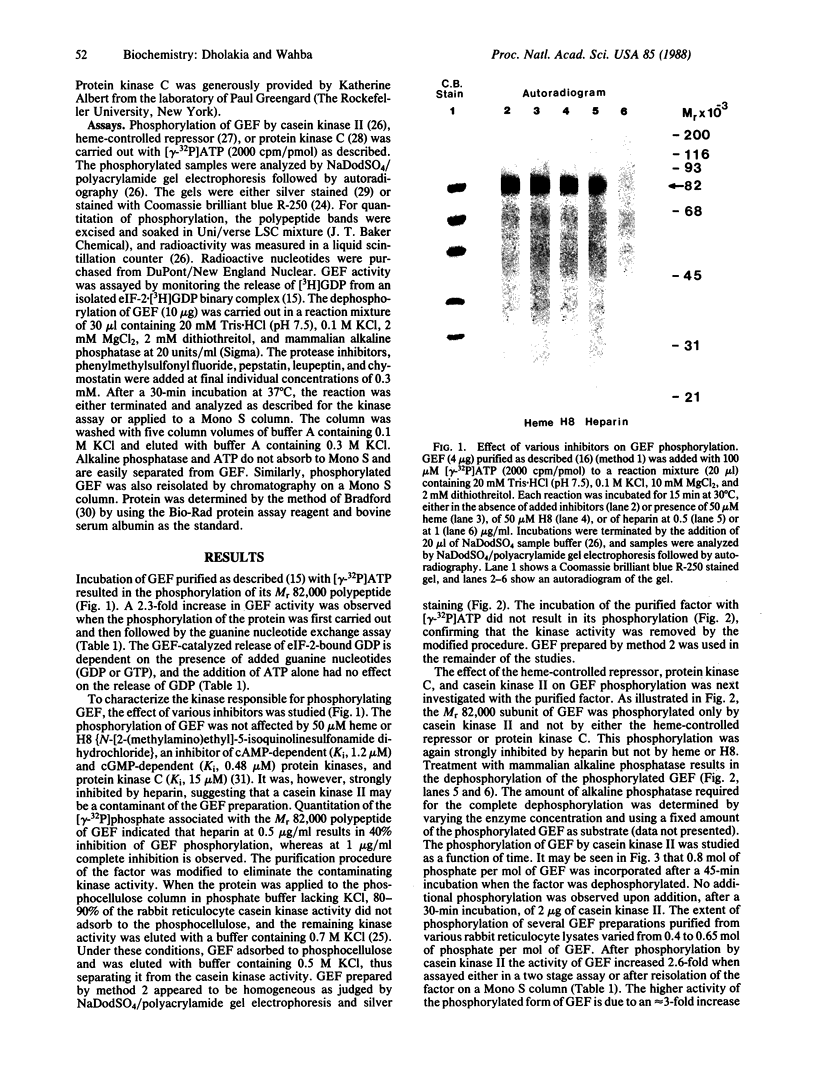
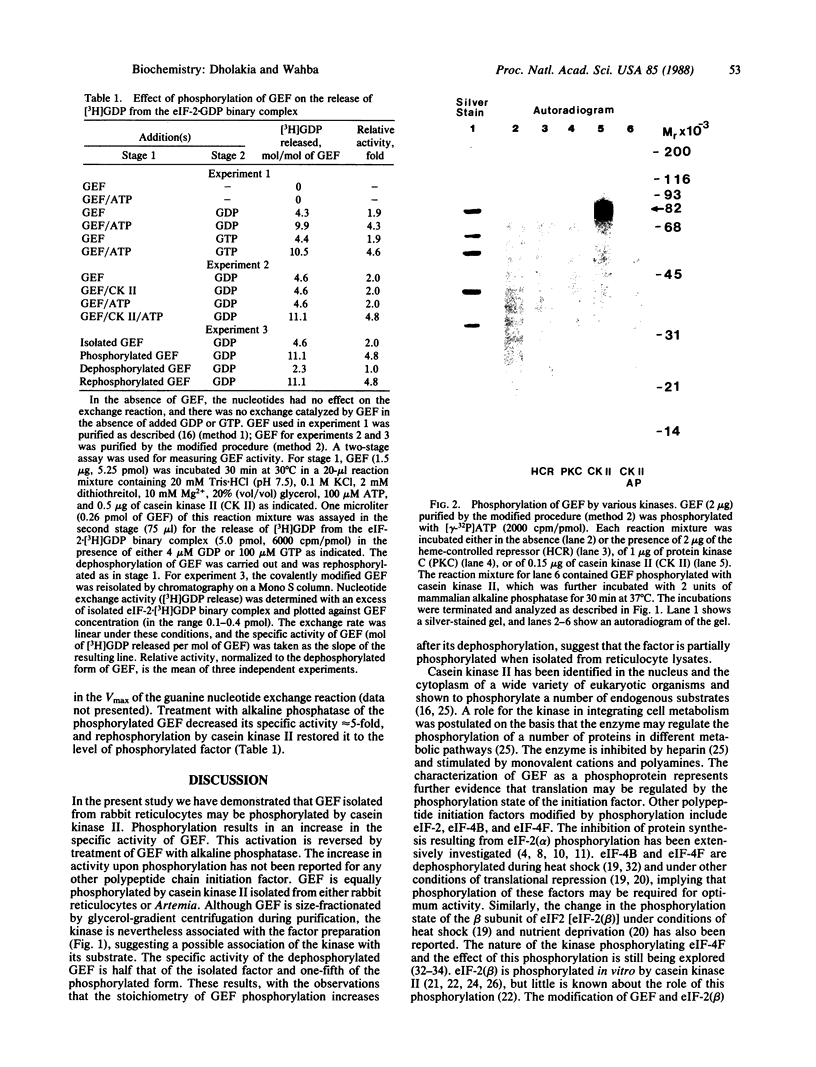
Abstract
We have demonstrated that the purified guanine nine nucleotide exchange factor (GEF) may be isolated as a complex with NADPH. Complete inhibition of the GEF-catalyzed exchange of eukaryotic initiation factor 2-bound GDP for GTP was observed in the presence of either 0.5-0.75 mM NAD+ or NADP+. Incubation of GEF with ATP results in the phosphorylation of its Mr 82,000 polypeptide. This phosphorylation is strongly inhibited by heparin but is not affected by heme or H8 (N-[2-(methylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride), an inhibitor of cAMP- and cGMP-dependent protein kinases and protein kinase C. The purification of GEF was modified to eliminate any contaminating kinase activity and the isolated protein appears to be homogeneous as judged by NaDodSO4/polyacrylamide gel electrophoresis and silver staining. The Mr 82,000 subunit of GEF is phosphorylated only upon addition of ATP and casein kinase II. The extent of phosphorylation is approximately equal to 0.55 mol of phosphate per mol of GEF, and this results in a 2.3-fold increase in the guanine nucleotide exchange activity. Following treatment of the phosphorylated GEF with alkaline phosphatase, the activity of the protein is reduced by a factor of 5. Rephosphorylation of GEF increases its specific activity to that of the phosphorylated protein. The results of this study suggest that phosphorylation/dephosphorylation of GEF plays a role in regulating polypeptide chain initiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert K. A., Walaas S. I., Wang J. K., Greengard P. Widespread occurrence of "87 kDa," a major specific substrate for protein kinase C. Proc Natl Acad Sci U S A. 1986 May;83(9):2822–2826. doi: 10.1073/pnas.83.9.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Edman J., Traut R. R., Hershey J. W. Phosphorylation of eukaryotic protein synthesis initiation factors. Proc Natl Acad Sci U S A. 1978 Jan;75(1):108–112. doi: 10.1073/pnas.75.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Pain V. M., Wong S. T., Henshaw E. C. Phosphorylation inhibits guanine nucleotide exchange on eukaryotic initiation factor 2. Nature. 1982 Mar 4;296(5852):93–95. doi: 10.1038/296093a0. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Dholakia J. N., Mueser T. C., Woodley C. L., Parkhurst L. J., Wahba A. J. The association of NADPH with the guanine nucleotide exchange factor from rabbit reticulocytes: a role of pyridine dinucleotides in eukaryotic polypeptide chain initiation. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6746–6750. doi: 10.1073/pnas.83.18.