Abstract
Alcadeins (Alcs) constitute a family of neuronal type I membrane proteins, designated Alcα, Alcβ, and Alcγ. The Alcs express in neurons dominantly and largely colocalize with the Alzheimer amyloid precursor protein (APP) in the brain. Alcs and APP show an identical function as a cargo receptor of kinesin-1. Moreover, proteolytic processing of Alc proteins appears highly similar to that of APP. We found that APP α-secretases ADAM 10 and ADAM 17 primarily cleave Alc proteins and trigger the subsequent secondary intramembranous cleavage of Alc C-terminal fragments by a presenilin-dependent γ-secretase complex, thereby generating “APP p3-like” and non-aggregative Alc peptides (p3-Alcs). We determined the complete amino acid sequence of p3-Alcα, p3-Alcβ, and p3-Alcγ, whose major species comprise 35, 37, and 31 amino acids, respectively, in human cerebrospinal fluid. We demonstrate here that variant p3-Alc C termini are modulated by FAD-linked presenilin 1 mutations increasing minor β-amyloid species Aβ42, and these mutations alter the level of minor p3-Alc species. However, the magnitudes of C-terminal alteration of p3-Alcα, p3-Alcβ, and p3-Alcγ were not equivalent, suggesting that one type of γ-secretase dysfunction does not appear in the phenotype equivalently in the cleavage of type I membrane proteins. Because these C-terminal alterations are detectable in human cerebrospinal fluid, the use of a substrate panel, including Alcs and APP, may be effective to detect γ-secretase dysfunction in the prepathogenic state of Alzheimer disease subjects.
Introduction
Alcadein (Alc)5 proteins comprise a family of evolutionarily conserved, type I membrane proteins that are predominantly expressed in neuronal tissues. Alc has been independently identified as a binding protein for the neuron-specific adaptor protein X11L (X11-like) (1) and as a postsynaptic Ca2+-binding protein, where it is known by the name calsyntenin (2). Alc functions as a cargo-receptor for the kinesin-1 motor that mediates anterograde transport of APP (3, 4), and a mutation in a nematode ortholog of the Alc gene is reported to cause a defect in associative learning (5, 6). Thus, Alc plays important roles in vesicular transport at the subcellular level and in learning behavior at the organismal level. Alc exists as four isoforms in mammals: Alcα1 (971 amino acids in humans), Alcα2 (981 amino acids in humans), Alcβ (956 amino acids in humans), and Alcγ (955 amino acids in humans) (1). Alcα, Alcβ, and Alcγ are encoded by independent genes, whereas Alcα1 and Alcα2 are splice variants derived from the Alcα gene.
In neurons, Alc proteins are complexed to X11L molecules, which, in turn, are complexed with the amyloid β-precursor protein (APP), a type I transmembrane protein that is believed to play a seminal role in the pathogenesis of familial and sporadic Alzheimer disease (reviewed for AD in Refs. 7–9 and for X11L in Refs. 10 and 11). In the absence of X11L, both Alc and APP proteins are rapidly cleaved in a coordinated manner (12). Levels of the endogenous APP metabolite, amyloid-β protein (Aβ), are elevated in the brains of X11L-deficient mice, indicating that the APP-X11L interaction is physiologically important in the regulation of APP metabolism in the brain in vivo (13, 14). Alc proteins are also cleaved successively by secretases and release soluble Alc ectodomain (sAlc, corresponding to the soluble APP ectodomain (sAPP)) and p3-Alc (corresponding to the APP fragment, p3) (12). Taken together with similarities and/or identities in their structure, cellular distribution, and neural function, the physiological and pathophysiological metabolic fate of Alc would be predicted to parallel that of APP (1, 3, 12).
In this study, we report that all three members of the Alc family (Alcα, Alcβ, and Alcγ) are cleaved by ADAM 10 and ADAM 17, which have been identified as the α-secretases for APP (15–17). Subsequent cleavage of the remaining Alc C-terminal fragments involves the presenilin-1 (PS1)-dependent γ-secretase, and this reaction liberates into cell-conditioned medium and into cerebrospinal fluid (CSF) a short peptide, p3-Alc, previously designated “β-Alc.” Our other analysis using CSF from three groups of human subjects (n = 158) indicates that p3-Alcα variant ratio (minor p3-Alcα38/major p3-Alcα35) correlated with the Aβ42/40 ratio in the sporadic AD (clinical dementia rating 0.5 + 1 patients) but not elderly non-demented and other neurological disease controls.6 Therefore, the detailed biochemical analysis for the cleavages of Alc proteins is significant for understanding the features of p3-Alc peptides in human subjects. We found that various FAD-linked PS1 mutations appeared, at different magnitudes, with the alterations of C termini of p3-Alcs, suggesting that one type of γ-secretase dysfunction appears in various phenotypes upon cleavage of Alc proteins and APP. In other words, one type of γ-secretase dysfunction largely alters the cleavage of one Alc species and APP, but the same dysfunction slightly alters the cleavage of another Alc species. When the cleavage phenotypes appear on APP to increase pathogenic Aβ42, the subject may experience the onset of AD. Testing the hypothesis that AD-related variant processing of p3-Alc peptide might yield surrogate markers for γ-secretase dysfunction is important. In this study, we characterized all p3-Alc species generated from Alcα, Alcβ, and Alcγ in detail.
EXPERIMENTAL PROCEDURES
Plasmid Construction and Stable Cell Lines Expressing PS1
The human Alcadein cDNAs we used were hAlcα1 (GenBankTM accession number AY753301), hAlcβ (NM_014718), and hAlcγ (NM_022131). The FLAG sequence was inserted between Leu38 and Glu39 to generate pcDNA3-FLAG-hAlcα1, between Lys26 and Pro27 to generate pcDNA3.1-FLAG-hAlcβ and between Gln29 and Arg30 to generate pcDNA3.1-FLAG-hAlcγ. The pcDNA3-FLAG-APP695 (18), pcDNA3-ADAM10-HA, and pcDNA3-ADAM17-HA (19) have been described previously. The plasmid encoding human PS1 cDNA, pcDNA3- PS1wt, was described previously (12). FAD-linked mutations were introduced by PCR-based site-directed mutagenesis to generate pcDNA4-PS1M146L, pcDNA3.1-PS1L166P, pcDNA4- PS1A246E, pcDNA4-PS1R278T, pcDNA4-PS1L286V, and pcDNA4-PS1A434C. HEK293 cells were transfected with these plasmids, and cells stably expressing PS1 were cloned.
Cells, Transfection, and Western Blot Assay
Mouse embryonic fibroblasts (MEFs) derived from ADAM 10 homozygous (−/−) and heterozygous (+/−) gene knock-out mice were described previously (20). HEK293, Neuro 2a, and MEFs (0.3–1.0 × 106) were subjected to gene transfection with the indicated amounts of various combinations of plasmids in Lipofectamine 2000 or Lipofectamine according to the manufacturer's protocol (Invitrogen). After transfection for 24 h, the medium was changed, and the cells were cultured for a further ∼24 h. In order to analyze secreted proteins, sAlc and sAPP were recovered from the conditioned medium by immunoprecipitation with an anti-FLAG antibody and Protein G-Sepharose. To analyze cellular proteins, the cells were harvested and lysed in Hepes-buffered saline with Triton X-100 (12). The cell lysates and the immunoprecipitates were analyzed by Western blotting with the indicated antibodies, detected by ECL (GE Healthcare), and quantified using the VersaDoc imaging System (Bio-Rad).
