Abstract
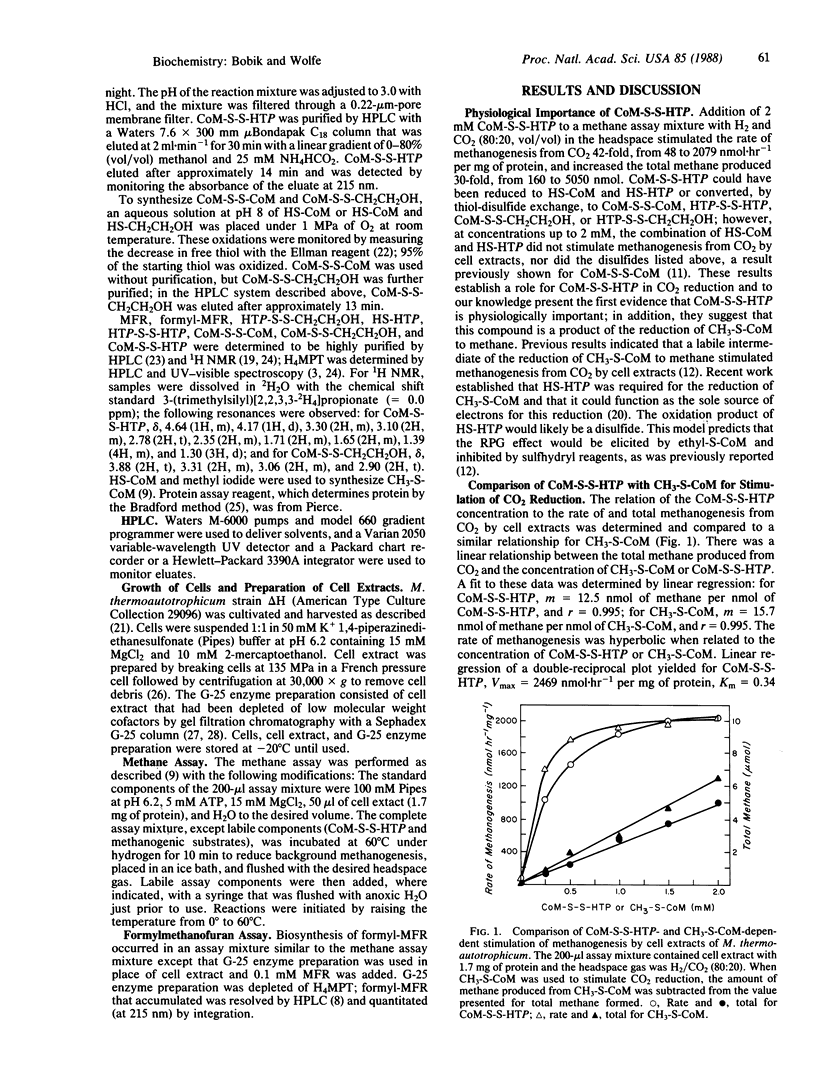
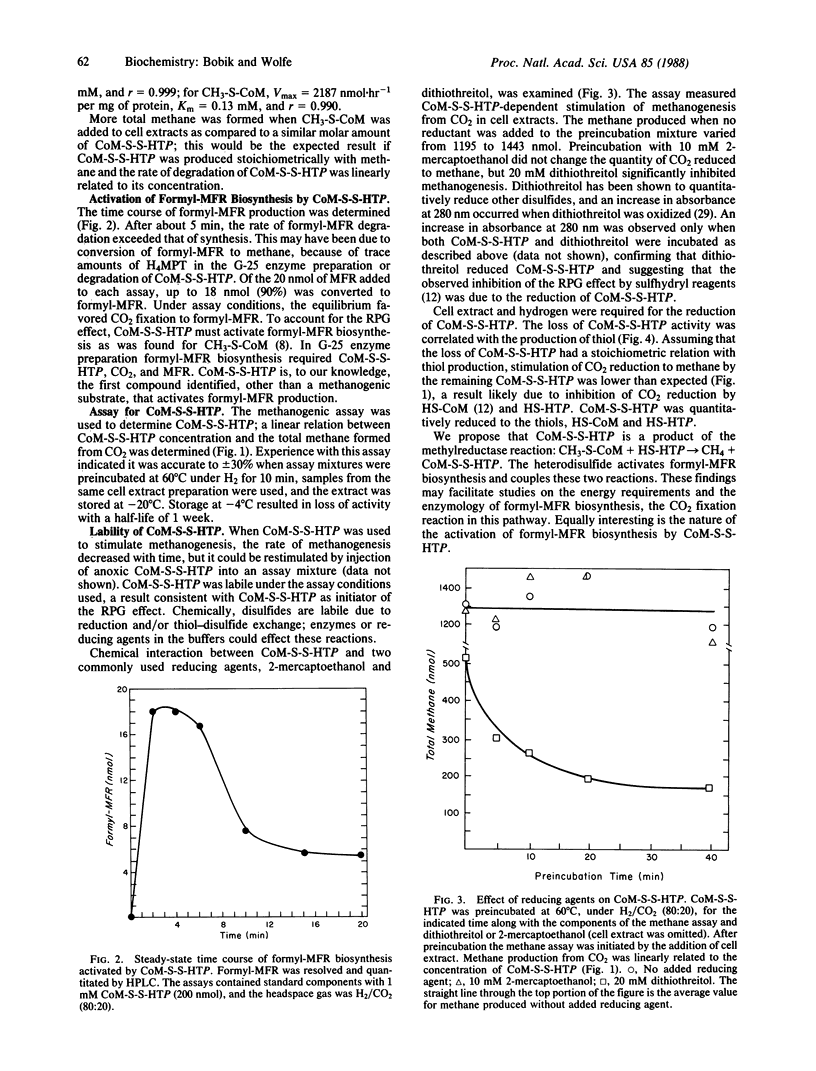
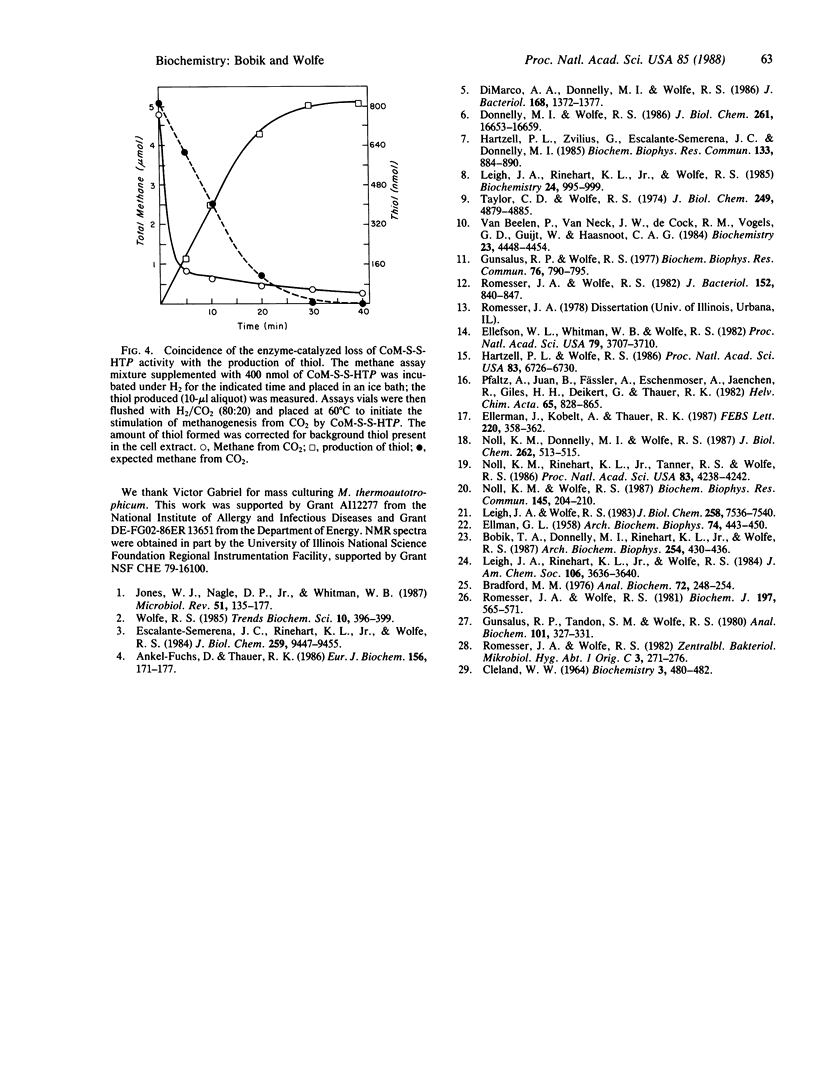
The heterodisulfide of the two coenzymes 2-mercaptoethanesulfonic acid (coenzyme M, HS-CoM) and N-(7-mercaptoheptanoyl)threonine O3-phosphate (HS-HTP) increased the rate of CO2 reduction to methane by cell extracts 42-fold. The stimulation resulted from activation of the initial step of methanogenesis, the production of formylmethanofuran from methanofuran and CO2. These results establish a role for this heterodisulfide (CoM-S-S-HTP) in the reduction of CO2 to formylmethanofuran. Evidence indicates that CoM-S-S-HTP is the labile intermediate that accounts for the coupling of the reduction of 2-(methylthio)ethanesulfonic acid by the methylreductase to formylmethanofuran biosynthesis, the "RPG effect." The heterodisulfide was found to be labile in cell extracts due to enzyme-catalyzed reduction and possibly thioldisulfide exchange.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankel-Fuchs D., Thauer R. K. Methane formation from methyl-coenzyme M in a system containing methyl-coenzyme M reductase, component B and reduced cobalamin. Eur J Biochem. 1986 Apr 1;156(1):171–177. doi: 10.1111/j.1432-1033.1986.tb09563.x. [DOI] [PubMed] [Google Scholar]
- Bobik T. A., Donnelly M. I., Rinehart K. L., Jr, Wolfe R. S. Structure of a methanofuran derivative found in cell extracts of Methanosarcina barkeri. Arch Biochem Biophys. 1987 May 1;254(2):430–436. doi: 10.1016/0003-9861(87)90121-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- DiMarco A. A., Donnelly M. I., Wolfe R. S. Purification and properties of the 5,10-methenyltetrahydromethanopterin cyclohydrolase from Methanobacterium thermoautotrophicum. J Bacteriol. 1986 Dec;168(3):1372–1377. doi: 10.1128/jb.168.3.1372-1377.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M. I., Wolfe R. S. The role of formylmethanofuran: tetrahydromethanopterin formyltransferase in methanogenesis from carbon dioxide. J Biol Chem. 1986 Dec 15;261(35):16653–16659. [PubMed] [Google Scholar]
