Abstract
All mitochondrial tRNAs in Trypanosoma brucei derive from cytosolic tRNAs that are in part imported into mitochondria. Some trypanosomal tRNAs are thiolated in a compartment-specific manner. We have identified three proteins required for the thio modification of cytosolic tRNAGln, tRNAGlu, and tRNALys. RNA interference-mediated ablation of these proteins results in the cytosolic accumulation non-thio-modified tRNAs but does not increase their import. Moreover, in vitro import experiments showed that both thio-modified and non-thio-modified tRNAGlu can efficiently be imported into mitochondria. These results indicate that unlike previously suggested the cytosol-specific thio modifications do not function as antideterminants for mitochondrial tRNA import. Consistent with these results we showed by using inducible expression of a tagged tRNAGlu that it is mainly the thiolated form that is imported in vivo. Unexpectedly, the imported tRNA becomes dethiolated after import, which explains why the non-thiolated form is enriched in mitochondria. Finally, we have identified two genes required for thiolation of imported tRNATrp whose wobble nucleotide is subject to mitochondrial C to U editing. Interestingly, down-regulation of thiolation resulted in an increase of edited tRNATrp but did not affect growth.
Introduction
Most protozoa, many fungi, plants, and a few animals lack a variable number of mitochondrial tRNA genes. It has been shown in these organisms that the missing genes are compensated for by import of a small fraction of the corresponding cytosolic tRNAs (1, 2). Among all organisms that import tRNAs trypanosomatids such as Trypanosoma brucei and Leishmania are extreme in that their mitochondrial genomes have lost all tRNA genes. Trypanosomatids therefore need to import the entire set of mitochondrial tRNAs. As a consequence all mitochondrial tRNAs in trypanosomatids derive from eukaryotic-type cytosolic tRNAs that need to function in the context of the bacterial-type translation system of mitochondria (3). Two tRNAs, the initiator tRNAMet and the tRNASec, are cytosol-specific. It has been shown that in T. brucei the tRNA import specificity is mediated by binding to cytosolic translation elongation factor 1a (4). The fact that initiator tRNAMet and tRNASec do not bind to elongation factor 1a therefore explains their exclusive cytosolic localization.
The extent of mitochondrial localization of different trypanosomal tRNAs varies by at least an order of magnitude (5, 6). The same is true for other organisms that import tRNAs (7). Cells therefore need to determine both the tRNA import specificity as well as the extent of mitochondrial localization of each imported tRNA species. How this is achieved is not known in any species.
In trypanosomatids mitochondrial tRNAs and their cytosolic counterparts derive from the same nuclear genes. However, due to compartment-specific post-transcriptional nucleotide modifications, the cytosolic and the corresponding imported tRNAs are often physically different. Mitochondria-specific nucleotide modifications (probably methylations) were found at the cytidine 32 of tRNALys(CUU), tRNALeu(CAA), and tRNATyr (8, 9). The tRNATrp of trypanosomatids is subject to extensive mitochondria-specific modifications, which include methylation of the pseudouridine at position 32, 2-thiolation of the uridine at position 33, as well as methylation and C to U editing of the wobble nucleotide (10–12). The wobble nucleotide of tRNAGlu(UUC) and tRNAGln(UUG) of Leishmania tarentolae consists of 5-methoxycarbonylmethyl uridine (mcm5U).2 In addition to this modification the same nucleotide also contains a cytosol-specific thio modification at position 2 of the uracil (resulting in mcm5S2U) and a mitochondria-specific 2′-O-methyl group in the ribose (resulting in mcm5Um) (13). Little is known about the function of these compartment-specific modifications. It has been shown that the cytidine 32 modifications are not required for import of the affected tRNAs but rather are a consequence of the mitochondrial localization of the tRNAs (9). Moreover, C to U editing of the imported tRNATrp is required for correct decoding of the UGA codon, because in trypanosomatid mitochondria this codon has been re-assigned to tryptophan (11, 12). Finally, it has been proposed that in L. tarentolae the cytosol-specific thio modifications of the tRNAGlu(UUC) and tRNAGln(UUG) might be antideterminants for mitochondrial import (13). According to this model the extent of import of the two tRNAs would be regulated by the extent of the thiolation. Evidence for this hypothesis is based on the essentially exclusive cytosolic localization of the thio-modified molecules. Moreover, it has been shown that isolated thio-modified tRNAGlu(UUC) is less efficiently imported into isolated mitochondria than the corresponding in vitro transcript lacking any modifications (13).
2-Thiolation of the wobble nucleotide of cytosolic tRNAGlu(UUC) and tRNAGln(UUG) is not restricted to trypanosomatids but occurs in all eukaryotes. Moreover, the same appears to be the case for the corresponding mitochondrial encoded tRNAs (14). Recent work in the yeast Saccharomyces cerevisiae has shown that thiolation of tRNAs depends on components of the Fe/S cluster (ISC) biogenesis pathway (15–17). ISCs of iron-sulfur proteins are essential for a wide variety of cellular processes, and their assembly requires complex and interconnected machineries that are localized in both mitochondria and the cytosol (17). Interestingly, thiolation of cytosolic tRNAs required components of both the cytosolic and the mitochondrial ISC assembly pathways, whereas thiolation of mitochondrial tRNAs needed only components of the mitochondrial ISC assembly pathway (15).
Recently, there has been great progress in the identification and characterization of the numerous factors that are required for 2-thiolation of eukaryotic tRNAs (18–21). Of special importance in the context of this study is the identification of yeast and human Mtu1, a mitochondria-specific tRNA 2-thiouridylase, responsible for the last step in the cascade leading to thiolation of mitochondrial tRNAs (22).
Here we show by using individual RNAi-mediated ablation of three components of the ISC biogenesis pathway that reduction of the thio-modified fraction of tRNAGln(UUG), tRNAGlu(UUC), and tRNALys(UUU) does not lead to increased import of the unmodified tRNA species. Moreover, in vitro import experiments indicate that both thio-modified and non-thio-modified tRNAs are efficiently imported into mitochondria. We present evidence that the enrichment of non-thio-modified tRNAGlu in mitochondria can best be explained by a mitochondrial dethiolation activity that removes the thio modification from the imported tRNAs. Finally, we identify two gene products required for thiolation of imported tRNATrp. We show that thiolation of the tRNATrp is not essential for normal growth of T. brucei and that its extent is negatively correlated with the extent C to U editing.
EXPERIMENTAL PROCEDURES
Cell Culture
T. brucei 427 and T. brucei 29-13 were grown at 27 °C in SDM79 supplemented with 5 and 15% of fetal calf serum, respectively. In the case of T. brucei 29-13 the medium was supplemented with 25 μg/ml hygromycin and 15 μg/ml G-418.