Antibodies
Anti-Alcα polyclonal rabbit UT135 antibody was raised against a peptide that was composed of Cys plus the sequence between positions 839 and 851 (NPHPFAVVPSTAT+C) of human Alcα1. The anti-Alcα monoclonal antibody 3B5 was raised against a peptide that was composed of Cys plus the sequence between positions 821 and 826 (C+FVHPEH). The anti-Alcβ polyclonal rabbit UT143 antibody was raised against a GST-fusion protein containing the sequence between 819 and 847 (FLHRGHQPPPEMAGHSLASSHRNSMIPSA) of human Alcβ. The anti-Alcγ polyclonal antibody UT166 was raised against a peptide composed of Cys plus the sequence between positions 823 and 834 (C+IQHSSVVPSIAT) of human Alcγ. These Alc-specific antibodies were raised against the extracellular juxtamembrane region of Alc family proteins and were specific for their respective p3-Alc targets with the exception of UT166, which exhibited cross-reactivity to p3-Alcα (data not shown). These antibodies were used to isolate and detect p3-Alc. The regions recognized by the specific antibodies are shown in supplemental Fig. S1. The anti-Alcα and anti-Alcβ cytoplasmic domain antibodies UT83 and UT99 were described previously (12). The monoclonal anti-FLAG (M2, Sigma) and anti-HA (12CA5, BD Biosciences) antibodies were purchased from vendors as noted. Anti-mouse and anti-rabbit IgG peroxidase-linked species-specific whole antibodies were purchased from GE Healthcare.
MALDI-TOF/MS and -MS/MS Analysis of p3-Alc Secreted into the Cultured Medium and Human CSF
HEK293 cells (8–9 × 106) were transfected with the plasmids (6 μg) pcDNA3-hAlcα1, pcDNA3.1-hAlcβ, or pcDNA3.1-hAlcγ in Lipofectamine 2000 for 24 h. The p3-Alcα, p3-Alcβ, and p3-Alcγ that were secreted into the medium (6 ml) were recovered by immunoprecipitation with the polyclonal anti-p3-Alcα U135 (4 μg, affinity-purified), polyclonal anti-p3-Alcβ UT143 (100 μl of serum), and polyclonal anti-p3-Alcγ UT166 (100 μl of serum) antibodies, respectively, and Protein G-Sepharose beads. The beads were sequentially washed with Wash buffer I (10 mm Tris-HCl (pH 8.0), 140 mm NaCl, 0.1% (w/v) n-octyl-d-glucoside, 0.025% (w/v) sodium azide) and Wash buffer II (10 mm Tris-HCl (pH 8.0), 0.025% (w/v) sodium azide), and then samples were eluted with trifluoroacetic acid/acetonitrile/water (1:20:20) saturated with sinapinic acid. The dissolved samples were dried on a target plate, and MALDI-TOF/MS analysis was performed using a UltraflexII TOF/TOF (Bruker Daltonics, Bremen, Germany). Molecular masses were calibrated using the peptide calibration standard (Bruker Daltonics).
For experiments with human samples (CSF was furnished by Choju Medical Institute and Tottori University), a mixture of human CSF (0.3–1.0 ml) from 5–10 individuals (70–90-year-old AD patients with clinical dementia rating 0.5, 1, or 2) was subjected to immunoprecipitation with the above antibodies. The beads were washed, and samples were eluted as described above. The dissolved samples were dried on a target plate, and MALDI-TOF/MS analysis was performed as described above.
The quantitative accuracy of mass spectrometric analysis with immunoprecipitation was confirmed by studies with a mixture of synthetic p3-Alcα35 and p3-Alcα39 peptides and a mixture of synthetic p3-Alcβ37 and p3-Alcβ40 peptides (supplemental Fig. S2). Molecular masses of p3-Alc species measured with MALDI-TOF/MS were compared with theoretical values to show the accuracy of mass spectrometric analysis (supplemental Table 1).
In all immunoprecipitation studies prior to MALDI-TOF/MS analysis, protease inhibitor mixture (5 μg/ml chymostatin, 5 μg/ml leupeptin, and 5 μg/ml pepstatin) was added in samples (cell media, CSF, cell/brain lysates) to prevent nonspecific proteolysis (12).
Aβ40 and Aβ42 levels were quantified with sandwich enzyme-linked immunosorbent assay systems (Immuno- Biological Laboratories, Takasaki, Japan). Informed consent for the use of human CSF in this study was obtained from the patients and their families.
RESULTS
Identification of the Proteinases That Initiate Processing of Alcα, Alcβ, and Alcγ
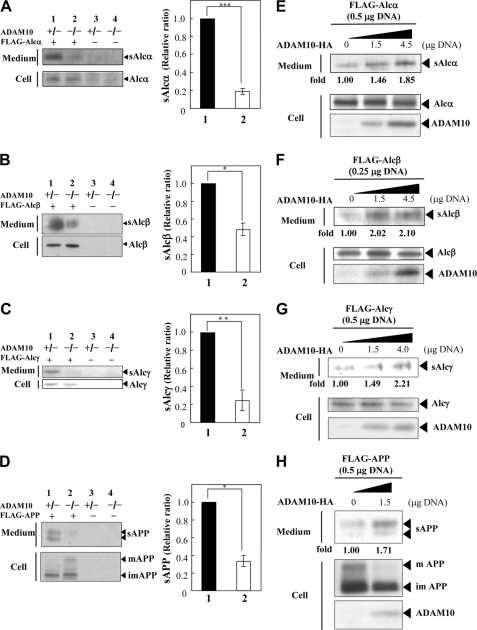
We have previously shown that the initial cleavage of Alc occurs in the extracellular juxtamembrane region and liberates the Alc ectodomain (sAlc) (12). The cell-associated C-terminal Alc fragment (AlcCTF) is proteolyzed by an intramembrane-cleaving protease, and both Alc cleavages are apparently catalyzed by the identical secretase enzymes that process APP (12). ADAM 10 is the enzyme that usually underlies the constitutive α-site cleavage of APP (16, 17); therefore, we initially examined whether this ADAM also cleaved Alc. FLAG-Alcα, FLAG-Alcβ, FLAG-Alcγ, or FLAG-APP was expressed in MEFs from ADAM 10-deficient, homozygous (−/−) and heterozygous (+/−) knock-out mice (20). We recovered sAlc isoforms by immunoprecipitation of conditioned media with an anti-FLAG antibody; the immunoprecipitates and cell lysates were analyzed by Western blotting with M2 (Fig. 1, A–D).
FIGURE 1.
Evidence that Alc family proteins and APP undergo identical processing by ADAM 10 α-secretase. A–D, generation of sAlc and sAPP in ADAM 10-deficient cells. ADAM 10 homozygous (−/−, lanes 2 and 4) and heterozygous (+/−, lanes 1 and 3) deficient MEFs were transiently transfected with 1.5 μg of pcDNA3-FLAG-hAlcα1 (+ in A), pcDNA3.1-FLAG-hAlcβ (+ in B), pcDNA3.1-FLAG-hAlcγ (+ in C), and pcDNA3-FLAG-APP695 (+ in D), or vector alone (− in A–D). Culture medium (1 ml) was immunoprecipitated with an anti-FLAG M2 antibody. The immunoprecipitates of conditioned medium (Medium) and cell lysate (Cell; 20 μg of protein) were analyzed by Western blotting with M2 (left panels). The levels of sAlc and sAPP in lane 2 are indicated as ratios relative to the levels shown in lane 1, which was assigned a reference value of 1.0 (values represent means ± S.E.). The asterisks indicate statistical significance as determined by Student's t test (n = 3; *, p < 0.05; **, p < 0.01; ***, p < 0.005) (right panels). E–H, rescue of the primary α-secretase type cleavage of Alc family proteins and APP in ADAM 10-deficient cells by expression of exogenous ADAM 10. ADAM 10-deficient MEFs were transiently transfected with the indicated amount of pcDNA3-ADAM10-HA in the presence of the indicated amounts of pcDNA3-FLAG-hAlcα1 (E), pcDNA3.1-FLAG-hAlcβ (F), pcDNA3.1-FLAG-hAlcγ (G), or pcDNA3-FLAG-APP695 (H). Empty vector was also added to standardize the amount of plasmid used. Conditioned culture medium (Medium; 1 ml) was immunoprecipitated with the anti-FLAG M2 antibody and analyzed by Western blotting with the same antibody. Cell lysates (Cell; 20 μg of protein) were analyzed by Western blotting with M2 for Alc family proteins and APP or with the anti-HA antibody for ADAM 10. The levels of sAlc and sAPP are indicated as -fold changes with respect to the levels detected when ADAM 10-HA was not expressed; this was assigned a reference value of 1.0. mAPP, mature APP; imAPP, immature APP.
