Abstract
For S-nitrosothiols and peroxynitrite to interfere with the activity of mitochondrial complex I, prior transition of the enzyme from its active (A) to its deactive, dormant (D) state is necessary. We now demonstrate accumulation of the D-form of complex I in human epithelial kidney cells after prolonged hypoxia. Upon reoxygenation after hypoxia there was an initial delay in the return of the respiration rate to normal. This was due to the accumulation of the D-form and its slow, substrate-dependent reconversion to the A-form. Reconversion to the A-form could be prevented by prolonged incubation with endogenously generated NO. We propose that the hypoxic transition from the A-form to the D-form of complex I may be protective, because it would act to reduce the electron burst and the formation of free radicals during reoxygenation. However, this may become an early pathophysiological event when NO-dependent formation of S-nitrosothiols or peroxynitrite structurally modifies complex I in its D-form and impedes its return to the active state. These observations provide a mechanism to account for the severe cell injury that follows hypoxia and reoxygenation when accompanied by NO generation.
Introduction
The mechanisms underlying the cellular response to hypoxia and their consequences are not completely understood. Because the mitochondrial respiratory chain is the major consumer of oxygen, mitochondria are likely to play a significant role in regulating its distribution in cells and tissues (1). Cells have the ability to decrease oxygen demand at low [O2] (2, 3), and the affinity of cytochrome c oxidase for oxygen is considered to be the most important factor in the decrease of mitochondrial oxygen consumption during hypoxia (4, 5). The interaction of nitric oxide (NO)2 with cytochrome c oxidase has been shown to be a significant determinant of the affinity of this enzyme for oxygen and is responsible for reducing cellular consumption of oxygen at low [O2]. Nitric oxide also plays a role in the early reduction of mitochondrial cytochromes that occurs as the [O2] decreases (6, 7). A direct consequence of such reduction is a backlog of electrons at all the redox centers of the respiratory chain, including cytochromes and ubiquinone, as well as the intramitochondrial pool of NAD(P)H.
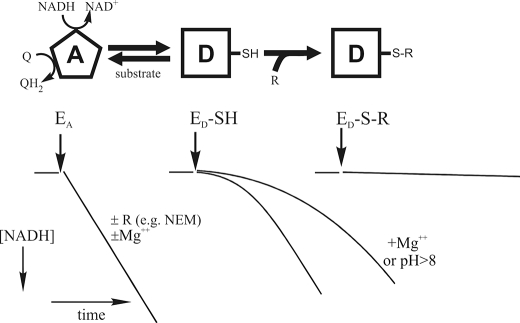
Mitochondrial complex I (EC 1.6.5.3, proton-translocating NADH:ubiquinone oxidoreductase) is responsible for oxidation of matrix NADH by membrane-bound ubiquinone and is the major entry point for electrons to the respiratory chain (8). It is also a major source of mitochondrial reactive oxygen species (ROS) (9–11). Two catalytically and structurally distinct forms of complex I have been identified in partially purified preparations in vitro: one is a fully competent, “active” A-form, and the other is a dormant, silent, “deactivated” D-form (12). In such systems a so-called pseudo-reversible A/D transition has been described in mammalian and other vertebrate complex I (13). The turnover number of active complex I in submitochondrial particles (SMP) is ∼104 min−1 at 25 °C, is almost pH-independent between 5.5 and 8.5, and is not significantly affected by the presence of divalent cations (12) (Fig. 1). Incubation of the enzyme at 37 °C in the absence of substrate results in its conversion to the D-form. The D-form of complex I is converted back to the A-form in the presence of substrate (NADH and ubiquinone) as a result of the slow (4 min−1) turnover(s) of the enzyme. This is manifest as a considerable lag phase during continuous assay of the NADH:ubiquinone oxidoreductase reaction catalyzed by deactivated enzyme. Reversible A/D transition has been demonstrated in intact mitochondria (11) and ex vivo in the perfused Langendorf rat heart during hypoxia/reoxygenation (14).
FIGURE 1.
Scheme illustrating the A/D transition of mitochondrial complex I. The A-form of the enzyme (EA) catalyzes the rapid energy-generating NADH:ubiquinone (Q) oxidoreductase reaction. The A-form of complex I is not sensitive to cysteine-modifying reagents (R) such as NEM. Incubation of the enzyme at 37 °C in the absence of substrate results in its conversion to the D-form (ED-SH), which can be reactivated by several enzyme turnovers during oxidation of a small pulse of NADH. There is a considerable lag phase in the NADH:Q oxidoreductase reaction during reactivation of the deactivated enzyme. The final rates reached during the reaction are, however, equal for the A- and D-form preparations. Alkalization of the medium and divalent cations acids significantly prolong the lag phase. If the critical cysteine residue (SH) of complex I that becomes exposed when the enzyme converts to the D-form is modified by treatment with NEM, 5,5′-dithiobis(nitrobenzoic acid), or NO-derived species, then the modified enzyme (ED-S-R) is prevented from undergoing turnover-dependent reactivation.
The A-form of complex I is insensitive to sulfhydryl reagents such as N-ethylmaleimide (NEM) or Ellman's reagent (Fig. 1), whereas treatment of the D-form with such reagents irreversibly abolishes the ability of the enzyme to respond to activation (15, 16). The critical cysteine 39 of the mitochondrially encoded ND3 subunit has been shown to become exposed on the surface of the D-form and to be responsible for the sensitivity of this form to SH reagents (17). This cysteine residue is not accessible to any covalent modification in the A-form.
