Abstract
Mechanistic details of mammalian metabolism in vivo and dynamic metabolic changes in intact organisms are difficult to monitor because of the lack of spatial, chemical, or temporal resolution when applying traditional analytical tools. These limitations can be addressed by sensitivity enhancement technology for fast in vivo NMR assays of enzymatic fluxes in tissues of interest. We apply this methodology to characterize organ-specific short chain fatty acid metabolism and the changes of carnitine and coenzyme A pools in ischemia reperfusion. This is achieved by assaying acetyl-CoA synthetase and acetyl-carnitine transferase catalyzed transformations in vivo. The fast and predominant flux of acetate and propionate signal into acyl-carnitine pools shows the efficient buffering of free CoA levels. Sizeable acetyl-carnitine formation from exogenous acetate is even found in liver, where acetyl-CoA synthetase and acetyl-carnitine transferase activities have been assumed sequestered in different compartments. In vivo assays of altered acetate metabolism were applied to characterize pathological changes of acetate metabolism upon ischemia. Coenzyme pools in ischemic skeletal muscle are reduced in vivo even 1 h after disturbing muscle perfusion. Impaired mitochondrial metabolism and slow restoration of free CoA are corroborated by assays employing fumarate to show persistently reduced tricarboxylic acid (TCA) cycle activity upon ischemia. In the same animal model, anaerobic metabolism of pyruvate and tissue perfusion normalize faster than mitochondrial bioenergetics.
Introduction
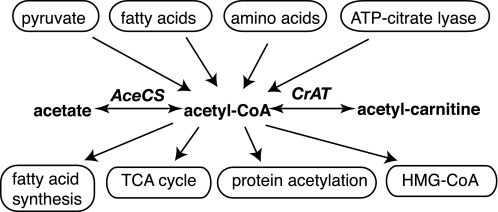
Acetyl coenzyme A (acetyl-CoA)2 is a central metabolite that connects metabolic paths such as fatty acid degradation and synthesis, cholesterol biosynthesis, glycolysis, and the TCA cycle (Fig. 1). Thus, acetyl-CoA is among the key molecules of energy and intermediary metabolism. Acetyl-CoA is generated by a number of enzymes in mammals including pyruvate dehydrogenase, β-ketothiolase, and ATP citrate-lyase (1). A less well-explored metabolic pathway forming acetyl-CoA in mammals is the acetyl-CoA synthetase (AceCS)-catalyzed catabolism of acetate (Fig. 1), which accounts for up to 10% of the energy expenditure in humans (2).
FIGURE 1.
Simplified account of the main metabolic routes involving acetyl-CoA. Acetyl-CoA formation from exogenous acetate is catalyzed by acetyl-CoA synthetase (AceCS), whereas carnitine acetyltransferase (CrAT) regenerates free CoA and forms acetyl-carnitine.
Esterification of carboxylic acids is a common means of generating activated molecules for entrance into metabolic pathways and renders the CoA-activated metabolite largely membrane impermeable. Pools of acyl-CoA esters in different cellular compartments require tight regulation because of their metabolic activity and because of the signaling function of some CoA esters (3). Under normal conditions, free CoA is regenerated by the metabolism esters, whereas large amounts of CoA esters may accumulate under stress conditions (4). Sufficient pools of free CoA are ensured under these conditions by buffering acyl-CoA:CoA ratios through transesterifications of acyl-CoA with carnitine. Carnitine also provides a shuttle for the flux of CoA-activated metabolites between intracellular compartments (5). Transesterification between CoA and carnitine esters by carnitine acyltransferases is freely reversible and thus occurs without wasting free energy on hydrolysis and resynthesis of the esters. The short fatty-acid specific carnitine acetyltransferase CrAT ensures compartmental buffering of CoA and acetyl-CoA pools in mitochondria, endoplasmic reticulum, and peroxisomes (3, 6).
The overall importance of the carnitine system is evidenced by the severe clinical manifestations of primary genetic carnitine deficiency diseases leading to myopathies, heart attacks, and death (4, 7, 8). Metabolic disturbance of the carnitine/CoA system occurs among others secondary to ischemia and hypoxia, cardiac failure, fatty acid oxidation disorders, ketosis, and diabetes (3, 4, 9). This suggests assaying metabolism of exogenous acetate or propionate by AceCS and CrAT (Fig. 1) in vivo for detection of pathological CoA and carnitine levels.
The precise physiological role of mammalian acetate metabolism has remained elusive and has attracted renewed interest, as mammalian acetate metabolism is modulated by sirtuins implicated in organism longevity (10, 11). Functional in vivo data on tissue acetate metabolism is required because of shortcomings in extrapolating in vitro and ex vivo data on pathway regulation to physiological settings. Previous use of acetate tracers in vivo has assessed oxidative tissue metabolism by NMR isotopomer analysis of steady-state 13C incorporation into metabolites and by radiolabel assays of acetate uptake and clearance (12–21). These approaches are limited in temporal and spatial resolution, in their use under non-steady-state conditions (22) or in the lack of chemical detail provided. Instantaneous measurements of metabolic pathway activity in vivo or in perfused organs have become feasible with polarization enhanced (i.e. hyperpolarized) NMR tracers (23), but have largely been limited to the use of pyruvate (24–33).
