Abstract
Nitric oxide exerts a plethora of biological effects via protein S-nitrosylation, a redox-based reaction that converts a protein Cys thiol to a S-nitrosothiol. However, although the regulation of protein S-nitrosylation has been the subject of extensive study, much less is known about the systems governing protein denitrosylation. Most recently, thioredoxin/thioredoxin reductases were shown to mediate both basal and stimulus-coupled protein denitrosylation. We now demonstrate that protein denitrosylation by thioredoxin is regulated dynamically by thioredoxin-interacting protein (Txnip), a thioredoxin inhibitor. Endogenously synthesized nitric oxide represses Txnip, thereby facilitating thioredoxin-mediated denitrosylation. Autoregulation of denitrosylation thus allows cells to survive nitrosative stress. Our findings reveal that denitrosylation of proteins is dynamically regulated, establish a physiological role for thioredoxin in protection from nitrosative stress, and suggest new approaches to manipulate cellular S-nitrosylation.
Introduction
It has become increasingly appreciated that protein S-nitrosylation, the covalent attachment of a nitroso group to a cysteine thiol side chain, is a principle mechanism by which nitric oxide modulates numerous cellular functions and phenotypes (1, 2). These include G-protein-coupled receptor signaling (3–5), death receptor-mediated apoptosis (6–9), vesicular trafficking (10–12), stimulation of prostaglandin synthesis (13–15), hypoxia-dependent control of blood flow (16–18), and the unfolded protein response (19). In addition, aberrant S-nitrosylation is implicated in pathologies such as tumor initiation and growth (20, 21), neurodegeneration (19, 22, 23), and pulmonary hypertension (24).
The three isoforms of nitric-oxide synthase (neuronal NOS/NOS1, iNOS/NOS2,2 and endothelial NOS/NOS3) are well established mediators of S-nitrosylation, and numerous studies have demonstrated that their localization is critical for S-nitrosylation of target proteins. For example, binding of iNOS to COX2 is required for S-nitrosylation and activation of prostaglandin synthesis (13), whereas the subcellular localization of endothelial NOS is a major determinant of S-nitrosylation-mediated protein trafficking (25). Moreover, although it is generally assumed that S-nitrosylation reactions are nonenzymatic, there is precedence for hemoglobin-dependent S-nitrosylation of the anion exchanger protein AE1 (26) and ceruloplasmin-dependent S-nitrosylation of glypican-1 (27) and GSH (28), consistent with a recent report that metalloproteins may play a general role in SNO synthesis (29).
By contrast with progress in elucidating the enzymatic determinants of S-nitrosylation, the intracellular mediators of denitrosylation and their possible contributions to overall S- nitrosylation status have only recently gained attention. By analogy to phosphorylation (where kinases and phosphatases together regulate phosphorylation), the steady-state level of S-nitrosylation is the net difference between nitrosylation and denitrosylation. The regulation of denitrosylation systems may therefore play a pivotal role in determining the levels of protein S-nitrosylation (protein-SNO) (30).
Two major pathways for enzymatic denitrosylation have recently been described (31). The first involves S-nitrosoglutathione (GSNO) reductase (GSNOR) (32, 33), an NADH-dependent oxidoreductase that specifically breaks down GSNO (protein-SNOs are not direct substrates). Because GSH/GSNO and protein-SH/protein-SNOs are in equilibrium via rapid transnitrosation reactions (34), GSNOR lowers the levels of protein S-nitrosothiols (35, 36) reactive toward GSH, including those that are S-nitrosylated under conditions of SNO signaling (5, 36) and nitrosative stress (35).
It has recently been demonstrated that the thioredoxins (Trx) constitute a second class of broad spectrum denitrosylase (6, 37, 38). Maintained Trx activity requires thioredoxin reductase (TrxR) (thioredoxin system), an NADPH-dependent selenoprotein. Benhar et al. (6) showed that the mitochondrial isoform of thioredoxin-2 (Trx2) mediates stimulus-coupled denitrosylation of caspase-3, thereby facilitating death signaling by Fas (7, 9). Also demonstrated was a role for cytosolic Trx1 in mediating constitutive denitrosylation, thus maintaining low levels of S-nitrosylation in response to exogenous and endogenous NO. The Trx system has therefore been shown to control the level of S-nitrosylation in the context of SNO-based signaling, but the role of Trx1-mediated denitrosylation in resisting nitrosative stress remains unknown.
Thioredoxin-interacting protein (Txnip, VDUP1, TBP-2) was originally described as a vitamin D3-up-regulated protein (39). Subsequent studies by Yodoi and co-workers (40) identified an interaction between Txnip and Trx1 that inhibits Trx1 in vivo (40–43). Schulze et al. (44) recently reported that exogenous GSNO attenuates the expression of Txnip, thereby activating Trx. We therefore considered the possibility that Txnip might serve as a locus of control over S-nitrosylation. Here we report that Trx1-mediated denitrosylation of proteins is augmented by endogenously derived nitric oxide via repression of Txnip. This feedback-regulated system confers protection against iNOS-mediated cell death.
EXPERIMENTAL PROCEDURES
Materials and Reagents
All materials were from Sigma unless otherwise indicated. [3H]Thymidine was from Cambridge Isotopes. 1400W, DETA-NO, and ODQ were from Cayman Chemical. Sources of antibodies were: monoclonal α-Txnip/Vdup1 (MBL International, catalogue number K0205-3); monoclonal α-iNOS (BD Biosciences, catalogue number 610431); monoclonal α-GAPDH (Millipore, catalogue number MAB374); human-specific monoclonal α-Trx1 (BD Biosciences, catalogue number 559969); monoclonal anti-GST (Santa Cruz, catalogue number sc-138); mouse-specific polyclonal α-Trx1 (Cell Signaling, catalogue number 2298); polyclonal α-TrxR1 (Abcam, catalogue number ab16840-100). All of the antibodies were used in 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween 20. CysNO was synthesized as described (45) and used immediately.