Transgenic Cell Lines
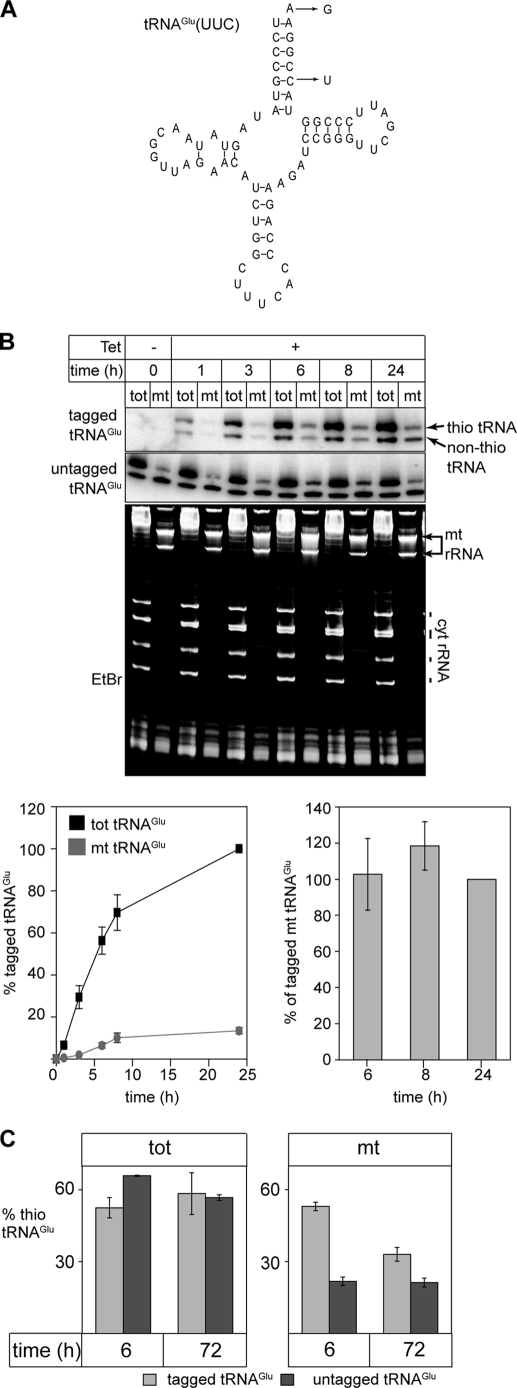
All transgenic cell lines used in this study are based on T. brucei strain 29-13. RNAi directed against the Tb-Nbp35 (Tb10.70.6210), Tb-Cfd1 (Tb927.7.1500), Tb-Nfs1 (Tb11.55.0013), and Tb-Mtu1 (Tb927.8.1830) mRNAs was performed using pLew-100 (23)-based stem loop constructs containing the puromycin resistance gene as described previously (24). As inserts we used a 505-bp fragment (nucleotides 217–722) for Tb-Nbp35, a 475-bp fragment (nucleotides 345–820) for Tb-Cfd1, a 952-bp fragment (nucleotides 107–1059) for Tb-Nfs1, and a 492-bp fragment (nucleotides 577–1069) for Tb-Mtu1. The cell line allowing inducible expression of a tagged tRNAGlu (Fig. 6) was established using the same strategy that had been successfully used in the case of the tagged elongator tRNAMet (4). Two tetracycline operators were fused in tandem to the immediate 5′-end of the tagged tRNAGlu gene. The tetracycline operator-tRNAGlu gene cassette was flanked on the 5′-side by 261 nucleotides corresponding to the 5′-flanking region of the tRNALeu gene, a sequence shown to be compatible with high levels of tRNA expression (25), and on the 3′-side by 25 nucleotides of its endogenous 3′-flanking region. Sequence alignments of the trypanosomal tRNAGlu with other eukaryotic tRNAsGlu allowed to identify conservative nucleotide replacements of which A72 to G72 and G5:C67 to G5:U67 were chosen as tags (Fig. 6A). The tags were introduced by PCR-directed mutagenesis and verified by sequencing. All constructs were linearized with NotI prior to transfection; selection with puromycin cloning and induction with 1 μg/ml tetracycline were done as described previously (26).
FIGURE 6.
Evidence for dethiolation of tRNAGlu after import. A, predicted secondary structure of the trypanosomal tRNAGlu. The nucleotide changes that were introduced to tag the tRNAGlu that can be specifically detected by oligonucleotide hybridization are indicated. B, inducible expression of tagged tRNAGlu. Appearance of tagged tRNAGlu (upper panel) as well as the endogenous wild-type tRNAGlu (middle panel) in the cytosol (tot) and in digitonin-extracted mitochondria (mt) was monitored by Northern analysis. The RNA samples were separated on APM gels to distinguish thio-modified from non-thio-modified tRNAsGlu. The bottom panel shows the corresponding ethidium bromide-stained gel (EtBr). Positions of the mitochondrial rRNAs (mt rRNA) and the cytosolic (cyt rRNA) as well as the tRNA region are indicated. Graph, bottom left: quantitative analysis of four independent experiments of the type shown on the top panels. The signal corresponding to the tagged tRNAGlu in the total RNA fraction at 24 h of induction was set to 100%. Graph, bottom right: comparison of relative amounts of tagged tRNAGlu (both thio-modified and non-thio-modified) that are recovered in mitochondria after 4, 6, and 24 h of induction, respectively. The fraction of the mitochondrial tagged tRNAGlu after 24 h of induction was set to 100%. Standard errors are indicated. C, percentages of thiolated tRNAGlu* (light gray bars) and of thiolated endogenous wild-type tRNAGlu (dark gray bars) were determined 6 and 72 h after induction in both the cytosolic (tot, left panel) and the mitochondrial fractions (mt, right panel). The graphs depict the mean of three independent experiments (standard errors are indicated).
Northern Analyses
Isolated RNA fraction were separated and analyzed on a short 8 m urea/10% polyacrylamide sequencing gel containing 0.5% of ((N-acryloylamino)phenyl)mercuric chloride (APM) to separate non-thio-modified from thio-modified tRNAs. Northern hybridizations using radioactively labeled oligonucleotide probes were done as described before (5). The following tRNAs were detected using the indicated oligonucleotide probe: tRNAGlu(UUC), 5′-GTGGTTCCGGTACCGGGA-3′; tRNAGln(UUG), 5′-GTGGTGGTCCTACCAGGAT-3′; tRNATrp(CCA), 5′-TGAGGACTGCAGGGATTG-3′; and tRNALys(UUU), 5′-GTGGCGCCTTCCGTGGGGATC-3′. The tagged tRNAGlu(UUC) could be specifically detected using the oligonucleotide 5′-TGGCTCCGATACCGGGA-3′. Imported tRNAPhe in the in vitro import assays was detected using oligonucleotide 5′-GTGGTGCGAATTCTGTGGATC-3′.
