Abstract
The signaling mechanisms facilitating cardiomyocyte (CM) differentiation from bone marrow (BM)-derived mesenchymal stem cells (MSCs) are not well understood. 5-Azacytidine (5-Aza), a DNA demethylating agent, induces expression of cardiac-specific genes, such as Nkx2.5 and α-MHC, in mouse BM-derived MSCs. 5-Aza treatment caused significant up-regulation of glycogen synthase kinase (GSK)-3β and down-regulation of β-catenin, whereas it stimulated GSK-3α expression only modestly. The promoter region of GSK-3β was heavily methylated in control MSCs, but was demethylated by 5-Aza. Although overexpression of GSK-3β potently induced CM differentiation, that of GSK-3α induced markers of neuronal and chondrocyte differentiation. GSK-3 inhibitors, including LiCl, SB 216743, and BIO, abolished 5-Aza-induced up-regulation of CM-specific genes, suggesting that GSK-3 is necessary and sufficient for CM differentiation in MSCs. Although specific knockdown of endogenous GSK-3β abolished 5-Aza-induced expression of cardiac specific genes, surprisingly, that of GSK-3α facilitated CM differentiation in MSCs. Although GSK-3β is found in both the cytosol and nucleus in MSCs, GSK-3α is localized primarily in the nucleus. Nuclear-specific overexpression of GSK-3β failed to stimulate CM differentiation. Down-regulation of β-catenin mediates GSK-3β-induced CM differentiation in MSCs, whereas up-regulation of c-Jun plays an important role in mediating CM differentiation induced by GSK-3α knockdown. These results suggest that GSK-3α and GSK-3β have distinct roles in regulating CM differentiation in BM-derived MSCs. GSK-3β in the cytosol induces CM differentiation of MSCs through down-regulation of β-catenin. In contrast, GSK-3α in the nucleus inhibits CM differentiation through down-regulation of c-Jun.
Introduction
Ischemic cardiomyopathy and myocardial infarction are accompanied by an irreversible loss of cardiomyocytes, endothelial cells, and smooth muscle cells, essential components of the heart (1). Cell-based cardiac repair offers the promise of rebuilding the injured heart from its component parts (reviewed in Refs. 2 and 3). Although remarkable progress in the field has clearly proven the concept of “cell-based cardiac repair,” initial clinical studies using adult stem cells have shown that the salutary effects mediated by cell transplantations are generally modest (4–7). A major challenge for cardiac regeneration therapy using adult stem cells may be to enhance stem cell differentiation into cardiomyocytes.
Among several important signaling mechanisms generally involved in cardiomyocyte differentiation, Wnt/β-catenin (canonical Wnt) and non-canonical Wnt signaling have been suggested to have a critical role in cardiogenesis (8). Glycogen synthase kinase (GSK)2 -3 is a key component of the canonical Wnt signaling pathway. GSK-3 phosphorylates β-catenin, and phosphorylated β-catenin is then subjected to ubiquitin proteasome degradation. However, upon Wnt binding to its receptors, Frizzled and low-density lipoprotein receptor-related protein, β-catenin phosphorylation by GSK-3β is inhibited and β-catenin is stabilized. Stabilized β-catenin translocates into the nucleus and induces target gene expression. Although both the canonical and non-canonical Wnt pathways are important in mediating cardiomyocyte differentiation in stem cells and cardiac progenitor cells (9–15), the role of downstream components of the Wnt pathway, and, in particular, the role of GSK-3, in mediating cardiomyocyte differentiation is not yet fully understood.
GSK-3 is a serine/threonine kinase that has a wide variety of functions in cells. GSK-3 phosphorylates many known intracellular targets, including β-catenin, glycogen synthase, elF2Bϵ, GATA4, myocardin, c-Jun, cyclin D1, and N-Myc, thereby regulating various cellular functions, including hypertrophy and apoptosis in cardiomyocytes (16). GSK-3 has two major isoforms, GSK-3α and GSK-3β, which have 97% identical amino acids in the catalytic domain but differ substantially in the N and C termini. Increasing lines of evidence suggest that GSK-3α and GSK-3β both have common and non-overlapping functions (17). For example, both GSK-3α and GSK-3β phosphorylate/degrade β-catenin in embryonic stem cells, but GSK-3α and GSK-3β play distinct roles in cardiac development in mice (18, 19). Importantly, the isoform-specific functions of GSK-3α and GSK-3β during cardiomyocyte differentiation are not well understood in mesenchymal stem cells (MSCs).
5-Azacytidine (5-Aza) is a chemical analogue of cytidine that removes methyl groups from DNA, thereby inducing gene expression. 5-Aza is a potent inducer of cardiomyocyte differentiation in bone marrow-derived MSCs (20). MSCs show potential for clinical application with evidence of tissue regeneration, including myocardial regeneration (21). We reasoned that by studying the function of signaling molecules modulated during cardiac differentiation, we should be able to elucidate the signaling mechanisms involved in stimulating cardiomyocyte differentiation in adult stem cells. Through initial screening of signaling molecules modulated by 5-Aza during cardiomyocyte differentiation in MSCs, we found that GSK-3 plays an important role in regulating this process.
Thus, the goals in this study were to elucidate whether GSK-3α/β is affected during differentiation of MSCs into the cardiomyocyte lineage in response to 5-Aza treatment, and if so, to examine whether modulation of GSK-3 plays a causative role in mediating cardiomyocyte differentiation in MSCs. Furthermore, we evaluated whether GSK-3α and GSK-3β play distinct roles in mediating cardiomyocyte differentiation and whether regulation of β-catenin is involved in modulation of cardiomyocyte differentiation by GSK-3α/β.
EXPERIMENTAL PROCEDURES
Transgenic Mice and MSC Culture
MSCs were isolated from bone marrow aspirates of 2–3-week-old C57BL/6 mice. Wild type mice, Tet-inducible GSK-3β transgenic mice (Tg-Tet-GSK-3β), Tg- Tet-GSK-3β mice cross-bred with CMV-tTA transgenic mice (Tg-Tet-GSK-3β-tTA) (16), and transgenic mice harboring the mouse Nkx2.5 (9.0 kb) promoter-LacZ (see below) were used. Tg-Tet-GSK-3β-tTA mice were fed doxycycline (Dox)-containing chow to suppress GSK-3β transgene expression. MSCs were cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium/F-12 (Invitrogen) and mesenchymal basal medium (Stem Cell) supplemented with 10% fetal bovine serum (Atlanta Biologicals) and 1% l-glutamine (Invitrogen). At passage three, cells were positive for CD105, CD29, and CD44, and negative for CD45. MSCs passaged 3–5 times were used.
Plasmid Constructs and Adenoviral Vectors
Adenoviruses harboring GSK-3α, GSK-3β, dominant-negative GSK-3β (DN-GSK-3β), β-catenin, and LacZ have been described previously (16, 22, 23). To make GSK-3β with a nuclear localization signal (GSK-3β-NLS), cDNA encoding GSK-3β was subcloned into pEF/myc/nuc (Invitrogen). cDNA encoding Wnt11 was amplified by reverse transcription-polymerase chain reaction from mouse heart mRNA, subcloned into pCR2.1-TOPO (Invitrogen), and sequenced to confirm the correct sequence. cDNA encoding Wnt3a was purchased from Origene. Complementary hairpin sequences for GSK-3α, GSK-3β, β-catenin, c-Jun, and scramble (supplemental Table S1) were commercially synthesized and cloned into pSilencer 2.0 under the U6 promoter (Ambion). Adenovirus vectors harboring GSK-3β-NLS, Wnt3a, Wnt11, shRNA-GSK-3α, shRNA-GSK-3β, shRNA-β-catenin, shRNA-c-Jun, and shRNA-scramble were generated using AdMax (Microbix).
