Abstract
Alcohol is a physical and behavioural teratogen. Fetal alcohol syndrome (FAS) is a common yet under-recognized condition resulting from maternal consumption of alcohol during pregnancy. While preventable, FAS is also disabling.
Although FAS is found in all socioeconomic groups in Canada, it has been observed at high prevalence in select First Nations and Inuit communities in Canada.
This statement addresses FAS prevention, diagnosis, early identification and management for health care professionals.
Prevention of FAS must occur at two levels. Primary prevention involves eliminating FAS through classroom or community education, and encouraging women to avoid consuming alcohol before conception and throughout pregnancy. Secondary prevention involves identifying women who are drinking while pregnant and reducing their consumption. This statement describes a variety of screening strategies including Tolerance-Annoyance, Cut Down, Eye Opener (T-ACE). Medical practitioners should recommend abstinence starting with the first prenatal visit. Prompt referral for alcohol treatment is recommended for pregnant individuals who are unable to stop drinking alcohol.
This statement describes the diagnosis of FAS, partial or atypical FAS, alcohol-related birth defects and alcohol-related neurodevelopmental disorder. With a history of in-utero alcohol exposure, a diagnosis of FAS should be considered with current or previous growth deficiency, select facial abnormalities involving the upper lip and eyes, and neurodevelopmental abnormalities. These features are best quantified with the use of a four-digit diagnostic method.
Strategies for early identification of possible alcohol-related abnormalities are outlined.
Intervention focuses on optimizing development, managing behavioural difficulties and providing appropriate school programming. Of prime importance is earliest possible childhood intervention to prevent secondary disabilities that may result from delay while awaiting a definitive diagnosis of FAS.
Keywords: Development, Fetal alcohol syndrome, Pregnancy
It is only since 1973, when Jones and Smith (1) reported the classic descriptions of the malformations associated with fetal exposure to alcohol, that the full scope of the devastation brought on by alcohol use during pregnancy was understood. A diagnosis of fetal alcohol syndrome (FAS) is based on a history of prenatal alcohol consumption by the mother, combined with a group of characteristics in the infant: poor growth, characteristic facial features and neurological abnormalities. Fetal alcohol effect (atypical FAS) originally described alcohol exposure with an incomplete picture of nonspecific physical and psychological manifestations. This nomenclature was then largely replaced by a classification system that specifies whether the effects are physical (alcohol-related birth defects [ARBD]) or related to the development of the nervous system or brain (alcoholrelated neurodevelopmental defects [ARND]) (2). Although ARBD and ARND do not necessarily accompany full-blown FAS, their effects can be just as severe (3). More recently, Astley and Clarren (4) suggested limiting nomenclature to the use of FAS and atypical FAS.
PREVALENCE
The exact prevalence of FAS/atypical FAS is unknown. Abel (5) estimated the overall incidence of FAS at 0.97/1000 (0.097%) live births and 43/1000 (4.3%) among babies of heavy drinkers. More recently, on the basis of three population studies, Sampson et al (6) estimated the incidence of FAS to be between 2.8/1000 and 4.8/1000 live births, and the incidence of a combination of FAS and ARND to be at least 9.1/1000 live births. Although all races are susceptible, FAS is disproportionately higher among American Indian offspring (7).
There is increasing awareness of the extent of FAS and atypical FAS in native communities in Canada (8), especially the relationship of these conditions with developmental delay and difficulty in learning (9,10). The few studies available suggest a very high incidence among Canadian Aboriginal people. Robinson et al (11) identified 22 (16%) of 116 children as having FAS in one Aboriginal community in British Columbia. A report by MacDonald (12) in 1991 suggested a rate of FAS in British Columbia of 3.3/1000 children and a rate of atypical FAS of up to five times as high if older children with the syndrome were included. Based on 42,909 births per year in 1998 (13), this predicted number of infants suggests that at least 142 infants are born with FAS and 710 infants are born with partial FAS per year in British Columbia. In northern Manitoba, based on physical findings at birth, Williams et al (14) estimated the incidence of FAS to be 7.2/1000 children, but suggested a number of cases may have been missed. FAS in newborns tends to be under-recognized (15).
ETIOLOGY
Alcohol is both a physical and behavioural teratogen. It is one of the leading causes of mental deficiency in the world. Autopsy and magnetic resonance imaging studies have demonstrated microcephaly, with evidence of tissue loss, cerebral dysgenesis, and abnormalities of glial and neuronal migration (16). Holoprosencephaly is characteristic of FAS. It is a condition that is associated with failure of the brain to divide into two hemispheres, and is usually associated with neurodevelopmental and facial abnormalities. There may also be associated abnormalities of the corpus callosum (eg, agenesis, hypoplasia), the brainstem and the cerebellum, especially the anterior portion of the vermis. Other findings may include absent olfactory lobes, hypoplasia of the hippocampus and abnormal or absent basal ganglia; commonly hypoplastic or absent caudate nuclei. Positron emission tomography scans have demonstrated abnormalities in glucose metabolism, especially in the anterior caudate nucleus and the vermis of the cerebellum, even in the absence of overt structural abnormalities. Studies are being done to relate brain abnormalities with neurobehavioural outcomes.
The variability of brain lesions is thought to result from differences in the amount of alcohol ingested, the pattern and timing of drinking, or the mother’s genetic ability to metabolize alcohol.
MATERNAL FACTORS
Maternal age and the amount of alcohol consumed were directly related to cognitive defects in a group of alcoholexposed infants (17). There was no relation between maternal drinking and neurodevelopmental outcome with a threshold intake of less than 15 mL (0.5 ounces) of alcohol per day, but above this level, infants of mothers older than 30 years of age were two to five times more likely to be functionally impaired than those of younger mothers. Functionally significant defects were seen primarily in infants whose mothers drank more than five drinks per occasion on an average of at least once a week. However, even in known alcohol-abusing mothers, FAS in newborns continues to be under-recognized (15).