- ELLMAN G. L. A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys. 1958 Apr;74(2):443–450. doi: 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- Ellefson W. L., Whitman W. B., Wolfe R. S. Nickel-containing factor F430: chromophore of the methylreductase of Methanobacterium. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3707–3710. doi: 10.1073/pnas.79.12.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermann J., Kobelt A., Pfaltz A., Thauer R. K. On the role of N-7-mercaptoheptanoyl-O-phospho-L-threonine (component B) in the enzymatic reduction of methyl-coenzyme M to methane. FEBS Lett. 1987 Aug 17;220(2):358–362. doi: 10.1016/0014-5793(87)80846-3. [DOI] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Rinehart K. L., Jr, Wolfe R. S. Tetrahydromethanopterin, a carbon carrier in methanogenesis. J Biol Chem. 1984 Aug 10;259(15):9447–9455. [PubMed] [Google Scholar]
- Gunsalus R. P., Tandon S. M., Wolfe R. S. A procedure for anaerobic column chromatography employing an anaerobic Freter-type chamber. Anal Biochem. 1980 Jan 15;101(2):327–331. doi: 10.1016/0003-2697(80)90195-5. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Stimulation of CO2 reduction to methane by methylcoenzyme M in extracts Methanobacterium. Biochem Biophys Res Commun. 1977 Jun 6;76(3):790–795. doi: 10.1016/0006-291x(77)91570-4. [DOI] [PubMed] [Google Scholar]
- Hartzell P. L., Wolfe R. S. Requirement of the nickel tetrapyrrole F430 for in vitro methanogenesis: reconstitution of methylreductase component C from its dissociated subunits. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6726–6730. doi: 10.1073/pnas.83.18.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell P. L., Zvilius G., Escalante-Semerena J. C., Donnelly M. I. Coenzyme F420 dependence of the methylenetetrahydromethanopterin dehydrogenase of Methanobacterium thermoautotrophicum. Biochem Biophys Res Commun. 1985 Dec 31;133(3):884–890. doi: 10.1016/0006-291x(85)91218-5. [DOI] [PubMed] [Google Scholar]