As expected, we found that the primary cleavage of APP was deficient in homozygous (−/−) but not in heterozygous (+/−) ADAM 10-deficient MEF cells (Fig. 1D); secretion of sAPP by −/− cells was 40% below the level of sAPP in ADAM 10+/− cells (compare lane 2 with lane 1 in D). Release of sAlcα, sAlcβ, and sAlcγ was deficient in ADAM 10−/− cells to a similar extent (Fig. 1, A–C). These observations strongly suggest that Alcα, Alcβ, and Alcγ are subjected to primary cleavage by ADAM 10 in a fashion similar to the α-secretase processing of APP.
The cleavage of Alc family proteins by ADAM 10 was rescued following expression of HA-tagged ADAM 10 in ADAM 10−/− cells (Fig. 1, E–H). In this experiment, we initially confirmed that sAPP secretion was restored by the expression of an exogenous cDNA for ADAM 10 (Fig. 1H). Secretion of sAPP from ADAM 10−/− cells that were expressing exogenous ADAM 10 increased ∼1.7-fold as compared with the level of sAPP that was secreted from ADAM 10−/− cells. As expected, we also found that the level of intracellular mature APP holoprotein (which is the substrate of ADAM 10 that gives rise to sAPP) was diminished in ADAM 10−/− cells following expression of exogenous ADAM 10. The wild type secretion patterns of sAlcα, sAlcβ, and sAlcγ were restored in ADAM 10−/− cells following expression of exogenous ADAM 10 (Fig. 1, E–G). In a dose-dependent fashion, these cells exhibited a 1.8–2.2-fold increase in sAlc in the medium when compared with sAlc secretion from ADAM 10−/− cells (Fig. 1, E–G). Taken together, these results suggest that ADAM 10 is an important secretase that proteolyzes Alcα, Alcβ, and Alcγ.
APP is also cleaved by another α-secretase, ADAM 17 (15, 16, 21), although ADAM 17 is largely expressed in glial cells rather than neurons (22). Cleavage of the Alc family was examined in Neuro 2a cells overexpressing an HA-tagged ADAM 17. We initially confirmed that ADAM 17 cleaved APP in these cells (supplemental Fig. S3). As expected, increased expression of ADAM 17 enhanced sAPPα secretion, indicating that, in our cells, exogenously expressed ADAM17 was active in proteolysis of APP. The secretion of sAlcα, sAlcβ, and sAlcγ from Neuro 2a cells expressing exogenous ADAM 17 was then assayed and quantified using experimental conditions that were identical to those used for the analysis of APP. We found that the secretion of sAlc (sAlcα, sAlcβ, and sAlcγ) increased in a dose-dependent manner in response to the exogenous expression of exogenous ADAM 17. These observations suggest that, like ADAM 10, ADAM 17 also cleaves Alcα, Alcβ, and Alcγ as well as APP. Taken together, these data show that Alc and APP are metabolized by the same two APP α-secretases.
Identification of the Primary and Secondary Cleavage Sites of Alc
ADAM 10 and ADAM 17 have been identified as the α-secretases that cleave the peptide bond between Lys612 and Leu613 of APP695, thereby destroying the Aβ domain (Fig. 2). This cleavage at the juxtamembranous region triggers a secondary intramembranous γ-cleavage, which results in the secretion of a nonamyloidogenic 3-kDa peptide (p3) composed of 24–26 amino acids (for a review, see Refs. 7 and 8). We have previously reported that Alc family proteins, like APP family proteins, are also cleaved within the intramembranous region. In our earlier study, we showed that the small peptide, previously designated “β-Alc,” was secreted by the secondary cleavage of alcadein C-terminal fragments (CTFs) by γ-secretase, but the exact cleavage sites were not previously identified (12). Now we have demonstrated that the peptide previously designated “β-Alc” is generated by the successive action of α- and γ-secretases. Furthermore, β-secretase/β-APP site-cleaving enzyme (BACE) (23) is not likely to be involved in the primary cleavage of Alcα, Alcβ, and Alcγ (supplemental Fig. S4). In HEK293 cells overexpressing human BACE1, the generation of sAPPβ and CTFβ, the products of APP cleaved by BACE, increased (supplemental Fig. S4A), whereas no remarkable changes were observed in the cleavages of Alcα, Alcβ, and Alcγ (supplemental Fig. S4B) in quality. The results suggest that Alcs are not a preferential substrate for BACE. Therefore, the β-Alc peptide has been renamed “p3-Alc” so as to maintain consistency with an APP-based nomenclature for the peptides produced by the proteolytic processing of Alc.
FIGURE 2.
Amino acid sequence of p3-Alcα, p3-Alcβ, and p3-Alcγ. The amino acid sequences of the major p3-Alc species are shown. p3-Alcα35, p3-Alcβ37, and p3-Alcγ31, are indicated as double-underlined letters along with the sequences of p3 and Aβ40 of APP and the sequence of p3-Alcα2N+35. The major primary (closed arrowheads) and secondary (open arrowheads) cleavage sites of Alcα1, Alcβ, and Alcγ are indicated together with those of APP (α, the cleavage site by α-secretase or ADAM 10/17; β, the cleavage site by β-secretase or BACE). Another primary cleavage site of Alcα1 is also indicated (arrow). Numbers on amino acids indicate their positions. Gray letters with a broken underline along with the sequence indicate the putative transmembrane region suggested by the Swiss-Prot protein knowledge base. The N terminus of p3-Alcα is Ala817 as determined by MALDI-TOF/MS analysis; however, that of p3-Alcα2N+“X” is Met815. The p3-Alcα35 with N-terminal Ala817 is a major species in CSF, whereas the p3-Alcα2N+35 with N-terminal Met815 is a major species in HEK293 cells (see supplemental Figs. S5A, S6A, and S7A). The N terminus of p3-Alcβ is Val813, which was determined by MALDI-MS/MS analysis (see supplemental Figs. S5B and S6B). The N terminus of p3-Alcγ is Leu804 (supplemental Fig. S5C); this coincides with the N-terminal sequence of Alcγ CTF that was determined using a gas phase peptide sequencer.