5-Aza Treatment
MSCs were treated with 5-Aza (Sigma, 5 μm) for 24 h without serum and then cultured in serum containing culture medium. Identically prepared MSCs without 5-Aza treatment were used as a control. Inhibition of GSK-3 kinase activity was achieved by maintaining MSCs in culture medium containing LiCl (10 mm), BIO (0.5 μg/ml), or SB216743 (2 μg/ml).
GSK-3β Conditional Overexpression Using Tet-On or Tet-Off System
Adenovirus vector harboring tTA or rtTA was introduced into Tet-MSCs isolated from Tg-Tet-GSK-3β mice. tTA- or rtTA-transduced MSCs were cultured with or without Dox (0.5 μg/ml, Clontech). Tet/tTA-MSCs isolated from Tg-Tet-GSK-3β-tTA mice were maintained in Dox containing medium and changed to normal culture medium for induction of GSK-3β transgene.
Western Blots
Total cell extracts were prepared, using cell lysis buffer containing 20 mm Tris-Cl (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 0.5 μg/ml of leupeptin, and 0.5 mm 4-(2-aminoethyl)benzenesulfonyl fluoride. For immunoblot analyses, polyvinylidene difluoride membranes were incubated with 5% nonfat milk buffer containing primary antibody overnight, followed by incubation with anti-mouse IgG or anti-rabbit IgG (Cell Signaling Technology, 1:2500 dilution) for 3 h. The following antibodies were used as primary antibodies: GSK-3α (Cell Signaling Technology, 1:5000), GSK-3β (BD Biosciences, 1:5000), phospho-GSK-3α/β (Cell Signaling Technology, 1:5000), phospho-GSK-3β (Cell Signaling Technology, 1:5000), β- catenin (BD Biosciences, 1:10000), Wnt3a (Santa Cruz Biotechnology, 1:2500), Wnt11 (R&D Systems, 1:2000), phospho-protein kinase C (Pan) (Cell Signaling Technology, 1:2500), sarcomeric α-actinin (Sigma, 1:10000), GATA4 (Santa Cruz Biotechnology, 1:5000), c-Jun (Cell Signaling Technology, 1:2500), troponin I (Santa Cruz Biotechnology, 1:5000), β-actin (Sigma, 1:10000), and GAPDH (Sigma, 1:5000).
Immunocytochemistry
MSCs were washed with phosphate-buffered saline, fixed with 4% paraformaldehyde for 10 min, permeabilized in 0.3% Triton X-100 for 10 min, and blocked in 3% bovine serum albumin for 1 h. The following antibodies were used as primary antibodies: Oct3/4 (Santa Cruz Biotechnology, 1:200 dilution), troponin I (Santa Cruz Biotechnology, 1:1000), cardiac troponin I (Abcam, 1:200), sarcomeric α-actinin (Sigma, 1:500), GSK-3β (BD Biosciences, 1:500), GSK-3α (Abcam, 1:500), and c-Jun (Cell Signaling Technology, 1:500).
Reverse Transcriptase-PCR
Total RNA was extracted using TRIzol (Invitrogen) and 1 μg of RNA was used for cDNA synthesis (Thermoscriptase®, Ambion). The RT-PCR mixture (Promega) was incubated at 95 °C for 5 min followed by 95 °C for 30 s, 59 °C for 1 min, and 72 °C for 30 s for 34 cycles and then incubated at 72 °C for 7 min. PCR primers used in this study are shown in supplemental Table S1.
Methylation Specific PCR
Genomic DNA was extracted from MSCs using the Wizard Genomic DNA Purification Kit (Promega) and then treated with the CpGenome Fast DNA Modification Kit (Millipore). CpGenome-modified DNA (1 μg) was subjected to PCR with methylation- or non-methylation-specific primers (supplemental Table S1) (24).
Transgenic Mice Harboring Nkx2.5-LacZ
cDNA containing mouse Nkx2.5 promoter-LacZ, which directs cardiac specific activation of luciferase (25), was kindly provided by Dr. K. Yutzey (The Children's Hospital Research Foundation, Cincinnati, OH). Transgenic mice harboring the mouse Nkx2.5 (9.0 kb) promoter-luciferase were generated on FVB background.
Luciferase Assay
Plasmids harboring TOP flash (TCF-luciferase plasmid, Millipore) and FOP flash (mutant TCF-luciferase plasmid, Millipore) were transfected into MSCs using Lipofectamine reagent (Invitrogen), and the luciferase activity was measured with the Luciferase Assay System (Promega) after cell lysis with Passive Lysis Buffer (Promega).
Statistical Analyses
All values are expressed as mean ± S.E. Statistical analyses were performed using analysis of variance and Newman-Keuls multiple comparison test with a p < 0.05 considered significant.
RESULTS
Murine Bone Marrow-derived MSCs Express Pluripotent Markers and 5-Aza Induces Expression of Cardiac Marker Genes in MSCs
MSCs at the third passage expressed Oct4 and Rex1 mRNA, pluripotent stem cell markers (Fig. 1A). Ninety-five percent of MSCs at the third passage were Oct3/4 positive (Fig. 1B). Although untreated bone marrow-derived mouse MSCs do not express cardiac marker genes, 5-Aza treatment (5 μm for 24 h), an established method of inducing bone marrow MSC differentiation into cardiomyocytes (20), induced mRNA expression of Nkx2.5, one of the earliest cardiac markers, and α-myosin heavy chain (α-MHC), a contractile protein (Fig. 1C). Although no cardiac troponin I (cTnI) positive cells were observed in control MSCs, 5-Aza induced a premature but clear striation pattern of cTnI in MSCs (Fig. 1D).
FIGURE 1.
Basal characteristics of mouse BM-derived MSCs. A, total RNA was prepared from MSCs at the 3rd and 10th passages. mRNA expression of Oct4 and Rex1, pluripotent stem cell markers, was evaluated by RT-PCR. B, protein expression of Oct3/4 (red) in MSCs at the 3rd passage was evaluated by immunostaining. Cells were co-stained with 4′,6-diamidino-2-phenylindole (DAPI). C and D, MSCs were treated with 5-Aza (5 μm) and cultured for 5 days. C, total RNA was prepared and mRNA expression of Nkx2.5, α-MHC, and GAPDH (internal control) evaluated by RT-PCR. D, protein expression of cTnI (green) in MSCs treated with or without 5-Aza was evaluated by immunostaining. Cells were co-stained with DAPI. The results are representative of at least 4 experiments.