Mammalian Cell Culture and Transfection
All of the cells were cultured at 37 °C in a 5% CO2 atmosphere. Where indicated, the culture incubator (Sanyo MCO-18M) was purged with moistened N2 gas to achieve lower O2 concentrations. Cell lines were obtained via the Duke Cell Culture Facility and grown in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. The cells were transfected with Lipofectamine 2000 (Invitrogen) per the manufacturer's instructions. To generate stable cell transfectants, HEK293 cells in a 6-well dish were transfected with 3 μg of the indicated pcDNA3.1 plasmid and 7.5 μl of Lipofectamine 2000, and the media was changed after 8 h. Twenty-four hours later, G418 (Invitrogen) was added to a final concentration of 1 mg/ml of active antibiotic. After 2 weeks, individual colonies were picked by gentle aspiration with a wide boar pipette tip and transferred to 24-well dishes. The cells were maintained with G418 for another 3 weeks. Bone marrow-derived macrophages (BMDMs) were isolated as described (46) and grown in Dulbecco's modified Eagle's medium containing 20% L-929 conditioned medium, 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin for 7 days prior to performing the experiments.
Cloning and DNA Manipulation
All of the PCRs were performed with Advantage Taq DNA polymerase (Clontech), and the products were verified by DNA sequencing (Duke DNA Sequencing Facility). The cDNA encoding human iNOS (GenBankTM accession number L09210) was excised from pCWori-iNOS by restriction digestion at the NdeI and XbaI sites. This 3.5-kb fragment was ligated into pDNR-1r donor plasmid (Clontech). The iNOS E377Q mutation was introduced via site-directed mutagenesis with a QuikChange kit (Stratagene) according to the manufacturer's instructions using the following primers: 5′-GTACATGGGCACACAGATCGGAGTCC-3′ and 5′-GGACTCCGATCTGTGTGCCCATGTAC-3′. The human iNOS WT and E377Q cDNAs were then transferred into pLP-IRES-neo via the Creator system (Clontech) according to the manufacturer's instructions. An IMAGE clone of mouse Txnip cDNA in pCMV-SPORT6 (BC031850) was acquired from Open Biosystems. To generate stable cell transfectants overexpressing Txnip, the cDNA of Txnip was subcloned into the 5′-EcoRI and 3′-XhoI sites in pcDNA3.1 via PCR with the following primers: 5′-TATTGAATTCACCATGGACTACAAAGACGATGACGACAAGGTGATGTTCAAGAAGATCAAGTC-3′ and 5′-TAATCTCGAGTCATTTCCTGCAGGCTCACTGCACGTT-3′. Mammalian expression plasmids encoding GST-tagged Trx1 (pEBG-Trx1) were kindly provided by Ki-Sun Kwon (Korea Research Institute of Bioscience and Biotechnology, Daejeon, South Korea) (47). Txnip reporter plasmids were created by PCR amplification of the Txnip promoter from human genomic DNA (isolated from HEK293 cells) and ligation into pGL4.14 at the 5′-XhoI and 3′-HindIII sites. To generate the 845-bp fragment (524 bp of promoter and 321 bp of 5′-UTR), the following primers were used: 5′-ATTACTCGAGCAGCCCCAAACCTGAAAGTATTCTTMGGAGC-3′ and 5′-ATATAAGCTTCTGAGTTGGTTTTAAGAGTTAGAAATGACGG-3′. To generate only the 524 bp of promoter (lacking the 5′-UTR), the following primers were used: 5′-ATTACTCGAGCAGCCCCAAACCTGAAAGTATTCTTGGAGC-3′ and 5′-AATTAAGCTTAAGCACTCCTTTGGAGAA-3′.
Quantitative Reverse Transcription-PCR and Luciferase Reporter Assays of Txnip
Total RNA was extracted with the RNeasy kit (Qiagen), and 500 ng of product was converted to cDNA with random hexamer oligonucleotides and Moloney murine leukemia virus reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Gene-specific primers (described below) were used for reverse transcription-PCR in a MyiQ reverse transcription-PCR detection system (Bio-Rad) using 1 μl of cDNA as template and 2× iQ SYBR green supermix (Bio-Rad). The expression of β-actin RNA in each sample was used to normalize the expression of Txnip. The size of each PCR product was verified by agarose gel electrophoresis. Fold change in expression was calculated using the comparative Ct method. For murine (RAW264.7) cells, the following primers were used for Txnip: 5′-CATGAGGCCTGGAAACAAAT-3′ and 5′-ACTGGTGCCATTAGGTCAGG-3′. For murine β-actin, the following primers were used: 5′-AGCCATGTACGTAGCCATCC-3′ and 5′-CTCTCAGCTGTGGTGGTGAA-3′. For human (HEK293) cells, the following primers were used for Txnip: 5′-TTCGGGTTCAGAAGATCAGG-3′ and 5′-TTGGATCCAGGAACGCTAAC-3′. For human β-actin, the following primers were used: 5′-TCAAGATCATTGCTCCTCCTGAGC-3′ and 5′-TTGCTGATCCACATCTGCTGGAAG-3′.
For analysis of Txnip promoter activity, HEK293 cells in 6-well dishes were transfected with the indicated pGL4.14 plasmid (1 μg each) and pRL-SV40 plasmid (0.1 μg each). The cells were co-transfected with 2 μg of either empty pRL, pRL- iNOS(WT), or pRL-iNOS(E377Q). Alternatively, the cells were treated with the indicated concentration of DETA-NO. After 24 h, the cells were harvested, and both firefly and Renilla luciferase activities were measured with a dual luciferase reporter assay (Promega) according to the manufacturer's instructions. All of the experiments were performed in triplicate.