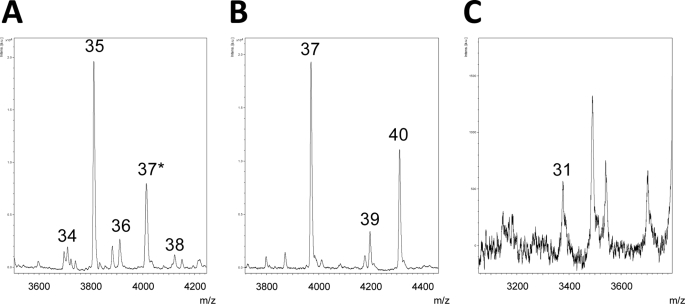
The recent production of anti-Alcα, anti-Alcβ, and anti-Alcγ antibodies raised against the respective extracellular juxtamembrane sequences has enabled us to recover p3-Alcα, p3-Alcβ, and p3-Alcγ secreted into the culture medium by HEK293 cells expressing Alcα, Alcβ, and Alcγ. The molecular masses of p3-Alcα, p3-Alcβ, and p3-Alcγ were determined by analyses with MALDI-TOF/MS. The spectrum of p3-Alcα showed two major peaks with molecular masses of 3804.8 and 4007.0 (supplemental Fig. S5A, indicated by arrows). The spectrum of p3-Alcβ showed two major peaks with molecular masses of 3964.0 and 4303.3 (supplemental Fig. S5B). The spectrum of p3-Alcγ showed one major peak with a molecular mass of 3377.8 Da and a minor peak with a molecular mass of 3689.2 Da (supplemental Fig. S5C).
The amino acid sequences of the respective major peak products were determined by MALDI-MS/MS analysis; the amino acid sequence of p3-Alcα was composed of 35 and 37 amino acids, that of p3-Alcβ was composed of 37 and 40 amino acids, and that of p3-Alcγ was composed of 31 and 34 amino acids (supplemental Fig. S5, middle and right; the sequences are also double-underlined in Fig. 2). Thus, the major p3-Alcα species, p3-Alcα35 and p3-Alcα2N+35, that were secreted from HEK293 cells expressing Alcα1 were peptides that include the sequence from Ala817 to Thr851 and from Met815 to Thr851 of human Alcα1. The major p3-Alcβ species, p3-Alcβ37 and p3-Alcβ40, that were secreted from HEK293 cells expressing Alcβ were peptides that included the sequences from Val813 to Thr849 and from Val813 to Ile852 of human Alcβ. The major p3-Alcγ species, p3-Alcγ31 and p3-Alcγ34, that were secreted from HEK293 cells expressing Alcγ were peptides that included the sequences from Leu804 to Thr834 and from Leu804 to Ile837 of human Alcγ. These p3-Alcα35, p3-Alcβ37, and p3-Alcγ31 species are also the major species in human CSF (see supplemental Fig. S6).
In our previous report, we used a gas phase protein sequencer to identify Ala816 (numbering for the Alcα1 isoform) as the N-terminal amino acid of the Alcα CTF (12). We therefore expected that ADAM 10 and ADAM 17 would cleave Alcα at the peptide bond between Met815 and Ala816. In the present study, we used MALDI-MS/MS to show that the N-terminal amino acid is Ala817 for p3-Alcα35 and Met815 for p3-Alcα2N+35) (supplemental Fig. S5A). The N-terminal Met815 and/or Ala816 of Alcα CTF generated by primary cleavage may be removed by an N-terminal exopeptidase during p3-Alcα secretion from cells.
We were unable to determine the N-terminal amino acid sequence of the Alcβ CTF using a gas phase protein sequencer; however, Val813, which was identified by MALDI-MS/MS analysis of p3-Alcβ37 in this study (supplemental Figs. S5B and S6B), is likely to be the N-terminal amino acid of Alcβ CTF. We also determined the N-terminal sequence of Alcγ CTF (Leu804–Ile-Val-Gln-Pro-Pro-Phe-Leu-Gln812)using a gas phase protein sequencer. This result was identical to the result obtained from the MALDI-MS/MS analysis (supplemental Fig. S5C), indicating that the primary cleavage site of Alcγ is the peptide bond between His803 and Leu804. The primary cleavage sites for the Alc family are shown in Fig. 2 (black arrowhead and arrow); these sites are also compared with the primary cleavage sites (α- and β- sites) of APP695.
MALDI-TOF/MS and -MS/MS analyses enabled us to determine the secondary cleavage sites of Alcα, Alcβ, and Alcγ (supplemental Figs. S5 and S6). The major secondary cleavage site of Alcα is the peptide bond between Thr851 and Val852; cleavage at this site generates p3-Alcα35 and p3-Alcα2N+35. The major secondary cleavage site of Alcβ is the peptide bond between Thr849 and Leu850; cleavage at this site generates p3-Alcβ37. Another secondary cleavage site of Alcβ is the peptide bond between Ile852 and Val853, which generates p3-Alcβ40. The major secondary cleavage site of Alcγ is the peptide bond between Thr834 and Val835, which generates p3-Alcγ31. Another secondary cleavage site of Alcγ includes the peptide bond between Ile837 and Ile838, which generates p3-Alcγ34. Secondary cleavage sites (open arrowheads) of Alc family proteins, determined by MALDI-MS/MS analysis, are also shown in Fig. 2, together with the major secondary γ-secretase-dependent cleavage sites of APP that generate Aβ40 and Aβ42.
Modulation of the γ-Cleavage Sites of Alc by FAD-linked PS1 Mutations
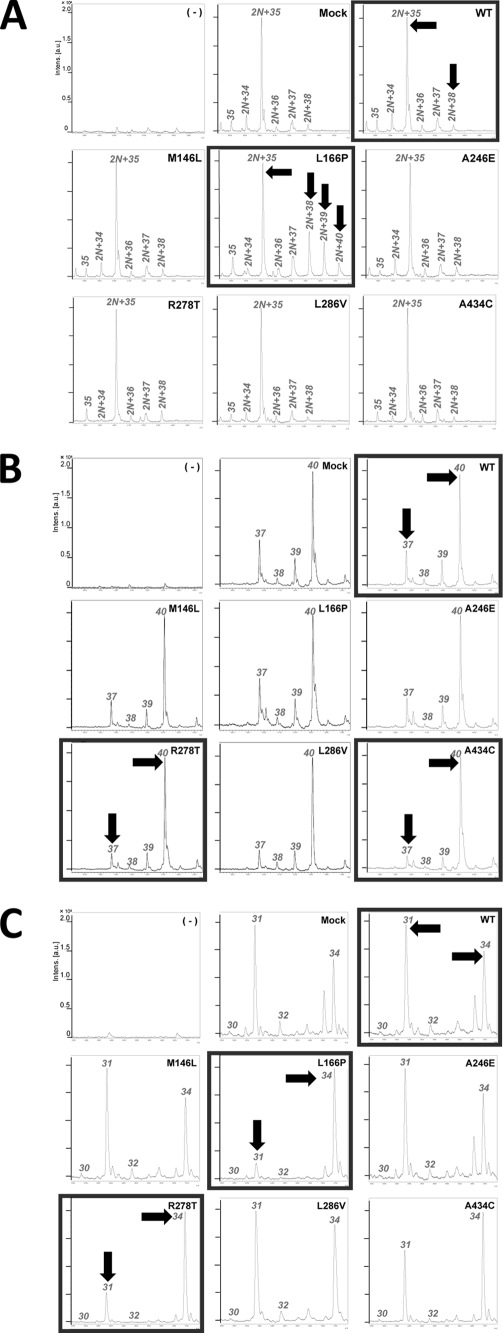
We have previously reported that secondary cleavage of Alcα is performed by a PS1-dependent γ-secretase (12). PS1 is known to be the catalytic component of the γ-secretase complex, and several mutations in the PS1 and PS2 genes cause FAD. Several FAD-linked PS1 mutations are known to alter the cleavage of APP β CTFs and increase the generation of C-terminally altered Aβ species, such as Aβ42 (24–27). Because Alc and APP are cleaved by the identical γ-secretase, we sought to determine whether Alc family proteins demonstrate displaced secondary cleavage sites in the presence of FAD-linked PS1 mutations. HEK293 cells stably expressing wild type PS1 (WT in Fig. 3) or PS1 carrying either FAD-linked M146L, L166P, A246E, A278T, L286V, or A434C mutations or vector alone (Mock) were co-transfected with Alcα, Alcβ, and Alcγ or without plasmid (−).
