen consisting of intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. Childs Nerv Syst. 1998;14:587–9. doi: 10.1007/s003810050277. [DOI] [PubMed] [Google Scholar]
- 13.Chang CH, Housepian EM, Herbert C., Jr An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93:1351–9. doi: 10.1148/93.6.1351. [DOI] [PubMed] [Google Scholar]
- 14.Geyer R, Levy M, Berger MS, Milstein J, Griffin B, Bleyer WA. Infants with medulloblastoma: a single institution review of survival. Neurosurgery. 1991;29:707–11. [PubMed] [Google Scholar]
- 15.Schmandt S, Kühl J. Chemotherapy as prophylaxis and treatment of meningosis in children less than 3 years of age with medulloblastoma. J Neurooncol. 1998;38:187–92. doi: 10.1023/a:1005924017460. [DOI] [PubMed] [Google Scholar]
- 16.Di Rocco C, Iannelli A, Papacci F, Tamburrini G. Prognosis of medulloblastoma in infants. Childs Nerv Syst. 1997;13:388–96. doi: 10.1007/s003810050106. [DOI] [PubMed] [Google Scholar]
- 17.Rivera–Luna R, López E, Rivera–Marquez H, et al. Survival of children under 3 years old with medulloblastoma: a study from the Mexican Cooperative Group for Childhood Malignancies (amohp) Childs Nerv Syst. 2002;18:38–42. doi: 10.1007/s00381-001-0527-2. [DOI] [PubMed] [Google Scholar]
- 18.Dhall G, Grodman H, Ji L, et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” i and ii protocols. Pediatr Blood Cancer. 2008;50:1169–75. doi: 10.1002/pbc.21525. [DOI] [PubMed] [Google Scholar]
- 19.Eberhart CG, Kepner JL, Goldthwaite PT, et al. Histopathologic grading of medulloblastomas: a Pediatric Oncology Group study. Cancer. 2002;94:552–60. doi: 10.1002/cncr.10189. [DOI] [PubMed] [Google Scholar]
- 20.Rutkowski S, Gerber NU, von Hoff K, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncol. 2008;11:201–10. doi: 10.1215/15228517-2008-084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutkowski S, von Hoff K, Emser A, et al. Prognostic factors and survival of young children with medulloblastoma: an international meta-analysis [abstract] Neuro Oncol. 2008;10:437. [Google Scholar]
- 22.Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase iii study. J Clin Oncol. 1999;17:832–45. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 23.Halperin EC, Watson DM, George SL. Duration of symptoms prior to diagnosis is related inversely to presenting disease stage in children with medulloblastoma. Cancer. 2001;91:1444–50. doi: 10.1002/1097-0142(20010415)91:8<1444::aid-cncr1151>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 24.Sanders RP, Onar A, Boyett JM, et al. M1 medulloblastoma: high risk at any age. J Neurooncol. 2008;90:351–5. doi: 10.1007/s11060-008-9671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorarinsdottir HK, Rood B, Kamani N, et al. Outcome for children < 4 years of age with malignant central nervous system tumors treated with high-dose chemotherapy and autologous stem cell rescue. Pediatr Blood Cancer. 2007;48:278–84. doi: 10.1002/pbc.20781. [DOI] [PubMed] [Google Scholar]
- 26.Chi SN, Gardner SL, Levy AS, et al. Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol. 2004;22:4881–7. doi: 10.1200/JCO.2004.12.126. [DOI] [PubMed] [Google Scholar]
- 27.Fouladi M, Gilger E, Kocak M, et al. Intellectual and functional outcome of children 3 years old or younger who have cns malignancies. J Clin Oncol. 2005;23:7152–60. doi: 10.1200/JCO.2005.01.214. [DOI] [PubMed] [Google Scholar]
- 28.Mulhern RK, Reddick WE, Palmer SL, et al. Neurocognitive deficits in medulloblastoma survivors and white matter loss. Ann Neurol. 1999;46:834–41. doi: 10.1002/1531-8249(199912)46:6<834::aid-ana5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.Dennis M, Spiegler BJ, Hetherington CR, Greenberg ML. Neuropsychological sequelae of the treatment of children with medulloblastoma. J Neurooncol. 1996;29:91–101. doi: 10.1007/BF00165522. [DOI] [PubMed] [Google Scholar]
- 30.Packer RJ, Sutton LN, Atkins TE, et al. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg. 1989;70:707–13. doi: 10.3171/jns.1989.70.5.0707. [DOI] [PubMed] [Google Scholar]
- 31.Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–13. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]