Biochemical markers in the mother may help to gauge the amount of alcohol that is consumed. Stoler et al (18) measured four blood markers during pregnancy: carbohydrate-deficient transferrin, gamma-glutamyl transpeptidase, mean red blood cell volume and whole blood-associated acetaldehyde. All mothers who consumed at least 29.6 mL of alcohol per day had at least one marker. All mothers with two or more markers had infants whose heights, weights and head circumferences were less than those of normal babies.
CLINICAL MANIFESTATIONS
The effects of prenatal alcohol exposure range from death or FAS at one end of the spectrum, to relative normality at the other end. The diagnosis of FAS is based on a triad of features in an individual exposed to alcohol in utero: preand postnatal growth deficiency, a characteristic pattern of facial abnormalities and central nervous system dysfunction.
Abnormal facial features include short palpebral fissures, increased intercanthal distance, a flattened face with a short nose, absent or hypoplastic filtrum, and a bow-shaped mouth with a thin upper lip. Standards for these features have been established (4).
The most devastating sequelae of fetal alcohol exposure are neurobehavioural, associated with alcohol’s effect on the central nervous system (16). In addition to microcephaly, central nervous system dysfunction may affect intelligence, activity and attention, learning and memory, language and motor abilities, and behaviour (Table 1).
TABLE 1.
Age-related diagnostic criteria for fetal alcohol syndrome and/or atypical fetal alcohol syndrome
| Infants |
| History of prenatal alcohol exposure |
| Facial abnormalities |
| Growth retardation – height, weight, head circumference |
| Hypotonia, increased irritability |
| Jitteriness, tremulousness, weak suck |
| Difficulty ‘habituating’, getting used to stimulation |
| Preschool |
| History of alcohol exposure, growth retardation, facial abnormalities |
| Friendly, talkative and alert |
| Temper tantrums and difficulty making transitions |
| Hyperactive; may be oversensitive to touch or over-stimulation |
| Attention deficits, developmental delays – speech, fine motor difficulties |
| Apparent skill levels may appear to be higher than their tested levels of ability |
| Middle childhood |
| History of alcohol exposure, growth retardation, facial abnormalities |
| Hyperactivity, attention deficit, impulsiveness |
| Poor abstract thinking |
| Inability to foresee consequences of actions |
| Lack of organization and sequencing |
| Inability to make choices |
| Lack of organizational skills |
| Inappropriate behaviour |
| Overly affectionate – does not discriminate between family and strangers |
| Lack of inhibitions |
| Communication problems |
| Lack of social skills to make and keep friends |
| Unresponsive to social clues |
| Uses behaviour as communication |
| Difficulty making transitions |
| Academic problems – reading and mathematics |
| Behaviour problems – ‘stretched toddler’ |
| Adolescent and adult |
| History of alcohol exposure, growth retardation, facial abnormalities |
| Intelligence Quotient – average to mildly retarded with wide range; continued school difficulties |
| Difficulty with adaptive and living skills |
| Attention deficits, poor judgment, impulsivity lead to problems with employment, stable living and the law |
| Serious life adjustment problems – depression, alcoholism, crime, pregnancy and suicide |
NEWBORN
The features described above may not be readily apparent at birth because many of the manifestations of fetal alcohol exposure appear with age (Table 1). The most consistent physical finding in newborns with FAS, apart from the characteristic facial appearance, which may be difficult to recognize, is growth retardation, especially a small head circumference (19,20). Increased motor activity, and alterations in motor tone and orientation behaviour have also been found (21–24) and tend to be relatively nonspecific. Hearing disorders (25), eye abnormalities (26) and assorted congenital abnormalities may also be found.
EARLY CHILDHOOD
Throughout early childhood, other behavioural manifestations become evident, such as delayed motor and speech development (27,28), and decreased cognitive abilities (29,30), with more serious defects occurring in children whose mothers drank heavily throughout their pregnancies (8,11,19,31,32). Difficulties with interpersonal relationship skills (33) are characteristic. Attention deficits, hyperactivity and impulsive behaviours similar to those found in children with attention deficit hyperactivity disorder (ADHD) have also been documented in children with FAS and/or atypical FAS (10,33). As the children get older, specific learning impairments in language and number processing may become evident (10,34,35). Abnormalities of hearing and speech (25,36), and olfactory difficulties may also be noted.
Although heavy drinking, and especially ‘binge’ drinking, appears to have an effect on cognition, behaviour and development, several studies suggest that behavioural abnormalities and language deficits may vary greatly. Greene et al (37) followed, up to the age of three years, a cohort of infants who were exposed to alcohol prenatally and found no significant relationship between alcohol exposure and language difficulties, unless craniofacial effects of FAS were present. Similarly, Russell et al (38) found no significant difference in the intellectual development or auditory processing in children of moderate or ‘social’ drinkers who had no stigmata of FAS or atypical FAS. Abel (3) gave convincing arguments that low alcohol consumption levels are unlikely to cause FAS, that effects depend on high blood alcohol levels, and that the number of drinks consumed at a time is more important than the ‘average’ alcohol consumption. Similarly, Godel et al (20) found that moderate drinking (fewer than five drinks, less than once per week) had no measurable effect on the newborn size compared with frequent or binge drinking, which was associated mainly with microcephaly.
Central nervous system dysfunction affects mainly intelligence, activity and attention, learning and memory, language, and motor abilities.
Effects on activity and attention include tremulousness, hyperactivity, irritability (hallmarks), attention deficits (increased nonalert state) and impulsivity. Unlike children with ADHD, who may show a similar spectrum of activity, children with FAS and/or atypical FAS scored more like normal controls on tests of vigilance and reaction time. Ingestion of alcohol in the ‘social drinker’ range — 13.3 mL of absolute alcohol per day (AAP) — was associated with a poorer attention span, even when controlled for parity, smoking, home environment and the sex of the child. In these cases, hyperactivity was not the issue.