Detection of Protein-SNOs with the Biotin Switch Technique (BST)
The BST was performed as described (45), with minor modifications. For determination of total protein-SNOs, the streptavidin-agarose was eluted under nonreducing conditions by boiling in 60 μl of 25 mm HEPES, 0.1 mm EDTA, 0.01 mm neocuproine, pH 8.0, containing 1% SDS. Eluants were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and incubated overnight with streptavidin-horseradish peroxidase (Amersham Biosciences/GE) at 1:1000 dilution in 5% nonfat dry milk in TBST.
Detection of Protein-SNOs with Mercury-coupled Photolysis Chemiluminescence
Protein-SNOs were measured essentially as described (48), with minor modifications. Following lysis, the proteins were isolated by passage through P-6 desalting gel (Bio-Rad) equilibrated and de-gassed in HEN buffer. This material was mixed 1:10 with either phosphate-buffered saline or phosphate-buffered saline containing 50 mm HgCl2 and incubated for 20 min at room temperature in the dark. The samples and GSNO standards were sequentially injected into the photolysis chamber. Protein-SNO content was determined by subtracting the “mercury-quenchable” signal from the total signal and normalizing to a GSNO standard curve.
Measurement of Cellular Damage and Proliferation
Cellular release of lactate dehydrogenase was measured with the Cytotox-96 assay kit (Promega) as described by the manufacturer; quantification was performed by normalization to a lactate dehydrogenase standard curve. [3H]Thymidine (PerkinElmer Life Sciences) uptake was measured following dilution of [3H]thymidine stock solution (1 μCi/μl) 1:250 into culture medium. After 4 h, the cells were gently collected with 1 ml of phosphate-buffered saline and centrifuged at 5000 × g, and the remaining phosphate-buffered saline was removed. The cell pellet was washed once with 10% trichloroacetic acid and then fixed for 1 h at 4 °C in 10% trichloroacetic acid. This material was centrifuged at 5000 × g, supernatant was discarded, and the pellet was resuspended in 1.0 ml of 0.2 m NaOH. After overnight incubation, measurements were made by scintillation counting.
RESULTS
Txnip Expression Is Negatively Regulated by Endogenous NO
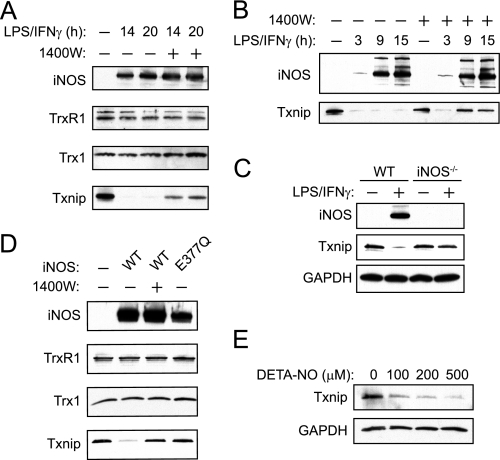
To initially examine the effects of endogenous NO on the Trx1 system, we employed the RAW264.7 murine macrophage cell line, which expresses iNOS following exposure to LPS and interferon-γ (IFN-γ) (49). These conditions also represent an established model of endogenous S-nitrosylation (50, 51). As shown in Fig. 1A, stimulation of macrophages with LPS and IFN-γ led to increased iNOS expression without affecting the levels of cytosolic Trx1 or TrxR1. The expression of Txnip, however, was markedly decreased, and this effect was partially reversed by the iNOS-specific inhibitor 1400W. Txnip suppression was observed as early as 2 h post-stimulation and continued for many hours thereafter (Fig. 1B and data not shown). Inhibition of iNOS with 1400W increased Txnip expression, but only at later time points corresponding with iNOS expression. Thus, Txnip undergoes two phases of suppression, early and late, but only the later phase is dependent on iNOS. We focused on the suppression of Txnip that is mediated by iNOS.
FIGURE 1.
Thioredoxin-interacting protein is suppressed in response to iNOS-derived NO in both murine and human cells. A, RAW264.7 cells were left untreated or exposed to LPS and IFN-γ (500 ng/ml and 100 units/ml, respectively) for the indicated times with or without 100 μm 1400W (iNOS inhibitor). Cellular extracts were subjected to SDS-PAGE and Western blotting. B, RAW264.7 cells were stimulated with LPS and IFN-γ for the indicated time periods in the absence or presence of 1400W, and Txnip or iNOS expression was assessed by Western blotting. C, BMDMs from WT and iNOS−/− mice were left untreated or stimulated with LPS and IFN-γ (100 ng/ml and 100 units/ml, respectively) for 12 h, and the expression of Txnip or iNOS was assessed by Western blotting. D, HEK293 cells were transfected with either empty pLP, pLP-iNOS(WT), or pLP-iNOS(E377Q) vectors. After 20 h, the cells were harvested, and the levels of various proteins were assessed by Western blotting. E, HEK293 cells were incubated with DETA-NO, a slow releasing NO donor (t½ = ∼20 h), for 18 h and subjected to Western blotting for Txnip.
To confirm the physiological relevance of Txnip suppression by NO, we examined the levels of Txnip in BMDMs harvested from wild type and iNOS−/− mice. As shown in Fig. 1C, BMDMs from wild type mice exhibited cytokine-dependent expression of iNOS and pronounced repression of Txnip, whereas BMDMs from iNOS−/− mice exhibited only a slight decrease in Txnip expression. Further, in human embryonic kidney (HEK293) cells, transfection of iNOS attenuated the expression of Txnip but had no effect on Trx1 or TrxR1 (Fig. 1D). Suppression of Txnip was reversed by 1400W, whereas an inactive iNOS E377Q mutant (52) had no effect on Txnip. Exogenous NO closely replicated the effects of iNOS, suppressing Txnip expression (Fig. 1E).