Recently we have shown in bovine SMP that the A/D conformational state of complex I is an important factor for the interaction of the enzyme with nitrosothiols and peroxynitrite, so that the D-form, but not the A-form, is susceptible to inhibition by these agents (18). Therefore it is likely that the previously observed NO-dependent persistent inhibition of mitochondrial complex I (19–23) takes place only when the enzyme is in the D-form and when NO can interact with ROS to form peroxynitrite (18).
We now show that deactivation of complex I occurs in human epithelial kidney cells during prolonged hypoxic incubation. Furthermore, accumulation of the covalently modified D-form is responsible for the persistent inhibition of cellular respiration that occurs in the presence of NO. We propose that transition from A to D, which is initially protective, may become an early step in the initiation of pathophysiology as a result of the modification of complex I by nitrosating agents, such as peroxynitrite, making this enzyme an early mitochondrial target for nitrosative or oxidative stress. This has major implications in understanding the susceptibility of cells to hypoxic damage when accompanied by the generation of NO.
MATERIALS AND METHODS
Isolation and Measurement of Activity of SMP
Bovine heart SMP were prepared according to standard procedure (24) and stored in liquid nitrogen. Activation and deactivation of complex I in SMP, and spectrophotometric measurement of complex I activity, were carried out at 340 nm as described previously (18). NADH:fumarate oxidoreductase was measured in the same way but using 100–200 μg of protein of SMP/ml because of the very slow rates of the reaction.
Determination of A/D Ratio
The previously described diagnostic test for conformational changes of complex I, utilizing the different sensitivity of the A- and D-forms to the SH reagent NEM, was used to determine the A/D ratio (17, 18). Briefly, in a heterogeneous (A and D) preparation of the enzyme, treatment of a sample with NEM results in irreversible inhibition of the D-form, so that any residual activity that can be measured is attributable to the A-form. The total activity of the enzyme (A+D) can be measured by converting all of a sample to the A-form, usually by a pulse of 5–10 μm NADH. Thus the proportion of complex I in the D-form in any preparation can be calculated.
To evaluate the time course of deactivation of the oxidized enzyme, SMP (final concentration, 20–30 μg/ml protein) were placed in a spectrophotometric cuvette containing buffer (0.25 m sucrose, 50 mm Tris-HCl, pH 8.0, 0.2 mm EDTA) supplemented with 1 mm MgCl2, 0.3 μm carbonyl cyanide p-trifluoromethoxyphenylhydrazone, and 150 units/ml catalase, and the reaction was initiated by adding 50 μm NADH. After consumption of all added NADH, complex I in the SMP became activated (i.e. all in the A-form) and was incubated for different time intervals (30 s to 5 min) in an oxidized state. NEM (1 mm) was then added, and NADH oxidase activity was assayed following the addition of 150 μm NADH. The rate of enzyme activity was used to calculate the percentage of complex I in the active form, as described above.
To evaluate the time course of deactivation of the reduced enzyme, buffer was added to a sealed spectrophotometrical cuvette and deaerated with argon to a final oxygen concentration of 25 μm O2. SMP (final concentration, 20–30 μg/ml protein) and 200 μm NADH were added, and after an initial short burst of NADH oxidase activity, the SMP were incubated anaerobically. No NADH oxidation was detected during anaerobic incubation, indicating full anaerobiosis and a lack of activity of complex I. After different time intervals (20 s to 5 min), 1 mm NEM was added, and the medium was reoxygenated. This was achieved by the addition of 200–400 μm hydrogen peroxide, which is decomposed by catalase in less than 0.5 s, with the generation of oxygen (25, 26). Similar results were obtained if the medium was reoxygenated by opening the cuvette and fast pipetting. NADH oxidase activity following NEM treatment was used to calculate the percentage of the enzyme in the A-form, as described previously.
Cell Culture
HEK293 cells transfected with an NO synthase under the control of a tetracycline-inducible promoter (27) were grown and harvested as described (5). Cells containing an empty vector were used in control experiments.
Oxygen, Cytochrome Redox State, and NO Measurements
Simultaneous measurement of cytochrome redox states and [O2] during cellular respiration were carried out in modified Hanks' solution using a Clark-type oxygen electrode (Rank Bros., Cambridge, UK) equipped with an NO-sensitive electrode (Innovative Instruments Inc., Tampa, FL) and with a spectrophotometrical system as described previously (7, 28).
To minimize back diffusion of oxygen into the chamber from the air during anaerobic incubation, the oxygen electrode base was placed in a plastic bag filled with nitrogen. The solutions of alamethicin, digitonin, and NEM added at the end of anaerobic incubation were deaerated with argon to avoid introduction of oxygen to the cell suspension. The same catalase/hydrogen peroxide system was used for rapid reoxygenation experiments. Similar results were obtained by creating a gaseous head space, flushing the chamber with oxygen for a short time, and then resealing the chamber. A specific inhibitor of NO synthase, SEITU (1 mm), was used to stop production of NO. The experiments were repeated four or five times, and the results are expressed as the means ± S.E.