Here, we report in vivo measurements of acetate metabolism by AceCS and CrAT (Fig. 1) to gain insight (i) on the capacity of different organs in healthy metabolic states to catabolize acetate and (ii) on alterations in acetate metabolism secondary to energy imbalance and altered CoA and carnitine pools upon muscle ischemia. We find that [1-13C]acetate, [1-13C]acetyl-CoA, and [1-13C]acetyl-carnitine signals in myocardium reflect high reliance on fatty acid metabolism under normoxic conditions and the capacity to feed acetate into the TCA cycle. Unexpectedly, sizeable flux of exogenous acetate to acetyl-carnitine is also detected in liver, where cytosolic AceCS and non-cytosolic CrAT activity have been assumed spatially sequestered (3, 34). Upon skeletal muscle ischemia, strongly reduced flux through the AceCS and CrAT catalyzed reactions indicates reduced CoA and carnitine pools even 1 h after ischemia has been released. Use of hyperpolarized [1-13C]pyruvate on the other hand shows that increased lactate signal caused by anaerobic glycolysis under conditions of high [NADH]:[NAD+] and pyruvate dehydrogenase inhibition normalizes substantially faster than acetate metabolism. These findings point toward kinetic selection against acetate metabolism during recovery because of the high Km value of AceCS for CoA (17). Persistently impaired mitochondrial bioenergetics in ischemia reperfusion is supported by use of hyperpolarized [1,4-13C2]fumarate to assay inhibition of TCA cycle enzymes in vivo.
EXPERIMENTAL PROCEDURES
Materials
Sodium [1-13C]acetate, [1-13C1]acetic acid, and sodium [1-13C]propionate were obtained from Cambridge Isotope Laboratories. GE Healthcare provided [1-13C]pyruvic acid, and trityl radical OX063 (Tris(8-carboxy-2,2,6,6-(tetra(hydroxyethyl)-benzo-[1,2-4,5′]-bis-(1,3)-dithiole-4-yl)- methyl sodium salt) and AH111501 (Tris(8-carboxy-2,2,6,6 (tetra(methoxyethyl) benzo-[1,2-4,5′]bis-(1,3)dithiole-4- yl)methyl sodium salt). The gadolinium complex 3-Gd (1,3,5-tris-(N-(DO3A-acetamido)-N-methyl-4-amino-2-methylphenyl)-[1,3,5]tria-zinane-2,4,6-trione) was synthesized in house. All other chemicals were obtained from Sigma Aldrich. Inbred laboratory mice of strain C57BL/6 were obtained from Charles River Laboratories International and given free access to standard mice chow and water.
Preparation and Hyperpolarization of 13C-labeled Substances
Acetate was prepared for hyperpolarization as previously described (35). A detailed optimization protocol is given in the supplemental Methods. For propionate preparation, the same protocol was used as for acetate. Pyruvic acid was prepared by adding 24.8 mg trityl radical OX063 and 1.1 mg of Gd complex to 1035 mg [1-13C]pyruvic acid, which results in a [1-13C]pyruvic acid preparation containing 15 mm OX063 and 0.5 mm Gd complex. [1,4-13C2]Fumaric acid was prepared by dissolving 40 mg (0.34 mmol) in 72 mg of a DMSO solution containing 19 mm AH111501 and 0.8 mm Gd complex.
DNP preparations were hyperpolarized in a polarizer as described by Ardenkjær-Larsen et al. (23). The hyperpolarized sample was subsequently dissolved in a phosphate buffer (40 mm, osmolality adjusted with NaCl to 210 mOsm) to provide a 50 mm solution of hyperpolarized substance at at pH of 7.1 ± 0.1, an osmolality of 274 ± 8 mOsm and a polarization of 17 ± 1% for acetate, 22 ± 1% for propionate, 23 ± 2% for pyruvate and 30 ± 2% for fumarate during the administration. In the dissolution buffer of pyruvic acid and fumaric acid equivalent amounts of NaOH were added to neutralize the acids. A dose of 0.44 mmol/kg was infused during 6 s resulting in an approximate blood concentration of 5 mm tracer.
13C MRS in Vivo
A catheter was placed in the tail vein, and animals were positioned in a 2.35 T Bruker Biospec Avance II MR scanner. Anesthesia was maintained in the MR system by a gas mixture of oxygen and nitrous oxide with 2% isoflurane. Gas flow was 200 ml/min O2 and 200 ml/min N2O. Body temperature of the animals was controlled to vary within 37.0 ± 0.2 °C. ECG, breathing, and rectal temperature were all monitored with a S. A. Instrument 1025 MR compatible animal monitoring system. Injection of the substances did not lead to abnormal effects on the physiological parameters that were monitored.