In Vitro Import of tRNAs
Import of tRNAs into isolated mitochondria was done as described before (4). Mitochondria from uninduced and induced (72 h) Tb-Nbp35 RNAi cell lines (Fig. 6A) or from wild-type T. brucei 427 (Fig. 6B) were isolated using the isotonic lysis procedure as described (27). For the experiments shown in Fig. 6A, 4.2 pmol of in vitro transcribed tRNAPhe from yeast was used substrate. For the experiments shown in Fig. 6B the native tRNAsGlu present in the isolated total tRNA fraction from uninduced and induced (72 h) Tb-Nbp35 RNAi cells were used as import substrates. To be able to detect the imported tRNAGlu it was specifically labeled at its 3′-end with radioactive dCTP using the oligonucleotide-directed 3′-splint labeling technique (9, 28). The oligonucleotide that was used, 5′-GTGGTGGTCCTACCAGGAT-3′, hybridizes to the 3′-end of the tRNAGlu and leaves a 5′-G overhang. Using this technique the tRNAGlu could be highly efficiently labeled. The labeled tRNAGlu was then either gel-purified or directly used in the import assay yielding identical results.
RESULTS
Thiolation of Cytosolic tRNAs of T. brucei Requires Tb-Nbp35, Tb-Cfd1, and Tb-Nfs1
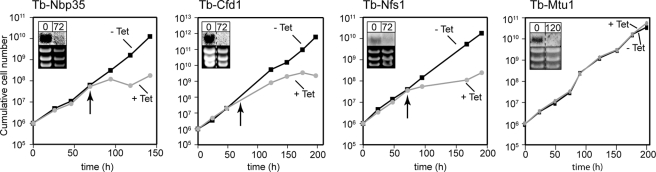
To investigate the role thio modifications may play in regulating mitochondrial tRNA import in T. brucei we produced cell lines allowing inducible RNAi-mediated ablation of components of the trypanosomal ISC biogenesis pathways. The selected factors were the orthologues of yeast Nbp35 and Cfd1, components of the cytosolic Fe/S protein assembly machinery, and of yeast Nfs1, a component of the mitochondrial ISC biogenesis pathway (17). Fig. 1 shows that ablation of any of these three factors leads to a growth arrest 3 days after induction of RNAi. This is expected, because ISC biogenesis is essential in all eukaryotes.
FIGURE 1.
Tb-Nbp35, TbCfd1, and Tb-Nfs1 but not Tb-Mtu1 are essential for normal growth of procyclic T. brucei. Growth curve in the presence and absence of tetracycline (+, -Tet) of representative clonal Tb-Nbp35, Tb-Cfd1, Tb-Nfs1, and Tb-Mtu1 RNAi cell lines. Insets: Northern blots of the corresponding mRNAs. The RNA from induced cells was isolated at 72 h, the time of the growth arrest (arrows). The rRNAs in the lower panel of the insets serve as loading controls.
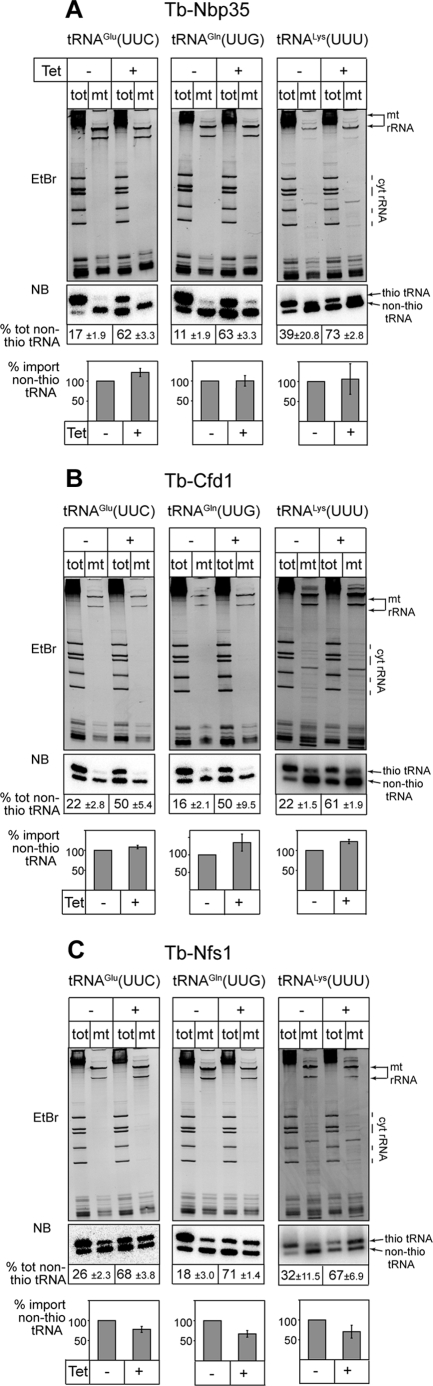
The wobble nucleotide of tRNAGlu(UUC) and tRNAGln(UUG) of Leishmania, a close relative of T. brucei, contains a thio group at position 2 of the uridine (13). To investigate whether this base modification requires the ISC biogenesis pathway we isolated total RNA from uninduced and induced RNAi cell lines and analyzed them by Northern blots. The RNA was separated on acrylamide gels containing APM, which causes a retardation in the migration of tRNAs that contain a thio carbonyl group (29, 30). Fig. 2 shows that ∼80% of total trypanosomal tRNAGlu(UUC) and tRNAGln(UUG) is thio-modified (Fig. 2, -Tet, tot samples). Moreover, down-regulation of either Tb-Nbp35, Tb-Cfd1, or Tb-Nfs1 led to a 2- to 5-fold increase of non-thio-modified tRNAGlu(UUC) and tRNAGln(UUG) (Fig. 2, +Tet, tot lanes) indicating that, as expected based on results in yeast (15), thiolation of cytosolic tRNAs requires all three proteins. The wobble base of tRNALys(UUU) is thought to be universally modified by a variety of modifications all of which include a thio group. Northern analysis shows that also in T. brucei the tRNALys(UUU) is thio-modified and that this modification depends on Tb-Nbp35, Tb-Cfd1, and Tb-Nfs1. This is different from the tRNALys(UUU) of the closely related L. tarentolae, which was reported to lack the thio modification (13).
FIGURE 2.