FIGURE 3.
Displacement of the intramembranous cleavage sites of Alcα, Alcβ, and Alcγ in cells expressing FAD-linked mutations in PS1. A, representative MS spectra of p3-Alcα secreted from cells expressing wild type PS1 or FAD-linked PS1 mutants. HEK293 cells with (WT) or without (Mock) the stable expression of wild type PS1, FAD-linked PS1 M146L, L166P, A246E, R278T, L286V, and A434C mutations were transfected with or without (−) pcDNA3-hAlcα1. The culture medium (6 ml) of cells expressing Alcα1 was immunoprecipitated with UT135, and the immunoprecipitates were subjected to MALDI-TOF-MS analysis. 35, p3-Alcα35; 2N+34, p3-Alcα2N+34; 2N+35, p3-Alcα2N+35; 2N+36, p3-Alcα2N+36; 2N+37, p3-Alcα2N+37; 2N+38, p3-Alcα2N+38; 2N+39, p3-Alcα2N+39; 2N+40, p3-Alcα2N+40. B, representative MS spectra of p3-Alcβ secreted from cells expressing wild type PS1 or FAD-linked PS1 mutants. HEK293 cells were transfected as described above with or without (−) pcDNA3-hAlcβ. The culture medium (6 ml) of cells expressing Alcβ was immunoprecipitated with UT143, and the immunoprecipitates were subjected to MALDI-TOF-MS analysis. 37, p3-Alcβ37; 38, p3-Alcβ38; 39, p3-Alcβ39; 40, p3-Alcβ40. C, representative MS spectra of p3-Alcγ secreted from cells expressing wild type PS1 or FAD-linked PS1 mutants. HEK293 cells were transfected as described above with or without (−) pcDNA3-hAlcγ. The culture medium (6 ml) of cells expressing Alcγ was immunoprecipitated with UT166, and the immunoprecipitates were subjected to MALDI-TOF-MS analysis. 30, p3-Alcγ30; 31, p3-Alcγ31; 32, p3-Alcγ32; 34, p3-Alcγ34.
p3-Alcα, p3-Alcβ, and p3-Alcγ were recovered from the culture medium by immunoprecipitation with anti-Alcα (UT135), anti-Alcβ (UT143), and anti-Alcγ (UT166) antibodies and analyzed by MALDI-TOF/MS (Fig. 3). The expression of PS1 was confirmed by Western blotting with anti-PS1 N- and C-terminal antibodies (data not shown), and the effect of the FAD-linked mutations on the Aβ42/Aβ40 ratio was examined in the medium of HEK293 cell lines, each of which expressed PS1 stably plus APP695 transiently (Fig. 4A, lower right).
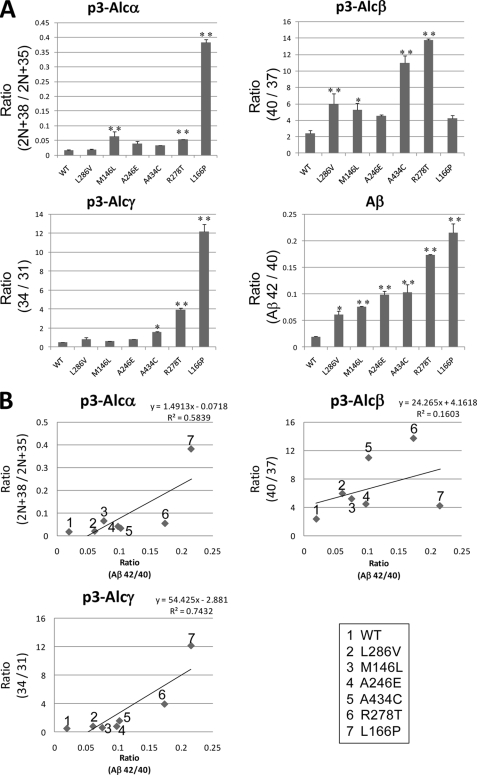
FIGURE 4.
Analysis of conditioned media of cultured cells. Covariant relationships linking the Aβ42/40 ratio with the ratios of minor p3-Alc species to major p3-Alc species. A, changes of the p3-Alcα2N+38 (minor) to p3-Alcα2N+35 (major) ratio. HEK293 cells with wild type PS1 or FAD-linked PS1 mutants were transfected with pcDNA3-hAlcα1, and p3-Alcα peptides were analyzed by MALDI-TOF-MS analysis in duplicate studies as described in the legend to Fig. 3. The peak area of p3-Alcα2N+38 was compared with that of p3-Alcα2N+35, and ratios (p3-Alcα2N+38/p3-Alcα2N+35) are indicated (upper left panel). Changes of p3-Alcβ40 to p3-Alcβ37 ratio are shown. HEK293 cells with wild type PS1 or FAD-linked PS1 mutants were transfected with pcDNA3-hAlcβ, and p3-Alcβ peptides were analyzed. In cultured cells, p3-Alcβ40 is a major peptide rather than p3-Alcβ37, whereas p3-Alcβ37 is major in CSF (compare Fig. 3B with Fig. 5B). Therefore, we sought the p3-Alcβ40/p3-Alcβ37 ratio as a minor/major ratio. The peak area of p3-Alcβ40 was compared with that of p3-Alcβ37, and ratios (p3-Alcβ40/p3-Alcβ37) are indicated (upper right panel). Changes of the p3-Alcγ34 to p3-Alcγ31 ratio are shown. HEK293 cells with wild type PS1 or FAD-linked PS1 mutants were transfected with pcDNA3-hAlcγ, and p3-Alcγ peptides were analyzed. We sought the p3-Alcγ34/p3-Alcγ31 ratio as a minor/major ratio. The peak area of p3-Alcγ34 was compared with that of p3-Alcγ31, and ratios (p3-Alcγ34/p3-Alcγ31) are indicated (lower left panel). Changes of the Aβ42/Aβ40 ratio (Aβ42/40) in the conditioned media of cells expressing PS1 (WT) and FAD-linked PS1 mutant, L286V, M146L, A246E, A434C, R278T, and L166P, are shown. HEK293 cells with wild type PS1 (WT) or FAD-linked PS1 mutants were transfected with pcDNA3-APP695. The amounts of Aβ40 and Aβ42 secreted into the medium were quantified by sandwich enzyme-linked immunosorbent assay, and the Aβ42/Aβ40 ratio was determined (lower right panel). Duplicate assays (n = 2) were performed, and the significance of the effects of FAD-linked mutants of PS1 versus the wild type PS1 was examined by Dunnett's multiple comparisons test. *, p < 0.05; **, p < 0.01. B, covariant analysis of Aβ42/Aβ40 ratio with ratios of certain minor p3-Alc to the major p3-Alc species. Graphs showing the relation of ratios of p3-Alcα2N+38/p3-Alcα2N+35 (upper left), p3-Alcβ40/p3-Alcβ37 (upper right), and p3-Alcγ34/p3-Alcγ31 (lower left) with the Aβ42/40 ratio were indicated. Numbers in panels indicate the PS1 mutation (WT indicates wild type PS1) as shown in the lower right panel. y, inclination; R2, correlation coefficient.
