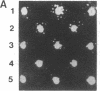
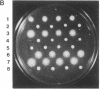
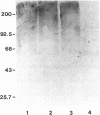
Abstract
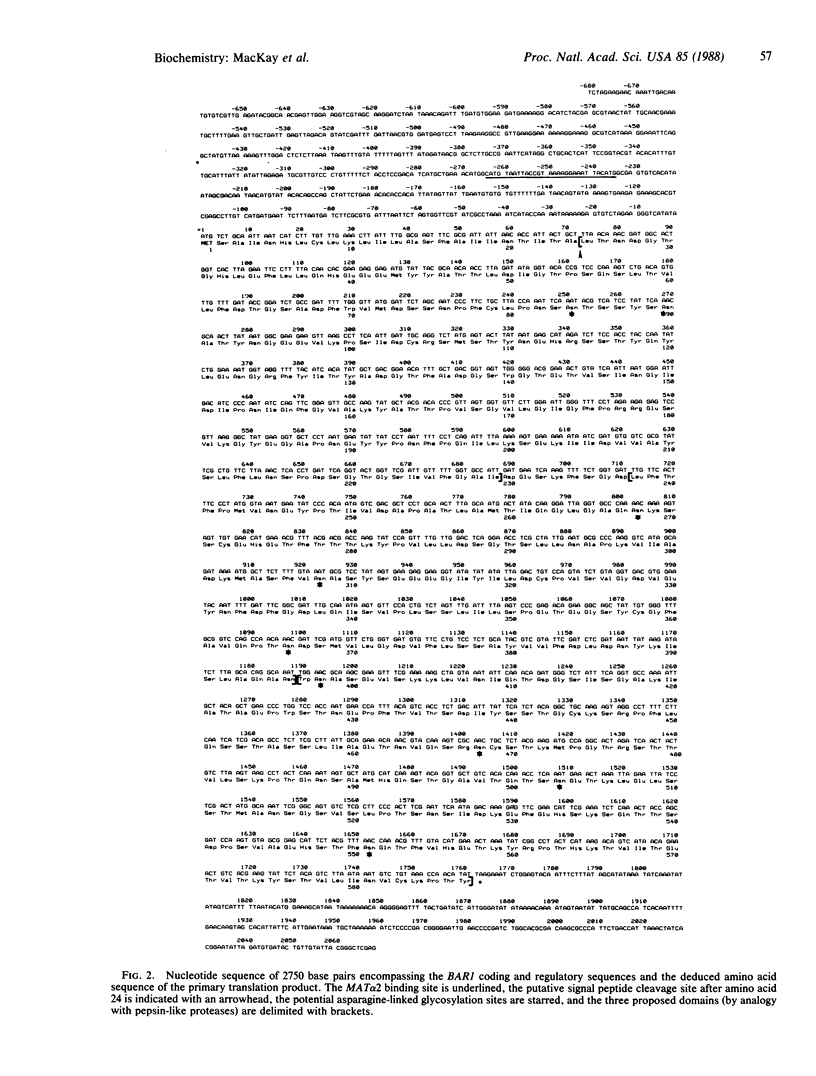
Saccharomyces cerevisiae a cells secrete an extracellular protein, called "barrier" activity, that acts as an antagonist of alpha factor, the peptide mating pheromone produced by mating-type alpha cells. We report here the DNA sequence of BAR1, the structural gene for barrier activity. The deduced primary translation product of 587 amino acids has a putative signal peptide, nine potential asparagine-linked glycosylation sites, and marked sequence similarity of the first two-thirds of the protein with pepsin-like proteases. Barrier activity was abolished by in vitro mutation of an aspartic acid predicted from this sequence homology to be in the active site. Therefore, barrier protein is probably a protease that cleaves alpha factor. The sequence similarity suggests that the first two-thirds of the barrier protein is organized into two distinct structural domains like those of the pepsin-like proteases. However, the BAR1 gene product has a third carboxyl-terminal domain of unknown function; deletion of at least 166 of the 191 amino acids of this region has no significant effect on barrier activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T., Kawasaki G. Nucleotide sequence of the triose phosphate isomerase gene of Saccharomyces cerevisiae. J Mol Appl Genet. 1982;1(5):419–434. [PubMed] [Google Scholar]
- Ammerer G. Expression of genes in yeast using the ADCI promoter. Methods Enzymol. 1983;101:192–201. doi: 10.1016/0076-6879(83)01014-9. [DOI] [PubMed] [Google Scholar]
- Ammerer G., Hunter C. P., Rothman J. H., Saari G. C., Valls L. A., Stevens T. H. PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol. 1986 Jul;6(7):2490–2499. doi: 10.1128/mcb.6.7.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudys M., Kostka V. Covalent structure of chicken pepsinogen. Eur J Biochem. 1983 Oct 17;136(1):89–99. doi: 10.1111/j.1432-1033.1983.tb07709.x. [DOI] [PubMed] [Google Scholar]
- Bayliss R. S., Knowles J. R., Wybrandt G. B. An aspartic acid residue at the active site of pepsin. The isolation and sequence of the heptapeptide. Biochem J. 1969 Jun;113(2):377–386. doi: 10.1042/bj1130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Betz R., MacKay V. L., Duntze W. a-Factor from Saccharomyces cerevisiae: partial characterization of a mating hormone produced by cells of mating type a. J Bacteriol. 1977 Nov;132(2):462–472. doi: 10.1128/jb.132.2.462-472.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Chan R. K., Otte C. A. Isolation and genetic analysis of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982 Jan;2(1):11–20. doi: 10.1128/mcb.2.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W. J., Takahashi K. The structure and function of acid proteases. III. Isolation and characterization of the active-site peptides from bovine rennin. J Biochem. 1974 Sep;76(3):467–474. doi: 10.1093/oxfordjournals.jbchem.a130590. [DOI] [PubMed] [Google Scholar]