Ablation of Tb-Nbp35, Tb-Cfd1, and Tb-Nfs1 causes accumulation of non-thio-modified cytosolic tRNAs but does not influence mitochondrial import. A, RNA isolated from total cell (tot) and digitonin-extracted mitochondrial pellets (mt) (5) of uninduced (-Tet) and induced (+Tet) Tb-Nbp35-RNAi cell line were separated on APM-containing polyacrylamide gels that allow to separate thio-modified (thio-tRNA) from non-thio-modified tRNA (non-thio-tRNA). The top panel shows the ethidium bromide-stained gel. The bottom panel shows the corresponding Northern blot probed for the indicated tRNAs. The numbers at the bottom indicate the percentage (±S.E.) of non-thio-modified cytosolic tRNA before (-Tet) and after (+Tet) induction of RNAi for 72 h. The graph compares the extent of mitochondrial localization of the non-thio-modified tRNAs in uninduced cells (-Tet, set to 100%) to the one in induced cells (+Tet). Standard errors are indicated. B, same analysis as in A but for the Tb-Cfd1 RNAi cell line. C, same analysis as in A and B but for the Tb-Nfs1 RNAi cell line.
Accumulation of Non-thio-modified tRNA Does Not Increase Import
Similar to the situation in L. tarentolae (13), the thio-modified tRNAGlu(UUC) and tRNAGln(UUG) of T. brucei are highly enriched in the cytosolic fraction (Fig. 2, -Tet, tot lanes) and essentially absent in mitochondria (Fig. 2, -Tet, mt lanes). Thus, to test whether thio modifications act as anti-import determinants in T. brucei we analyzed the localization of the non-thio-modified tRNAs that accumulate after down-regulation of Tb-Cfd1, Tb-Nbp35, and Tb-Nfs1. Should the thio modification indeed function as an antideterminant for mitochondrial tRNA import one would expect an increase in the import of the non-thio-modified tRNA fraction. The cell fractionation in Fig. 2 (+Tet, tot and mt lanes) shows that this is not the case: in all three cell lines the non-thio-modified tRNAs that accumulate after induction of RNAi remain in the cytosol. Moreover, the same is the case for the non-thio-modified fraction of the tRNALys(UUU). Northern analyses of enriched nuclear and cytosolic RNA fractions (4) showed that the non-thio-modified tRNA accumulated in the cytosol and was not retained in the nucleus (data not shown).
Thiolation of Mitochondrial tRNATrp of T. brucei Requires Nfs1 and Mtu1
The fraction of leishmanial and trypanosomal tRNATrp that is imported into mitochondria is thiolated at the ultimate nucleotide before the anticodon (10). Thus, we decided to test whether Tb-Nbp35, Tb-Cfd1, or Tb-Nfs1, three components of Fe/S protein assembly machinery, besides being required for 2-thiolation of cytosolic tRNAs (Fig. 2) are also needed for thiolation of the imported mitochondrial tRNATrp. Moreover, we did the same test for the trypanosomal Mtu1 orthologue. Mtu1 is a highly conserved mitochondria-specific tRNA 2-thiouridylase whose bacterial MNMA homolog functions in 2-thiolation of bacterial tRNAs (22).
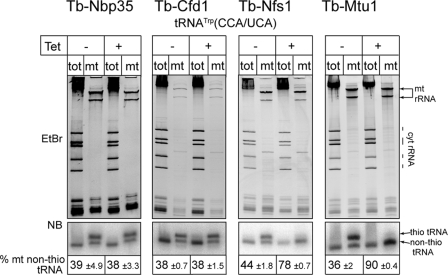
Accumulation of non-thio-modified tRNATrp in induced RNAi cell lines shows that mitochondria-specific thiolation of tRNATrp depends on Tb-Nfs1 and Tb-Mtu1 but, unlike thiolation of the cytosolic tRNAs and in line with results from yeast (16), does not require Tb-Nbp35 or Tb-Cfd1 (Fig. 3). However, unlike Tb-Nfs1, Tb-Mtu is not involved in thiolation of cytosolic tRNAs (data not shown). Interestingly, ablation of Tb-Mtu1 in contrast to the other three tested proteins does not impair growth (Fig. 1, rightmost panel). Because it is known that mitochondrial translation is essential in insect stage T. brucei this shows that functional mitochondrial proteins can be synthesized in the absence of thiolated tRNATrp.
FIGURE 3.
Ablation of Tb-Nfs1 and Tb-Mtu1 but not of Nbp35 and Tb-Cfd1 causes accumulation of non-thio-modified mitochondrial tRNATrp. RNA isolated from total cell (tot) and digitonin-extracted mitochondrial pellets (mt) of the indicated uninduced (-Tet) and induced (+Tet, 72 h for Tb-Nfs1, Tb-Nbp35, Tb-Cfd1, and 120 h for Tb-Mtu1) RNAi cell lines were separated on APM-containing polyacrylamide gels. Positions of thio-modified (thio-tRNA) and non-thio-modified tRNAs (non-thio-tRNA) are indicated. The top panel shows the ethidium bromide-stained gel. The bottom panel shows the corresponding Northern blot probed for the tRNATrp. The numbers at the bottom indicate the percentage (±S.E.) of non-thio-modified mitochondrial tRNATrp before (-Tet) and after (+Tet) induction of RNAi.
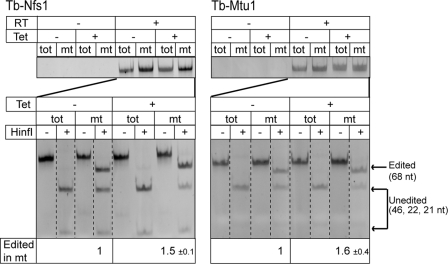
Removal of the Thiolation Stimulates tRNATrp Editing
The fraction of leishmanial and trypanosomal tRNATrp that is imported into mitochondria is not only thiolated at ultimate nucleotide before the anticodon but also undergoes C to U RNA editing at the wobble position to recognize the UGA codon that has been reassigned to tryptophan (11). RNAi-mediated down-regulation of Tb-Nfs1 and Tb-Mtu1 results in an accumulation of non-thio-modified tRNATrp (Fig. 3). The two RNAi cell lines can therefore be used to investigate whether and how the mitochondria-specific thiolation is connected to the C to U editing. The extent of RNA editing was analyzed by HinfI restriction digests of reverse transcription-PCR amplified tRNATrp. Fig. 4 shows that there is a 1.5- to 1.6-fold increase of the percentage of edited tRNATrp in induced Tb-Nfs1 and Tb-Mtu1 RNAi cells. This increase is due to accumulation of non-thio-modified tRNATrp, because it is not seen in the induced Tb-Nbp35 and Tb-Cfd1 RNAi cell lines (data not shown). Thus, it appears that thiolation at position 33 has an inhibitory effect on the C to U editing of the adjacent uridine. These results are in complete agreement with the recently published study by Wohlgamuth-Benedum et al. (31), which demonstrated the same inhibitory effect of thiolation on editing of trypanosomal tRNATrp by using a poisoned primer extension assay. Moreover, the results obtained with the Tb-Mtu1 RNAi cell line extend this study by showing that the degree of tRNATrp editing does not influence the growth rate of T. brucei (Fig. 1, rightmost panel).