The Intelligence Quotient (IQ) in children with FAS is highly variable, ranging from 50 to 115. In the six-year-old offspring of mothers with ‘problem’ drinking during pregnancy, a mean decrease of seven IQ points was found (38,39). Streissguth et al (40) found a similar decrease in IQ in six-year-old children who had been exposed to ‘binge’ drinking (greater than five drinks at one time) in utero. In a study by LaDue et al (41), adolescents and adults with FAS were found to have intellectual functioning in the mild to moderate range of impairment, with 46% scoring an IQ of less than 69. There was a marked discrepancy between a mean verbal IQ of 65 and a performance IQ of 79, with significant specific deficits in academic and adaptive function.
Even with a normal IQ, learning tended to be compromised in alcohol-exposed children. Features included poor short term memory with intact long term memory, difficulty establishing routines in infants (Brazelton Scale) (42), decreased academic performance, especially with ‘binge’ drinking, problems with verbal memory (recalling Word List) (43), and defects in spatial memory, with poor retention of learned tasks. Defects identified by testing include defects in replicating shapes from memory (clock drawing), and recalling and copying details. Problems with reading and mathematics are common (10).
Speech delay and language deficits such as difficulties in word comprehension, naming ability, articulation, expressive and receptive language skills, and articulation disorders are also typical.
Interpersonal skills tend to be impaired (34) and behaviour problems are common (Table 2). Difficulties include the inability to make and keep friends. Children with FAS and/or atypical FAS are excessively friendly, even to strangers, and lack the ability to discriminate between friends, family and strangers.
TABLE 2.
Cognitive and behavioural profile of children with fetal alcohol syndrome and/or atypical fetal alcohol syndrome
| Lack of organization |
| Sequencing |
| Inability to make choices |
| Poor abstract thinking |
| Inability to foresee consequences |
| Impulsive |
| Inappropriate behaviour |
| Excessive friendliness |
| Lack of inhibitions |
| Inability to learn from previous experiences |
| Communication problems |
| Unresponsive to social clues |
| Inability to make and keep friends |
| Use behaviour as communication |
| Difficulty with adaptive living skills |
Cognitive problems are also common in children with FAS. Attention, short term memory, flexibility and planning, auditory memory (tapping memory and number sequences), and spatial visualization all may be affected (24). These children may also have motor problems, including delayed motor development, impaired fine motor skills and difficulties with balance (25). Problems that can be exposed by testing include delay in motor development and fine motor coordination, uncoordinated motor patterns, ataxia, hemiplegia, defects in motor speed, precision, finger tapping speed and grip strength (10).
Individuals exposed to alcohol in utero may have long term sequelae that requires life-long care and attention. LaDue et al (41), Olson et al (44) and Streissguth et al (45) have established a profile of psychological and behavioural manifestations of FAS and atypical FAS that do not improve with age. Poor judgment and the inability to appreciate the possible consequences of an action are common. If these characteristics are combined with frustration by poor school performance or a tendency toward impulsive behaviour, conduct leading to conflict with society may result. Furthermore, excessive familiarity and friendliness even toward strangers, combined with a lack of inhibitions, can also lead to exploitation and abuse.
Other problems with long term implications, such as poor social skills (31), difficulty with organization, and difficulty with recognizing and setting boundaries, make dayto-day living difficult and hazardous (46). These continuing defects distinguish individuals with fetal exposure to alcohol from individuals with attention deficit disorders and learning disabilities not associated with alcohol exposure. There is evidence that adequate and early intervention can minimize the effects of behaviours related to FAS or atypical FAS (28,30). Thus, it is important to recognize individuals with FAS or atypical FAS early.
DIAGNOSIS AND MANAGEMENT
Management of FAS needs a proactive approach.
The first aim is prevention: changing attitudes toward drinking in young people of school age.
The second aim is to identify the at-risk drinker, if possible, before pregnancy occurs, allowing for early intervention in the drinking habits.
The third aim is to identify the at-risk infant, either at birth or in early infancy.
The fourth aim is to start intervention as soon as possible to prevent secondary problems.
The last aim is to make a more precise and definitive diagnosis, either of FAS or of comorbid conditions that require treatment so that specific services can be accessed and specific problems can be addressed.
Identifying the at-risk drinker
As a part of the traditional Aboriginal society, mother and baby are considered parts of a larger circle that involves partners, families and communities (47). Identifying the atrisk women should be done in this context so that sympathetic support and treatment can be mobilized easily.
All women who are seen by primary care physicians, midwives or nurse practitioners should be asked about their drinking habits, whether pregnant or not pregnant. This line of questioning should be done respectfully as part of history taking, in the context of a culturally-based, traditional approach. The extent of drinking can be characterized as follows:
Abstainers: Consume no alcohol.
Low-risk drinkers: Consume one to two standard drinks per day, three times a week or less. Alcohol has no effect on their health. They do not use alcohol while driving, while pregnant, when breastfeeding or with certain medications.
At-risk drinkers: Consume seven to 21 standard drinks per week. Consume more than three to four standard drinks per occasion, or drink in high-risk situations.
Problem drinkers: Consume more than 21 standard drinks per week and may experience negative consequences (behavioural, family, medical, mental health, employment, legal, etc) from such drinking.
There are a number of ways to approach the subject of drinking. Questions should be part of a complete history that includes dietary intake, smoking habits and the extent of drinking. The questioner should be supportive and nonconfrontational, because merely asking the questions about drinking may elicit a defensive response. Nevertheless, it is important that all women, whether nonpregnant (Appendix 1) or pregnant (Appendix 2) be asked about their drinking habits.
Answers to these questions help to assess the degree of problem drinking and can lead to one of four possible conclusions:
patient is an at-risk drinker;
patient is a problem drinker;
patient may be alcohol-dependent; and
patient is not at risk (48).