As expected, HEK293 cells stably expressing wild type PS1 (WT) or vector alone (Mock) plus Alcα generated p3-Alcα species with C-terminal end of Thr851 (p3-Alcα2N+35) as the major species (Fig. 3A). We determined the amino acid sequences of this p3-Alcα species with MALDI-MS/MS analysis and confirmed that this is p3-Alcα2N+35 and not p3-Alcα37. This p3-Alcα2N+35 was a minor p3-Alcα species in human CSF in which p3-Alcα35 was major (left panel in supplemental Figs. S5A and S6A). In any case, HEK293 cells expressing PS1 (WT) generated largely p3-Alcα species with the C-terminal Thr851 (right panel in supplemental Figs. S5A and S6A).
In contrast, HEK293 cells expressing FAD-linked PS1 mutants generated qualitatively altered p3-Alcα (Fig. 3A). This was especially remarkable in the medium of cells expressing PS1 carrying the L166P mutation; there were increases in the levels of p3-Alcα2N+38 that included a peptide of Met815–Ilu854 (Fig. 4A, upper left). The p3-Alcα2N+39 that included a peptide of Met815–Val855, and p3-Alcα2N+40 that included a peptide of Met815–Val856 were also increased (arrows in Fig. 3A). This L166P mutation is known to increase Aβ42/40 ratio greatly (Fig. 4A, lower right). Other FAD-linked PS1 mutations, such as R278T, A434C, and A246E, showed a moderate effect to increase Aβ42/40, whereas these mutations appeared to have little or almost no effect on the increase of minor species, including p3-Alcα2N+38 (Fig. 4A, compare upper left to lower right).
We then investigated the generation of p3-Alcβ and p3-Alcγ from HEK293 cells expressing Alcβ or Alcγ together with PS1 carrying FAD-linked mutations (Fig. 3, B and C, and Fig. 4A). HEK293 cells stably expressing wild type PS1 (WT) or vector alone (Mock) plus Alcβ generated major p3-Alcβ37 and p3-Alcβ40 (see Fig. 2 for the amino acid sequence). HEK293 cells expressing FAD-linked PS1 mutants, especially R278T and A434C, demonstrated remarkable decreases in levels of p3-Alcβ37 (arrows in Fig. 3B). We then created minor species/major species ratios for p3-Alcβ40 to p3-Alcβ37, which were compared with the Aβ42/40 ratio (Fig. 4A, compare upper right to lower right). In cultured cells, p3-Alcβ40 is a major species rather than p3-Alcβ37, whereas p3-Alcβ37 is a major species on CSF (compare Fig. 3B to Fig. 5B). Therefore, in the case of p3-Alcβ, we sought the p3-Alcβ40/p3-Alcβ37 ratio as minor/major ratio. In contrast to the ratio p3-Alcα2N+38/p3-Alcα2N+35, L166P did not show remarkable change for the ratio p3-Alcβ40/p3-Alcβ37.
FIGURE 5.
Representative MS spectra of p3-Alc peptides in human CSF. Human p3-Alcα (A), p3-Alcβ (B), and p3-Alcγ (C) species in CSF. The 300 μl (A and B) or 1 ml (C) of human CSF mixture was subjected to immunoprecipitation with UT135 (A, 8 μg of IgG fraction), UT143 (B, 100 μl of serum), and UT166 (C, 100 μl of serum) antibodies, respectively. The precipitates were analyzed for molecular mass with MALDI-TOF/MS. A, 34, p3-Alcα34; 35, p3-Alcα35; 36, p3-Alcα36; 37*, a mixture of p3-Alcα37 and p3-Alcα2N+35 (see supplemental Fig. S7B); 38, p3-Alcα38; 39, p3-Alcα39. B, 35, p3-Alcβ35; 36, p3-Alcβ36; 37, p3-Alcβ37; 38, p3-Alcβ38; 39, p3-Alcβ39; 40, p3-Alcβ40. C, 31, p3-Alcγ31.
HEK293 cells stably expressing wild type PS1 or vector alone (mock) plus Alcγ generated p3-Alcγ31 and p3-Alcγ34 as major peptide metabolites (see Fig. 2 for the amino acid sequence). HEK293 cells expressing FAD-linked PS1 mutants, especially L166P and R278T, demonstrated remarkable decreases in the levels of p3-Alcγ31 along with increases in the levels of p3-Alcγ34 (arrows in Fig. 3C). The alteration of the p3-Alcγ34/p3-Alcγ31 ratio showed some similarity to that of the p3-Alcα2N+38/p3-Alcα2N+35 ratio in various PS1 mutations but differed from the alteration of the p3-Alcβ40/p3-Alcβ37 ratio (Fig. 4A, compare lower left to upper right).
Six FAD-linked PS1 mutants (L166P, R278T, A434C, A246E, M146L, and L286V) increased the Aβ42/40 ratio at various magnitudes (Fig. 4A, lower right), whereas some of them did not show a similar effect to alter the minor species/major species ratio in the p3-Alc species. To analyze the correlation coefficients of γ-cleavage alteration between APP and Alc, the Aβ42/40 ratio was plotted to certain p3-Alc minor/major ratios (Fig. 4B). We have confirmed that the p3-Alc minor/major ratio of peak area detected by MALDI-TOF/MS analysis correlated well to those of theoretically calculated values in quantity (supplemental Fig. S2). As expected from the Fig. 4A on the basis of visual inspection, p3-Alcβ showed a property different from p3-Alcα and p3-Alcγ in correlation coefficient to Aβ42/40. The p3-Alcα2N+38/p3-Alcα2N+35 and p3-Alcγ34/p3-Alcγ31 ratios showed a strong correlation to the Aβ42/40 ratio (R2 > 0.5), whereas the p3-Alcβ40/p3-Alcβ37 ratio showed a positive but weak correlation to the Aβ42/40 ratio. These results suggest that phenotypes of γ-secretase dysfunction appeared in the altered cleavages of APP and/or Alc family proteins in variety. The magnitudes of C-terminal alteration of p3-Alcα, p3-Alcβ, and p3-Alcγ along with Aβ were not equivalent, suggesting that one type of γ-secretase dysfunction does not appear in the phenotype equivalently in the cleavage of type I membrane proteins.
The p3-Alc Species in Human CSF
Secreted Aβ species are detectable in human CSF, and it has been proposed that they might be useful as possible biomarkers or endophenotypes for the diagnosis and classification of AD (28–31). Above, we discussed how p3-Alc speciation and Aβ42/40 were modulated by certain FAD mutant PS1 molecules, which reflect γ-secretase dysfunction (Figs. 3 and 4). In our separate study, we sought to characterize CSF p3-Alc/Aβ relationships with a special interest in determining whether sporadic AD CSF might display a covariance between p3-Alc and Aβ as a potential indicator of underlying γ-secretase dysfunction.6 We herein examined whether the p3-Alcα, p3-Alcβ, and p3-Alcγ species that were identified in cell study were present in human CSF. The p3-Alc species were recovered from pooled human CSF samples by immunoprecipitation with anti-p3-Alcα 3B5, anti-p3-Alcβ UT143, and anti-p3-Alcγ UT166 antibodies, followed by analyses with MALDI-TOF/MS (Fig. 5 and supplemental Fig. S6).