MR imaging was performed using a dual-tuned 1H-13C birdcage resonator with inner diameter of 72 mm and a standard proton MR imaging sequence to retrieve anatomic information. 13C spectra of myocardial and liver metabolism were acquired with a 12-mm 13C surface coil placed on the sternum or abdomen of the mouse, respectively. Spectra in myocardium and liver were recorded either with a 90° flip angle 15 s after the start of the injection or with a time series of 30 one-dimensional spectra obtained with 30° flip angles and a repetition time of 3 s. For the ischemic model, an 8-mm 13C surface coil was placed around the hind leg of the mouse, and a time series of 30 one-dimensional spectra was obtained with 25° flip angles and a repetition time of 4 s for acetate and pyruvate injections. Spectra of fumarate injections were recorded with a time series of 60 one-dimensional spectra obtained with 10° flip angles and a repetition time of 2 s.
Ischemia
Hindlimb ischemia was introduced by occlusion with a string for 30 min as described by in 't Zandt et al. (36). Occlusion was performed at a constant force for 30 min and was released 5 min before injection of 13C-labeled substrate. After data acquisition, the mouse was kept in the scanner for further 55 min before a second injection of 13C-labeled substrate. Data were analyzed in Jmrui (37). Eight spectra were summed starting from the first spectrum where the acetate peak appears. Sum spectra were then analyzed in batch. The same methodology was applied for pyruvate metabolism. Fumarase activities were indexed by signal area ratios of malate and fumarate 22 s after fumarate injection.
13C DNP Enzymatic Assays
5-mm NMR tubes were prepared with 4.7 units/ml acetyl-CoA synthetase, 125 units/ml carnitine acetyltransferase, 6 mm coenzyme A, 10 mm carnitine, 10 mm ATP, 75 mm Tris buffer of pH 7.6, 7.5 mm MgCl2, and 2 mm dithiothreitol for enzymatic in vitro DNP assays (35). The assay mixture was kept at 37 °C in a water bath for 15 min prior to experiments. Hyperpolarized short chain fatty acid ([1-13C]acetate or [1-13C]propionate) was added to a final concentration of 2 mm. Real-time reaction kinetics was followed by acquiring low flip angle (10°) 13C NMR spectra every 2 s. In vitro assays were perfomed on a 400 MHz Varian Inova spectrometer.
RESULTS
Fast Activation of Acetate to Esters
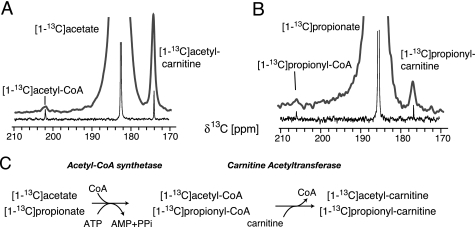
In vivo NMR spectra of acetate metabolism were recorded in mouse heart, liver, and skeletal muscle supplied with hyperpolarized [1-13C]acetate. Hyperpolarized in vivo NMR provides sufficient sensitivity to detect conversion of the marker to different molecular species. In the case of acetate, two metabolites were detected at 202.1 ppm and 174 ppm in the heart (Fig. 2A) in addition to the dominating substrate signal (at 182.6 ppm). Signal assignments to acetyl-CoA (202.1 ppm) and acetyl-carnitine (174 ppm) were supported in vitro in a reaction with purified AceCS and CrAT, which yields acetyl-CoA and acetyl-carnitine NMR signals at the spectral positions detected in vivo (Fig. 2, A and C).
FIGURE 2.
In vivo and in vitro metabolism of hyperpolarized [1-13C]acetate and [1-13C]propionate (A and B). In vivo NMR spectra (bold gray lines) of myocardium are recorded with a 90° flip angle pulse after injecting 175-μl hyperpolarized substrate. Spectra of reactions conducted with 2 mm hyperpolarized substrate and isolated AceCS and CrAT in vitro according to the scheme in C are shown as thin black lines. Identity of in vivo metabolite signals with acyl-CoA and acyl-carnitine adducts is validated by spectral frequencies in vivo for both substrates.
Metabolism of Exogenous Propionate
AceCS and CrAT activity in vivo and in vitro were additionally assessed by the use of hyperpolarized [1-13C]propionate, which also is a substrate for AceCS (38) and CrAT (5) catalyzed reactions. As in the case of hyperpolarized [1-13C]acetate, two reaction products can be detected in vivo (206.1 and 176.9 ppm, Fig. 2B), which can be ascribed to CoA and carnitine esters of propionate in an in vitro reaction with purified AceCS and CrAT (Fig. 2, B and C).
Propionate is found to be a substrate for short chain fatty acid metabolism in vivo in agreement with reports of similar activities and Michaelis constants of CrAT toward acetyl-CoA and propionyl-CoA in vitro (39, 40). Lower substrate preference of AceCS for propionate is reflected by lower intensities of [1-13C]propionyl-CoA and [1-13C]propionyl-carnitine signals as compared with respective acetyl esters in DNP-NMR assays in vivo (Fig. 2). This finding is in agreement with in vitro data indicating that propionate is a less preferred substrate of AceCS than acetate (38).