An alternative approach to risk assessment is called Tolerance-Annoyance, Cut Down, Eye Opener (T-ACE).
How much alcohol do you drink before you feel its effects? (Tolerance)
Has anyone Annoyed you by saying you should cut down on your drinking?
Have you ever thought you should Cut Down?
Have you ever had a drink to get going in the morning? (Eye Opener)
If the woman answered the tolerance question with two or more drinks, the score is 2. A positive response to the other questions yields a score of 1 for each question. A total score of 2 or more indicates ‘at-risk’ drinking behaviour (49).
At-risk drinkers who are not pregnant should be advised to cut down or abstain from alcohol use. Dependent drinkers should be asked to abstain and should be referred to an alcohol specialist. The goal for a pregnant woman should be complete abstinence. Advice should be given with the support of the spouse, family and friends who are closest to the person at risk. The object is to make them allies for supporting the change in behaviour (47). Strategies for intervention are outlined in the booklet, A Guide for Primary-Care Providers (48). Close follow-up and sympathetic support are essential.
Early identification of the at-risk child
The importance of early diagnosis:
The earlier that FAS and its associated problems are identified, the sooner effective management can begin. The doctor or the midwife is usually the first to be confronted with a potentially affected baby and has an important role to play in diagnosis and management. With early diagnosis, anticipatory guidance and support can be provided to the mother. Alcohol-affected infants may be very difficult to manage and there is potential for child abuse. It is also important to prevent further exposure of the infant to alcohol through breastfeeding (50). Assessments and early intervention programs should be mobilized early so that plans can be made for the child’s future educational needs. There is evidence that early intervention may help to prevent and minimize secondary FAS and/or atypical FAS behavioural effects.
Identification of the at-risk newborn
Appendix 3 is a useful screen to help the primary care practitioner identify the newborn at risk for FAS (51). Suspicion of FAS is based on physical signs, growth retardation, especially of the head (4,20), characteristic facial features (1,2,52) and evidence of central nervous system dysfunction. Affected newborn infants tend to sleep poorly; be irritable; be hypersensitive to touch, light and sound; have difficulty establishing routines; and be poor feeders.
Early management:
Identified at-risk infants should be referred to early childhood intervention programs without delay to prevent damaging behaviours that may develop, and to deal with developmental problems. The mother should be given support and help in dealing with a difficult infant. Often, the mother has FAS and may have problems coping with a difficult child.
Identification of possible FAS in the toddler or preschool child
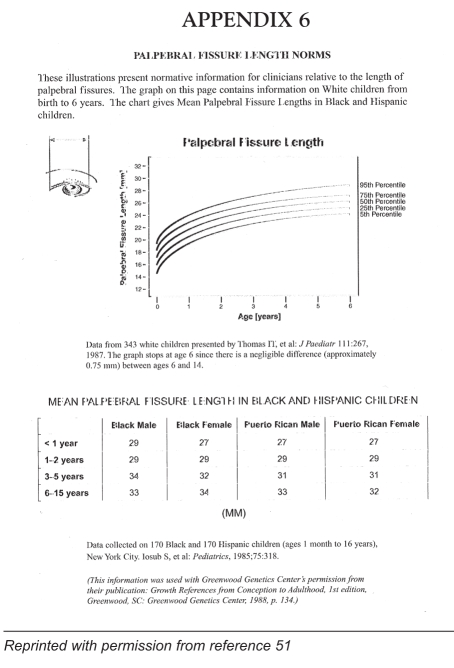
The older at-risk child will often present with more definite and specific signs than the newborn child: developmental delay, especially of speech, poor growth and behavioural abnormalities. Characteristic behavioural manifestations of FAS, such as hyperactivity, poor judgement, inability to appreciate consequences of actions, excessive friendliness, difficulties with sequencing, poor short term memory and learning difficulties, may become apparent at this stage. Screening of the 18-to 24-month-old child (Appendix 4) and of the four-to five-year-old child (Appendix 5) can help the paediatrician, physician or public health nurse in the diagnosis and management of FAS (51). Appendix 6 gives standards for measurements of palpebral fissure. No standards have been established for Canadian Aboriginal people.
Early management:
If behavioural, physical or learning difficulties typical of FAS are identified, the child should be referred not only for further diagnostic work-up by an FAS team, but also for help in managing behaviours. However, it is important to begin intervention even in the absence of a definitive diagnosis.
Identification of FAS in the school-aged child
The school-aged child, if not previously identified, will usually be referred for diagnostic work-up because of learning problems — especially with reading and mathematics — or with behavioural abnormalities. The full spectrum of behavioural abnormalities (Tables 1, 2) may be evident as well as secondary, usually negative, behavioural manifestations. This child should be referred for a full work-up, which requires cooperation among the teacher, parent, school psychologist and physician.
Making a definitive diagnosis
A definitive diagnosis of FAS is difficult to make because there are no biochemical markers and the manifestations of this condition are so variable. Making the diagnosis depends on identifying a spectrum of clinical characteristics that are static and not due to postnatal factors. The participation of a team of skilled physicians and psychologists may be needed to carry out the detailed physical examination, developmental assessment, cognitive tests and parental and school behaviour questionnaires to provide the precision required for diagnosis.
The 4-digit Diagnostic Code (4) provides a reproducible, objective, consistent and precise method for the diagnosis of FAS. Four criteria are assessed, quantified and assigned a rating of 1 to 4 for each criteria, depending on the degree of abnormality:
impaired growth;
facial abnormalities (52);
abnormal brain function; and
degree of maternal drinking.
Precise criteria for evaluating maternal drinking, growth and facial characteristics are provided with the diagnostic guide (4). Although brain dysfunction is the most significant disability caused by prenatal exposure to alcohol, it is also the most difficult to assess because it includes parameters, such as IQ, cognition, and neurological and behavioural abnormalities, that vary considerably among individuals. Dysfunction is rated on a scale of 1 to 4, depending on severity.