Confocal Microscopy
All of the imaging experiments were conducted using Hanks' buffer supplemented with 5% calf serum, as in previous experiments with cells. Confocal images of NADH autofluorescence were obtained using a Zeiss 510 UV-visible confocal laser scanning microscope equipped with a META detection system and a 40× fluorite oil immersion objective, as described previously (29, 30). Illumination intensity was kept to a minimum (at 0.1–0.2% of laser output) to avoid phototoxicity. The pinhole was set to give an optical slice of ∼2 μm. NADH autofluorescence was excited at 351 and measured at 375–470 nm. The data were generated from a minimum of three independent experiments, using at least 20 cells/experiment. Statistical analysis and exponential curve fitting were performed using Origin 8 (Microcal Software Inc., Northampton, MA) software. The data were analyzed by parametric Student's t tests, and significance was expressed (**, p < 0.005). For all graphs, the bars represent the means ± S.E.
Materials
All of the chemicals were purchased from Sigma. Cell culture medium and serum were from Invitrogen. Protein content was determined by BCA assay.
RESULTS
A/D Transition in SMP in Oxidized and Reduced Conditions
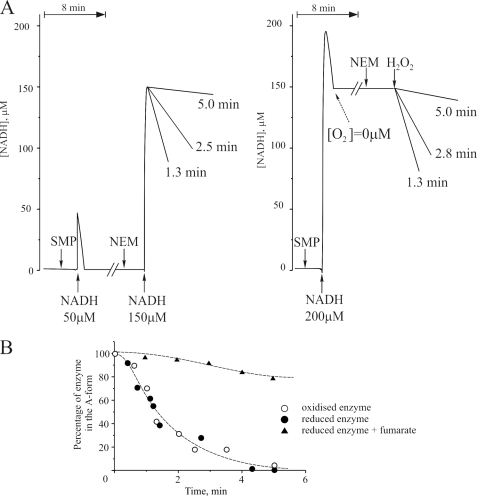
Reversible deactivation of complex I in SMP is usually achieved by incubation at 30–35 °C for 30–60 min at ambient [O2] without adding any substrate (18, 24). In such aerobic conditions and in the absence of electron donors, conformational changes in complex I occur within the oxidized enzyme; very little is known, however, about the ability of enzyme to undergo deactivation in its reduced form. We compared deactivation of complex I at 37 °C in its oxidized state (SMP in aerobic conditions without substrate; Fig. 2A, left trace) and its reduced state (anoxia in the presence of NADH; Fig. 2A, right trace) for different times. The percentage of complex I in the A-form after each period of anoxia was determined by calculating the residual rate of NADH oxidation after NEM treatment as a percentage of the NADH oxidase activity catalyzed by fully activated enzyme (2.1 ± 0.2 μmol NADH × min−1 × mg−1). We found that the time-dependent conversion of enzyme into the D-form took place at a similar rate in oxidized and reduced complex I (Fig. 2B). The t½ of the deactivation process at 37 °C in the conditions of our experiment was 1.8 min. When SMP were incubated in the reduced conditions in the presence of fumarate, the time-dependent deactivation of complex I was prevented (Fig. 2B).
FIGURE 2.
Deactivation of complex I in bovine SMP in oxidized and reduced conditions at 37 °C. A, NADH oxidase activity of SMP after their incubation in oxidized (left panel) and reduced (right panel) conditions. NEM was added to block the D-form prior to the activity measurements (see “Materials and Methods” for details). B, time course of the deactivation of oxidized complex I (open circles), reduced complex I (closed circles), and reduced enzyme in the presence of 1 mm fumarate (triangles).
Effect of Anoxia on A/D Transition in Cells
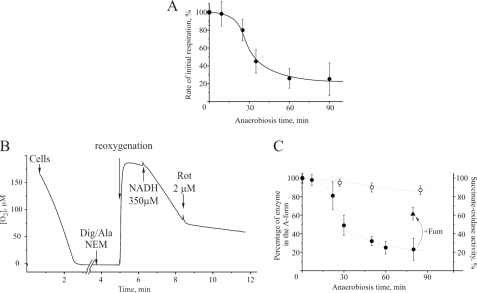
We next investigated the effect of incubation of HEK293 cells in anaerobic conditions for different times on the initial rate of oxygen consumption after reoxygenation. In these experiments the cells were resuspended in Hanks' solution, placed in an oxygen electrode chamber, and supplemented with an excess of catalase (150 units/ml). Oxygen was consumed by the cells, which were then incubated anaerobically for different time intervals (1–90 min). The solution was then rapidly reoxygenated by adding hydrogen peroxide, which was converted into oxygen by the catalase. The rate of oxygen consumption was slow initially, increased as reoxygenation progressed, and eventually become linear. The initial rate of respiration during reoxygenation was measured and calculated as a percentage of the steady state respiration rate prior to anaerobiosis (16.3 ± 2.9 nmol of O2 × min−1/107 cells). The drop in the initial respiration rate after anaerobiosis was found to be dependent on the duration of anaerobic incubation (Fig. 3A).
FIGURE 3.