Tissue Differences of Acetate Activation
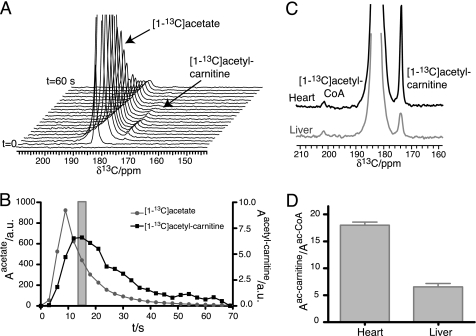
Acetate was accordingly employed as the in vivo biomarker of choice for assaying short chain fatty acid metabolism in different organs and in ischemic tissue. A time series showing myocardial metabolism of hyperpolarized [1-13C]acetate in vivo is given in Fig. 3. Signal build-up or decay because of chemical transformation is convoluted with signal decay due to relaxation of the hyperpolarized spin ensemble. Maximum [1-13C]acetyl-carnitine signal in the dynamic series was used to define the time after infusion, where metabolism of hyperpolarized [1-13C]acetate is most sensitively detected. Metabolic differences of liver and myocardium in living animals can be assessed from the obtained hyperpolarized NMR spectra, which show a ∼3-fold higher acetyl-carnitine:acetyl-CoA signal ratio in myocardium than in liver. Notwithstanding, both acetyl-carnitine and acetyl-CoA signal can be detected in liver (Fig. 3, C and D).
FIGURE 3.
Signal intensities of acetate and acetyl-carnitine in myocardium. A time series of spectra was recorded by applying a 30° flip angle pulse every 3 s (A). Signal intensities reflect chemical transformation and relaxation of the hyperpolarized spin ensemble. Acetyl-carnitine and acetyl-CoA signals were measured in heart and liver with single 90° flip angle pulses after 15 s, where maximum acetyl-carnitine signal is observed in the time series (B). C and D, different ratios of acetyl-carnitine and acetyl-CoA signal directly indicate different acetate metabolism in liver and heart (p < 0.01; n = 6). Data values are displayed as mean ± S.D. throughout.
Acetate Metabolism in Ischemia-Reperfusion
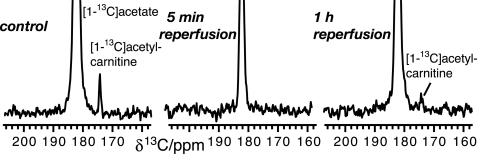
Pathology-related changes to the cellular metabolism were assessed in skeletal muscle by the use of hyperpolarized [1-13C]acetate. A well-characterized model of skeletal muscle ischemia was employed, where ischemia is induced by occluding blood flow in hindlimb skeletal muscle. This model has previously been characterized by in vivo 31P NMR, which shows recovery of phosphocreatine to ∼90% of preischemic values within 16 min after occluding blood flow for 30 min, while ATP signal is only weakly reduced during ischemia because of the regeneration of ATP from phosphocreatine (36). Metabolic pathway activities were assayed in this model by [13C]DNP-NMR experiments performed before occlusion of blood flow for 30 min (control), after 5 min of reperfusion and after 1 h of reperfusion. Resultant spectra show a reduction of acetyl-carnitine formation from exogenous acetate following ischemia. Most notably, the effect was pronounced both after 5 min and after 1 h of reperfusion (Fig. 4).
FIGURE 4.
In vivo13C NMR spectra of acetate metabolism in skeletal muscle 5 min and 1 h after ischemia, as compared with control. Spectra were recorded as a time series (Fig. 3) and 8 traces recorded 4–32 s after the injection were summed. Acetate metabolism does not recover to preischemic values within 1 h.
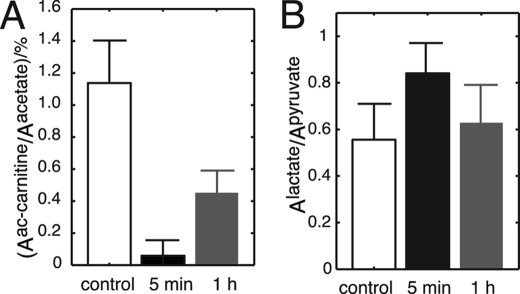
Statistical analysis of hyperpolarized [1-13C]acetate metabolism in mice shows that the ischemic group (n = 3) is significantly separate from the control group (Fig. 5A). Potential effects of altered tissue perfusion after ischemia were accounted for by relating [1-13C]acetyl-carnitine signal areas to [1-13C]acetate signal areas inside the muscle (Fig. 5A). This ratio is decreased from 1.14 ± 0.26% (n = 3) in the control group to 0.06 ± 0.10% (n = 3) in the second group with 5 min of reperfusion. After 1 h of reperfusion, the ratio is 0.42 ± 0.16% (n = 3) (Fig. 5A). Thus, the formation of acetyl-carnitine from exogenous [1-13C]acetate is reduced more than 10-fold right after the ischemic period (p < 0.01) and is still significantly decreased (p < 0.02) after 1 h of reperfusion.
FIGURE 5.
Formation of acetyl-carnitine is significantly reduced in ischemic mice, and disturbances of mitochondrial energy metabolism are easily detectable in vivo with hyperpolarized acetate for 1 h. Increased lactate fermentation is detectable in vivo with hyperpolarized [1-13C]pyruvate but is less persistent.