A rating of 4 (definite brain dysfunction) defines a situation of ‘static encephalopathy’ and depends on definite findings of brain damage — microcephaly, abnormalities of brain imaging, persistent neurological findings of prenatal origin and/or an IQ score of 60 or lower.
At the other end of the scale, a rating of 1 (absent) is given when no brain problems are demonstrated.
A rating of 3 (probable brain dysfunction), also characterized as ‘static encephalopathy’, is based on abnormalities in three of four areas of brain function affecting cognition, achievement, adaptation, neurological ‘soft’ signs and language.
A rating of 2 (possible brain dysfunction), referred to as ‘neurobehavioural disorder’, is based on personal observations and historical information about behaviour, suggesting the possibility of brain damage.
Defining these abnormalities may depend on extensive observation, checklists and testing. The easily administered and specific fetal alcohol behaviour scale, developed and standardized by Streissguth et al (43), may be useful in quantifying behaviours. It is based on scoring a simple “yes/no” on 36 items and the results are valid regardless of age, race, sex or IQ.
Table 3 lists a number of tests that may also be useful in quantifying behaviours, and cognitive and neurodevelopmental abnormalities, but may require the services of a psychologist (42,43,53,54).
TABLE 3.
Tests available to delineate neurodevelopmental problems
| Tests used to measure intelligence may include: |
| Bayley Scales of Infant Mental and Motor Development (Bayley) resulting in a Mental Development Index |
| Stanford Binet – yields Intelligence Quotient (IQ) |
Wechsler Scales – yield IQ
|
| Tests used to measure attention and hyperactivity include: |
| Taland Letter cancelling test |
| WISC-R digit span |
| Wisconsin Card Sorting Test (WCST) – indicates shifting attention |
| Attention deficit hyperactivity disorder comprehensive Teacher’s Rating Scale (ACTeRS) (54) |
| Tests of learning and memory include: |
| Brazelton Scale – habituation (42) |
| Pediatric Early Elementary Examination (PEEX) (60) |
| Pediatric Examination of Educational Readiness (PEER) (61) |
| Brigance (53,62) |
| Tests of language include: |
| Denver Development Screening Test (DDST) |
| Word Span |
| Naming |
| Word comprehension |
| Woodstock Reading Mastery |
| Tests of motor abilities include: |
| DDST |
| WISC-R |
| PEEX (60) |
| PEER (61) |
| Tests of social skills and behaviour include: |
| Vineland Adaptive Behaviour Scales (VABS) (31) |
| Fetal Alcohol Behavior Scale (FABS) (43) |
| FAS\atypical FAS Scale (10) |
| ACTeRS (54) |
| Tests of Visual-spatial difficulties include: |
| Beery Developmental Test of Visual-Motor Integration |
| Frostig Developmental Test of Visual Perception |
| PEEX (60) |
| PEER (61) |
The numbers obtained in the four categories are then slotted into a four-digit diagnostic code. Codes vary from 1111 (normal) to 4444 (unequivocal FAS) (4). Grouping results in 22 code combinations, all of which lead to different diagnostic possibilities. Only three possibilities refer to FAS (FAS, alcohol exposed; FAS, alcohol exposure unknown; and atypical FAS, alcohol exposed). Advantages of this system include precision and reproducibility, consistency of diagnosis and consideration of other possible diagnoses. Disadvantages include the possibility of false negatives. For instance, if a definite history of alcohol exposure and behaviours typical of FAS are found (Table 2), but growth failure and typical FAS facial features are absent, the resultant scores (1134 or 1143) would be designated as ‘static encephalopathy’ and not FAS, even though fetal alcohol exposure may be the likely cause. This difference in labelling may be important because funding for intervention services may hinge on a stated diagnosis of FAS.
The use of the four-digit diagnostic scale is recommended for making the diagnosis of FAS. It is fairly simple and straightforward, and can be carried out by a well-trained physician with a minimum of sophisticated tests. Testing by a psychologist may be useful in further defining disabilities and in planning intervention.
INTERVENTION
Intervention should be based on need, and should not be delayed because of long waiting lists or delay in accessing definitive diagnostic services. The consequences of delaying treatment can be serious for children with FAS. Children with FAS who are excessively friendly may be at risk for abuse, and those who lack a sense of consequence may get into trouble with the law. Indeed, a high percentage of youth in the criminal justice system have been identified with FAS and/or atypical FAS (55). Delays in dealing with behavioural and cognitive problems at this stage can result in secondary disabilities (56) and problems such as school failure, loss of self-esteem, frustration and acting out. While abnormalities associated with FAS are permanent and life-long, some can be modified with early intervention. Indeed, the literature on FAS is full of reports of successes associated with early intervention (57).
If neonatal or infant screening identifies behavioural or neurodevelopmental abnormalities, treatment should start as soon as possible. Health care providers should not wait for a more definitive diagnosis, but should begin working on the child’s interpersonal behaviours and learning in a way that promotes self-worth and self-esteem. This approach means identifying the child’s strong points and building on them.
Referral for a more specific diagnosis is important to establish an etiology. Because not all children with FAS and/or atypical FAS have the same spectrum of abnormalities, identification permits the planning an intervention program that is more specific to the individual.
Infants with FAS and/or atypical FAS are very difficult to manage, and because of this problem, they are at risk for abuse from caregivers. Caregivers must be given information about what to expect from the infant and should be provided with guidance in managing behaviours. They may need respite care.
Parents and caregivers can learn to take cues from the baby. Infants should be handled and stroked gently, and cuddled frequently with frequent eye contact and soft, soothing words. Sudden movements and bouncing should be avoided. Infants and children with FAS and/or atypical FAS handle transitions poorly, so it is important to establish a strict routine.
Long term objectives of early childhood intervention and education include:
establishing and maintaining a sense of self-worth;
establishing acceptable interpersonal behaviour;
fostering independence; and
teaching children how to make acceptable decisions.