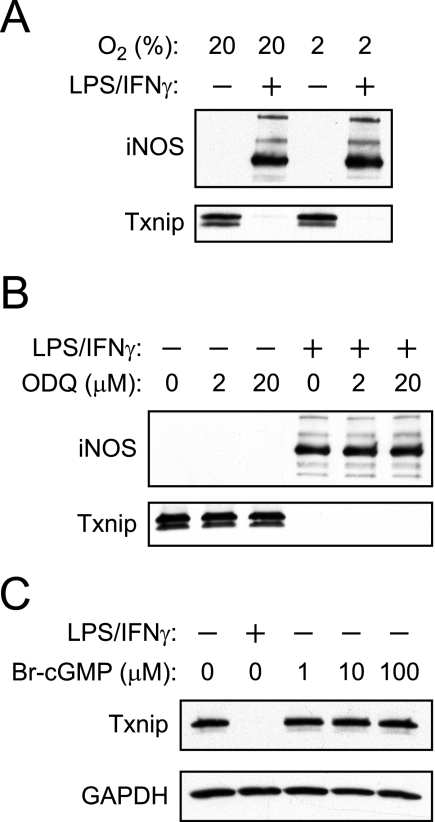
Mammalian cell culture is routinely performed under atmospheric 21% O2, whereas most perivascular tissues experience 2–5% O2 (53, 54). Because 21% O2 imposes an oxidative stress that is known to affect leukocyte stimulation (55), we examined the effects of physiological O2 concentrations (i.e. 2%) on NO-mediated Txnip suppression. As shown in Fig. 2A, the degree of iNOS-mediated suppression of Txnip at 2% O2 is comparable with that observed in room air.
FIGURE 2.
Suppression of Txnip is independent of cGMP synthesis or ambient O2 concentration. A, RAW264.7 cells were stimulated with LPS/IFN-γ for 16 h in either ambient O2 (21%) or physiological O2 (2%), and the expression of Txnip and iNOS was assessed by Western blotting. B, RAW264.7 cells were stimulated with LPS/IFN-γ for 16 h in the absence or presence of the soluble guanylyl cyclase inhibitor ODQ, and the expression of Txnip/iNOS was assessed by Western blotting. C, RAW264.7 cells were stimulated with LPS/IFN-γ for 16 h or treated with 8-bromo-cGMP.
Regulation of protein function by NO is typically mediated by either S-nitrosylation or cGMP. To evaluate a role for cGMP, we employed ODQ (an inhibitor of soluble guanylyl cyclase) and 8-bromo-cGMP (a cell-permeable and stable analogue of cGMP). Suppression of Txnip was neither inhibited by ODQ (Fig. 2B) nor replicated by 8-bromo-cGMP (Fig. 2C). Collectively, our experiments establish that both endogenous and exogenous NO suppresses Txnip via a cGMP-independent pathway. Regulation of Txnip is thus likely to be linked to protein S-nitrosylation.
Txnip Suppression Involves Transcriptional Deactivation
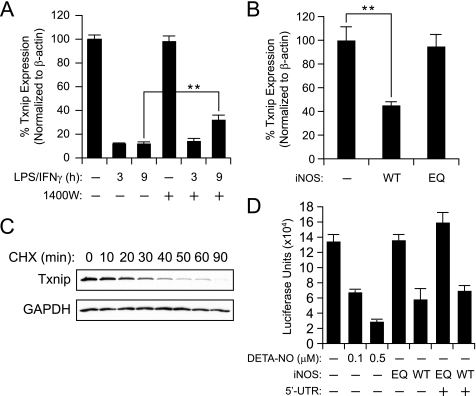
Schulze et al. (44) previously demonstrated that exogenous GSNO inhibits the Txnip promoter, and we therefore reasoned that endogenously synthesized NO may operate similarly. As shown in Fig. 3A, cytokine stimulation leads to a robust decrease in Txnip mRNA to ∼10% of the basal levels, and where iNOS expression is robust (e.g. 9 h), these effects are partially reversed by 1400W. Residual iNOS activity (∼10% as assessed by Griess assay) may account for the continued suppression of Txnip. Notably, mRNA levels over time correlate with Txnip protein expression (Fig. 1B). In a confirmatory experiment, transfection of wild type iNOS, but not the inactive E377Q mutant, led to a reduction in Txnip mRNA (Fig. 3B).
FIGURE 3.
NO-dependent Txnip suppression involves transcriptional deactivation of the Txnip promoter. A, RAW264.7 cells were stimulated with LPS/IFN-γ with or without 1400W for the indicated time periods; total cellular RNA was extracted and subjected to quantitative reverse transcription-PCR. Txnip RNA levels were normalized to β-actin. Where indicated, iNOS was inhibited (∼90%) with 100 μm 1400W. **, p < 0.01. B, HEK293 cells were transfected with either empty pLP-IRES-Neo, pLP-iNOS(WT), or pLP-iNOS(E377Q) (EQ) plasmids for 20 h, and cellular RNA was subjected to quantitative reverse transcription-PCR with normalization to β-actin. C, a time course of Txnip protein stability in RAW264.7 macrophages. Intracellular protein synthesis was inhibited with 100 μm cycloheximide (CHX), cells were harvested at the indicated time points, and Txnip protein levels were assessed by Western blotting. D, effects of exogenous NO and iNOS activity on human Txnip promoter activity. HEK293 cells were transfected with pGL4.14-Txnip (524 bp of promoter with or without 321 bp of 5′-UTR) along with pRL-SV40 for internal normalization of luciferase activity. The cells were either treated with DETA-NO for 12 h or co-transfected with pLP-iNOS(WT) or pLP-iNOS(E377Q). Twenty-four hours after transfection, the cells were harvested and assayed for firefly and Renilla luciferase activities.