Different Recovery of Different Pathways
Persistent metabolic changes in this ischemic model were subsequently assayed by alternative hyperpolarized substrates of mitochondrial and cytosolic reactions. Use of hyperpolarized [1,4-13C2]fumarate was employed to assess TCA cycle activity with a substrate that is not reliant on co-substrates for conversion to its product malate. Formation of [1,4-13C2]malate from [1,4-13C2]fumarate is significantly increased from 0.74 ± 0.19% to 2.85 ± 0.07% (p < 0.05, n = 2) after 5 min and to 7.50 ± 0.42% (p < 0.05, n = 2) after 60 min of reperfusion. The increased formation of [1,4-13C2]malate shows reduced rates of malate clearance relative to its synthesis, thus suggesting a reduced progression of subsequent reactions in the TCA cycle and overall reduced TCA cycle flux.
Hyperpolarized [1-13C]pyruvate as substrate for the cytosolic lactate dehydrogenase reaction on the other hand shows increased conversion to lactate upon ischemia. This is expected from increasing NADH:NAD+ ratios under hypoxic conditions (41). Pyruvate reduction to lactate is, however, significantly increased (p < 0.01) only shortly after ischemia has been released with an increase of the lactate:pyruvate ratio from 0.56 ± 0.15 (control; n = 5) to 0.84 ± 0.12 (n = 5), whereas deviations from control levels are insignificant (p > 0.2) after 1 h of reperfusion (lactate:pyruvate ratio 0.63 ± 0.15 (n = 5)) (Fig. 5B). This normalization in cytosolic pyruvate usage after 1 h is in contrast to abnormally low acetate metabolism by AceCS and CrAT as well as TCA cycle activity in mitochondria.
DISCUSSION
Acetate is a well-suited substrate for in vivo spectroscopy because of its fast cellular resorption and simple metabolism. Acetate supposedly is lipophilic enough to diffuse freely across membranes that separate subcellular compartments and thus sensitively reports on fluxes through AceCS- and CrAT-catalyzed reactions, even when this activity is sequestered in organelles.
Organ specific pathway activity of acetate metabolism as obtained in this study shows a larger flux of exogenous acetate to acetyl-carnitine in myocardium than in the liver. In the heart, the predominant AceCS2 isozyme activity implies uptake of exogenous acetate mostly into the mitochondrial acetyl-CoA pool and subsequent free equilibration with carnitine, thus allowing an increased flux of marker signal into the acetyl-carnitine pool. This is in principal agreement with the notion that fatty acids are major substrates of energy metabolism in myocardium (42) and that the mitochondrial isoforms AceCS2 predominates in the heart (1). In myocardium, skeletal muscle, and other tissues, AceCS2 supposedly functions to feed acetate into the TCA cycle as acetyl-CoA (43).
In liver, less acetyl-CoA flux to acetyl-carnitine is found than in the heart (Fig. 3). This reflects that the prevalence of different AceCS isoforms in both tissues results in organ-specific differences in acetate metabolism. In the liver, cytosolic AceCS1 provides acetyl-CoA for lipid synthesis. The putative absence both of mitochondrial AceCS2 and cytosolic CrAT supposedly avoids the use of acetate for acetyl-carnitine formation, mitochondrial import, and degradation in competition to lipogenesis (3, 34). The detection of acetyl-carnitine synthesis by in vivo DNP-NMR is thus surprising and indicates that fatty acid synthesis and oxidative metabolism compete for acetate in liver. The finding of acetyl-carnitine synthesis indicates either (i) the presence of non-negligible acetyl-CoA synthetase activity in liver peroxisomes, endoplasmic reticulum or mitochondria, (ii) presence of non-negligible CrAT activity in the cytosol, or (iii) specific uptake mechanisms of acetyl-CoA into cellular organelles (3). The possibility that acetyl-carnitine signal in liver results from synthesis outside the liver and subsequent resorption from plasma seems unlikely, as no significant increase in plasma levels of [1-13C]acetyl-carnitine was observed following injection of hyperpolarized [1-13C]acetate.
The capacity to detect tissue differences of hyperpolarized [1-13C]acetate metabolism in vivo allows the use of hyperpolarized [1-13C]acetate as noninvasive disease biomarker. Reduced acetate metabolism as observed by 13C DNP-NMR in vivo up to 1 h after ischemia suggests that co-substrate pools (ATP, CoA, carnitine, see Fig. 2C) or enzyme activities for acetyl-carnitine synthesis from exogenous acetate are reduced following ischemia and normalize only slowly. Earlier studies suggest that ischemic muscle exhibits a cellular build-up of free fatty acids and their activated acyl-CoA and acyl-carnitine esters (8, 42, 44, 45). Free carnitine and CoA pools on the other hand decrease and get limiting to acetate metabolism, fatty acid oxidation, and pyruvate decarboxylation under conditions of low TCA cycle flux in ischemia, acute myocardial infarction and cardiac failure (9, 17, 46–49).
Reduced pools of free carnitine and increased pools of acetyl-carnitine have previously been observed ex vivo after 30 min of recovery from anaerobic metabolism (48). ATP on the other hand retains preischemic levels because of buffering by phosphocreatine in the current ischemic model (36), while limited literature data on AceCS and CrAT expression give no indication of reduced enzyme levels under hypoxic conditions (50–52). Overall, this suggests that reduced, slowly recovering pools of free CoA and carnitine rather than ATP or enzyme levels are assayed by reduced acetate activation in vivo for more than 1 h after ischemia.