Within an early childhood intervention program, these children may be taught to function within their limitations, learn how to make proper choices, develop acceptable interpersonal skills, master basic life skills and, above all, maintain self-esteem. Such educational intervention often means scaling down academic expectations and emphasizing training for future self-sufficiency. Programming for success can result in improved learning and enhanced self-image, which in turn can decrease ‘acting out’ behaviours.
As children get older, their difficult behaviours may be related to limited short term memory, problems with sequencing, difficulties making choices and a lack of appreciation of consequences of actions. As a result, they have difficulty remembering simple routines and instructions. It is important to keep tasks simple, to use concrete examples and to give instructions one at a time. They may have difficulty recognizing and reacting to dangerous situations, so may need to be protected at all times. Limits should be simple and consistent and explanations should be given calmly. Tantrums may represent attempts at communication and should be handled with short time-outs. Children with FAS and/or atypical FAS need to be taught effective means for making their wants known.
Strategies for dealing with difficult behaviours include the following.
Keep tasks simple.
Use concrete examples.
Keep instructions simple and give them one at a time.
Concentrate on life skills.
More specific strategies depend on the problems that are uncovered. Hinde (58) outlined approaches to specific behaviours in the one-to three-year-old child with FAS or atypical FAS.
Close communication and cooperation is necessary between parents and professionals. Parents should be taught how to analyze tasks by identifying a desired outcome, then breaking the task down into small steps. Specific measures can be developed to help modify attention and hyperactivity problems. Teaching the child to distinguish family and friends from strangers can diminish the problem of excessive friendliness. Poor sequencing can be addressed by establishing routines and using pictures to reinforce them. The goal is to help children learn skills that will eventually lead to independent living.
Similar strategies can be extended to the preschool-and school-aged child (59). Teachers need to be trained in effective techniques and need to work in cooperation with parents and school psychologists.
It is important to train specialists to deal with behavioural abnormalities in early childhood intervention programs. Intervention by community-based specialists working directly with parents or foster-parents should be carried out in the context of the family and the community. Because experts in early childhood education are few, their services could be supplemented by well-trained volunteers who could do home visits and supply family support. Training could occur in the community in a fashion that is modelled on the training of crisis-line volunteers.
If possible, affected children should remain with their birth families. Parenting courses should be made available and parents should be encouraged to participate. However, if the mother has been affected by FAS or is unable to cope, or if the family situation threatens the well-being of the child, foster care may be necessary. Foster families should have specific training or be experienced with FAS children. The frustration level is often very high and caregivers need respite. Multiple foster homes should be avoided because they are damaging to attachment and the child’s selfesteem. Some individuals with FAS may not be able to develop the skills to live independently and may require long term group home placement.
Ongoing research is desirable to see whether measures taken are effective and should be criteria-based. Using the 4-digit Diagnostic Code assures that criteria for diagnosis are consistent. It also allows comparisons of prevalence, outcomes and the effectiveness of preventive and intervention measures.
FUNDING
Children across Canada do not have equal access to diagnostic and intervention services. For example, status Indians are funded for more comprehensive services than nonstatus individuals in northern Saskatchewan, despite having the same range of problems. Training of early childhood education specialists, school psychologists, occupational and physiotherapists should also be covered globally.
Funding should not depend on a formal diagnosis of FAS. Because of waiting lists and the lack of a definitive diagnosis, the ‘window of opportunity’ for dealing with behavioural abnormalities and preventing secondary disabilities is often missed. Currently, only children who are labelled as ‘disabled’ can access funding. FAS and related developmental and physical conditions should be considered disabilities that are eligible for financial help.
Funding for FAS must be global, with each jurisdiction contributing to a ‘pot’ from which all children can benefit. This would require unprecedented cooperation between the federal government, provincial governments, social services, native bands and departments of education.
RECOMMENDATIONS
The Canadian Paediatric Society recommends that the following measures be taken to prevent, diagnose and manage FAS.
Primary prevention of FAS should involve schoolbased educational programs; early recognition; treatment of at-risk women; and communitysponsored, culturally-centred programs. Health care providers should ask women about their drinking habits, whether or not they are pregnant.
Health care providers play an important role in identifying babies or children with FAS. They should become familiar with the screening tools that are available to diagnose the condition in children at various ages.
If behavioural or physical abnormalities consistent with FAS are identified, intervention should begin without delay, even before a definitive diagnosis is made.
Intervention programs should involve the child’s family and community.
FAS diagnostic and treatment services require a multidisciplinary approach, involving physicians, psychologists, early childhood educators, teachers, social service professionals, family therapists, nurses and community support circles.
Diagnostic and treatment services should be publicly funded and available to all Canadians, regardless of their ethnicity, status (eg, status and nonstatus aboriginals), place of residence or income.
Interventions should continue to be evaluated for effectiveness.
To ensure that all children have access to the appropriate services and support, cooperation is required at various levels and across various sectors: federal government; provincial ministries of health, social services and education; and local community groups.
Individuals and groups providing diagnostic and treatment services should take a culturally based, holistic approach.