Txnip protein should be sensitive to decreases in mRNA if the protein is rapidly turned over. Indeed in RAW264.7 cells incubated with cycloheximide (to inhibit ribosomal protein synthesis), Txnip protein undergoes complete turnover within 90 min (Fig. 3C). Based on these findings, we reasoned that iNOS-derived NO might suppress Txnip via inhibition of promoter activity. Firefly luciferase reporter plasmids containing 524 bp of the human Txnip promoter with or without the 321 bp of the 5′-UTR (845 bp of DNA total) were transfected into HEK293 cells together with empty control or iNOS-containing plasmid. Txnip reporter activity was inhibited by active iNOS or exogenous NO, whereas the iNOS(E377Q) mutant was without effect (Fig. 3D). Promoter inhibition was not dependent on the Txnip 5′-UTR. These data suggest that endogenous NO represses Txnip expression, at least in part, via inhibition of promoter activity.
Txnip Is an Endogenous Regulator of Protein S-Nitrosylation
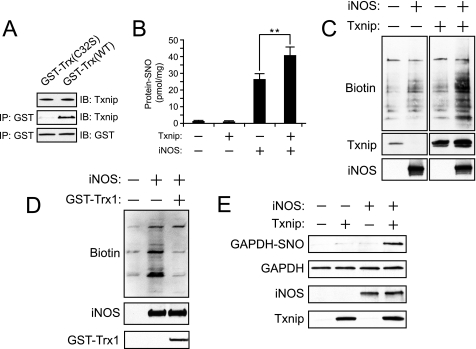
We sought to confirm that Txnip binds to the active site Cys32 of Trx1, which should inhibit Trx1-mediated denitrosylation. To this end, HEK293 cells were transfected with cDNAs encoding GST-tagged Trx1 (wild type and C32S) and examined for interactions with endogenous Txnip. Whereas affinity isolation of wild type Trx1 pulled down Txnip, the active site C32S mutant of Trx1 did not (Fig. 4A). Thus, Txnip binds directly to the active site Cys32 of Trx1.
FIGURE 4.
Thioredoxin-interacting protein binds to the active site Cys of Trx1 and facilitates protein S-nitrosylation. A, HEK293 cells were transfected with pEBG-GST-Trx1(WT) and C32S active site mutant for 20 h, Trx1 was isolated with GSH-agarose and eluted with 50 mm dithiothreitol to release disulfide-linked Txnip. B, HEK293 cells stably transfected with either pcDNA3.1 or pcDNA3.1-Txnip were co-transfected with pLP or pLP-iNOS for 20 h. Protein-SNO was measured by mercury-coupled photolysis-chemiluminescence. **, p < 0.01. C, protein-SNOs were assayed by the BST following co-transfections as in B. Total protein S-nitrosylation (biotinylation) was detected with avidin-horseradish peroxidase. Indicated is a single representative experiment with in-gel cropping. D, HEK293 cells stably transfected with pCDNA3.1-Txnip were co-transfected ± iNOS and either empty pEBG or pEBG-GST-Trx1 and subjected to the BST as in C. E, S-nitrosylation of GAPDH was detected by the BST using Western blotting for GAPDH. IP, immunoprecipitation; IB, immunoblot.
Given the recent demonstrations that Trx1 is a constitutively active denitrosylase (6, 37, 38), we sought to examine the effects of Txnip on intracellular S-nitrosylation. HEK293 cells transfected with iNOS exhibit increased intracellular levels of protein-SNOs (Fig. 4B) as measured by mercury-coupled photolysis-chemiluminescence, which employs UV-based photolysis of the S-NO bond (and ozone-based detection of the released NO (48). Co-transfection of Txnip with iNOS further increased protein S-nitrosylation by ∼40% (n = 6, p < 0.05). These findings were confirmed with the BST, in which protein-SNOs are chemically converted to disulfide-linked biotin moieties and detected with avidin-based reagents (45) (Fig. 4C). Numerous protein-SNOs are increased by Txnip co-expression, suggesting that they are substrates for Trx1-mediated denitrosylation. Further, the Txnip-dependent potentiation of protein S-nitrosylation was reversed by co-transfection of GST-Trx1 (Fig. 4D). Because Trx-1 is reported to denitrosylate GAPDH-SNO (6), a mediator of NO-based cell death (51, 56), we reasoned that Txnip may regulate nitrosative stress. Indeed, S-nitrosylation of GAPDH in iNOS-transfected HEK293 cells was markedly potentiated by Txnip co-transfection (Fig. 4E). These findings suggest that Txnip is a broad spectrum regulator of S-nitrosylation and that Trx1-mediated denitrosylation may protect against nitrosative stress.
Txnip Repression Protects from Nitrosative Stress
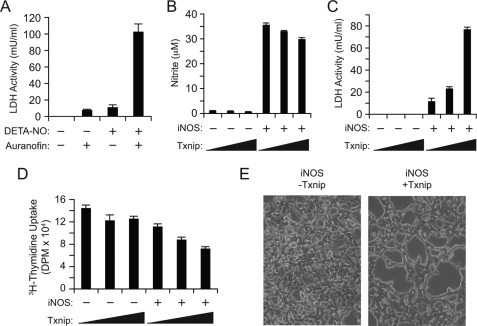
To examine the importance of the Trx1 system in nitrosative stress resistance, HEK293 cells were exposed to DETA-NO and/or auranofin (a potent TrxR inhibitor) and assayed for cell damage. Although either compound alone elicited some degree of injury, the two agents combined showed marked synergy effects (Fig. 5A). Thus, the Trx system evidently protects against NO. We therefore posited that Txnip repression serves to protect the cell from nitrosative stress by facilitating Trx1-dependent denitrosylation and examined the effects of Txnip expression on NO-mediated cell damage. HEK293 cells were transfected with plasmids encoding Txnip and/or iNOS and assessed for NO production and cellular integrity. Although Txnip expression had no effect on iNOS activity (Fig. 5B), it markedly potentiated iNOS-mediated cellular injury (Fig. 5C). Thus, Txnip opposes the cellular defense against endogenous NO without affecting NO production (Fig. 6).