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dholakia J. N., Wahba A. J. The isolation and characterization from rabbit reticulocytes of two forms of eukaryotic initiation factor 2 having different beta-polypeptides. J Biol Chem. 1987 Jul 25;262(21):10164–10170. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Heat shock-induced translational alterations in HeLa cells. Initiation factor modifications and the inhibition of translation. J Biol Chem. 1984 Oct 10;259(19):11882–11889. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Regulation of initiation factors during translational repression caused by serum depletion. Covalent modification. J Biol Chem. 1985 May 10;260(9):5493–5497. [PubMed] [Google Scholar]
- Duncan R., Milburn S. C., Hershey J. W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987 Jan 5;262(1):380–388. [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Goss D. J., Parkhurst L. J., Mehta H. B., Woodley C. L., Wahba A. J. Studies on the role of eukaryotic nucleotide exchange factor in polypeptide chain initiation. J Biol Chem. 1984 Jun 25;259(12):7374–7377. [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinase II. Methods Enzymol. 1983;99:317–331. doi: 10.1016/0076-6879(83)99067-5. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinases--multipotential protein kinases. Curr Top Cell Regul. 1982;21:101–127. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Petryshyn R., London I. M. Characterization of double-stranded-RNA-activated kinase that phosphorylates alpha subunit of eukaryotic initiation factor 2 (eIF-2 alpha) in reticulocyte lysates. Proc Natl Acad Sci U S A. 1980 Feb;77(2):832–836. doi: 10.1073/pnas.77.2.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matts R. L., Levin D. H., London I. M. Effect of phosphorylation of the alpha-subunit of eukaryotic initiation factor 2 on the function of reversing factor in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1983 May;80(9):2559–2563. doi: 10.1073/pnas.80.9.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta H. B., Dholakia J. N., Roth W. W., Parekh B. S., Montelaro R. C., Woodley C. L., Wahba A. J. Structural studies on the eukaryotic chain initiation factor 2 from rabbit reticulocytes and brine shrimp Artemia embryos. Phosphorylation by the heme-controlled repressor and casein kinase II. J Biol Chem. 1986 May 25;261(15):6705–6711. [PubMed] [Google Scholar]
- Mehta H. B., Woodley C. L., Wahba A. J. Protein synthesis in brine shrimp embryos and rabbit reticulocytes. The effect of Mg2+ on binary (eukaryotic initiation factor 2 X GDP) and ternary (eukaryotic initiation factor 2 X GTP X met-tRNAf) complex formation. J Biol Chem. 1983 Mar 25;258(6):3438–3441. [PubMed] [Google Scholar]