Effect of hypoxia on the deactivation state of complex I in HEK293 cells. A, the initial rate of cellular respiration after reoxygenation following incubation in anaerobic conditions for different time periods; 100% corresponds to a steady state rate of respiration of 16.3 ± 2.9 nmol of O2 × min−1/107 cells. B, determination of the percentage of complex I in the A-form in permeabilized cells. C, the time course of complex I deactivation after different periods of anoxia. The cells were permeabilized with digitonin (Dig) and alamethicin (Ala) as described under “Materials and Methods,” and NEM-insensitive and rotenone (Rot)-sensitive NADH oxidase (closed circles) or succinate oxidase (open circles) activity was measured. The triangle shows NADH oxidase activity when fumarate was present in the incubation medium.
In another series of experiments, we tested the A/D state of mitochondrial complex I in cells subjected to anaerobic incubation for different time intervals (1–90 min), as described above. The cells were then permeabilized with digitonin/alamethicin, and NEM was added immediately after. Rotenone-sensitive NADH oxidase activity was then assessed after reoxygenation by hydrogen peroxide and the addition of NADH (Fig. 3B). After permeabilization, mitochondrial complex I can oxidize the added NADH in aerobic conditions. Because NEM blocks the D-form of complex I irreversibly, all of the activity observed was attributable to the A-form. As shown in Fig. 3C, the NADH oxidase activity of permeabilized and NEM-treated cells, reflecting the final percentage of the A-form of complex I, decreased with the duration of anoxic incubation. Succinate oxidase activity was not affected by anaerobic incubation, indicating that the primary effect of the absence of oxygen was at the level of complex I. A significant amount of complex I remained in the active form if 1 mm fumarate was added immediately before permeabilization and NEM treatment (Fig. 3C).
Persistent Inhibition of Mitochondrial Respiration by Endogenously Generated NO
In Cells in an Oxygen Electrode Chamber
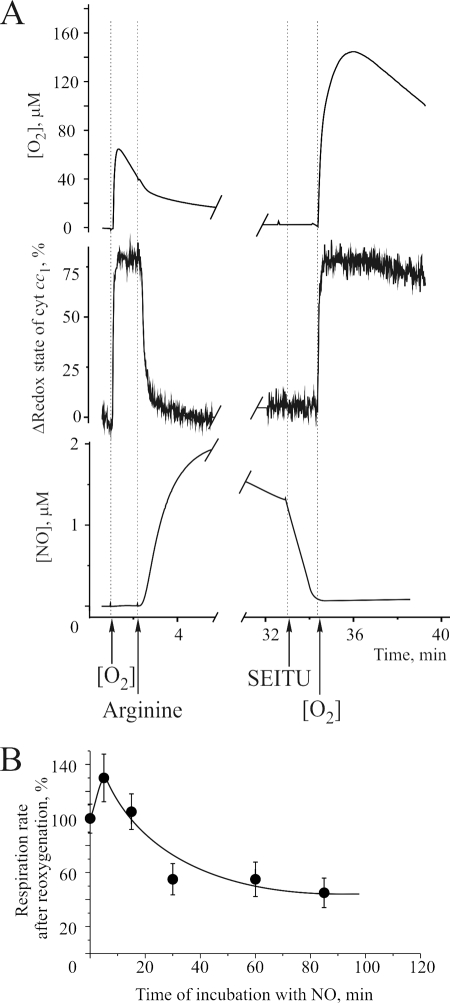
To evaluate the effects of endogenous NO on cellular respiration, we used HEK293 cells transfected with an NO synthase under the control of a tetracycline-inducible promoter. These cells produce NO when l-arginine is added. The cells were allowed to respire to anaerobiosis (<0.5 μm O2) in an oxygen electrode chamber. The cell suspension was then reoxygenated, resulting in oxidation of the cytochromes (Fig. 4A). After a linear respiration rate was achieved, 0.5 mm l-arginine was added at an [O2] of 50 μm. This resulted in the rapid production of NO, full reduction of cytochromes, and inhibition of mitochondrial respiration. The cells were then incubated for up to 90 min in the presence of endogenously generated NO. At different time points 1 mm SEITU was added to inhibit NO synthase, and after complete disappearance of NO from the cell medium, the suspension was reoxygenated to initiate oxygen consumption. The rate of oxygen consumption was measured and calculated as a percentage of the oxygen consumption prior to anaerobiosis. As shown in Fig. 4B, the reduction in respiration rate was dependent on the duration of incubation in the presence of NO.
FIGURE 4.
Effect of prolonged incubation of cells in the presence of endogenous NO. A, detection of oxygen consumption (top trace), cytochrome c redox state (middle trace), and NO in the medium (bottom trace) after the addition of 500 μm l-arginine to HEK293 cells transfected with an NO synthase. After different time intervals, the specific NO synthase inhibitor SEITU (1 mm) was added, and respiration was reinitiated after complete disappearance of NO from the medium. The rate of oxygen consumption was measured and calculated as a percentage of the oxygen consumption prior to anaerobiosis. B, dependence of the rate of oxygen consumption on the duration of incubation in the presence of NO.