FIGURE 4.
Down-regulation of thio-modified tRNATrp stimulates C to U editing. Extent of tRNATrp editing in induced and uninduced Tb-Nfs1 and Tb-Mtu1 RNAi cell lines was quantified by an reverse transcription-PCR based assay as described before (12). The top panel shows that the cytosolic and mitochondrial RNA fractions that were used as templates for RT are free of DNA. The extent of RNA editing was analyzed by using a HinfI restriction digest (lower panel). RNA editing destroys a HinfI site that is only present in the cDNA derived from the unedited tRNATrp (14). Introduction of a synthetic HinfI at the 5′-end of the 5′ reverse transcription-PCR primer provides an internal control for the HinfI digestion. cDNA amplified from unedited tRNATrp contains two HinfI sites and, thus, will be digested into three fragments (46, 22, and 21 nt; unedited). The cDNA derived from edited tRNATrp contains the synthetic HinfI site only and will be digested into two fragments (68 and 21 nt; edited). Measuring the intensities of the diagnostic bands for non-edited (46 nt) and edited (68 nt) tRNATrp allows, after correcting for their different molecular mass, determination of the fraction of edited tRNATrp in T. brucei mitochondria. The mean of the increase, including standard error of tRNATrp editing in induced RNAi cells, is indicated. The restriction digests have been analyzed on a single gel for each RNA cell line, but the lanes have been rearranged electronically for clarity (dashed lines).
Tb-Nbp35 RNAi Does Not Affect the tRNA Import Machinery
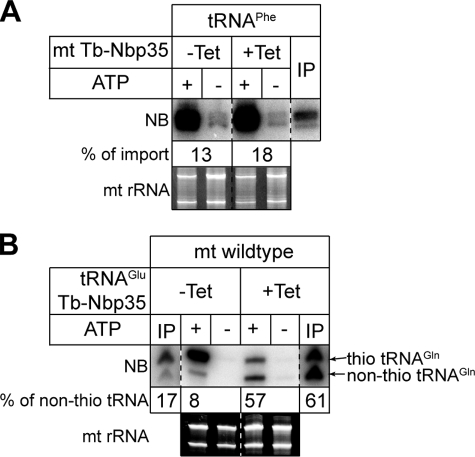
It would in principle be possible that the ISC machinery itself, or an unknown ISC-protein depending on it, is directly involved in mitochondrial tRNA import. Should this be the case the cytosolic accumulation of non-thio-modified tRNA observed in Fig. 2 could still be consistent with a putative role of thio modifications as antideterminants for mitochondrial tRNA import. To exclude this possibility we performed in vitro import assays: isolated mitochondria from the uninduced and induced Tb-Nbp35-RNAi cell line were incubated with in vitro transcribed substrate tRNA in the presence and absence of ATP. As a model substrate we were using in vitro transcribed tRNAPhe of yeast, because it had been shown by using transgenic cells that heterologous tRNAs of yeast and human origin are efficiently imported into T. brucei mitochondria in vivo (32).
Fig. 5A shows that in vitro transcribed tRNAPhe was efficiently imported into mitochondria isolated from uninduced cells and from cells induced for 72 h, the time point cytosolic accumulation of non-thio-modified tRNAs was observed. This shows that the cytosolic accumulation of non-thio-modified tRNAs caused by down-regulation of Tb-Nbp35 is not due to an impaired tRNA import machinery.
FIGURE 5.
In vitro import assays. A, Nbp35 is not required for tRNA import. In vitro import assays in the presence and absence of ATP using mitochondria isolated from the uninduced (-Tet) and induced (+Tet) Nbp35-RNAi cell line and in vitro transcribed yeast tRNAPhe as a substrate. The input lane (IP) depicts 1% of the added substrate. The imported tRNAPhe was detected by Northern blot and specific oligonucleotide hybridization. The percentages of the added substrate that is imported are indicated. B, in vitro import does not show preference for thio- or non-thio-modified tRNAGlu. In vitro import assay in the presence and absence of ATP using wild-type mitochondria and substrate tRNAGlu isolated from the uninduced (-Tet) and induced (+Tet) Nbp35-RNAi cell line into wild-type mitochondria. The substrate tRNAGlu was radioactively labeled using the 3′-end splint labeling technique (9, 28) and detected by using a PhosphorImager. The samples were analyzed on an APM gel. IP depicts 1% of the added substrate. The percentage of non-thio-modified tRNAGlu before and after import is indicated. All reactions shown in A and B were treated with micrococcus nuclease. The ethidium bromide-stained panels show the two mitochondrial rRNAs and serve as loading controls.
Thio-modified tRNAs Are Efficiently Imported in Vitro
If the thio modifications in cytosolic tRNAs do not function as import antideterminants both thio-modified and non-thio-modified tRNAs should be imported into isolated mitochondria with equal efficiency. To test this, we prepared tRNAs from induced and uninduced Tb-Nbp35 RNAi cell lines. The tRNAGlu present in the two fractions was specifically labeled by oligonucleotide-directed 3′-splint labeling (9, 28). Subsequently the two tRNA fractions were resolved on a polyacrylamide gel, and the labeled tRNA was visualized on the wet gel, cut out, and eluted. The eluted radioactive tRNAGlu(UUC) was then used as substrate for in vitro import assays. Fig. 5B shows that the ratio of thio-modified to non-thio-modified tRNAGlu(UUC) as monitored on APM gels did not significantly change during import into isolated wild-type mitochondria.
Our in vitro import assay faithfully reproduces the membrane translocation step of mitochondrial tRNA import. However, as described before, it does not show the same specificity that is observed in vivo (4). This can be explained by the absence of eukaryotic elongation factor 1a, which has been shown to mediate the specificity of tRNA import in T. brucei. Thus, our results show that at the level of the membrane translocation step there is no selectivity for either thio-modified or non-thio-modified tRNAs.
Imported tRNAGlu Is Subject to Dethiolation
If thio-modified tRNAs can be imported into mitochondria in vitro (Fig. 5B) why do they accumulate in the cytosol in vivo? A possible explanation would be that thio-modified tRNAs are indeed imported into mitochondria in vivo but that after import the thio modification is removed. Such a scenario could explain the highly enriched cytosolic localization of the thio-modified tRNAGlu(UUC) and tRNAGln(UUG) that is seen at steady state.