5-Aza Induces Cardiomyocyte Differentiation through an Increase in GSK-3β Protein and mRNA Expression
GSK-3β and β-catenin are important components of the canonical Wnt signaling pathway. To examine the effect of 5-Aza on the canonical Wnt signaling pathway, protein expression of GSK-3 isoforms and β-catenin was evaluated in 5-Aza-treated (5 μm for 24 h) and control MSCs. Expression of GSK-3α and GSK-3β was detectable but low. On the other hand, β-catenin was expressed abundantly in unstimulated MSCs. 5-Aza treatment increased expression of GSK-3α and GSK-3β in a time-dependent manner in MSCs, although induction of GSK-3α by 5-Aza was milder than that of GSK-3β (Fig. 2, A and B). Expression of GSK-3β at day 5 was significantly greater in 5-Aza-treated MSCs than in untreated MSCs (supplemental Fig. S1A). 5-Aza treatment did not induce up-regulation of GSK-3β in COS-7 cells, suggesting that the effect of 5-Aza is cell type-specific (supplemental Fig. S1B). Up-regulation of GSK-3α and GSK-3β by 5-Aza was also observed at the mRNA level (supplemental Fig. S1C). The promoter regions of GSK-3α and GSK-3β contain CpG islands (supplemental Fig. S2). The CpG islands are methylated in untreated MSCs, but are demethylated after 5-Aza treatment (Fig. 2C). Although protein expression of β-catenin gradually increased in control MSCs, a progressive decrease in β-catenin was observed in 5-Aza-treated MSCs (Fig. 2, A and D), accompanied by decreases in TCF/LEF transcriptional activity as determined by TOP flash/FOP flash reporter gene assays (Fig. 2E).
FIGURE 2.
The effect of 5-Aza treatment upon expression of GSK-3α, GSK-3β, and β-catenin in MSCs. MSCs were treated with or without 5-Aza (5 μm) and then cultured for the indicated durations. A, protein expression of GSK-3β, phosphorylated GSK-3β, GSK-3α, β-catenin, and β-actin (internal control) was evaluated by immunoblot analyses. B, expression of GSK-3β was quantitated by densitometric analyses of the immunoblots. The level of GSK-3β was normalized by that in MSCs without 5-Aza at day 1. The results are mean ± S.E. from 5 experiments. C, genomic DNA was extracted from MSCs with or without 5-Aza and the methylation status in the GSK-3β and GSK-3α promoter region was examined by methylation-specific PCR. U, demethylated; M, methylated. D, protein expression of β-catenin/β-actin was evaluated by densitometric analyses. In B and D, *, p < 0.05; **, p < 0.01; ***, p < 0.001. The experiments were conducted 5 times. Protein expression in MSCs on day 1 without 5-Aza treatment was set as 1. The error bars show S.E. E, transcriptional activity of TCF/LEF was evaluated by reporter gene assays. MSCs were transfected with the TOP-FOP flash reporter genes and then treated with or without 5-Aza. The luciferase activity was evaluated on day 3 and the experiments were conducted 3 times.
GSK-3β Induces Cardiomyocyte Differentiation in MSCs
To examine whether expression of GSK-3 isoforms mimics the effect of 5-Aza upon cardiomyocyte differentiation, GSK-3α or GSK-3β were overexpressed via adenovirus transduction in MSCs (Fig. 3A). Although GSK-3β overexpression reduced expression of β-catenin in MSCs, GSK-3α overexpression did not (Fig. 3A, see supplemental Fig. S6B). Adenovirus vectors harboring LacZ (Ad-LacZ) and DN-GSK-3β (Ad-DN-GSK-3β) were used as controls. As expected, Ad-DN-GSK-3β increased expression of β-catenin (Fig. 3B). Transduction of MSCs with adenovirus harboring GSK-3β (Ad-GSK-3β), but not Ad-LacZ or Ad-DN-GSK-3β, induced mRNA expression of mesoderm markers, including Flk-1 (26) (Fig. 3C and data not shown). Ad-GSK-3β-induced mRNA expression of cardiomyocyte markers, including Nkx2.5, cardiac troponin C (cTnC), α-MHC, and atrial natriuretic factor (Fig. 3C). Although Ad-GSK-3α also slightly induced mRNA expression of Flk-1, cTnC, and α-MHC, the extent of their up-regulation was less than that by Ad-GSK-3β. Transduction of Ad-LacZ or Ad-DN-GSK-3β did not significantly induce expression of the cardiomyocyte markers (Fig. 3C). To obtain genetic evidence of cardiomyocyte differentiation, we generated transgenic mice harboring a LacZ gene driven by the Nkx2.5 promoter (9.0 kb), a cardiac specific promoter (Tg-Nkx2.5-LacZ). Transduction of MSCs isolated from Tg-Nkx2.5-LacZ mice with Ad-GSK-3β increased the number of β-galactosidase positive cells, whereas transduction with Ad-DN-GSK-3β, Ad-GSK-3α, or Ad-LacZ did not (Fig. 3D and data not shown). Sarcomeric α-actinin protein expression was observed in MSCs transduced with Ad-GSK-3β, but not in MSCs transduced with Ad-DN-GSK-3β. On the other hand, only weak or negligible expression of sarcomeric α-actinin was observed in GSK-3α-overexpressing MSCs (Fig. 3E, see also Fig. 8D). Ad-GSK-3β exhibited stronger induction of α-actinin expression than 5-Aza treatment (supplemental Fig. S3). Taken together, these results suggest that GSK-3β induces cardiomyocyte differentiation in bone marrow-derived MSCs and that overexpression of GSK-3β induces cardiomyocyte differentiation more potently than that of GSK-3α.
FIGURE 3.
GSK-3α, GSK-3β, and DN-GSK-3β overexpression in MSCs. A, protein expression of GSK-3α, GSK-3β, β-catenin, and β-actin was evaluated by immunoblot analyses in Ad-LacZ-, Ad-GSK-3α-, and Ad-GSK-3β-transduced MSCs. B, protein expression of β-catenin and β-actin was evaluated by immunoblot analyses in Ad-DN-GSK-3β-transduced MSCs. C, mRNA expression of Flk-1, Nkx2.5, atrial natriuretic factor, cTnC, α-MHC, GSK-3α, and GSK-3β was evaluated by RT-PCR. Expression of GAPDH was evaluated as an internal control. D, MSCs were prepared from transgenic mice harboring Nkx2.5-LacZ. MSCs were subjected to 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) staining after GSK-3α, GSK-3β, or DN-GSK-3β overexpression. MSCs without adenovirus transduction were used as a negative control for this experiment. E, MSCs were subjected to immunostaining with sarcomeric α-actinin and 4′,6-diamidino-2-phenylindole (DAPI). The results are representative of at least 4 experiments.
FIGURE 8.
GSK-3α and GSK-3β have distinct subcellular localization in MSCs. A, subcellular localization of endogenous GSK-3α and GSK-3β in MSCs was evaluated by immunostaining. B–D, MSCs were transduced with Ad-LacZ, Ad-GSK-3α, Ad-GSK-3β, or Ad-GSK-3β-NLS. B, MSCs were subjected to staining with anti-GSK-3α antibody, anti-GSK-3β antibody, and 4′,6-diamidino-2-phenylindole (DAPI). C, mRNA expression of Flk-1, Nkx2.5, α-MHC, cTnC, and GAPDH (internal control) was evaluated by RT-PCR. D, protein expression of GSK-3α, GSK-3β, α-actinin, cTnI, and GAPDH (internal control) was evaluated by immunoblots. * indicates GSK-3β-NLS.