We find that acetate provides unique and sensitive information on cellular energy metabolism as compared with markers of glucose metabolism, because acetate metabolism is altered for a longer period of time after ischemia than e.g. pyruvate reduction (Fig. 5), which is affected by altered NADH and acetyl-CoA pools. The finding that acetate metabolism is more persistently affected by altered cosubstrate pools than pyruvate metabolism seems plausible in light of the high Michaelis-Menten constant Km (0.4 × 10−3 m) of AceCS to CoA in cardiac muscle as compared with Km values for CoA of 0.02 × 10−3 m for pyruvate dehydrogenase, 0.05 × 10−3 m for thiolase and 0.1 × 10−6 m for α-ketoglutarate dehydrogenase (17). This implies that CoA depletion following ischemia or high work loads will turn off AceCS activity faster than the other aforementioned CoA-dependent enzymes. Accordingly, AceCS activity is turned on later during recovery from CoA depletion than e.g. pyruvate dehydrogenase, thus suggesting the use of hyperpolarized [1-13C]acetate as a persistent marker of altered CoA and carnitine pools.
Free carnitine and CoA are indispensable for cellular energy metabolism and depend on the regeneration of CoA by the TCA cycle. We detect long-term alteration of TCA cycle flux upon ischemia-reperfusion in form of reduced clearance of malate by the cycle. Reduced flux of hyperpolarized [1-13C]pyruvate and [2-13C]pyruvate into the TCA cycle has been described in ischemic myocardium in vivo (25) and ex vivo (24, 33). The recovery of pyruvate dehydrogenase flux into the TCA cycle during reperfusion has however remained unclear, presumably because of the absence of long-chain fatty acids and ketones in ex vivo perfusion.
Reduced in vivo clearance of [1,4-13C2]malate by the TCA cycle in ischemia-reperfusion in the current study directly shows that reaction fluxes in the cycle are reduced relative to the formation of malate by fumarase. Inhibition of TCA cycle enzymes α-ketoglutarate dehydrogenase, succinate dehydrogenase, and aconitase accordingly has been associated with the presence of reactive oxygen species when tissue is reoxygenated upon ischemia (53, 54), and enzymatic fluxes under oxidative stress await further studies in vivo. The submicromolar Km of α-ketoglutarate dehydrogenase toward CoA suggests that TCA cycle activity in recovering skeletal muscle is not limited by CoA. Acetyl-CoA is unlikely to be limiting under conditions of increased beta oxidation following ischemia (55), especially as the Km of citrate synthase toward acetyl-CoA (0.2 mm) indicates saturation (56, 57). Stable ATP levels (36) and fast recovery of reduction equivalents as judged from recovering LDH flux in the current ischemia-reperfusion model thus indicate that altered fluxes in the TCA cycle result from inhibition of TCA cycle enzymes rather than limiting acetyl-CoA or cosubstrate pools. Overall, instantaneous measurements of acetate metabolism, TCA cycle, and anaerobic glycolysis in vivo demonstrate long lasting disturbance of mitochondrial bioenergetics following tissue hypoxia.
This work was supported by a commercial research grant from GE HealthCare.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Methods” and Fig. S1.
- acetyl-CoA
- acetyl coenzyme A
- AceCS
- acetyl-CoA synthetase
- CrAT
- carnitine acetyltransferase
- DNP
- dynamic nuclear polarization
- ECG
- electrocardiogram
- MR
- magnetic resonance
- MRS
- magnetic resonance spectroscopy
- NMR
- nuclear magnetic resonance
- OX063
- Tris(8-carboxy-2,2,6,6-(tetra(hydroxyethyl)-benzo-[1,2-4,5′]-bis-(1,3)-dithiole-4-yl)-methyl sodium salt
- TCA cycle
- tricarboxylic acid cycle
- GFP
- green fluorescent protein.