Estimation of NADH/NAD+ Dynamics
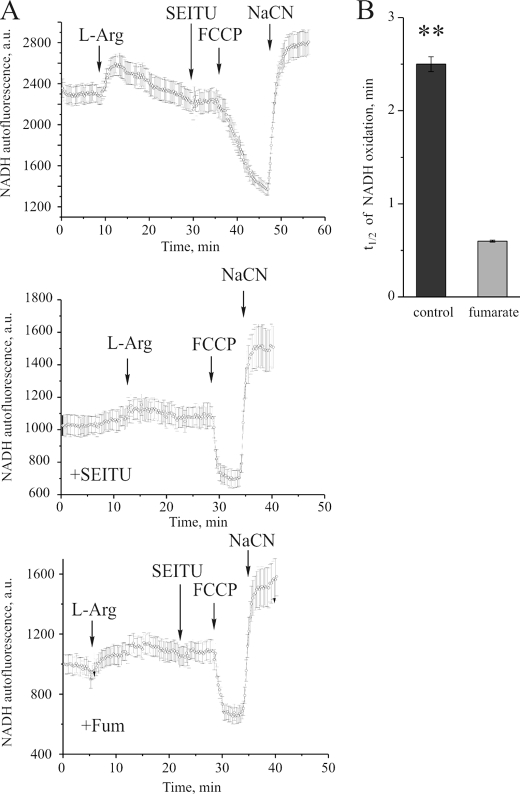
The redox state of matrix NAD(P) was assessed by confocal microscopy of HEK293 cells transfected with NO synthase under the control of a tetracycline-inducible promoter. The addition of l-arginine (500 μm) caused a 38 ± 3.2% increase in NAD(P)H autofluorescence because of the production of NO with consequent inhibition of cytochrome c oxidase (n = 65 cells; Fig. 5A, top panel). After 25 min of active NO production by cells, 1 mm SEITU was added to inhibit NO synthase. Subsequent addition of carbonyl cyanide p-trifluoromethoxyphenylhydrazone resulted in oxidation of matrix NADH, which was slow (half-life = ∼2.5 min), indicating that the major fraction of complex I was inhibited. Sodium cyanide (0.5 mm) was added to estimate the fluorescence signal from fully reduced matrix nucleotides. Treatment of the cells with SEITU prior to the addition of l-arginine prevented the production of NO and an increase in NAD(P)H autofluorescence (Fig. 5A, center panel). The presence of fumarate (0.5 mm) in the medium during incubation with NO resulted in a 5.8-fold acceleration of the rate of oxidation of NADH compared with such treatment in the absence of fumarate, indicating that NADH oxidase activity was not compromised by NO in the presence of fumarate (Fig. 5, A, bottom panel, and B).
FIGURE 5.
Measurement of intrinsic fluorescence of NAD(P)H in HEK293 cells transfected with a tetracycline-inducible NO synthase. The images were acquired on a confocal imaging system as described under “Materials and Methods.” A, the time course of NAD(P)H autofluorescence after generation of NO (top panel), in the absence of NO (SEITU-treated, center panel), and in the presence of NO and fumarate (bottom panel). B, the t½ of NADH oxidation after incubation with NO in the absence and presence of fumarate.
DISCUSSION
We show that in bovine SMP, turnover-dependent deactivation of complex I can occur in both reduced and oxidized enzyme at a similar rate. This indicates that complex I can undergo deactivation when all iron sulfur clusters and bound FMN of the enzyme are in the reduced state. Therefore we concluded that complex I can potentially undergo deactivation when oxygen is lacking, i.e. when the respiratory chain is reduced and turnover of the enzyme is decreased. It should be stressed that reduction of the respiratory chain in hypoxic conditions would result in a significant reduction of the ubiquinone pool, which is important for the turnover of complex I (31). Fumarate prevented the deactivation of reduced complex I in anaerobic conditions. The likely explanation for this is that in the presence of excess fumarate, complex II can act in reverse mode, reducing fumarate to succinate and thus reoxidizing ubiquinol (32). In this way, an electron acceptor for complex I is regenerated. This NADH:fumarate oxidoreductase reaction will maintain a slow turnover of complex I sufficient to support the enzyme in the active state. The rate of the NADH:fumarate oxidoreductase reaction was ∼12 nmol NADH × min−1 × mg−1 protein, which is only ∼1% of the NADH oxidase activity of SMP.
Using a HEK293 cell line, we have also shown that there is a time-dependent lag phase in cellular respiration upon reoxygenation after prolonged anaerobic incubation. To determine the involvement of the A- and D-forms of complex I in this response, we measured NEM-insensitive oxidation of NADH in cells after anaerobic incubation in an oxygen electrode chamber. The cell plasma membrane was permeabilized with digitonin, and alamethicin was used to make the inner mitochondrial membrane permeable to exogenously added NADH (33). Subsequent treatment with NEM irreversibly blocks the accumulated D-form so that the rotenone-sensitive NADH oxidase activity is provided by the NEM-insensitive A-form of complex I left in the cell. Our results show that A/D transition occurred in cells during prolonged anaerobic incubation and that the amount of conversion was dependent on the duration of anaerobiosis. In our conditions the decrease in the A-form content occurred at a much slower rate in cells than in SMP (t½ = 30–40 and 1.8 min, respectively). This difference is probably due to the different environment of the enzyme in SMP in buffer and in intact mitochondria, where complex I is exposed to high concentrations of matrix proteins, metabolites, high osmolarity, and electrical field. The rate of deactivation can be affected by slow turnover of complex I during oxidation of NADH in a side reaction catalyzed by the enzyme in the presence of high concentrations of reduced nucleotides that accumulate in the matrix in hypoxia/anoxia. For example, the decrease in the A-form in cells during anaerobic incubation could be prevented by the addition of fumarate, probably because of the NADH:fumarate oxidoreductase activity that provides a turnover of the enzyme (32). Despite earlier reports to the contrary (34), there is some evidence for the ability of fumarate to cross membranes into the mitochondrial matrix, because dicarboxylic acid transport activity has been found in the plasma membrane (35, 36) as well as in the inner mitochondria membrane of some cell types (37, 38).