- Moldave K. Eukaryotic protein synthesis. Annu Rev Biochem. 1985;54:1109–1149. doi: 10.1146/annurev.bi.54.070185.005333. [DOI] [PubMed] [Google Scholar]
- Pain V. M. Initiation of protein synthesis in mammalian cells. Biochem J. 1986 May 1;235(3):625–637. doi: 10.1042/bj2350625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panniers R., Henshaw E. C. A GDP/GTP exchange factor essential for eukaryotic initiation factor 2 cycling in Ehrlich ascites tumor cells and its regulation by eukaryotic initiation factor 2 phosphorylation. J Biol Chem. 1983 Jul 10;258(13):7928–7934. [PubMed] [Google Scholar]
- Ranu R. S. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and initial characterization of the double stranded RNA activated protein kinase. Biochem Biophys Res Commun. 1980 Nov 17;97(1):252–262. doi: 10.1016/s0006-291x(80)80162-8. [DOI] [PubMed] [Google Scholar]
- Rychlik W., Gardner P. R., Vanaman T. C., Rhoads R. E. Structural analysis of the messenger RNA cap-binding protein. Presence of phosphate, sulfhydryl, and disulfide groups. J Biol Chem. 1986 Jan 5;261(1):71–75. [PubMed] [Google Scholar]
- Salimans M., Goumans H., Amesz H., Benne R., Voorma H. O. Regulation of protein synthesis in eukaryotes. Mode of action of eRF, an eIF-2-recycling factor from rabbit reticulocytes involved in GDP/GTP exchange. Eur J Biochem. 1984 Nov 15;145(1):91–98. doi: 10.1111/j.1432-1033.1984.tb08526.x. [DOI] [PubMed] [Google Scholar]
- Siekierka J., Mauser L., Ochoa S. Mechanism of polypeptide chain initiation in eukaryotes and its control by phosphorylation of the alpha subunit of initiation factor 2. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2537–2540. doi: 10.1073/pnas.79.8.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Staehelin T. Binding and release of eukaryotic initiation factor eIF-2 and GTP during protein synthesis initiation. Proc Natl Acad Sci U S A. 1978 Jan;75(1):204–208. doi: 10.1073/pnas.75.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuazon P. T., Merrick W. C., Traugh J. A. Site-specific phosphorylation if initiation factor 2 by three cyclic nucleotide-independent protein kinases. J Biol Chem. 1980 Nov 25;255(22):10954–10958. [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Nucleotide regulation of a eukaryotic protein synthesis initiation complex;. Biochim Biophys Acta. 1975 May 1;390(2):231–245. doi: 10.1016/0005-2787(75)90344-5. [DOI] [PubMed] [Google Scholar]
- Woodley C. L., Roychowdhury M., MacRae T. H., Olsen K. W., Wahba A. J. Protein synthesis in brine shrimp embryos. Regulation of the formation of the ternary complex (Met-tRNAf X eIF-2 X GTP) by two purified protein factors and phosphorylation of Artemia eIF-2. Eur J Biochem. 1981 Jul;117(3):543–551. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]