FIGURE 5.
Txnip facilitates nitrosative stress in iNOS-transfected HEK293 cells. A, HEK293 cells were incubated with 2 mm DETA-NO and/or 2 μm auranofin for 16 h, and lactate dehydrogenase (LDH) release was measured. B–E, HEK293 cells were transfected with either empty pCMV or pCMV-Txnip plasmid (0, 2, or 4 μg) and empty pLP or pLP-iNOS(WT) plasmid (3 μg). After 28 h, media nitrite (NO2−) (B) and lactate dehydrogenase release (C) were measured. D, cells were transfected for 20 h as in B, and cellular uptake of [3H]thymidine was assayed. E, samples from above were visualized by light microscopy prior to lactate dehydrogenase measurement. Shown are cells transfected with pLP-iNOS(WT) and either empty pCMV (left panel) or pCMV-Txnip (right panel) plasmid.
FIGURE 6.
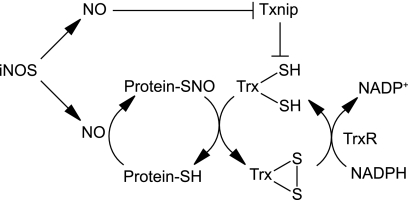
Feedback regulation of protein denitrosylation by Txnip. iNOS-derived NO mediates protein S-nitrosylation. Coincident down-regulation of Txnip enables denitrosylation by Trx1, thereby alleviating nitrosative stress.
To determine the effects of Txnip and iNOS on cellular growth and DNA synthesis, [3H]thymidine uptake was assayed in HEK293 cells expressing Txnip and/or iNOS. Cells transfected with Txnip or iNOS alone exhibited slight or no decrease in [3H]thymidine uptake, consistent with previous reports (41, 42) (Fig. 5D). By contrast, co-expression of iNOS and Txnip led to significant suppression of [3H]thymidine uptake (Fig. 5D). Cells transfected with both iNOS and Txnip also exhibited decreased intercellular adhesion and increased swelling, whereas cells transfected with iNOS alone appeared grossly normal (Fig. 5E).
DISCUSSION
The molecular mechanisms governing protein S-nitrosylation and denitrosylation are active areas of investigation. Two enzymatic systems, GSNOR and Trx, have been shown to impact protein denitrosylation (31), but whereas GSNOR has well established functions in cellular signaling and defense, the roles of the Trx1 system are less clear. In particular, Benhar et al. (6) demonstrated that Trx2 mediates denitrosylation of proteins in response to Fas signaling, whereas Trx1 reverses S-nitrosylation by iNOS or exogenous NO. Here we show that denitrosylation is induced by NO through transcriptional repression of Txnip, thus allowing Trx1 to protect against nitrosative stress (Fig. 6). More generally, inducible denitrosylation by Trx1 may complement the constitutive function of GSNOR in resisting nitrosative stress. Protein denitrosylation is thus evidently regulated at multiple levels.
Txnip can apparently form a stable disulfide with Trx1 within the reducing cytosolic environment (redox potential, approximately −300 mV (57, 58)), where intra- and intermolecular disulfides are generally considered thermodynamically unstable. The Txnip-Trx1 intermolecular disulfide is presumably supported by a kinetic (e.g. steric) barrier to reduction. Future studies on the structural aspects of Txnip (and its association with Trx1) will hopefully address this issue, as well as other outstanding questions raised by our studies, particularly the molecular mechanism by which Txnip is suppressed by NO. It is important to note in this regard that many transcription factors and DNA-regulatory proteins have been shown to undergo regulatory S-nitrosylation, consistent with our finding that Txnip suppression is independent of cGMP. We therefore speculate that nitrosative stress, imposed by excessive S-nitrosylation, might be sensed by a specific SNO regulatory system that then transduces the nitrosative defense response. In this scenario, Txnip suppression may represent only a small part of the adaptive response to nitrosative stress.
Inducible protection is a strategy employed by both microbes and mammals exposed to high levels of NO. But whereas microbes rely principally on inducible enzymes to consume NO (e.g. flavohemoglobins), mammals are evidently devoid of major NO detoxifying activities. Instead, protection is conferred by the consumption and removal of SNOs. Thiol-based mechanisms of protection against SNO have thus supplanted the bacterial strategy of enzymatic NO consumption (31), and in this regard, the dithiol-containing Trx plays a key role. This conceptualization strengthens the notion that protein denitrosylation is a central function of the Trx system.
Footnotes
- iNOS
- inducible nitric-oxide synthase
- 1400W
- N-[[3-(aminomethyl)phenyl]methyl]-ethanimidamide, dihydrochloride
- LPS
- lipopolysaccharide
- IFN-γ
- interferon-γ
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- 8-bromo-cGMP
- 8-bromoguanosine-3′,5′-cyclic monophosphate
- DETA-NO
- diethylenetriamine NONOate
- Trx
- thioredoxin(s)
- TrxR
- thioredoxin reductase
- Txnip
- thioredoxin-interacting protein
- SNO
- S-nitrosothiol
- GSNO
- S-nitrosoglutathione
- GSNOR
- GSNO reductase
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GST
- glutathione S-transferase
- BMDM
- bone marrow-derived macrophage
- WT
- wild type
- UTR
- untranslated region
- BST
- biotin switch technique.