We have previously reported that the D-form of complex I is susceptible to inhibition by NO-related species (18). To determine the effect of NO adducts on cells exposed to hypoxia, we used HEK293 cells transfected with an NO synthase under the control of a tetracycline-inducible promoter. The addition of 100–500 μm l-arginine resulted in the continuous formation of large quantities of NO and full reduction of the respiratory chain, as evident from the maximal reduction of mitochondrial cytochromes and absence of cyanide-sensitive oxygen consumption. Incubation of cells in hypoxic conditions for a short period (5–10 min) in the presence of NO resulted in a slight increase in the respiration rate, possibly because of an increase in mitochondrial activity in response to the drop in cellular ATP. After prolonged hypoxic incubation in the presence of NO, however, the cellular respiration was inhibited in a time-dependent manner, even after removal of NO from the medium. In the conditions described above, complex I is likely to be susceptible to NO-dependent persistent inhibition because of the accumulation of the D-form, known to be inhibited by NO-related species (18).
Confocal microscopy for monitoring the autofluorescence of intramitochondrial NAD(P)H was used as a semiquantitative approach to estimate complex I in live cells. The fluorescence signal reflects the reduction level of mitochondrial nucleotides, which is the balance between NADH-generating activities of NAD-dependent dehydrogenases and NADH-consuming activities of complex I. In HEK293-tet-inducible nitric-oxide synthase cells, initiation of NO production by the addition of l-arginine caused an increase in the amount of NADH in the matrix, because of inhibition of the respiratory chain at the level of cytochrome c oxidase (5). For estimation of complex I activity, the rapid oxidation of matrix NADH was initiated by uncoupling the mitochondria by carbonyl cyanide p-trifluoromethoxyphenylhydrazone after inhibition of NO production by SEITU. In such a situation, accumulated NADH is rapidly oxidized by complex I so that the initial rate is largely proportional to the amount of active enzyme. Incubation in the presence of NO for 25 min resulted in inhibition of complex I activity. The presence of fumarate prevented the loss of activity of complex I after prolonged incubation with NO.
It has been suggested that fumarate decreases mitochondrial damage because the proton translocating activity of complex I is maintained even in the absence of oxygen, providing ATP synthesis and therefore preventing mitochondrial dysfunction (39, 40). The observed NADH:fumarate oxidoreductase reaction catalyzed by SMP in the presence of an excess of electron donor and acceptor is slow (0.5–1% of NADH oxidase reaction). Nevertheless, this reaction has been shown to support uncoupler-sensitive ATP synthesis, although the amount of ATP formed in that reaction was ∼8% of basal myokinase activity of the assay (32). Therefore, we hypothesize that the observed mitochondrial protection is due to the slow turnover of the enzyme in the presence of fumarate but the absence of oxygen. In such conditions complex I is maintained in the A-form during hypoxia, and this may be beneficial for the cells in certain tissues.
Our results show the occurrence of mitochondrial complex I A/D transition in cells and provide a biochemical basis for observations, by our group and others, of interactions of the enzyme with NO metabolites (19–21, 23, 41). As shown previously, partial inhibition of complex I during ischemic incubation would protect mitochondria from damage after reperfusion (42, 43). Therefore, it is possible that the reversible deactivation of complex I in the absence of oxygen may function as a protective valve and reduce the burst of respiration downstream of complex I upon tissue reoxygenation. This delayed activation of complex I would decrease the generation of ROS by the mitochondrial respiratory chain and lessen the oxidative damage after reoxygenation. The hypoxic deactivation of complex I might initially act as an intrinsic protective mechanism against overproduction of ROS and provide a safe way for returning the cellular bioenergetic function to normal after the reintroduction of oxygen. Recently it was found that treatment by mitochondrially targeted S-nitrosothiols (44) or nitrite (45) protects against ischemia-reperfusion injury, most likely via S-nitrosation of complex I. We propose that such protection is probably due to reversible S-nitrosation of the cysteine residue(s) of complex I prolonging the time required for reactivation of complex I. However, in conditions leading to increased occurrence of the D-form (inhibition of cytochrome c oxidase by NO, absence of oxygen, elevated temperature, or increased fatty acids (46) or Ca2+ concentration (12)), the susceptibility of complex I to reactive nitrogen species will change significantly (18). If the D-form accumulates in the presence of NO or in conditions of nitrosative stress, it may have severe pathophysiological consequences, depending on the duration of exposure, type of tissue, and presence of natural effectors of A/D transition and of ROS. The D-form of complex I would be persistently modified by NO adducts, arresting or delaying its activation. Because NO interacts with superoxide anion even faster than the latter can be metabolized by superoxide dismutase (47), it is tempting to speculate that superoxide production in the presence of NO (7) would result in the formation of peroxynitrite, followed by augmentation of irreversible inhibition of the D-form of complex I and of matrix enzymes like aconitase or α-ketoglutarate dehydrogenase (48, 49). Complex I modified in this way does not catalyze the physiological NADH:ubiquinone oxido-reduction, making this enzyme an early mitochondrial target for nitrosative stress and initiating a so-called “vicious cycle” of damage (50–52). The detrimental effect of such irreversible locking of complex I in the D-form could be due, in part, to the fact that the D-form of the enzyme generates superoxide radicals at a higher rate than the A-form (53). This is in agreement with a recent finding that NO-inhibited enzyme also generates more ROS (54). We now aim to establish whether the transition from A to D, which is initially protective, can become a pathophysiological trigger in certain circumstances.