- Ciejek E., Thorner J. Recovery of S. cerevisiae a cells from G1 arrest by alpha factor pheromone requires endopeptidase action. Cell. 1979 Nov;18(3):623–635. doi: 10.1016/0092-8674(79)90117-x. [DOI] [PubMed] [Google Scholar]
- Duntze W., MacKay V., Manney T. R. Saccharomyces cerevisiae: a diffusible sex factor. Science. 1970 Jun 19;168(3938):1472–1473. doi: 10.1126/science.168.3938.1472. [DOI] [PubMed] [Google Scholar]
- Duntze W., Stötzler D., Bücking-Throm E., Kalbitzer S. Purification and partial characterization of -factor, a mating-type specific inhibitor of cell reproduction from Saccharomyces cerevisiae. Eur J Biochem. 1973 Jun;35(2):357–365. doi: 10.1111/j.1432-1033.1973.tb02847.x. [DOI] [PubMed] [Google Scholar]
- Hicks J. B., Herskowitz I. Evidence for a new diffusible element of mating pheromones in yeast. Nature. 1976 Mar 18;260(5548):246–248. doi: 10.1038/260246a0. [DOI] [PubMed] [Google Scholar]
- James M. N., Sielecki A. R. Molecular structure of an aspartic proteinase zymogen, porcine pepsinogen, at 1.8 A resolution. Nature. 1986 Jan 2;319(6048):33–38. doi: 10.1038/319033a0. [DOI] [PubMed] [Google Scholar]
- Johnson A. D., Herskowitz I. A repressor (MAT alpha 2 Product) and its operator control expression of a set of cell type specific genes in yeast. Cell. 1985 Aug;42(1):237–247. doi: 10.1016/s0092-8674(85)80119-7. [DOI] [PubMed] [Google Scholar]
- Kronstad J. W., Holly J. A., MacKay V. L. A yeast operator overlaps an upstream activation site. Cell. 1987 Jul 31;50(3):369–377. doi: 10.1016/0092-8674(87)90491-0. [DOI] [PubMed] [Google Scholar]
- Manney T. R. Expression of the BAR1 gene in Saccharomyces cerevisiae: induction by the alpha mating pheromone of an activity associated with a secreted protein. J Bacteriol. 1983 Jul;155(1):291–301. doi: 10.1128/jb.155.1.291-301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manney T. R., Jackson P., Meade J. Two temperature-sensitive mutants of Saccharomyces cerevisiae with altered expression of mating-type functions. J Cell Biol. 1983 Jun;96(6):1592–1600. doi: 10.1083/jcb.96.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D. Tests for comparing related amino-acid sequences. Cytochrome c and cytochrome c 551 . J Mol Biol. 1971 Oct 28;61(2):409–424. doi: 10.1016/0022-2836(71)90390-1. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller A. M., MacKay V. L., Nasmyth K. A. Identification and comparison of two sequence elements that confer cell-type specific transcription in yeast. Nature. 1985 Apr 18;314(6012):598–603. doi: 10.1038/314598a0. [DOI] [PubMed] [Google Scholar]
- Misono K. S., Chang J. J., Inagami T. Amino acid sequence of mouse submaxillary gland renin. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4858–4862. doi: 10.1073/pnas.79.16.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morávek L., Kostka V. Complete amino acid sequence of hog pepsin. FEBS Lett. 1974 Jul 15;43(2):207–211. doi: 10.1016/0014-5793(74)81001-x. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. Use of peptide tagging to detect proteins expressed from cloned genes: deletion mapping functional domains of Drosophila hsp 70. EMBO J. 1984 Dec 20;3(13):3087–3093. doi: 10.1002/j.1460-2075.1984.tb02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A., Reed S. I. Isolation of genes by complementation in yeast: molecular cloning of a cell-cycle gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2119–2123. doi: 10.1073/pnas.77.4.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong E. B., Perlmann G. E. The amino-terminal sequence of porcine pepsinogen. J Biol Chem. 1968 Dec 10;243(23):6104–6109. [PubMed] [Google Scholar]