The UT135 or 3B5 antibody recovered a peptide of molecular mass 3804.6, which was assigned the identity of p3-Alcα35 by MALDI-MS/MS analysis (Fig. 5A and supplemental Fig. S6A). This antibody also recovered another peptide, which contained two components, namely p3-Alcα37 (molecular mass 4003.0, a peptide composed of Ala817–Val853) and p3-Alcα 2N+35 (molecular mass 4006.9, a peptide composed of Met815–Thr851). The two components were distinguishable by reflector mode analysis (supplemental Fig. S7). p3-Alcα34, p3-Alcα36, and p3-Alcα38 were also identified in human CSF (supplemental Fig. S7A).
The antibody UT143 recovered peptides of molecular masses 3963.9 and 4303.2 (Fig. 5B and supplemental Fig. S6B), which were designated p3-Alcβ37 and p3-Alcβ40, respectively, according to MALDI-MS/MS analysis. The antibody UT166 recovered a peptide of molecular mass 3377.6 (Fig. 5C and supplemental Fig. S6C). The species with a molecular mass of 3377.6 (arrow in supplemental Fig. S6C) could not be analyzed by MALDI-MS/MS for amino acid sequence because the amount of peptide that was recovered by immunoprecipitation was not sufficient to obtain significant signals; however, the molecular mass coincided with that of p3-Alcγ31 (see supplemental Fig. S5C). These results demonstrate that the intramembrane cleavage sites of Alc in humans are identical to those determined by our cell-conditioned medium studies and that p3-Alcα35, p3-Alcα37, p3-Alcβ37, p3-Alcβ40, and p3-Alcγ31 are the major p3-Alc peptides recovered from human CSF.
To confirm the major p3-Alc peptide detected in CSF is the exact major product in brain, we compared p3-Alcα species in mouse CSF and brain (supplemental Fig. S8). The major p3-Alcα35 in CSF was also major product in the brain, along with the similar profile of p3-Alcα minor species between CSF and brain. Furthermore, the p3-Alcα species profile of mouse CSF was identical to those of human CSF (supplemental Fig. S8), suggesting that p3-Alcα35 is major product in mouse and human brain.
DISCUSSION
In this study, we show that the Alc family proteins Alcα, Alcβ, and Alcγ are cleaved by APP α-secretases ADAM 10 and ADAM 17. Alc is expressed largely in neurons (1); therefore, ADAM 10 is thought to be the most likely candidate for Alc cleavage in the central nervous system because ADAM 17 is predominantly expressed in glial cells (22). Thus, in neurons, Alc and APP are primarily cleaved by the same enzymes when both proteins are liberated from their individual or coordinated complexes with X11L. These results agree with our previous report that APP and Alc are likely to be metabolized in a coordinated fashion (12). Although APP is also cleaved by BACE, Alc proteins are not likely to be major substrates for this enzyme (supplemental Fig. S4).
In addition to the similar mechanisms regulating the primary cleavage of Alc family proteins and APP, we also found that Alc family proteins are cleaved by the same γ-secretase complex as is APP (12). In this study, we have demonstrated that p3-Alc species with altered C termini are secreted together with the increased ratio of Aβ42 to Aβ40 by cells expressing FAD-linked PS1 mutants, although the magnitude of alteration was diversified among Alc family proteins in cells expressing various FAD-linked PS1 mutants. p3-Alc species are not prone to aggregation7 and are detectable in human CSF; this raises the possibility that some p3-Alc changes in the pathological state might serve in clinical situations as markers of variant protein processing by the dysfunction of γ-secretase.
In the cell culture experiments, we observed that wild type and several pathogenic PS1 mutants modulated p3-Alcα, p-3-Alcβ, and p3-Alcγ C-terminal speciation at various magnitudes. This indicates that APP is not the only γ-secretase substrate that undergoes variant processing in association with PS1 mutations and suggests that some of the PS1 mutations caused more alteration in the cleavage of Alc protein(s) than APP and vice versa.
In studies with cells expressing FAD-linked PS1 mutant, a covariance was observed when Aβ42/40 and certain variant p3-Alc minor/major species ratios, such as p3-Alcα2N+38/p3-Alcα2N+35 or p3-Alcγ34/p3-Alcγ31, were plotted against each other (Fig. 4B). We observed similar phenomena in the CSF of subjects with sporadic AD, in that there existed disease-related covariant signature relationships between p3-Alcα38/p3-Alcα35 ratios and Aβ42/40 ratios.6 In the case of p3-Alcα38/35 ratios, the covariant signature showed a positive slope in AD subjects, whereas the signatures of both the aged non-demented control subjects and the other neurological disease control subjects were similar to each other and negative in slope, suggesting a pathogenic malfunction of the γ-secretase complex in sporadic AD patients,6 as observed in a pathogenic state of FAD-linked PS mutations in this study (Fig. 4B).
The biophysical mechanisms by which pathogenic PS1 mutations exert their effects are poorly understood, and it is unclear how p3-Alc ratios from the CSF of humans who do not harbor PS1 mutations are altered, as can be seen in this study with FAD-linked PS1 mutant. It is possible that wild type PS1 is behaving as if a PS1 mutation were present as a result of changes in some co-factors that modulate γ-secretase function, a result of the conformational changes of PS1, and/or a result of peripheral environmental changes at the place that γ-secretase is active. Therefore, the present results suggest that qualitative changes of p3-Alc species with altered C termini may be useful in diagnosing dysfunction of γ-secretase prior to the pathogenesis of sporadic Alzheimer disease. Taking these findings together with those of another study of human subjects,6 we propose a hypothesis that γ-secretase dysfunction may be a feature of the pathogenesis in some population of common sporadic AD and that measurement of unusual p3-Alc fragments may provide surrogate markers to detect clinical γ-secretase dysfunction.
Acknowledgment
S. H. thanks Dr. Hideaki Wakita (National Institute for Longevity Sciences, Obu, Japan) for technical advice.
This work was supported, in whole or in part, by National Institutes of Health, NIA, Grants R01 AG23611, P01 AG10491, and P50 AG005138 (to S. G.). This work was also supported in part by Grants-in-aid for Scientific Research on Priority Areas 20023001 (to T. S.) from the Ministry of Education, Science, Culture, Sports, and Technology, Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. S1–S8.
S. Hata, S. Fujishige, Y. Araki, M. Taniguchi, K. Urakami, E. Peskind, H. Akatsu, M. Araseki, R. Martins, M. Maeda, A. Levey, K. Chung, T. Montine, J. Leverenz, A. Fagan, A. Goate, R. Bateman, D. Holtzman, T. Yamamoto, T. Nakaya, S. Gandy, and T. Suzuki, submitted for publication.
Y. Araki and N. Takei, unpublished observations.
- Alc
- alcadein
- AD
- Alzheimer disease
- Aβ
- amyloid β-protein
- ADAM
- a disintegrin and metalloproteinase
- APP
- amyloid β-precursor protein
- sAPP
- soluble large extracellular N-terminal domain of APP truncated at the primary cleavage site
- sAlc
- soluble large extracellular N-terminal domain of Alc truncated at the primary cleavage site
- p3-Alc
- small peptide generated by serial primary and secondary cleavages of Alc
- CSF
- cerebrospinal fluid
- CTF
- C-terminal fragment of APP or Alc truncated at the primary cleavage site
- BACE
- β-site APP-cleaving enzyme
- MEF
- mouse embryonic fibroblast
- PS
- presenilin
- MALDI
- matrix-assisted laser desorption ionization
- TOF
- time-of-flight
- MS
- mass spectrometry
- MS/MS
- tandem MS.