- Jones W. J., Nagle D. P., Jr, Whitman W. B. Methanogens and the diversity of archaebacteria. Microbiol Rev. 1987 Mar;51(1):135–177. doi: 10.1128/mr.51.1.135-177.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Rinehart K. L., Jr, Wolfe R. S. Methanofuran (carbon dioxide reduction factor), a formyl carrier in methane production from carbon dioxide in Methanobacterium. Biochemistry. 1985 Feb 12;24(4):995–999. doi: 10.1021/bi00325a028. [DOI] [PubMed] [Google Scholar]
- Leigh J. A., Wolfe R. S. Carbon dioxide reduction factor and methanopterin, two coenzymes required for CO2 reduction to methane by extracts of Methanobacterium. J Biol Chem. 1983 Jun 25;258(12):7536–7540. [PubMed] [Google Scholar]
- Noll K. M., Donnelly M. I., Wolfe R. S. Synthesis of 7-mercaptoheptanoylthreonine phosphate and its activity in the methylcoenzyme M methylreductase system. J Biol Chem. 1987 Jan 15;262(2):513–515. [PubMed] [Google Scholar]
- Noll K. M., Rinehart K. L., Jr, Tanner R. S., Wolfe R. S. Structure of component B (7-mercaptoheptanoylthreonine phosphate) of the methylcoenzyme M methylreductase system of Methanobacterium thermoautotrophicum. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4238–4242. doi: 10.1073/pnas.83.12.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll K. M., Wolfe R. S. The role of 7-mercaptoheptanoylthreonine phosphate in the methylcoenzyme M methylreductase system from Methanobacterium thermoautotrophicum. Biochem Biophys Res Commun. 1987 May 29;145(1):204–210. doi: 10.1016/0006-291x(87)91307-6. [DOI] [PubMed] [Google Scholar]
- Romesser J. A., Wolfe R. S. Coupling of methyl coenzyme M reduction with carbon dioxide activation in extracts of Methanobacterium thermoautotrophicum. J Bacteriol. 1982 Nov;152(2):840–847. doi: 10.1128/jb.152.2.840-847.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romesser J. A., Wolfe R. S. Interaction of coenzyme M and formaldehyde in methanogenesis. Biochem J. 1981 Sep 1;197(3):565–571. doi: 10.1042/bj1970565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]