Acknowledgment
We are grateful to Annie Higgs for help in the preparation of this manuscript.
Footnotes
- NO
- nitric oxide
- ROS
- reactive oxygen species
- A/D transition
- active/deactive transition
- NEM
- N-ethyl maleimide
- SEITU
- 2-ethyl-2-thiopseudourea hydrobromide
- SMP
- submitochondrial particles.
REFERENCES
- 1.Hagen T., Taylor C. T., Lam F., Moncada S. (2003) Science 302, 1975–1978 [DOI] [PubMed] [Google Scholar]
- 2.Budinger G. R., Chandel N., Shao Z. H., Li C. Q., Melmed A., Becker L. B., Schumacker P. T. (1996) Am. J. Physiol. 270, L44–L53 [DOI] [PubMed] [Google Scholar]
- 3.Budinger G. R., Duranteau J., Chandel N. S., Schumacker P. T. (1998) J. Biol. Chem. 273, 3320–3326 [DOI] [PubMed] [Google Scholar]
- 4.Chandel N. S., Budinger G. R., Schumacker P. T. (1996) J. Biol. Chem. 271, 18672–18677 [DOI] [PubMed] [Google Scholar]
- 5.Palacios-Callender M., Hollis V., Frakich N., Mateo J., Moncada S. (2007) J. Cell Sci. 120, 160–165 [DOI] [PubMed] [Google Scholar]
- 6.Chance B. (1988) FEBS Lett. 226, 343–346 [DOI] [PubMed] [Google Scholar]
- 7.Palacios-Callender M., Quintero M., Hollis V. S., Springett R. J., Moncada S. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 7630–7635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandt U. (2006) Annu. Rev. Biochem. 75, 69–92 [DOI] [PubMed] [Google Scholar]
- 9.Galkin A., Brandt U. (2005) J. Biol. Chem. 280, 30129–30135 [DOI] [PubMed] [Google Scholar]
- 10.Fato R., Bergamini C., Bortolus M., Maniero A. L., Leoni S., Ohnishi T., Lenaz G. (2009) Biochim. Biophys. Acta 1787, 384–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy M. P. (2009) Biochem. J. 417, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinogradov A. D. (1998) Biochim. Biophys. Acta 1364, 169–185 [DOI] [PubMed] [Google Scholar]
- 13.Maklashina E., Kotlyar A. B., Cecchini G. (2003) Biochim. Biophys. Acta 1606, 95–103 [DOI] [PubMed] [Google Scholar]
- 14.Maklashina E., Kotlyar A. B., Karliner J. S., Cecchini G. (2004) FEBS Lett. 556, 64–68 [DOI] [PubMed] [Google Scholar]
- 15.Gavrikova E. V., Vinogradov A. D. (1999) FEBS Lett. 455, 36–40 [DOI] [PubMed] [Google Scholar]
- 16.Gostimskaya I. S., Cecchini G., Vinogradov A. D. (2006) Biochim. Biophys. Acta 1757, 1155–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galkin A., Meyer B., Wittig I., Karas M., Schägger H., Vinogradov A., Brandt U. (2008) J. Biol. Chem. 283, 20907–20913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galkin A., Moncada S. (2007) J. Biol. Chem. 282, 37448–37453 [DOI] [PubMed] [Google Scholar]
- 19.Clementi E., Brown G. C., Feelisch M., Moncada S. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 7631–7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borutaite V., Budriunaite A., Brown G. C. (2000) Biochim. Biophys. Acta 1459, 405–412 [DOI] [PubMed] [Google Scholar]
- 21.Riobó N. A., Clementi E., Melani M., Boveris A., Cadenas E., Moncada S., Poderoso J. J. (2001) Biochem. J. 359, 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearce L. L., Kanai A. J., Epperly M. W., Peterson J. (2005) Nitric Oxide 13, 254–263 [DOI] [PubMed] [Google Scholar]
- 23.Dahm C. C., Moore K., Murphy M. P. (2006) J. Biol. Chem. 281, 10056–10065 [DOI] [PubMed] [Google Scholar]
- 24.Kotlyar A. B., Vinogradov A. D. (1990) Biochim. Biophys. Acta 1019, 151–158 [DOI] [PubMed] [Google Scholar]
- 25.Cooper C. E., Davies N. A., Psychoulis M., Canevari L., Bates T. E., Dobbie M. S., Casley C. S., Sharpe M. A. (2003) Biochim. Biophys. Acta 1607, 27–34 [DOI] [PubMed] [Google Scholar]
- 26.Wingo W. J., Emerson G. M. (1975) Anal. Chem. 47, 351–352 [Google Scholar]
- 27.Mateo J., García-Lecea M., Cadenas S., Hernández C., Moncada S. (2003) Biochem. J. 376, 537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollis V. S., Palacios-Callender M., Springett R. J., Delpy D. T., Moncada S. (2003) Biochim. Biophys. Acta 1607, 191–202 [DOI] [PubMed] [Google Scholar]