Although transduction of MSCs with Ad-GSK-3α induced mRNA expression of both nestin, a neural marker, and Sox9, a chondrocyte marker, Ad-GSK-3β did not induce mRNA expression of either of them (supplemental Fig. S4). Transduction of MSCs with Ad-DN-GSK-3β induced mRNA expression of Sox9, but not nestin, suggesting that endogenous GSK-3β may inhibit chondrogenic differentiation in MSCs (supplemental Fig. S4).
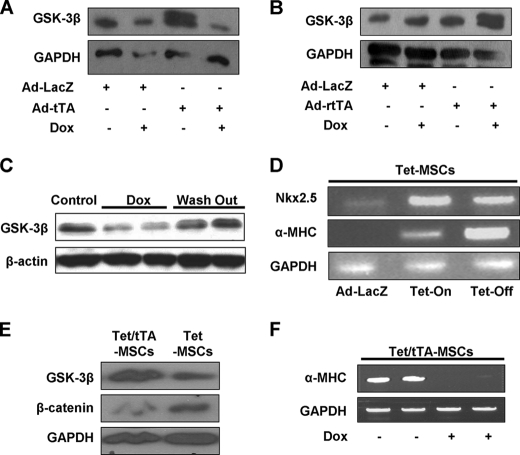
To achieve up-regulation of GSK-3β by an alternative method, MSCs derived from Tet-GSK-3β transgenic mice were transduced with Ad-tTA (the Tet-Off system) or Ad-rtTA (the Tet-On system), and then treated with or without Dox. GSK-3β expression was induced by withdrawing Dox in the Tet-Off system and by adding Dox in the Tet-On system (Fig. 4, A and B). The effect of Dox upon transgene expression was reversible (Fig. 4C), suggesting that GSK-3β expression can be regulated by Dox treatment in these MSCs. In both the Tet-On and Tet-Off systems, up-regulation of GSK-3β induced expression of Nkx2.5 and α-MHC mRNA in MSCs (Fig. 4D). Alternatively, Tet inducible GSK-3β transgenic mice were crossed with transgenic mice harboring CMV-tTA, and then MSCs were prepared from the bone marrow of bigenic mice. In the absence of Dox, MSCs prepared from the bigenic mice expressed more GSK-3β and less β-catenin than MSCs from control mice (Fig. 4E). Culturing MSCs prepared from Tet-GSK-3β and CMV-tTA bigenic mice in Dox-free medium induced α-MHC expression, which was completely suppressed in the presence of Dox (Fig. 4F). These results support the notion that cardiomyocyte differentiation of MSCs can be stimulated by drug-regulatable up-regulation of GSK-3β.
FIGURE 4.
GSK-3β overexpression, using Tet-On or Tet-Off systems. A and B, MSCs were prepared from Tg-Tet-GSK-3β mice. The GSK-3β transgene has a Myc tag. A, MSCs prepared from Tg-Tet-GSK-3β mice were transduced with adenovirus harboring tTA (Ad-tTA) and transgene expression was suppressed by Dox (0.5 μg/ml) treatment. Adenoviral vector LacZ (Ad-LacZ) was used as a control. B, MSCs prepared from Tg-Tet-GSK-3β mice were transduced with adenovirus harboring rtTA and transgene expression was induced by Dox (0.5 μg/ml) treatment. Ad-LacZ was used as a control. C, GSK-3β expression was decreased by Dox and recovered after Dox was washed out in MSCs prepared from Tg-Tet-GSK-3β-tTA mice. D, the effect of inducible GSK-3β overexpression in the Tet-On (rtTA) or Tet-Off (tTA) system upon mRNA expression of Nkx2.5 and α-MHC is shown. Ad-LacZ was used as a negative control. E and F, Tg-Tet-GSK-3β mice were crossed with transgenic mice harboring CMV-tTA and then MSCs were prepared from either Tg-Tet-GSK-3β (Tet) or Tg-Tet-GSK-3β-tTA (Tet/tTA) mice. E, protein expression of GSK-3β, β-catenin, and GAPDH (internal control) was evaluated by immunoblots. F, MSCs prepared from Tet/tTA mice were cultured with or without Dox. mRNA expression of α-MHC and GAPDH was evaluated by RT-PCR. Experiments were repeated at least 4 times.
Because both canonical and non-canonical Wnt pathways induce differentiation of stem cells into the cardiomyocyte lineage, we compared the effect of GSK-3β upon MSC differentiation with that of Wnt agonists. Using adenovirus transduction, we overexpressed either Wnt11, an agonist for the non-canonical Wnt pathway, or Wnt3a, an agonist for the canonical Wnt pathway, in MSCs (Fig. 5, A and B). Wnt11 induced activation of protein kinase C and Wnt3a caused a significant accumulation of β-catenin, suggesting that the non-canonical and canonical Wnt pathways were stimulated in MSCs in our experimental conditions (Fig. 5C). Up-regulation of GSK-3β induced the frequent appearance of tubular structures in MSCs (Figs. 5D and supplemental S5). Tubular structures are induced when MSCs are differentiated into the cardiomyocyte lineage (20, 27). Although up-regulation of GSK-3α and Wnt11 also induced some tubular structures, they were not as prominent as those induced by GSK-3β. Tubular structures were not observed in MSCs treated with Ad-LacZ, Ad-Wnt3a, or Ad-DN-GSK-3β (Figs. 5D and supplemental S5). Wnt11 induced mRNA expression of αMHC, but little or no expression of Flk-1, Nkx2.5, or cTnC, in MSCs (Fig. 5E), whereas Wnt3a did not induce mRNA expression of any of these marker genes (Fig. 5F). These results suggest that GSK-3β induces cardiomyocyte differentiation of MSCs more potently than the Wnt agonists.
FIGURE 5.
GSK-3β induces cardiomyocyte differentiation more potently than Wnt agonists. MSCs were transduced with adenoviruses harboring LacZ, Wnt3a, Wnt11, GSK-3α, GSK-3β, and DN-GSK-3β. A–C, protein expression of Wnt3a (A), Wnt11 (B), β-catenin (C), and phosphorylated pan-protein kinase C (Phospho-PKC (Pan)) (C) was evaluated by immunoblot analyses. The level of β-catenin protein expression was quantitated (C, right). GAPDH expression was evaluated as an internal control. D, the effects of LacZ, Wnt3a, Wnt11, GSK-3α, GSK-3β, and DN-GSK-3β expression upon branched myofibril formation were evaluated. Left, representative photos are shown. Right, the extent of branched myofibrils/cell surface (%) was quantitated. ***, p < 0.001. Pictures with a higher magnification are shown in supplemental Fig. S5. Error bars in B and D show S.E. E and F, mRNA expression of Flk-1, Nkx2.5, cTnC, and α-MHC in MSCs after transduction with Wnt11 (E) and Wnt3a (F) was evaluated by RT-PCR analyses. mRNA expression of GAPDH is shown as an internal control. Experiments were repeated at least 4 times.