REFERENCES
- 1.Yamamoto J., Ikeda Y., Iguchi H., Fujino T., Tanaka T., Asaba H., Iwasaki S., Ioka R. X., Kaneko I. W., Magoori K., Takahashi S., Mori T., Sakaue H., Kodama T., Yanagisawa M., Yamamoto T. T., Ito S., Sakai J. (2004) J. Biol. Chem. 279, 16954–16962 [DOI] [PubMed] [Google Scholar]
- 2.Pouteau E., Piloquet H., Maugeais P., Champ M., Dumon H., Nguyen P., Krempf M. (1996) Am. J. Physiol. 271, E58–E64 [DOI] [PubMed] [Google Scholar]
- 3.Ramsay R. R., Zammit V. A. (2004) Mol. Aspects Med. 25, 475–493 [DOI] [PubMed] [Google Scholar]
- 4.Rebouche C. J., Paulson D. J. (1986) Annu. Rev. Nutr. 6, 41–66 [DOI] [PubMed] [Google Scholar]
- 5.Bieber L. L. (1988) Annu. Rev. Biochem. 57, 261–283 [DOI] [PubMed] [Google Scholar]
- 6.Cordente A. G., López-Viñas E., Vázquez M. I., Swiegers J. H., Pretorius I. S., Gómez-Puertas P., Hegardt F. G., Asins G., Serra D. (2004) J. Biol. Chem. 279, 33899–33908 [DOI] [PubMed] [Google Scholar]
- 7.Engel A. G., Angelini C. (1973) Science 179, 899–902 [DOI] [PubMed] [Google Scholar]
- 8.Bahl J. J., Bressler R. (1987) Annu. Rev. Pharmacol. Toxicol. 27, 257–277 [DOI] [PubMed] [Google Scholar]
- 9.Stephens F. B., Constantin-Teodosiu D., Greenhaff P. L. (2007) J. Physiol. 581, 431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallows W. C., Lee S., Denu J. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 10230–10235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.North B. J., Sinclair D. A. (2007) Trends Biochem. Sci 32, 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyama N., Akino H., Kanamaru H., Suzuki Y., Muramoto S., Yonekura Y., Sadato N., Yamamoto K., Okada K. (2002) J. Nucl. Med. 43, 181–186 [PubMed] [Google Scholar]
- 13.Bertocci L. A., Jones J. G., Malloy C. R., Victor R. G., Thomas G. D. (1997) J Appl. Physiol. 83, 32–39 [DOI] [PubMed] [Google Scholar]
- 14.Gropler R. J., Geltman E. M., Sampathkumaran K., Pérez J. E., Schechtman K. B., Conversano A., Sobel B. E., Bergmann S. R., Siegel B. A. (1993) J Am. Coll. Cardiol. 22, 1587–1597 [DOI] [PubMed] [Google Scholar]
- 15.Malloy C. R., Sherry A. D., Jeffrey F. M. (1990) Am. J. Physiol. 259, H987–H995 [DOI] [PubMed] [Google Scholar]
- 16.Brown M., Marshall D. R., Sobel B. E., Bergmann S. R. (1987) Circulation 76, 687–696 [DOI] [PubMed] [Google Scholar]
- 17.Robitaille P. M., Rath D. P., Abduljalil A. M., O'Donnell J. M., Jiang Z., Zhang H., Hamlin R. L. (1993) J. Biol. Chem. 268, 26296–26301 [PubMed] [Google Scholar]
- 18.Szczepaniak L., Babcock E. E., Malloy C. R., Sherry A. D. (1996) Magn. Reson. Med. 36, 451–457 [DOI] [PubMed] [Google Scholar]
- 19.Pouteau E., Dumon H., Biourge V., Krempf M., Nguyen P. (1998) J. Nutr. 128, 2651S–2653S [DOI] [PubMed] [Google Scholar]
- 20.Bertocci L. A., Lujan B. F. (1999) J Appl. Physiol. 86, 2077–2089 [DOI] [PubMed] [Google Scholar]
- 21.Cerdan S., Künnecke B., Seelig J. (1990) J. Biol. Chem. 265, 12916–12926 [PubMed] [Google Scholar]
- 22.Malloy C. R., Thompson J. R., Jeffrey F. M., Sherry A. D. (1990) Biochemistry 29, 6756–6761 [DOI] [PubMed] [Google Scholar]
- 23.Ardenkjær-Larsen J. H., Fridlund B., Gram A., Hansson G., Hansson L., Lerche M. H., Servin R., Thaning M., Golman K. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10158–10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merritt M. E., Harrison C., Storey C., Sherry A. D., Malloy C. R. (2008) Magn. Reson. Med. 60, 1029–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golman K., Petersson J. S., Magnusson P., Johansson E., Akeson P., Chai C. M., Hansson G., Månsson S. (2008) Magn. Reson. Med. 59, 1005–1013 [DOI] [PubMed] [Google Scholar]
- 26.Chen A. P., Kurhanewicz J., Bok R., Xu D., Joun D., Zhang V., Nelson S. J., Hurd R. E., Vigneron D. B. (2008) Magn. Reson. Imaging 26, 721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albers M. J., Bok R., Chen A. P., Cunningham C. H., Zierhut M. L., Zhang V. Y., Kohler S. J., Tropp J., Hurd R. E., Yen Y. F., Nelson S. J., Vigneron D. B., Kurhanewicz J. (2008) Cancer Res. 68, 8607–8615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen A. P., Albers M. J., Cunningham C. H., Kohler S. J., Yen Y. F., Hurd R. E., Tropp J., Bok R., Pauly J. M., Nelson S. J., Kurhanewicz J., Vigneron D. B. (2007) Magn. Reson. Med. 58, 1099–1106 [DOI] [PubMed] [Google Scholar]