- Palfree R. G., Bussey H. Yeast killer toxin: purification and characterisation of the protein toxin from Saccharomyces cerevisiae. Eur J Biochem. 1979 Feb 1;93(3):487–493. doi: 10.1111/j.1432-1033.1979.tb12847.x. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Russell P. R. Evolutionary divergence of the mRNA transcription initiation mechanism in yeast. Nature. 1983 Jan 13;301(5896):167–169. doi: 10.1038/301167a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda P., Marciniszyn J., Jr, Liu D., Tang J. Primary structure of porcine pepsin. III. Amino acid sequence of a cyanogen bromide fragment, CB2A, and the complete structure of porcine pepsin. J Biol Chem. 1975 Jul 10;250(13):5082–5088. [PubMed] [Google Scholar]
- Sodek J., Hofmann T. Amino acid sequence around the active site aspartic acid in penicillopepsin. Can J Biochem. 1970 Sep;48(9):1014–1016. doi: 10.1139/o70-158. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Blair L. C., Thorner J. Cell interactions and regulation of cell type in the yeast Saccharomyces cerevisiae. Annu Rev Microbiol. 1983;37:623–660. doi: 10.1146/annurev.mi.37.100183.003203. [DOI] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Herskowitz I. Control of yeast cell type by the mating type locus. I. Identification and control of expression of the a-specific gene BAR1. J Mol Biol. 1981 Dec 5;153(2):305–321. doi: 10.1016/0022-2836(81)90280-1. [DOI] [PubMed] [Google Scholar]
- Stepanov V. M., Baratova L. A., Pugacheva I. B., Belyanova L. P., Revina L. P., Timokhina E. A. N-terminal sequence of swine pepsinogen and pepsin. The site of pepsinogen activation. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1164–1170. doi: 10.1016/0006-291x(73)90814-0. [DOI] [PubMed] [Google Scholar]
- Strazdis J. R., MacKay V. L. Reproducible and rapid methods for the isolation and assay of a-factor, a yeast mating hormone. J Bacteriol. 1982 Sep;151(3):1153–1161. doi: 10.1128/jb.151.3.1153-1161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H., Kikuno R., Hayashida H., Miyata T., Kugimiya W., Inouye S., Yuki S., Saigo K. Close structural resemblance between putative polymerase of a Drosophila transposable genetic element 17.6 and pol gene product of Moloney murine leukaemia virus. EMBO J. 1985 May;4(5):1267–1272. doi: 10.1002/j.1460-2075.1985.tb03771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford C. A., Daniels L. B., Park F. J., Jones E. W., Van Arsdell J. N., Innis M. A. The PEP4 gene encodes an aspartyl protease implicated in the posttranslational regulation of Saccharomyces cerevisiae vacuolar hydrolases. Mol Cell Biol. 1986 Jul;6(7):2500–2510. doi: 10.1128/mcb.6.7.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]