Expression of GSK-3 Is Required for Cardiomyocyte Differentiation in MSCs
Because GSK-3 was up-regulated by 5-Aza and up-regulation of GSK-3β potently induced cardiomyocyte differentiation in MSCs, we next examined whether GSK-3 is required for induction of cardiomyocyte differentiation by 5-Aza in MSCs. MSCs were stimulated with 5-Aza in the presence or absence of known inhibitors of GSK-3. Treatment of MSCs with LiCl suppressed 5-Aza-induced down-regulation of β-catenin, suggesting that LiCl suppresses GSK-3 activity under 5-Aza treatment in MSCs (Fig. 6A). Although treatment of MSCs with LiCl induced expression of GATA4, LiCl alone did not induce expression of other cardiomyocyte marker genes. LiCl did, however, inhibit 5-Aza-induced up-regulation of cardiomyocyte marker mRNAs, including Nkx2.5, atrial natriuretic factor, cTnC, and cTnI (Fig. 6B), as well as cTnI protein expression (Fig. 6C). Treatment of MSCs with other GSK-3 inhibitors, including 6-bromo-indirubin-3′-oxime (BIO) and SB216743, reversed 5-Aza-induced down-regulation of β-catenin (Fig. 6D). In addition, BIO and SB216743 inhibited 5-Aza-induced up-regulation of Flk-1, Nkx2.5, cTnC, and α-MHC mRNA (Fig. 6E) and sarcomeric α-actinin protein expression (supplemental Fig. S3). These results suggest that GSK-3 plays an essential role in mediating 5-Aza-induced cardiomyocyte differentiation in MSCs.
FIGURE 6.
GSK-3 is required for 5-Aza-induced cardiomyocyte differentiation in MSCs. MSCs were treated with 5-Aza (5 μm) for 24 h and cultured 4 more days in the presence or absence of GSK-3 inhibitors. A, the effect of LiCl (10 mm) upon β-catenin and β-actin protein expression was evaluated by immunoblot analyses. B, the effect of LiCl upon 5-Aza-induced up-regulation of cardiomyocyte marker mRNA was evaluated by RT-PCR. C, the effect of LiCl upon 5-Aza-induced up-regulation of cTnI protein was evaluated by immunocytochemistry. Double staining with 4′,6-diamidino-2-phenylindole (DAPI) is also shown. D, the effect of GSK-3 inhibitors, BIO and SB216743, upon expression of β-catenin and GAPDH (internal control) was evaluated by immunoblot (left) and densitometric analyses (right). The experiments were repeated 3 times, and the error bars show S.E. E, MSCs were treated with 5-Aza in the presence or absence of GSK-3 inhibitors. mRNA expression of Flk-1, Nkx2.5, cTnC, α-MHC, and GAPDH (internal control) was evaluated by RT-PCR. The results are representative of 3 to 4 experiments.
The Roles of the GSK-3 Isoforms in Mediating Cardiomyocyte Differentiation in MSCs
To evaluate the roles of endogenous GSK-3α and GSK-3β in mediating cardiomyocyte differentiation separately, we generated adenovirus vectors harboring shRNA-GSK-3α (Ad-sh-GSK-3α), shRNA-GSK-3β (Ad-sh-GSK-3β), and shRNA-scramble (Ad-sh-scramble) and confirmed that Ad-sh-GSK-3α and Ad-sh-GSK-3β selectively down-regulate GSK-3α and GSK-3β, respectively (Fig. 7A). Although Ad-sh-GSK-3β increased expression of β-catenin, Ad-sh-GSK-3α did not (Fig. 7A). Down-regulation of GSK-3β did not induce expression of Flk-1, a mesoderm marker, and the cardiomyocyte-specific genes, including αMHC, Nkx2.5, and cTnC (Fig. 7B). Unexpectedly, however, down-regulation of GSK-3α did induce mRNA expression of Flk-1 and cardiac specific genes (Fig. 7B). Down-regulation of GSK-3α, but not GSK-3β, also induced protein expression of α-actinin, cTnI, and GATA4, cardiomyocyte markers (Fig. 7C, see also Fig. 10C). Furthermore, although down-regulation of GSK-3β inhibited 5-Aza-induced up-regulation of mesoderm and cardiomyocyte markers, down-regulation of GSK-3α enhanced it (Fig. 7D). Essentially the same results were obtained with shRNA-GSK-3α targeting a distinct site (data not shown), suggesting that the effect of Ad-sh-GSK-3α was mediated by GSK-3α and not an off-target effect. These results suggest that endogenous GSK-3α and GSK-3β have opposite effects upon cardiomyocyte differentiation in MSCs. When GSK-3α was down-regulated in the presence of GSK-3β up-regulation, β-catenin remained down-regulated (Fig. 7E), whereas mesoderm and cardiomyocyte differentiation were enhanced (Fig. 7F), suggesting that up-regulation of GSK-3β and down-regulation of GSK-3α utilize distinct cellular mechanisms to induce cardiomyocyte differentiation in MSCs.
FIGURE 7.
The role of GSK-3α and GSK-3β in mediating cardiomyocyte differentiation of MSCs. A–D, MSCs were treated with Ad-shRNA-scramble (Ad-sh-scramble), Ad-shRNA-GSK-3α (Ad-sh-GSK-3α), or Ad-shRNA-GSK-3β (Ad-sh-GSK-3β) in the presence or absence of 5-Aza. A, protein expression of GSK-3α, GSK-3β, β-catenin, and GAPDH (internal control) was examined by immunoblot assays. A short exposure of an immunoblot is shown for β-catenin because the band with Ad-sh-GSK-3β was saturated after longer exposures. B, mRNA expression of Flk-1, Nkx2.5, α-MHC, cTnC, and β-actin (internal control) was examined by RT-PCR. C, protein expression of sarcomeric α-actinin, cTnI, GATA4, and β-actin (internal control) was examined by immunoblot assays. D, mRNA expression of Flk-1, Nkx2.5, α-MHC, cTnC, and GAPDH (internal control) was evaluated by RT-PCR. E and F, MSCs were transduced with Ad-GSK-3β together with Ad-sh-scramble or Ad-sh-GSK-3α. E, protein expression of GSK-3α, GSK-3β, β-catenin, and GAPDH (internal control) was evaluated by immunoblot assays. F, mRNA expression of Flk-1, Nkx2.5, α-MHC, cTnC, atrial natriuretic factor, and GAPDH was evaluated by RT-PCR. Please note that a smaller cycle number was used in RT-PCR to show that Ad-sh-GSK-3α enhances the effect of Ad-GSK-3β. In A–F, the results are representative of 3–4 experiments.
FIGURE 10.
Up-regulation of c-Jun plays a critical role in mediating shRNA-GSK-3α-induced cardiomyocyte differentiation in MSCs. A and B, MSCs were treated with Ad-shRNA-scramble (Ad-sh-scramble), Ad-shRNA-GSK-3α (Ad-sh-GSK-3α), or Ad-shRNA-GSK-3β (Ad-sh-GSK-3β). C–E, MSCs were transduced with Ad-sh-scramble, Ad-shRNA-c-Jun (Ad-sh-c-Jun), Ad-sh-GSK-3α, or Ad-sh-c-Jun plus Ad-sh-GSK-3α. A and C, protein expression of c-Jun and GAPDH (internal control) was evaluated by immunoblots. B, MSCs were subjected to staining with anti-c-Jun antibody, anti-sarcomeric α-actinin antibody, and 4′,6-diamidino-2-phenylindole (DAPI). D, mRNA expression of Flk-1, Nkx2.5, α-MHC, cTnC, and GAPDH (internal control) was evaluated by RT-PCR. E, mRNA expression of Nestin, Sox9, and GAPDH (internal control) was evaluated by RT-PCR. In A–E, the results are representative of 3 experiments.
