Abstract
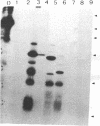
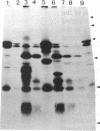
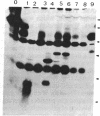
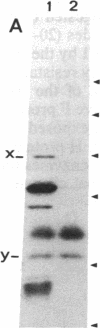
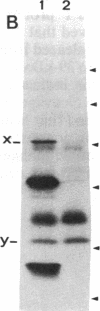
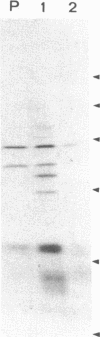
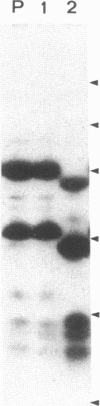
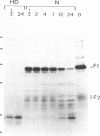
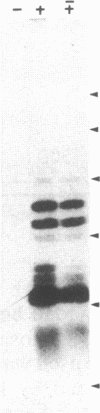
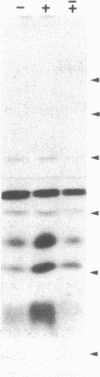
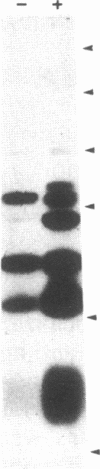
This study describes the use of limited proteolysis of monoclonal antibody (mAb)-bound antigens in the analysis of the two measles virus surface glycoproteins. This approach is dubbed protein "footprinting" in analogy with DNA "footprinting." Protein footprinting was superior to competitive-binding assays and as good as in vitro mAb-selected variant analysis in differentiating among mAbs with various specificities to a given protein. Proteolytic digestion of the antigen prior to mAb binding drastically reduced mAb binding resulting in poor differentiation among mAbs. In contrast, protein footprinting showed that some mAbs retained the ability to immunoprecipitate such fragments. Thus footprinting could be used for localization of mAb epitopes on a protein and proved also to be an effective means of distinguishing among mAb-selected variants differing in single epitopes. Conformational changes caused by heat-denaturation or the binding of anti-antibody to an antigen-antibody complex could also be detected by footprinting.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Teplow D. B., Hood L. E., Kent S. B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. J Biol Chem. 1986 Mar 25;261(9):4229–4238. [PubMed] [Google Scholar]
- Alkhatib G., Briedis D. J. The predicted primary structure of the measles virus hemagglutinin. Virology. 1986 Apr 30;150(2):479–490. doi: 10.1016/0042-6822(86)90312-0. [DOI] [PubMed] [Google Scholar]
- Bellini W. J., Silver G. D., McFarlin D. E. Biosynthesis of measles virus hemagglutinin in persistently infected cells. Arch Virol. 1983;75(1-2):87–101. doi: 10.1007/BF01314129. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Pereira L., Hampar B., Zweig M., Cohen G. H. Effect of monoclonal antibodies on limited proteolysis of native glycoprotein gD of herpes simplex virus type 1. J Virol. 1982 Feb;41(2):478–488. doi: 10.1128/jvi.41.2.478-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Scott A., Dittmar K. E., Owada M. Effect of p15-associated protease from an avian RNA tumor virus on avian virus-specific polyprotein precursors. J Virol. 1980 Feb;33(2):680–688. doi: 10.1128/jvi.33.2.680-688.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudallal Z. A., Briand J. P., Regenmortel M. H. A major part of the polypeptide chain of tobacco mosaic virus protein is antigenic. EMBO J. 1985 May;4(5):1231–1235. doi: 10.1002/j.1460-2075.1985.tb03765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rima B. K. The proteins of morbilliviruses. J Gen Virol. 1983 Jun;64(Pt 6):1205–1219. doi: 10.1099/0022-1317-64-6-1205. [DOI] [PubMed] [Google Scholar]
- Schwyzer M., Weil R., Frank G., Zuber H. Amino acid sequence analysis of fragments generated by partial proteolysis from large simian virus 40 tumor antigen. J Biol Chem. 1980 Jun 25;255(12):5627–5634. [PubMed] [Google Scholar]
- Sheshberadaran H., Chen S. N., Norrby E. Monoclonal antibodies against five structural components of measles virus. I. Characterization of antigenic determinants on nine strains of measles virus. Virology. 1983 Jul 30;128(2):341–353. doi: 10.1016/0042-6822(83)90261-1. [DOI] [PubMed] [Google Scholar]
- Sheshberadaran H., Norrby E. Characterization of epitopes on the measles virus hemagglutinin. Virology. 1986 Jul 15;152(1):58–65. doi: 10.1016/0042-6822(86)90371-5. [DOI] [PubMed] [Google Scholar]
- Sheshberadaran H., Norrby E., Rammohan K. W. Monoclonal antibodies against five structural components of measles virus. II. Characterization of five cell lines persistently infected with measles virus. Arch Virol. 1985;83(3-4):251–268. doi: 10.1007/BF01309921. [DOI] [PubMed] [Google Scholar]