REFERENCES
- 1.Foster M. W., Hess D. T., Stamler J. S. (2009) Trends Mol. Med. 15, 391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess D. T., Matsumoto A., Kim S. O., Marshall H. E., Stamler J. S. (2005) Nat. Rev. Mol. Cell Biol. 6, 150–166 [DOI] [PubMed] [Google Scholar]
- 3.Kokkola T., Savinainen J. R., Mönkkönen K. S., Retamal M. D., Laitinen J. T. (2005) BMC Cell Biol. 6, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nozik-Grayck E., Whalen E. J., Stamler J. S., McMahon T. J., Chitano P., Piantadosi C. A. (2006) Am. J. Physiol. Lung Cell Mol. Physiol. 290, L136–L143 [DOI] [PubMed] [Google Scholar]
- 5.Whalen E. J., Foster M. W., Matsumoto A., Ozawa K., Violin J. D., Que L. G., Nelson C. D., Benhar M., Keys J. R., Rockman H. A., Koch W. J., Daaka Y., Lefkowitz R. J., Stamler J. S. (2007) Cell 129, 511–522 [DOI] [PubMed] [Google Scholar]
- 6.Benhar M., Forrester M. T., Hess D. T., Stamler J. S. (2008) Science 320, 1050–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann J., Haendeler J., Zeiher A. M., Dimmeler S. (2001) J. Biol. Chem. 276, 41383–41387 [DOI] [PubMed] [Google Scholar]
- 8.Kim J. E., Tannenbaum S. R. (2004) J. Biol. Chem. 279, 9758–9764 [DOI] [PubMed] [Google Scholar]
- 9.Mannick J. B., Hausladen A., Liu L., Hess D. T., Zeng M., Miao Q. X., Kane L. S., Gow A. J., Stamler J. S. (1999) Science 284, 651–654 [DOI] [PubMed] [Google Scholar]
- 10.Matsushita K., Morrell C. N., Cambien B., Yang S. X., Yamakuchi M., Bao C., Hara M. R., Quick R. A., Cao W., O'Rourke B., Lowenstein J. M., Pevsner J., Wagner D. D., Lowenstein C. J. (2003) Cell 115, 139–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrell C. N., Matsushita K., Chiles K., Scharpf R. B., Yamakuchi M., Mason R. J., Bergmeier W., Mankowski J. L., Baldwin W. M., 3rd, Faraday N., Lowenstein C. J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3782–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G., Moniri N. H., Ozawa K., Stamler J. S., Daaka Y. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1295–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S. F., Huri D. A., Snyder S. H. (2005) Science 310, 1966–1970 [DOI] [PubMed] [Google Scholar]
- 14.Tian J., Kim S. F., Hester L., Snyder S. H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 10537–10540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L., Han C., Lim K., Wu T. (2008) J. Biol. Chem. 283, 3077–3087 [DOI] [PubMed] [Google Scholar]
- 16.Diesen D. L., Hess D. T., Stamler J. S. (2008) Circ. Res. 103, 545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamler J. S., Jia L., Eu J. P., McMahon T. J., Demchenko I. T., Bonaventura J., Gernert K., Piantadosi C. A. (1997) Science 276, 2034–2037 [DOI] [PubMed] [Google Scholar]
- 18.McMahon T. J., Moon R. E., Luschinger B. P., Carraway M. S., Stone A. E., Stolp B. W., Gow A. J., Pawloski J. R., Watke P., Singel D. J., Piantadosi C. A., Stamler J. S. (2002) Nat. Med. 8, 711–717 [DOI] [PubMed] [Google Scholar]
- 19.Uehara T., Nakamura T., Yao D., Shi Z. Q., Gu Z., Ma Y., Masliah E., Nomura Y., Lipton S. A. (2006) Nature 441, 513–517 [DOI] [PubMed] [Google Scholar]
- 20.Li F., Sonveaux P., Rabbani Z. N., Liu S., Yan B., Huang Q., Vujaskovic Z., Dewhirst M. W., Li C. Y. (2007) Mol. Cell 26, 63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim K. H., Ancrile B. B., Kashatus D. F., Counter C. M. (2008) Nature 452, 646–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung K. K., Thomas B., Li X., Pletnikova O., Troncoso J. C., Marsh L., Dawson V. L., Dawson T. M. (2004) Science 304, 1328–1331 [DOI] [PubMed] [Google Scholar]
- 23.Yao D., Gu Z., Nakamura T., Shi Z. Q., Ma Y., Gaston B., Palmer L. A., Rockenstein E. M., Zhang Z., Masliah E., Uehara T., Lipton S. A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10810–10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon T. J., Ahearn G. S., Moya M. P., Gow A. J., Huang Y. C., Luchsinger B. P., Nudelman R., Yan Y., Krichman A. D., Bashore T. M., Califf R. M., Singel D. J., Piantadosi C. A., Tapson V. F., Stamler J. S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 14801–14806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwakiri Y., Satoh A., Chatterjee S., Toomre D. K., Chalouni C. M., Fulton D., Groszmann R. J., Shah V. H., Sessa W. C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19777–19782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawloski J. R., Hess D. T., Stamler J. S. (2001) Nature 409, 622–626 [DOI] [PubMed] [Google Scholar]
- 27.Mani K., Cheng F., Havsmark B., David S., Fransson L. A. (2004) J. Biol. Chem. 279, 12918–12923 [DOI] [PubMed] [Google Scholar]