Differential Subcellular Localization of GSK-3 Isoforms May in Part Mediate Differential Effects of GSK-3α and GSK-3β upon Cardiomyocyte Differentiation in MSCs
Immunostaining with isoform-specific antibodies indicated that GSK-3α is localized primarily in the nucleus in MSCs. In contrast, GSK-3β is localized mainly in the cytosol but also partly in the nucleus (Fig. 8A). Transduction of MSCs with adenovirus harboring LacZ did not significantly change subcellular localization of endogenous GSK-3α or GSK-3β (Fig. 8B). Adenovirus-mediated overexpression of GSK-3α increased expression of GSK-3α in both the nucleus and cytoplasm and decreased expression of endogenous GSK-3β (Figs. 8B and supplemental S6A). Thus, overexpression of GSK-3α alters the subcellular distribution of GSK-3 isoforms. On the other hand, adenovirus-mediated overexpression of GSK-3β increased expression of GSK-3β in both the nucleus and cytoplasm, without significantly changing the subcellular distribution of GSK-3 isoforms (Fig. 8B).
To examine the role of GSK-3β in the cytosol in modulating cardiomyocyte differentiation, GSK-3β-NLS was expressed in MSCs. As expected, GSK-3β-NLS induced expression of GSK-3β predominantly in the nucleus (Fig. 8B). Overexpression of GSK-3β-NLS failed to induce mRNA and protein expression of cardiac markers (Fig. 8, C and D), suggesting that cytosolic expression is essential for cardiomyocyte differentiation of MSCs by GSK-3β. We also attempted to make GSK-3α-NES, GSK-3α exclusively expressed in the cytosol, but thus far we have not been successful.
GSK-3β Induces Cardiomyocyte Differentiation of MSCs through Down-regulation of β-Catenin
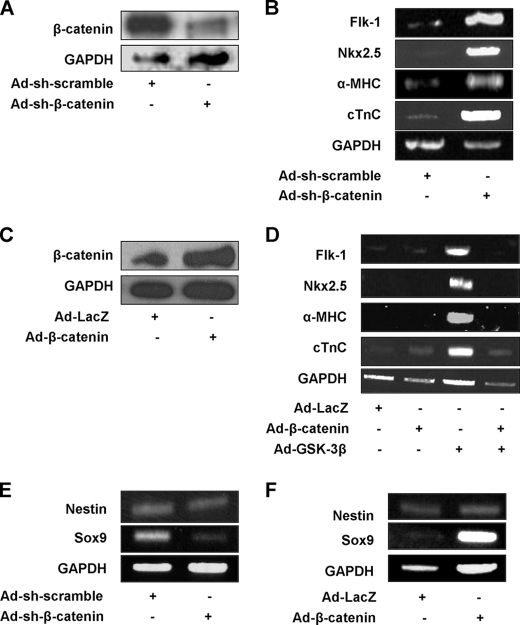
The signaling mechanisms by which GSK-3α and GSK-3β differentially affect cardiomyocyte differentiation in MSCs were investigated. Although overexpression of GSK-3β significantly down-regulated β-catenin expression, that of GSK-3α did not significantly reduce it (supplemental Fig. S6B). Down-regulation of GSK3β significantly increased β-catenin protein expression, whereas that of GSK3α did not affect it in MSCs (supplemental Fig. S6C). These results suggest that endogenous GSK-3β, but not GSK-3α, plays an essential role in regulating the cellular level of β-catenin in MSCs. To examine whether down-regulation of β- catenin is sufficient to induce expression of cardiomyocyte marker genes, β-catenin was down-regulated by adenovirus harboring shRNA-β-catenin (Ad-sh-β-catenin) (Fig. 9A). Down-regulation of β-catenin by Ad-sh-β-catenin potently induced mesoderm and cardiomyocyte markers, whereas shRNA-scramble had no effect (Fig. 9B). Up-regulation of β-catenin by adenovirus harboring β-catenin (Ad-β-catenin) (Fig. 9C) inhibited GSK-3β induced up-regulation of mesoderm and cardiomyocyte markers (Fig. 9D). These results suggest that down-regulation of β-catenin plays an important role in mediating induction of cardiomyocyte differentiation by GSK-3β. On the other hand, because down-regulation of GSK-3α did not affect β-catenin expression, induction of cardiomyocyte differentiation by down-regulation of GSK-3α is unlikely to be mediated by β-catenin-dependent mechanisms. Down-regulation of β-catenin inhibited, whereas up-regulation of β-catenin stimulated, expression of Sox9, suggesting that β-catenin mediates chondrocyte differentiation in MSCs (Fig. 9, E and F).
FIGURE 9.
Down-regulation of β-catenin plays a critical role in mediating GSK-3β-induced cardiomyocyte differentiation in MSCs. A and B, MSCs were transduced with Ad-shRNA-scramble (Ad-sh-scramble) or Ad-shRNA-β-catenin (Ad-sh-β-catenin). A, protein expression of β-catenin and GAPDH (internal control) was evaluated by immunoblots. B, mRNA expression of Flk-1, Nkx2.5, α-MHC, cTnC, and GAPDH (internal control) was evaluated by RT-PCR. C, MSCs were transduced with Ad-LacZ or Ad-β-catenin and protein expression of β-catenin and GAPDH (internal control) was evaluated by immunoblots. D, MSCs were transduced with Ad-LacZ, Ad-β-catenin, Ad-GSK-3β, or Ad-β-catenin plus Ad-GSK-3β. mRNA expression of Flk-1, Nkx2.5, α-MHC, cTnC, and GAPDH (internal control) was evaluated by RT-PCR. E and F, MSCs were transduced with either Ad-sh-scramble or Ad-sh-β-catenin (E), or either Ad-LacZ or Ad-β-catenin (F). mRNA expression of Nestin, Sox9, and GAPDH (internal control) was evaluated by RT-PCR. In A–F, the results are representative of 3 experiments.
Down-regulation of GSK-3a Induces Cardiomyocyte Differentiation of MSCs through Up-regulation of c-Jun
c-Jun plays an important role in mediating cardiomyocyte differentiation in bone marrow mononuclear cells (9). Down-regulation of GSK-3α, but not GSK-3β, up-regulated c-Jun expression in the nucleus in MSCs (Figs. 10, A and B, and supplemental S6D). Transduction with adenovirus harboring shRNA-c-Jun reversed the up-regulation of c-Jun, and mesoderm and cardiomyocyte markers induced by down-regulation of GSK-3α in MSCs (Fig. 10, C and D), suggesting that c-Jun plays an important role in mediating cardiomyocyte differentiation of MSCs induced by down-regulation of GSK-3α. Down-regulation of c-Jun up-regulated mRNA expression of nestin, suggesting that c-Jun negatively regulates neuronal differentiation in MSCs (Fig. 10E).
DISCUSSION
Expression of GSK-3 remains low when MSCs are uncommitted. However, GSK-3β is up-regulated when cardiomyocyte differentiation of MSCs is initiated by 5-Aza. Furthermore, up-regulation of GSK-3β is both necessary and sufficient for cardiomyocyte differentiation initiated by 5-Aza in MSCs. Unexpectedly, down-regulation, rather than up-regulation, of endogenous GSK-3α stimulated cardiomyocyte differentiation in MSCs. These results suggest that GSK-3β is an endogenous regulator of MSC differentiation and that GSK-3α and GSK-3β have opposite effects upon cardiomyocyte differentiation in MSCs.