- 29.Golman K., Zandt R. I., Lerche M., Pehrson R., Ardenkjaer-Larsen J. H. (2006) Cancer Res. 66, 10855–10860 [DOI] [PubMed] [Google Scholar]
- 30.Golman K., in 't Zandt R., Thaning M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11270–11275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Day S. E., Kettunen M. I., Gallagher F. A., Hu D. E., Lerche M., Wolber J., Golman K., Ardenkjaer-Larsen J. H., Brindle K. M. (2007) Nat. Med. 13, 1382–1387 [DOI] [PubMed] [Google Scholar]
- 32.Schroeder M. A., Cochlin L. E., Heather L. C., Clarke K., Radda G. K., Tyler D. J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12051–12056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroeder M. A., Atherton H. J., Ball D. R., Cole M. A., Heather L. C., Griffin J. L., Clarke K., Radda G. K., Tyler D. J. (2009) Faseb J 23, 2529–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zammit V. A. (1999) Prog. Lipid Res. 38, 199–224 [DOI] [PubMed] [Google Scholar]
- 35.Jensen P. R., Meier S., Ardenkjaer-Larsen J. H., Duus J. O., Karlsson M., Lerche M. H. (2009) Chem. Commun. 34, 5168–5170 [DOI] [PubMed] [Google Scholar]
- 36.in 't Zandt H. J., Oerlemans F., Wieringa B., Heerschap A. (1999) NMR Biomed. 12, 327–334 [DOI] [PubMed] [Google Scholar]
- 37.Naressi A., Couturier C., Devos J. M., Janssen M., Mangeat C., de Beer R., Graveron-Demilly D. (2001) Magma 12, 141–152 [DOI] [PubMed] [Google Scholar]
- 38.Fujino T., Kondo J., Ishikawa M., Morikawa K., Yamamoto T. T. (2001) J. Biol. Chem. 276, 11420–11426 [DOI] [PubMed] [Google Scholar]
- 39.Bloisi W., Colombo I., Garavaglia B., Giardini R., Finocchiaro G., Didonato S. (1990) Eur. J. Biochem. 189, 539–546 [DOI] [PubMed] [Google Scholar]
- 40.Farrell S. O., Fiol C. J., Reddy J. K., Bieber L. L. (1984) J. Biol. Chem. 259, 13089–13095 [PubMed] [Google Scholar]
- 41.Kobayashi K., Neely J. R. (1983) J Mol. Cell Cardiol. 15, 359–367 [DOI] [PubMed] [Google Scholar]
- 42.Whitmer J. T., Idell-Wenger J. A., Rovetto M. J., Neely J. R. (1978) J. Biol. Chem. 253, 4305–4309 [PubMed] [Google Scholar]
- 43.Wolfe A. J. (2005) Microbiol. Mol. Biol. Rev. 69, 12–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Idell-Wenger J. A., Grotyohann L. W., Neely J. R. (1978) J. Biol. Chem. 253, 4310–4318 [PubMed] [Google Scholar]
- 45.Calvani M., Reda E., Arrigoni-Martelli E. (2000) Basic Res. Cardiol. 95, 75–83 [DOI] [PubMed] [Google Scholar]
- 46.Iliceto S., Scrutinio D., Bruzzi P., D'Ambrosio G., Boni L., Di Biase M., Biasco G., Hugenholtz P. G., Rizzon P. (1995) J Am. Coll. Cardiol. 26, 380–387 [DOI] [PubMed] [Google Scholar]
- 47.Shug A. L., Thomsen J. H., Folts J. D., Bittar N., Klein M. I., Koke J. R., Huth P. J. (1978) Arch Biochem. Biophys. 187, 25–33 [DOI] [PubMed] [Google Scholar]
- 48.Minkler P. E., Brass E. P., Hiatt W. R., Ingalls S. T., Hoppel C. L. (1995) Anal. Biochem. 231, 315–322 [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi A., Fujisawa S. (1994) J. Mol. Cell Cardiol. 26, 499–508 [DOI] [PubMed] [Google Scholar]
- 50.Aharinejad S., Schäfer R., Hofbauer R., Abraham D., Blumer R., Miksovsky A., Traxler H., Pullirsch D., Alexandrowicz R., Taghavi S., Kocher A., Laufer G. (2001) Transplantation 72, 1043–1049 [DOI] [PubMed] [Google Scholar]
- 51.Yoshii Y., Furukawa T., Yoshii H., Mori T., Kiyono Y., Waki A., Kobayashi M., Tsujikawa T., Kudo T., Okazawa H., Yonekura Y., Fujibayashi Y. (2009) Cancer Sci. 100, 821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hülsmann W. C., Dubelaar M. L. (1992) Mol. Cell Biochem. 116, 125–129 [DOI] [PubMed] [Google Scholar]
- 53.Nulton-Persson A. C., Szweda L. I. (2001) J. Biol. Chem. 276, 23357–23361 [DOI] [PubMed] [Google Scholar]
- 54.Hoerter J., Gonzalez-Barroso M. D., Couplan E., Mateo P., Gelly C., Cassard-Doulcier A. M., Diolez P., Bouillaud F. (2004) Circulation 110, 528–533 [DOI] [PubMed] [Google Scholar]
- 55.Kudo N., Barr A. J., Barr R. L., Desai S., Lopaschuk G. D. (1995) J. Biol. Chem. 270, 17513–17520 [DOI] [PubMed] [Google Scholar]
- 56.Shepherd D., Garland P. B. (1969) Biochem. J. 114, 597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leonardi R., Zhang Y. M., Rock C. O., Jackowski S. (2005) Prog. Lipid Res. 44, 125–153 [DOI] [PubMed] [Google Scholar]