The dynamics of the modification can be studied by following the fate of the thiolation on newly synthesized tRNAGlu(UUC). For this purpose we produced a cell line that allows inducible expression of a tagged tRNAGlu(UUC). This was achieved by transfection of T. brucei 29-13, which expresses the tetracycline repressor, with a construct containing the tetracycline operator 5′ of the tagged tRNAGlu(UUC) gene. We have previously used the same approach to study import of newly synthesized tagged tRNAMet (4). The two nucleotide replacements that were used as tags for the tRNAGlu(UUC) (Fig. 6A) are not expected to interfere with the function of the tRNA since they occur naturally in tRNAsGlu of other eukaryotes (33). Fig. 5B shows that addition of tetracycline induces expression of the tagged tRNAGlu(UUC) in a time-dependent manner. Moreover, analysis of digitonin-extracted mitochondrial fractions showed that the tagged tRNAGlu(UUC) is thiolated and imported into mitochondria.
The RNA samples were separated on an APM-containing gel to determine the ratio of thiolated versus non-thiolated tagged tRNAGlu(UUC) at different time points. As expected the extent of thiolation of the newly synthesized tagged tRNAGlu(UUC) in the total RNA fraction was essentially identical to the one observed for the endogenous wild-type tRNAGlu(UUC) and remained constant over time (Fig. 6B, top and middle panel). However, for the newly synthesized tagged tRNAGlu(UUC) that was imported into mitochondria the situation was different. Whereas shortly after synthesis a large extent was thio-modified this fraction decreased at longer induction times (Fig. 6B, upper panel, compare 6 h and 24 h).
To analyze the dynamics of the thiolation more precisely we quantified its extent in total and mitochondrial tRNAGlu(UUC) 6 and 72 h after induction in three independent experiments. The extent of thiolation of the newly synthesized tRNAGlu(UUC) in the total RNA fraction was the same at 6 and 72 h of induction. The tagged tRNAGlu(UUC) that was imported into mitochondria, however, was thiolated to the same extent than its cytosolic counterpart when analyzed 6 h after induction but shows a significant lower level of thiolation when analyzed 72 h after induction. This lower level is close to the one of the endogenous imported tRNAGlu(UUC) (Fig. 6C, right panel). At this time point the tagged tRNAGlu(UUC) is at steady state and therefore does not represent the newly synthesized tRNAGlu(UUC) anymore.
In principle there are three ways of how to explain the time-dependent decline of the thiolation level of mitochondrial tRNAGlu: (i) higher import efficiency for non-thiolated tRNAGlu, (ii) intramitochondrial degradation that is specific for the thiolated species, or (iii) removal of the thiomodification after import. Different import efficiencies for the two tRNAGlu species can be excluded based on the in vitro import assays shown in Fig. 5B as well as based on data shown by Paris et al. (34). Moreover, should selective intramitochondrial degradation of the thiolated tagged tRNAGlu be responsible for the observed accumulation of non-thiolated tRNA in mitochondria, we would expect the percentage of the tagged mitochondrial tRNAGlu to decrease with time, because with time an increasingly larger fraction of the molecules would be degraded. The bottom right panel of Fig. 6B shows that this is not the case, because the fraction of tagged tRNA that is present in mitochondria is very similar at the 6, 8, and 24 h, respectively. However, during that time period we clearly see a shift in the ratio of thio-modified to non-modified tRNAGlu (Fig. 6B, top panel).
Thus the most parsimonious explanation for our results is that in vivo the tRNAGlu(UUC) is imported in the thiolated state and that after import it becomes partially dethiolated explaining the enrichment of dethiolated tRNAGlu(UUC) that is observed in mitochondria.
DISCUSSION
The involvement of components in the ISC biogenesis pathway for thio modification of eukaryotic tRNAs has so far mainly been analyzed in yeast (15, 16). In the present study we have extended this analysis to the parasitic protozoa T. brucei. We show that Tb-Nbp35 and Tb-Cfd1, components of the cytosolic Fe/S protein assembly machinery, as well as Nfs1, a component of the mitochondrial ISC biogenesis machinery, are required to thiolate cytosolic tRNAGlu(UUC), tRNAGln(UUG), and tRNALys(UUU) of T. brucei. Thiolation of the mitochondrially imported tRNATrp, however, required Tb-Nfs1 and Tb-Mtu1 but was independent of Tb-Nbp35 and Tb-Cfd1. According to the recently revised eukaryotic phylogeny eukaryotes are divided into six supergroups (35, 36). S. cerevisiae together with humans belongs to Ophistokonta, whereas T. brucei belongs to Excavata. The fact that tRNA thiolation in trypanosomes requires the same components as in yeast indicates that the tRNA thio modification pathway is conserved within the two supergroups and strongly suggests that it was already present in the common ancestor of all eukaryotes.
L. tarentolae and T. brucei are closely related, and most aspects of mitochondrial tRNA import that have been investigated in the two species are highly similar. Both lack mitochondrial tRNA genes (37, 38), show identical import specificity and edit the imported tRNATrp to accommodate the variant genetic code (11, 12). Whether the actual tRNA import machinery is also identical is unclear at present. In Leishmania tropica the tRNA import machinery has been reported to consist of a heterooligomeric protein complex composed mainly of components from different respiratory complexes (39), whereas in T. brucei it has not been characterized yet. Taking into account all the similarities described above and their shared evolutionary history we would expect the tRNA import mechanism to be conserved between the two groups of trypanosomatids.
However, our experiments in T. brucei show (i) that increasing the fraction of non-thio-modified tRNA in vivo does not increase the extent of mitochondrial partition of any of the three tested tRNAs and (ii) that thiolated tRNAs can be efficiently imported into mitochondria both in vitro and in vivo. This demonstrates that in T. brucei the thio modification of the wobble nucleotide in cytosolic tRNAs does not function as an antideterminant for mitochondrial tRNA import.
The same conclusion was reached in a recent publication by Paris et al. (34). They were able to show that ablation of Tb-Nfs1 by RNAi does not change the extent of mitochondrial localization of thio-modified or non-thio-modified trypanosomal tRNAs. Further experiments in the same study showed that a chemically dethiolated tRNAGlu was as efficiently imported into isolated mitochondria as its fully modified counterpart (34). These results are fully consistent with our results and support the conclusion that in T. brucei thio modifications do not act as antideterminants for mitochondrial tRNA import.