5-Aza is a cytosine analogue and a demethylating agent that induces changes in chromatin structure, gene expression, cellular morphology, and survival in mammalian cells. Because phosphorylation of GSK-3β is not significantly affected by 5-Aza, 5-Aza must increase the total activity of GSK-3β primarily through up-regulation of GSK-3β mRNA. The promoter region of GSK-3β contains a prominent CpG island that is methylated in unstimulated MSCs, suggesting that 5-Aza induces up-regulation of GSK-3β through epigenetic modification of the promoter. Because GSK-3β stabilizes DNA methyltransferases, 5-Aza may initiate a positive feedback loop of GSK-3β promoter demethylation (28). At present, whether or not demethylation of the GSK-3β promoter is an endogenous mechanism for differentiation of adult stem cells into the cardiomyocyte lineage remains to be elucidated. In any event, GSK-3β may substitute for 5-Aza for induction of cardiomyocyte differentiation in MSCs, because clinical use of 5-Aza would be limited due to its nonspecific effects and obvious teratogenic actions.
GSK-3β is a central component of the Wnt pathway and negatively regulates β-catenin through phosphorylation-dependent proteolytic degradation (29). Although previous studies have shown that stimulation and inhibition of the Wnt signaling mechanism affect cardiomyocyte differentiation (8), up-regulation of GSK-3β induced cardiomyocyte markers more strongly than stimulation of either the canonical or non-canonical Wnt signaling pathways with Wnt3a and Wnt11, respectively. Because down-regulation of β-catenin alone also potently induces cardiomyocyte markers, modulating downstream components of the Wnt signaling pathway may induce cardiomyocyte differentiation more efficiently than stimulating the Wnt pathways at the receptor level.
It should be noted that GSK-3β not only regulates the Wnt pathway but also modulates a wide variety of signaling pathways, including other signaling cascades known to regulate stem cell differentiation, such as the Notch (30) and Hedgehog (31) pathways. Thus, up-regulation of GSK-3β may have a broader effect than selective stimulation of the Wnt pathway by the Wnt receptor ligand.
Increasing lines of evidence suggest that GSK-3α and GSK-3β have distinct cellular functions, despite the fact that they share 97% identity in their kinase domains and 36% identity overall. Our results suggest that GSK-3α and GSK-3β have distinct effects upon cardiomyocyte differentiation. Up-regulation of GSK-3β has a stronger effect upon cardiomyocyte differentiation in MSCs than up-regulation of GSK-3α. Although overexpression of GSK-3α slightly induces expression of cardiomyocyte markers, this effect may be mediated through promiscuous phosphorylation of GSK-3β substrates due to overexpression. In fact, adenovirus-mediated overexpression of GSK-3α substantially altered subcellular localization of GSK-3α in MSCs (see below). Importantly, although down-regulation of GSK-3β inhibited 5-Aza-induced cardiomyocyte differentiation, down-regulation of GSK-3α stimulated cardiomyocyte differentiation in MSCs. Previous studies have suggested that GSK-3α and GSK-3β could differentially affect cardiac development. Although GSK-3β knock-out mice exhibit cardiac defects consisting of malformation of the cardiac outflow tract and markedly thickened ventricular walls, contributing to their early mortality, GSK-3α knock-out mice show no significant cardiac defects (18). However, to our knowledge, the fact that GSK-3α and GSK-3β have opposite effects upon differentiation of stem cells has not been shown previously.
One possible explanation for the potential difference in their functions is that GSK-3α and GSK-3β exist in distinct subcellular localizations. For example, in adult mouse hearts, GSK-3α is localized primarily in the nucleus, whereas GSK-3β exists primarily in the cytosol (17). GSK-3α localized in the nucleus phosphorylates and induces nuclear exit/proteolytic degradation of G1 cyclins, whereas endogenous GSK-3β localized in the cytosol does not induce nuclear exit of G1 cyclins in the mouse heart (17). Immunostaining of the GSK-3 isoforms suggest that GSK-3α is localized primarily in the nucleus, and GSK-3β primarily in the cytosol but also in the nucleus in MSCs. Forced expression of GSK-3β in the nucleus did not induce cardiomyocyte differentiation, whereas overexpression of GSK-3α induces cytosolic expression of GSK-3α and partially stimulates cardiomyocyte differentiation. We therefore speculate that endogenous GSK-3β more efficiently phosphorylates β-catenin in the cytosol, thereby inducing efficient proteolytic degradation, whereas endogenous GSK-3α primarily localized in the nucleus may regulate other targets, presumably transcription factors. Because GSK-3α and GSK-3β equally affect expression of β-catenin in some cell types, such as ES cells (19, 32), we speculate that subcellular localization of GSK-3α/β may be cell type- or developmental stage-dependent.
Importantly, up-regulation of GSK-3β and down-regulation of GSK-3α have additive effects upon cardiomyocyte differentiation in MSCs, consistent with the notion that they mediate cardiomyocyte differentiation through distinct cellular mechanisms. Our results suggest that down-regulation of β-catenin plays an important role in mediating the effect of GSK-3β upon MSC differentiation into the cardiomyocyte lineage. On the other hand, down-regulation of GSK-3α induces cardiomyocyte differentiation through up-regulation of c-Jun in MSCs. Because GSK-3 phosphorylates β-catenin and c-Jun (19, 33), thereby stimulating their degradation, it is likely that GSK-3β in the cytosol may phosphorylate β-catenin, whereas GSK-3α in the nucleus may phosphorylate c-Jun, thereby regulating cardiomyocyte differentiation in MSCs (supplemental Fig. S7). Interventions to selectively stimulate GSK-3β or inhibit GSK-3α may be considered independently or in combination with other methods to facilitate cardiomyocyte differentiation of MSCs for cell-based therapy in vivo.
We have successfully engineered MSCs that conditionally express GSK-3β through either withdrawal or application of Dox. Phasic modulation of the Wnt/β-catenin signaling mechanism effectively stimulates differentiation of progenitor cells into the cardiomyocyte lineage (10, 34). Activation of β-catenin is required to maintain and expand cardiac progenitor cells (11, 35) but must be repressed to induce cardiomyocyte differentiation from cardiac progenitor cells (8). Thus, it would be interesting to test whether ex vivo engineered MSCs, in which the timing of expression of GSK-3β can be regulated by Dox treatment, enhance the efficacy of cell therapy in vivo.
Acknowledgment
We thank Daniela Zablocki for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL 059139, HL067724, HL069020, AG023039, AG027211, and HL91469 from the United States Public Health Service and grants from the New Jersey Commission of Science and Technology Stem Cell Research Grant Program.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S7.
- GSK
- glycogen synthase kinase
- 5-Aza
- 5-azacytidine
- BIO
- 6-bromo-indirubin-3′-oxime
- MSC
- mesenchymal stem cell
- Dox
- doxycycline
- Tg
- transgenic
- NLS
- nuclear localization signal
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- RT
- reverse transcription
- α-MHC
- α-myosin heavy chain
- cTnI
- cardiac troponin I
- cTnC
- cardiac troponin C
- DN
- dominant-negative
- shRNA
- short hairpin RNA
- tTA
- tetracycline-controlled transactivator
- rtTA
- reverse tetracycline controlled transactivator.