APPENDIX 1
APPENDIX 2
APPENDIX 3
APPENDIX 4
APPENDIX 5
APPENDIX 6
Footnotes
INDIAN AND INUIT HEALTH COMMITTEE
Members: Drs Garth Bruce, Royal University Hospital, Saskatoon, Saskatchewan (director responsible); Jim Carson, University of Manitoba, Winnipeg, Manitoba (chair); James Irvine, La Ronge, Saskatchewan; Keith Menard, Stanton Medical Clinic, Yellowknife, Northwest Territories; Kent Saylor, Kahnawake, Quebec; Leigh Wincott, Thompson General Hospital, Thompson, Manitoba
Consultants: Dr Fred Baker, Calgary, Alberta; Mr Keith Conn, Medical Services Branch, Health Program Services, Health Canada, Ottawa, Ontario; Drs John Godel, Heriot Bay, British Columbia; Michael Moffatt, Winnipeg Children’s Hospital, Winnipeg, Manitoba; Gary Pekeles, The Montreal Children’s Hospital, Montreal, Quebec
Liaisons: Ms Claudette Dumont-Smith, Ottawa, Ontario (Aboriginal Nurses Association of Canada); Ms Reepa Evic-Carleton, Ottawa, Ontario (Inuit Women’s Association); Ms Melanie Morningstar, Ottawa, Ontario (Assembly of First Nations); Ms Margaret Horn, Kahnawake, Quebec (National Indian and Inuit Community Health representative); Drs David Grossman, Harborview Injury Prevention and Research Center, University of Washington, Seattle, Washington, USA (Committee on Native American Child Health, American Academy of Pediatrics); Vincent Tookenay, Ottawa, Ontario (Native Physicians Association of Canada)
Principal author: Dr John Godel, Heriot Bay, British Columbia
The recommendations in this statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate.
Internet addresses are current at the time of publication.
REFERENCES
- 1.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early pregnancy. Lancet. 1973;ii:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 2.Stratton K, Howe C, Battaglia FC, editors. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention and Treatment. Washington: National Academy Press; 1996. [Google Scholar]
- 3.Abel EL. What really causes FAS? Teratology. 1999;59:4–6. doi: 10.1002/(SICI)1096-9926(199901)59:1<4::AID-TERA3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Astley SJ, Clarren SK.Diagnostic Guide for Fetal Alcohol Syndrome and Related Conditions: The 4-Digit Diagnostic Code Seattle: University of Washington, FAS Diagnostic and Prevention Network; 1999. <http://depts.washington.edu/fasdpn> (Version current at February 12, 2002). [Google Scholar]
- 5.Abel EL. An update on Incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol Teratol. 1995;17:437–43. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- 6.Sampson PD, Streissguth AP, Bookstein FL, et al. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–26. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Aase JM. The fetal alcohol syndrome in American Indians: A high risk group. Neurobehav Toxicol Teratol. 1981;3:153–6. [PubMed] [Google Scholar]
- 8.Carney LJ, Chermak GD. Performance of American Indian children with fetal alcohol syndrome on the test of language development. J Commun Disord. 1991;24:123–34. doi: 10.1016/0021-9924(91)90016-c. [DOI] [PubMed] [Google Scholar]
- 9.Asante KO, Nelms-Matzke J. Report on the survey of children with chronic handicaps and fetal alcohol syndrome in the Yukon and Northwest British Columbia. Terrance: Mills Memorial Hospital; 1985. [Google Scholar]
- 10.Godel JC, Lee BE, McCallum DE, et al. Exposure to alcohol in utero: Influence on cognitive function and learning in a northern elementary population. Paediatr Child Health. 2000;5:93–100. doi: 10.1093/pch/5.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson GC, Conry JL, Conry RF. Clinical profile and prevalence of fetal alcohol syndrome in an isolated community in British Columbia. CMAJ. 1987;137:203–7. [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald . A Report to Communications and Education Branch BC Ministry of Health and Minister Responsible for Seniors. Vancouver: Ministry of Health; 1991. [Google Scholar]
- 13.BC Vital Statistics Annual Report. Victoria: Crown Publishers; 1998. [Google Scholar]
- 14.Williams RJ, Odaibo FS, McGee JM. Incidence of fetal alcohol syndrome in northeastern Manitoba. Can J Public Health. 1999;90:192–4. doi: 10.1007/BF03404505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoler JM, Holmes LB. Under-recognition of prenatal alcohol effects in infants of known alcohol abusing women. J Pediatr. 1999;135:430–6. doi: 10.1016/s0022-3476(99)70164-2. [DOI] [PubMed] [Google Scholar]
- 16.Roebuck TM, Mattson SN, Riley EP. A review of the neuroanatomical findings in children with Fetal Alcohol Syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:339–44. doi: 10.1111/j.1530-0277.1998.tb03658.x. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson JL, Jacobson SW, Sokol RJ, Ager JW., Jr Relationship of maternal age and pattern of drinking to functionally significant cognitive deficit in infancy. Alcohol Clin Exp Res. 1998;22:345–51. doi: 10.1111/j.1530-0277.1998.tb03659.x. [DOI] [PubMed] [Google Scholar]
- 18.Stoler JM, Huntington KS, Peterson CM, et al. The prenatal detection of significant alcohol exposure with maternal blood markers. J Pediatr. 1998;133:346–52. doi: 10.1016/s0022-3476(98)70267-7. [DOI] [PubMed] [Google Scholar]
- 19.Russell M, Skinner JB. Early measures of maternal alcohol misuse as predictors of adverse pregnancy outcomes. Alcohol Clin Exp Res. 1988;12:824–30. doi: 10.1111/j.1530-0277.1988.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 20.Godel JC, Pabst HF, Hodges PE, Johnson KE, Froese GJ, Joffres MR. Smoking and caffeine and alcohol intake during pregnancy in a northern population: Effect on fetal growth. CMAJ. 1992;147:181–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Walpole I, Zubrick S, Ponte J, Lawrence C. Low to moderate maternal alcohol use before and during pregnancy, and neurobehavioural outcome in the newborn Infant. Dev Med Child Neurol. 1991;33:875–83. doi: 10.1111/j.1469-8749.1991.tb14796.x. [DOI] [PubMed] [Google Scholar]