REFERENCES
- 1.Araki Y., Tomita S., Yamaguchi H., Miyagi N., Sumioka A., Kirino Y., Suzuki T. (2003) J. Biol. Chem. 278, 49448–49458 [DOI] [PubMed] [Google Scholar]
- 2.Vogt L., Schrimpf S. P., Meskenaite V., Frischknecht R., Kinter J., Leone D. P., Ziegler U., Sonderegger P. (2001) Mol. Cell Neurosci. 17, 151–166 [DOI] [PubMed] [Google Scholar]
- 3.Araki Y., Kawano T., Taru H., Saito Y., Wada S., Miyamoto K., Kobayashi H., Ishikawa H. O., Ohsugi Y., Yamamoto T., Matsuno K., Kinjo M., Suzuki T. (2007) EMBO J. 26, 1475–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig A., Blume J., Diep T. M., Yuan J., Mateos J. M., Leuthäuser K., Steuble M., Streit P., Sonderegger P. (2009) Traffic 10, 572–589 [DOI] [PubMed] [Google Scholar]
- 5.Ikeda D. D., Duan Y., Matsuki M., Kunitomo H., Hutter H., Hedgecock E. M., Iino Y. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5260–5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoerndli F. J., Walser M., Fröhli Hoier E., de Quervain D., Papassotiropoulos A., Hajnal A. (2009) PLoS One 4, e4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selkoe D. J. (2001) Physiol. Rev. 81, 741–766 [DOI] [PubMed] [Google Scholar]
- 8.Gandy S. (2005) J. Clin. Invest. 115, 1121–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Small S. A., Gandy S. (2006) Neuron 52, 15–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki T., Nakaya T. (2008) J. Biol. Chem. 283, 29633–29637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taru H., Suzuki T. (2009) J. Alzheimers Dis. 18, 253–265 [DOI] [PubMed] [Google Scholar]
- 12.Araki Y., Miyagi N., Kato N., Yoshida T., Wada S., Nishimura M., Komano H., Yamamoto T., De Strooper B., Yamamoto K., Suzuki T. (2004) J. Biol. Chem. 279, 24343–24354 [DOI] [PubMed] [Google Scholar]
- 13.Sano Y., Syuzo-Takabatake A., Nakaya T., Saito Y., Tomita S., Itohara S., Suzuki T. (2006) J. Biol. Chem. 281, 37853–37860 [DOI] [PubMed] [Google Scholar]
- 14.Saito Y., Sano Y., Vassar R., Gandy S., Nakaya T., Yamamoto T., Suzuki T. (2008) J. Biol. Chem. 283, 35763–35771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buxbaum J. D., Liu K. N., Luo Y., Slack J. L., Stocking K. L., Peschon J. J., Johnson R. S., Castner B. J., Cerretti D. P., Black R. A. (1998) J. Biol. Chem. 273, 27765–27767 [DOI] [PubMed] [Google Scholar]
- 16.Lammich S., Kojro E., Postina R., Gilbert S., Pfeiffer R., Jasionowski M., Haass C., Fahrenholz F. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3922–3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allinson T. M., Parkin E. T., Turner A. J., Hooper N. M. (2003) J. Neurosci. Res. 74, 342–352 [DOI] [PubMed] [Google Scholar]
- 18.Ando K., Oishi M., Takeda S., Iijima K., Isohara T., Nairn A. C., Kirino Y., Greengard P., Suzuki T. (1999) J. Neurosci. 19, 4421–4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endres K., Postina R., Schroeder A., Mueller U., Fahrenholz F. (2005) FEBS J. 272, 5808–5820 [DOI] [PubMed] [Google Scholar]
- 20.Hartmann D., de Strooper B., Serneels L., Craessaerts K., Herreman A., Annaert W., Umans L., Lübke T., Lena Illert A., von Figura K., Saftig P. (2002) Hum. Mol. Genet. 11, 2615–2624 [DOI] [PubMed] [Google Scholar]
- 21.Allinson T. M., Parkin E. T., Condon T. P., Schwager S. L., Sturrock E. D., Turner A. J., Hooper N. M. (2004) Eur. J. Biochem. 271, 2539–2547 [DOI] [PubMed] [Google Scholar]
- 22.Goddard D. R., Bunning R. A., Woodroofe M. N. (2001) Glia 34, 267–271 [DOI] [PubMed] [Google Scholar]
- 23.Vassar R., Bennett B. D., Babu-Khan S., Kahn S., Mendiaz E. A., Denis P., Teplow D. B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M. A., Biere A. L., Curran E., Burgess T., Louis J. C., Collins F., Treanor J., Rogers G., Citron M. (1999) Science 286, 735–741 [DOI] [PubMed] [Google Scholar]
- 24.Sherrington R., Rogaev E. I., Liang Y., Rogaeva E. A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K., Tsuda T., Mar L., Foncin J.-F., Bruni A. C., Montesi M. P., Sorbi S., Rainero I., Pinessi L., Nee L., Chumakov I., Pollen D., Brookes A., Sanseau P., Polinsky R. J., Wasco W., Da Silva H. A. R., Haines J. L., Pericak-Vance M. A., Tanzi R. E., Roses A. D., Fraser P. E., Rommens J. M., St George-Hyslop P. H. (1995) Nature 375, 754–760 [DOI] [PubMed] [Google Scholar]
- 25.Kwok J. B., Taddei K., Hallupp M., Fisher C., Books W. S., Broe G. A., Hardy J., Fulham M. J., Nicholson G. A., Stell R., St. George-Hyslop P. H., Fraser P. E., Kakulas B., Clarnette R., Relkin N., Gandy S. E., Schofield P. R., Martins R. N. (1997) Neuroreport 8, 1537–1542 [DOI] [PubMed] [Google Scholar]
- 26.Devi G., Fotiou A., Jyrinji D., Tycko B., DeArmand S., Rogaeva E., Song Y. Q., Medieros H., Liang Y., Orlacchio A., Williamson J., St George-Hyslop P., Mayeux R. (2000) Arch. Neurol. 57, 1454–1457 [DOI] [PubMed] [Google Scholar]
- 27.Moehlmann T., Winkler E., Xia X., Edbauer D., Murrell J., Capell A., Kaether C., Zheng H., Ghetti B., Haass C., Steiner H. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 8025–8030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanai M., Matsubara E., Isoe K., Urakami K., Nakashima K., Arai H., Sasaki H., Abe K., Iwatsubo T., Kosaka T., Watanabe M., Tomidokoro Y., Shizuka M., Mizushima K., Nakamura T., Igeta Y., Ikeda Y., Amari M., Kawarabayashi T., Ishiguro K., Harigaya Y., Wakabayashi K., Okamoto K., Hirai S., Shoji M. (1998) Ann. Neurol. 44, 17–26 [DOI] [PubMed] [Google Scholar]
- 29.Fagan A. M., Mintun M. A., Mach R. H., Lee S. Y., Dence C. S., Shah A. R., LaRossa G. N., Spinner M. L., Klunk W. E., Mathis C. A., DeKosky S. T., Morris J. C., Holtzman D. M. (2006) Ann. Neurol. 59, 512–519 [DOI] [PubMed] [Google Scholar]
- 30.Graff-Radford N. R., Crook J. E., Lucas J., Boeve B. F., Knopman D. S., Ivnik R. J., Smith G. E., Younkin L. H., Petersen R. C., Younkin S. G. (2007) Arch. Neurol. 64, 354–362 [DOI] [PubMed] [Google Scholar]
- 31.Kauwe J. S., Jacquart S., Chakraverty S., Wang J., Mayo K., Fagan A. M., Holtzman D. M., Morris J. C., Goate A. M. (2007) Ann. Neurol. 61, 446–453 [DOI] [PubMed] [Google Scholar]