Thus, it appears that while thio modifications mediate tRNA sorting in Leishmania they play no such role in T. brucei. However, in the light of the fact that T. brucei and Leishmania are closely related it should also be considered that the situation might be the same in both organisms. In fact, a detailed comparison of the in vitro import experiments done in T. brucei and Leishmania shows that the apparent contradiction between the in vitro import experiments can be explained. In the original study in L. tarentolae it was shown that in vitro import of the native fully modified tRNAGlu(UUC) was less efficient than the corresponding in vitro transcribed tRNAGlu(UUC) lacking any nucleotide modifications (13). However, an average tRNA has 13 modified residues. It is therefore possible that the difference in the in vitro import efficiencies of the tested substrates is not a consequence of the thio-modified uridine but of one or more of the other modifications. The experiments performed in our study and in the one by Paris et al. (34), on the other hand, compared in vitro import efficiencies of two tRNAsGlu(UUC) that differ only by the wobble nucleotide thio modification. Interestingly, in these cases both substrates were imported with equal efficiency.
The fact that thio-modified tRNAGlu(UUC) and tRNAGln(UUG) in L. tarentolae are essentially specific for the cytosol and that their non-thio-modified counterparts are highly enriched in mitochondria has been taken as evidence that thio-modified tRNAs are not imported in vivo (13). The thio-modified tRNAGlu(UUC) and tRNAGln (UUG) of T. brucei show essentially the same intracellular distribution than the corresponding tRNAs in L. tarentolae. However, we show that in T. brucei the mitochondrial enrichment of the non-thio-modified tRNAGlu(UUC) can best be explained by post import removal of the thio modification rather than by selective import of the non-thio-modified tRNA.
Dethiolation could either be due to non-enzymatic intramitochondrial oxidation or it could be an enzyme-mediated reaction. Our results do not allow us to distinguish between the two possibilities. However, the fact that a large fraction of mitochondrial tRNATrp is permanently 2-thio-modified (10, 12) shows that dethiolation is specific for a subset of tRNAs, which suggests that it is enzyme-catalyzed.
2-Thiolation is highly conserved in eukaryotes and occurs in tRNAs that read two degenerate codons ending in purine of split codon boxes where the pyrimidine- and purine-ending codons code for different amino acids. The 2-thiolation together with a methoxycarbonylmethyl group on position 5 (mcm5S2) were proposed (i) to restrict the wobble base pairing to purines and (ii) to stabilize the correct codon anticodon interaction (40–42). The fully modified wobble uridine (mcm5S2U) of cytosolic tRNAs was shown to be essential in S. cerevisiae (43). In nematodes and in fission yeast 2-thiolation of tRNAs has been implicated in maintaining genome integrity (18). Finally there is a link between 2-thiolation of tRNA and human diseases: a mutation in the human mitochondrial tRNALys gene, which abolishes the modification of the wobble uridine, including the 2-thiolation causes myoclonus epilepsy associated with ragged-red fibers by preventing efficient binding of the mutant tRNA to the ribosome (44).
As expected for eukaryotic tRNAs the wobble uridine of leishmanial tRNAGlu(UUC) and tRNAGln(UUG) is modified to mcm5U (13). Moreover, the cytosolic fraction of the wobble uridine contains a thio modification at position 2 of the uracil resulting in mcm5S2U. It is reasonable to assume that also in trypanosomatids these modifications serve to restrict wobble base pairing and to stabilize codon anticodon interactions. However, the wobble nucleotide of the mitochondrial fraction of trypanosomatid tRNAGlu(UUC) and tRNAGln(UUG) lacks the thio group and contains instead a 2′-O-methyl group on the ribose yielding mcm5Um (13). Thus, the mitochondrial 2′-O- methyl group probably serves an analogous function as the cytosolic 2-thio modification in the cytosol.
In addition to modulating decoding, thio modifications may have regulatory functions. It has been suggested that in T. brucei cytosol-specific thio modifications influence the stability of tRNAGlu and tRNAGln (31). We could confirm this observation in our RNAi cell lines, however, the down-regulation was more variable and less pronounced. Wohlgamuth-Benedum et al. (31) furthermore showed that down-regulation of thiolation by ablation of Nfs1 causes stimulation of tRNA editing. Based on this result they proposed that the mitochondria-specific thiolation of the tRNATrp plays a role in regulating the extent of editing. Tight regulation of tRNA editing might be required if unedited and edited tRNATrp have non-overlapping functions in translation assigned to UGG and UGA codons, respectively. We have shown that ablation of Mtu1 essentially abolishes thiolation of mitochondrial tRNATrp, which in agreement with the results by Wohlgamuth-Benedum et al. (31) leads to an increase in C to U editing of the wobble nucleotide. However, ablation of Mtu1 did not effect growth of insect stage T. brucei even though the energy metabolism of these cells depends on oxidative phosphorylation and therefore requires mitochondrial gene products. These results show that efficient synthesis of mitochondrial proteins tolerates significant changes in the ratio of unedited to edited tRNATrp. However, this is in cell culture and the situation might well be different in vivo and/or in the different stages of the life cycle.
In summary, we have shown that thio modification of the wobble nucleotide in cytosolic tRNAs of trypanosomatids, unlike previously suggested, are not antideterminants for mitochondrial tRNA import. Moreover, we demonstrate that thio-modified tRNAs can readily be imported both in vitro and in vivo. Finally, we provide evidence that the thio group gets removed after import, illustrating an unprecedented dynamics of a tRNA nucleotide modification.
Acknowledgments
We thank Ramon Kranaster, University of Konstanz, for the synthesis of APM, Elke K. Horn and Katharina Schmid-Lüdi for excellent technical assistance, and Marina Cristodero and Moritz Niemann for helpful comments on the manuscript.
This work was supported by Grant 3100A0_121937 (to A. S.) from the Swiss National Foundation.
- mcm5U
- methoxycarbonylmethyl uridine
- mcm5S2U
- mcm5U with a cytosol-specific thio modification at position 2 of uracil
- mcm5Um
- mcm5U with a mitochondria-specific 2′-O-methyl group in ribose
- ISC
- Fe/S cluster
- APM
- ((N-acryloylamino)phenyl)mercuric chloride
- RNAi
- RNA interference
- nt
- nucleotide(s).