- 29.Duchen M. R., Surin A., Jacobson J. (2003) Methods Enzymol. 361, 353–389 [DOI] [PubMed] [Google Scholar]
- 30.Abramov A. Y., Duchen M. R. (2008) Biochim. Biophys. Acta 1777, 953–964 [DOI] [PubMed] [Google Scholar]
- 31.Capaldi R. A. (1982) Biochim. Biophys. Acta 694, 291–306 [DOI] [PubMed] [Google Scholar]
- 32.Sanadi D. R., Fluharty A. L. (1963) Biochemistry 2, 523–528 [DOI] [PubMed] [Google Scholar]
- 33.Gostimskaya I. S., Grivennikova V. G., Zharova T. V., Bakeeva L. E., Vinogradov A. D. (2003) Anal. Biochem. 313, 46–52 [DOI] [PubMed] [Google Scholar]
- 34.Chappell J. B. (1968) Br. Med. Bull. 24, 150–157 [DOI] [PubMed] [Google Scholar]
- 35.Kimmich G. A., Randles J., Bennett E. (1991) Am. J. Physiol. 260, C1151–C1157 [DOI] [PubMed] [Google Scholar]
- 36.Hagos Y., Burckhardt B. C., Larsen A., Mathys C., Gronow T., Bahn A., Wolff N. A., Burckhardt G., Steffgen J. (2004) Am. J. Physiol. Renal Physiol 286, F86–93 [DOI] [PubMed] [Google Scholar]
- 37.Passarella S., Atlante A., Barile M., Quagliariello E. (1987) Neurochem. Res. 12, 255–264 [DOI] [PubMed] [Google Scholar]
- 38.Passarella S., Atlante A., Valenti D., de Bari L. (2003) Mitochondrion 2, 319–343 [DOI] [PubMed] [Google Scholar]
- 39.Pisarenko O., Studneva I., Khlopkov V., Solomatina E., Ruuge E. (1988) Biochim. Biophys. Acta 934, 55–63 [DOI] [PubMed] [Google Scholar]
- 40.Weinberg J. M., Venkatachalam M. A., Roeser N. F., Nissim I. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 2826–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burwell L. S., Nadtochiy S. M., Tompkins A. J., Young S., Brookes P. S. (2006) Biochem. J. 394, 627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Q., Moghaddas S., Hoppel C. L., Lesnefsky E. J. (2006) J. Pharmacol. Exp. Ther. 319, 1405–1412 [DOI] [PubMed] [Google Scholar]
- 43.Lesnefsky E. J., He D., Moghaddas S., Hoppel C. L. (2006) FASEB J. 20, 1543–1545 [DOI] [PubMed] [Google Scholar]
- 44.Prime T. A., Blaikie F. H., Evans C., Nadtochiy S. M., James A. M., Dahm C. C., Vitturi D. A., Patel R. P., Hiley C. R., Abakumova I., Requejo R., Chouchani E. T., Hurd T. R., Garvey J. F., Taylor C. T., Brookes P. S., Smith R. A., Murphy M. P. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 10764–10769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dezfulian C., Shiva S., Alekseyenko A., Pendyal A., Beiser D. G., Munasinghe J. P., Anderson S. A., Chesley C. F., Vanden Hoek T. L., Gladwin M. T. (2009) Circulation 120, 897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loskovich M. V., Grivennikova V. G., Cecchini G., Vinogradov A. D. (2005) Biochem. J. 387, 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kissner R., Nauser T., Kurz C., Koppenol W. H. (2003) IUBMB Life 55, 567–572 [DOI] [PubMed] [Google Scholar]
- 48.Andersson U., Leighton B., Young M. E., Blomstrand E., Newsholme E. A. (1998) Biochem. Biophys. Res. Commun. 249, 512–516 [DOI] [PubMed] [Google Scholar]
- 49.Park L. C., Zhang H., Sheu K. F., Calingasan N. Y., Kristal B. S., Lindsay J. G., Gibson G. E. (1999) J. Neurochem. 72, 1948–1958 [DOI] [PubMed] [Google Scholar]
- 50.Sanz A., Caro P., Gómez J., Barja G. (2006) J. Bioenerg. Biomembr. 38, 121–127 [DOI] [PubMed] [Google Scholar]
- 51.Merlo Pich M., Raule N., Catani L., Fagioli M. E., Faenza I., Cocco L., Lenaz G. (2004) FEBS Lett. 558, 19–22 [DOI] [PubMed] [Google Scholar]
- 52.Dlasková A., Hlavatá L., Jezek P. (2008) Int. J. Biochem. Cell Biol. 40, 1792–1805 [DOI] [PubMed] [Google Scholar]
- 53.Vinogradov A. D., Grivennikova V. G. (2005) Biochemistry 70, 120–127 [DOI] [PubMed] [Google Scholar]
- 54.Borutaite V., Brown G. C. (2006) Biochim. Biophys. Acta 1757, 562–566 [DOI] [PubMed] [Google Scholar]