REFERENCES
- 1.Pfeffer M. A. (1995) Annu. Rev. Med. 46, 455–466 [DOI] [PubMed] [Google Scholar]
- 2.Laflamme M. A., Murry C. E. (2005) Nat. Biotechnol. 23, 845–856 [DOI] [PubMed] [Google Scholar]
- 3.Passier R., van Laake L. W., Mummery C. L. (2008) Nature 453, 322–329 [DOI] [PubMed] [Google Scholar]
- 4.Dimmeler S., Zeiher A. M., Schneider M. D. (2005) J. Clin. Invest. 115, 572–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wollert K. C., Meyer G. P., Lotz J., Ringes-Lichtenberg S., Lippolt P., Breidenbach C., Fichtner S., Korte T., Hornig B., Messinger D., Arseniev L., Hertenstein B., Ganser A., Drexler H. (2004) Lancet 364, 141–148 [DOI] [PubMed] [Google Scholar]
- 6.Britten M. B., Abolmaali N. D., Assmus B., Lehmann R., Honold J., Schmitt J., Vogl T. J., Martin H., Schächinger V., Dimmeler S., Zeiher A. M. (2003) Circulation 108, 2212–2218 [DOI] [PubMed] [Google Scholar]
- 7.Schächinger V., Assmus B., Britten M. B., Honold J., Lehmann R., Teupe C., Abolmaali N. D., Vogl T. J., Hofmann W. K., Martin H., Dimmeler S., Zeiher A. M. (2004) J. Am. Coll. Cardiol. 44, 1690–1699 [DOI] [PubMed] [Google Scholar]
- 8.Cohen E. D., Tian Y., Morrisey E. E. (2008) Development 135, 789–798 [DOI] [PubMed] [Google Scholar]
- 9.Flaherty M. P., Abdel-Latif A., Li Q., Hunt G., Ranjan S., Ou Q., Tang X. L., Johnson R. K., Bolli R., Dawn B. (2008) Circulation 117, 2241–2252 [DOI] [PubMed] [Google Scholar]
- 10.Ueno S., Weidinger G., Osugi T., Kohn A. D., Golob J. L., Pabon L., Reinecke H., Moon R. T., Murry C. E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 9685–9690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qyang Y., Martin-Puig S., Chiravuri M., Chen S., Xu H., Bu L., Jiang X., Lin L., Granger A., Moretti A., Caron L., Wu X., Clarke J., Taketo M. M., Laugwitz K. L., Moon R. T., Gruber P., Evans S. M., Ding S., Chien K. R. (2007) Cell Stem Cell 1, 165–179 [DOI] [PubMed] [Google Scholar]
- 12.Koyanagi M., Haendeler J., Badorff C., Brandes R. P., Hoffmann J., Pandur P., Zeiher A. M., Kühl M., Dimmeler S. (2005) J. Biol. Chem. 280, 16838–16842 [DOI] [PubMed] [Google Scholar]
- 13.Naito A. T., Shiojima I., Akazawa H., Hidaka K., Morisaki T., Kikuchi A., Komuro I. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19812–19817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kundu M., Lindsten T., Yang C. Y., Wu J., Zhao F., Zhang J., Selak M. A., Ney P. A., Thompson C. B. (2008) Blood 112, 1493–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu W., Shiojima I., Ito Y., Li Z., Ikeda H., Yoshida M., Naito A. T., Nishi J., Ueno H., Umezawa A., Minamino T., Nagai T., Kikuchi A., Asashima M., Komuro I. (2008) Nature 454, 345–349 [DOI] [PubMed] [Google Scholar]
- 16.Hirotani S., Zhai P., Tomita H., Galeotti J., Marquez J. P., Gao S., Hong C., Yatani A., Avila J., Sadoshima J. (2007) Circ. Res. 101, 1164–1174 [DOI] [PubMed] [Google Scholar]
- 17.Matsuda T., Zhai P., Maejima Y., Hong C., Gao S., Tian B., Goto K., Takagi H., Tamamori-Adachi M., Kitajima S., Sadoshima J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 20900–20905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerkela R., Kockeritz L., Macaulay K., Zhou J., Doble B. W., Beahm C., Greytak S., Woulfe K., Trivedi C. M., Woodgett J. R., Epstein J. A., Force T., Huggins G. S. (2008) J. Clin. Invest. 118, 3609–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Force T., Woodgett J. R. (2009) J. Biol. Chem. 284, 9643–9647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino S., Fukuda K., Miyoshi S., Konishi F., Kodama H., Pan J., Sano M., Takahashi T., Hori S., Abe H., Hata J., Umezawa A., Ogawa S. (1999) J. Clin. Invest. 103, 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hare J. M., Chaparro S. V. (2008) Curr. Opin. Organ Transplant 13, 536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morisco C., Zebrowski D., Condorelli G., Tsichlis P., Vatner S. F., Sadoshima J. (2000) J. Biol. Chem. 275, 14466–14475 [DOI] [PubMed] [Google Scholar]
- 23.Zhai P., Gao S., Holle E., Yu X., Yatani A., Wagner T., Sadoshima J. (2007) J. Biol. Chem. 282, 33181–33191 [DOI] [PubMed] [Google Scholar]
- 24.Herman J. G., Graff J. R., Myöhänen S., Nelkin B. D., Baylin S. B. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 9821–9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanstein B., Eckner R., DiRenzo J., Halachmi S., Liu H., Searcy B., Kurokawa R., Brown M. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 11540–11545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ema M., Takahashi S., Rossant J. (2006) Blood 107, 111–117 [DOI] [PubMed] [Google Scholar]
- 27.Planat-Bénard V., Menard C., André M., Puceat M., Perez A., Garcia-Verdugo J. M., Pénicaud L., Casteilla L. (2004) Circ. Res. 94, 223–229 [DOI] [PubMed] [Google Scholar]
- 28.Sun L., Zhao H., Xu Z., Liu Q., Liang Y., Wang L., Cai X., Zhang L., Hu L., Wang G., Zha X. (2007) Cell. Signal. 19, 2255–2263 [DOI] [PubMed] [Google Scholar]
- 29.Hardt S. E., Sadoshima J. (2002) Circ. Res. 90, 1055–1063 [DOI] [PubMed] [Google Scholar]
- 30.Foltz D. R., Santiago M. C., Berechid B. E., Nye J. S. (2002) Curr. Biol. 12, 1006–1011 [DOI] [PubMed] [Google Scholar]
- 31.Jia J., Amanai K., Wang G., Tang J., Wang B., Jiang J. (2002) Nature 416, 548–552 [DOI] [PubMed] [Google Scholar]
- 32.MacAulay K., Doble B. W., Patel S., Hansotia T., Sinclair E. M., Drucker D. J., Nagy A., Woodgett J. R. (2007) Cell Metab. 6, 329–337 [DOI] [PubMed] [Google Scholar]
- 33.Wei W., Jin J., Schlisio S., Harper J. W., Kaelin W. G., Jr. (2005) Cancer Cell 8, 25–33 [DOI] [PubMed] [Google Scholar]
- 34.Tzahor E. (2007) Dev. Cell 13, 10–13 [DOI] [PubMed] [Google Scholar]
- 35.Kwon C., Arnold J., Hsiao E. C., Taketo M. M., Conklin B. R., Srivastava D. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 10894–10899 [DOI] [PMC free article] [PubMed] [Google Scholar]