REFERENCES
- 1.Salinas T., Duchêne A. M., Maréchal-Drouard L. (2008) Trends Biochem. Sci. 33, 320–329 [DOI] [PubMed] [Google Scholar]
- 2.Schneider A., Maréchal-Drouard L. (2000) Trends Cell Biol. 10, 509–513 [DOI] [PubMed] [Google Scholar]
- 3.Schneider A. (2001) Int. J. Parasitol. 31, 1403–1415 [DOI] [PubMed] [Google Scholar]
- 4.Bouzaidi-Tiali N., Aeby E., Charrière F., Pusnik M., Schneider A. (2007) EMBO J. 26, 4302–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan T. H., Pach R., Crausaz A., Ivens A., Schneider A. (2002) Mol. Cell. Biol. 22, 3707–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapushoc S. T., Alfonzo J. D., Rubio M. A., Simpson L. (2000) J. Biol. Chem. 275, 37907–37914 [DOI] [PubMed] [Google Scholar]
- 7.Vinogradova E., Salinas T., Cognat V., Remacle C., Maréchal- Drouard L. (2009) Nucleic Acids Res. 37, 1521–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider A. (1996) Nucleic Acids Res. 24, 1225–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider A., McNally K. P., Agabian N. (1994) Nucleic Acids Res. 22, 3699–3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crain P. F., Alfonzo J. D., Rozenski J., Kapushoc S. T., McCloskey J. A., Simpson L. (2002) RNA 8, 752–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alfonzo J. D., Blanc V., Estévez A. M., Rubio M. A., Simpson L. (1999) EMBO J. 18, 7056–7062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charrière F., Helgadóttir S., Horn E. K., Söll D., Schneider A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 6847–6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko T., Suzuki T., Kapushoc S. T., Rubio M. A., Ghazvini J., Watanabe K., Simpson L., Suzuki T. (2003) EMBO J. 22, 657–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki T. (2005) in Fine-tuning of RNA Functions by Modification and Editing (Grosjean H. ed) pp. 24–69, Springer, New York [Google Scholar]
- 15.Nakai Y., Nakai M., Lill R., Suzuki T., Hayashi H. (2007) Mol. Cell. Biol. 27, 2841–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakai Y., Umeda N., Suzuki T., Nakai M., Hayashi H., Watanabe K., Kagamiyama H. (2004) J. Biol. Chem. 279, 12363–12368 [DOI] [PubMed] [Google Scholar]
- 17.Lill R., Mühlenhoff U. (2008) Annu. Rev. Biochem. 77, 669–700 [DOI] [PubMed] [Google Scholar]
- 18.Dewez M., Bauer F., Dieu M., Raes M., Vandenhaute J., Hermand D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5459–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakai Y., Nakai M., Hayashi H. (2008) J. Biol. Chem. 283, 27469–27476 [DOI] [PubMed] [Google Scholar]
- 20.Huang B., Lu J., Byström A. S. (2008) RNA 14, 2183–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noma A., Sakaguchi Y., Suzuki T. (2009) Nucleic Acids Res. 37, 1335–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umeda N., Suzuki T., Yukawa M., Ohya Y., Shindo H., Watanabe K., Suzuki T. (2005) J. Biol. Chem. 280, 1613–1624 [DOI] [PubMed] [Google Scholar]
- 23.Wirtz E., Leal S., Ochatt C., Cross G. A. (1999) Mol. Biochem. Parasitol. 99, 89–101 [DOI] [PubMed] [Google Scholar]
- 24.Bochud-Allemann N., Schneider A. (2002) J. Biol. Chem. 277, 32849–32854 [DOI] [PubMed] [Google Scholar]
- 25.Crausaz Esseiva A., Maréchal-Drouard L., Cosset A., Schneider A. (2004) Mol. Biol. Cell 15, 2750–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCulloch R., Vassella E., Burton P., Boshart M., Barry J. D. (2004) Methods Mol. Biol. 262, 53–86 [DOI] [PubMed] [Google Scholar]
- 27.Schneider A., Charrière F., Pusnik M., Horn E. K. (2007) Methods Mol. Biol. 372, 67–80 [DOI] [PubMed] [Google Scholar]
- 28.Hausner T. P., Giglio L. M., Weiner A. M. (1990) Genes Dev. 4, 2146–2156 [DOI] [PubMed] [Google Scholar]
- 29.Igloi G. L., Kössel H. (1987) Methods Enzymol. 155, 433–448 [DOI] [PubMed] [Google Scholar]
- 30.Igloi G. L. (1988) Biochemistry 27, 3842–3849 [DOI] [PubMed] [Google Scholar]
- 31.Wohlgamuth-Benedum J. M., Rubio M. A., Paris Z., Long S., Poliak P., Lukes J., Alfonzo J. D. (2009) J. Biol. Chem. 284, 23947–23953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hauser R., Schneider A. (1995) EMBO J. 14, 4212–4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jühling F., Mörl M., Hartmann R. K., Sprinzl M., Stadler P. F., Pütz J. (2009) Nucleic Acids Res. 37, D159–D162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paris Z., Rubio M. A., Lukes J., Alfonzo J. D. (2009) RNA 15, 1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adl S. M., Simpson A. G., Farmer M. A., Andersen R. A., Anderson O. R., Barta J. R., Bowser S. S., Brugerolle G., Fensome R. A., Fredericq S., James T. Y., Karpov S., Kugrens P., Krug J., Lane C. E., Lewis L. A., Lodge J., Lynn D. H., Mann D. G., McCourt R. M., Mendoza L., Moestrup O., Mozley-Standridge S. E., Nerad T. A., Shearer C. A., Smirnov A. V., Spiegel F. W., Taylor M. F. (2005) J. Eukaryot. Microbiol. 52, 399–451 [DOI] [PubMed] [Google Scholar]
- 36.Simpson A. G., Roger A. J. (2004) Curr. Biol. 14, R693–R696 [DOI] [PubMed] [Google Scholar]
- 37.Hancock K., Hajduk S. L. (1990) J. Biol. Chem. 265, 19208–19215 [PubMed] [Google Scholar]
- 38.Simpson A. M., Suyama Y., Dewes H., Campbell D. A., Simpson L. (1989) Nucleic Acids Res. 17, 5427–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adhya S. (2008) Int. J. Biochem. Cell Biol. 40, 2681–2685 [DOI] [PubMed] [Google Scholar]
- 40.Johansson M. J., Esberg A., Huang B., Björk G. R., Byström A. S. (2008) Mol. Cell. Biol. 28, 3301–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Numata T., Ikeuchi Y., Fukai S., Suzuki T., Nureki O. (2006) Nature 442, 419–424 [DOI] [PubMed] [Google Scholar]
- 42.Ashraf S. S., Sochacka E., Cain R., Guenther R., Malkiewicz A., Agris P. F. (1999) RNA 5, 188–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Björk G. R., Huang B., Persson O. P., Byström A. S. (2007) RNA 13, 1245–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasukawa T., Suzuki T., Ishii N., Ohta S., Watanabe K. (2001) EMBO J. 20, 4794–4802 [DOI] [PMC free article] [PubMed] [Google Scholar]