- 28.Inoue K., Akaike T., Miyamoto Y., Okamoto T., Sawa T., Otagiri M., Suzuki S., Yoshimura T., Maeda H. (1999) J. Biol. Chem. 274, 27069–27075 [DOI] [PubMed] [Google Scholar]
- 29.Foster M. W., Liu L., Zeng M., Hess D. T., Stamler J. S. (2009) Biochemistry [DOI] [PubMed] [Google Scholar]
- 30.Forrester M. T., Thompson J. W., Foster M. W., Nogueira L., Moseley M. A., Stamler J. S. (2009) Nat. Biotechnol. 27, 557–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benhar M., Forrester M. T., Stamler J. S. (2009) Nat. Rev. Mol. Cell Biol. 10, 721–732 [DOI] [PubMed] [Google Scholar]
- 32.Jensen D. E., Belka G. K., Du Bois G. C. (1998) Biochem. J. 331, 659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L., Hausladen A., Zeng M., Que L., Heitman J., Stamler J. S. (2001) Nature 410, 490–494 [DOI] [PubMed] [Google Scholar]
- 34.Tsikas D., Sandmann J., Rossa S., Gutzki F. M., Frölich J. C. (1999) Anal. Biochem. 270, 231–241 [DOI] [PubMed] [Google Scholar]
- 35.Liu L., Yan Y., Zeng M., Zhang J., Hanes M. A., Ahearn G., McMahon T. J., Dickfeld T., Marshall H. E., Que L. G., Stamler J. S. (2004) Cell 116, 617–628 [DOI] [PubMed] [Google Scholar]
- 36.Ozawa K., Whalen E. J., Nelson C. D., Mu Y., Hess D. T., Lefkowitz R. J., Stamler J. S. (2008) Mol. Cell 31, 395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sengupta R., Ryter S. W., Zuckerbraun B. S., Tzeng E., Billiar T. R., Stoyanovsky D. A. (2007) Biochemistry 46, 8472–8483 [DOI] [PubMed] [Google Scholar]
- 38.Stoyanovsky D. A., Tyurina Y. Y., Tyurin V. A., Anand D., Mandavia D. N., Gius D., Ivanova J., Pitt B., Billiar T. R., Kagan V. E. (2005) J. Am. Chem. Soc. 127, 15815–15823 [DOI] [PubMed] [Google Scholar]
- 39.Chen K. S., DeLuca H. F. (1994) Biochim. Biophys. Acta 1219, 26–32 [DOI] [PubMed] [Google Scholar]
- 40.Nishiyama A., Matsui M., Iwata S., Hirota K., Masutani H., Nakamura H., Takagi Y., Sono H., Gon Y., Yodoi J. (1999) J. Biol. Chem. 274, 21645–21650 [DOI] [PubMed] [Google Scholar]
- 41.Junn E., Han S. H., Im J. Y., Yang Y., Cho E. W., Um H. D., Kim D. K., Lee K. W., Han P. L., Rhee S. G., Choi I. (2000) J. Immunol. 164, 6287–6295 [DOI] [PubMed] [Google Scholar]
- 42.Schulze P. C., De Keulenaer G. W., Yoshioka J., Kassik K. A., Lee R. T. (2002) Circ. Res. 91, 689–695 [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., De Keulenaer G. W., Lee R. T. (2002) J. Biol. Chem. 277, 26496–26500 [DOI] [PubMed] [Google Scholar]
- 44.Schulze P. C., Liu H., Choe E., Yoshioka J., Shalev A., Bloch K. D., Lee R. T. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 2666–2672 [DOI] [PubMed] [Google Scholar]
- 45.Forrester M. T., Foster M. W., Stamler J. S. (2007) J. Biol. Chem. 282, 13977–13983 [DOI] [PubMed] [Google Scholar]
- 46.Kurtz S., McKinnon K. P., Runge M. S., Ting J. P., Braunstein M. (2006) Infect. Immun. 74, 6855–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang C. Y., Ryu Y. S., Chung M. S., Kim K. D., Park S. S., Chae S. K., Chae H. Z., Kwon K. S. (2004) Oncogene 23, 8868–8875 [DOI] [PubMed] [Google Scholar]
- 48.Stamler J. S., Jaraki O., Osborne J., Simon D. I., Keaney J., Vita J., Singel D., Valeri C. R., Loscalzo J. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 7674–7677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyons C. R., Orloff G. J., Cunningham J. M. (1992) J. Biol. Chem. 267, 6370–6374 [PubMed] [Google Scholar]
- 50.Eu J. P., Liu L., Zeng M., Stamler J. S. (2000) Biochemistry 39, 1040–1047 [DOI] [PubMed] [Google Scholar]
- 51.Hara M. R., Agrawal N., Kim S. F., Cascio M. B., Fujimuro M., Ozeki Y., Takahashi M., Cheah J. H., Tankou S. K., Hester L. D., Ferris C. D., Hayward S. D., Snyder S. H., Sawa A. (2005) Nat. Cell Biol. 7, 665–674 [DOI] [PubMed] [Google Scholar]
- 52.Chen P. F., Tsai A. L., Berka V., Wu K. K. (1997) J. Biol. Chem. 272, 6114–6118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Intaglietta M., Johnson P. C., Winslow R. M. (1996) Cardiovasc Res. 32, 632–643 [PubMed] [Google Scholar]
- 54.Tsai A. G., Friesenecker B., Mazzoni M. C., Kerger H., Buerk D. G., Johnson P. C., Intaglietta M. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6590–6595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atkuri K. R., Herzenberg L. A., Herzenberg L. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3756–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sawa A., Khan A. A., Hester L. D., Snyder S. H. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 11669–11674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dooley C. T., Dore T. M., Hanson G. T., Jackson W. C., Remington S. J., Tsien R. Y. (2004) J. Biol. Chem. 279, 22284–22293 [DOI] [PubMed] [Google Scholar]
- 58.Østergaard H., Tachibana C., Winther J. R. (2004) J. Cell Biol. 166, 337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]