- 22.Coles CD, Smith I, Fernhoff PM, Falek A. Neonatal neurobehavioural characteristics as correlates of maternal alcohol use during gestation. Alcohol Clin Exp Res. 1985;9:454–60. doi: 10.1111/j.1530-0277.1985.tb05582.x. [DOI] [PubMed] [Google Scholar]
- 23.Roebuck TM, Simmons RW, Richardson C, Mattson SN, Riley EP. Neuromuscular responses to disturbances of balance in children with prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:1992–7. [PubMed] [Google Scholar]
- 24.Olson HC, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychologic deficits in adolescents with fetal alcohol syndrome: Clinical findings. Alcohol Clin Exp Res. 1998;22:1998–2012. [PubMed] [Google Scholar]
- 25.Church MW, Kaltenbach JA. Hearing, speech, language, and vestibular disorders and the fetal alcohol syndrome. Alcohol Clin Exp Res. 1997;21:495–512. doi: 10.1111/j.1530-0277.1997.tb03796.x. [DOI] [PubMed] [Google Scholar]
- 26.Hellstrom A, Chen Y, Stromland K. Fundus morphology assessed by digital image analysis in children with fetal alcohol syndrome. J Pediatr Ophthalmol Strabismus. 1997;34:17–24. doi: 10.3928/0191-3913-19970101-05. [DOI] [PubMed] [Google Scholar]
- 27.Roebuck TM, Simmons RW, Mattson SN, Riley EP. Prenatal exposure to alcohol affects the ability to maintain postural balance. Alcohol Clin Exp Res. 1998;22:252–8. [PubMed] [Google Scholar]
- 28.Nagahara AH, Handa RJ. Fetal alcohol exposure produces delaydependent memory deficits in juvenile and adult rats. Alcohol Clin Exp Res. 1997;21:710–5. [PubMed] [Google Scholar]
- 29.Kerns KA, Don A, Mateer CA, Streissguth AP. Cognitive defects in nonretarded adults with fetal alcohol syndrome. J Learn Disabil. 1997;30:685–93. doi: 10.1177/002221949703000612. [DOI] [PubMed] [Google Scholar]
- 30.Uecker A, Nadel L. Spatial but not object memory impairments in children with fetal alcohol syndrome. J Am Mental Retard. 1998;103:12–8. doi: 10.1352/0895-8017(1998)103<0012:SBNOMI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Conry J. Neuropsychological deficits in fetal alcohol syndrome and fetal alcohol effects. Alcohol Clin Exp Res. 1990;14:650–5. doi: 10.1111/j.1530-0277.1990.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 32.Becker M, Warr-Leeper GA, Leeper HA. Fetal alcohol syndrome: A description of oral motor, articulatory, short-term memory, grammatical, and semantic abilities. J Commun Disord. 1990;23:97–124. doi: 10.1016/0021-9924(90)90016-r. [DOI] [PubMed] [Google Scholar]
- 33.Nanson JL, Hiscock M. Attention deficits in children exposed to alcohol prenatally. Alcohol Clin Exp Res. 1990;14:656–61. doi: 10.1111/j.1530-0277.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 34.Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res. 1998;22:528–33. [PubMed] [Google Scholar]
- 35.Kopera-Frye K, Dehaene S, Streissguth AP. Impairments of number processing induced by fetal alcohol exposure. Neuropsychologia. 1996;34:1187–96. doi: 10.1016/0028-3932(96)00043-7. [DOI] [PubMed] [Google Scholar]
- 36.Church MW, Eldis F, Blakley BW, Bawle EV. Hearing, language speech, vestibular, and dentofacial disorders in fetal alcohol syndrome. Alcohol Clin Exp Res. 1997;21:227–37. [PubMed] [Google Scholar]
- 37.Greene T, Ernhart CB, Martler S, Sokol R, Ager J. Prenatal alcohol exposure and language development. Alcohol Clin Exp Res. 1990;14:937–45. doi: 10.1111/j.1530-0277.1990.tb01842.x. [DOI] [PubMed] [Google Scholar]
- 38.Russell M, Czarnecki DM, Cowan R, MePherson E, Mudar PJ. Measures of maternal alcohol use as predictors of development in early childhood. Alcohol Clin Exp Res. 1991;14:991–1000. doi: 10.1111/j.1530-0277.1991.tb05200.x. [DOI] [PubMed] [Google Scholar]
- 39.Larroque B, Kaminski M. Prenatal alcohol exposure and development at preschool age. Alcohol Clin Exp Res. 1998;22:295–30. doi: 10.1111/j.1530-0277.1998.tb03652.x. [DOI] [PubMed] [Google Scholar]
- 40.Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: Effects on child IQ and learning problems at age 7 1/2 years. Alcohol Clin Exp Res. 1990;14:662–9. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 41.Ladue RA, Streissguth AP, Randels SP. Clinical considerations pertaining to adolescents and adults with fetal alcohol syndrome. In: Sonderegger TB, editor. Perinatal Substance Abuse: Research Findings and Clinical Implications. Baltimore: The Johns Hopkins University Press; 1993. pp. 104–31. [Google Scholar]
- 42.Streissguth AP, Barr HM, Martin DC. Maternal alcohol use and neonatal habituation assessed with the Brazelton Scale. Child Dev. 1983;54:1109–18. [PubMed] [Google Scholar]
- 43.Streissguth AP, Bookstein HM, Barr HM, Sherman P. A fetal alcohol behaviour scale. Alcohol Clin Exp Res. 1998;22:325–33. doi: 10.1111/j.1530-0277.1998.tb03656.x. [DOI] [PubMed] [Google Scholar]
- 44.Olson HC, Burgess DM, Streissguth AP. Fetal alcohol syndrome (FAS) and fetal alcohol effects (ATYPICAL FAS): A life span view with implications for early Intervention. Vol. 13. Arlington: ZERO TO THREE/National Center for Clinical Infant Programs; 1992. pp. 24–9. [Google Scholar]
- 45.Streissguth AP, Aasfe JM, Claren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. JAMA. 1991;265:1961–7. [PubMed] [Google Scholar]
- 46.Burgess DM, Streissguth AP. Fetal alcohol syndrome and fetal alcohol effects: Principles for educators. Phi Delta Kappan. 1992;74:24–9. [Google Scholar]
- 47.Van Bibber M, editor. Aboriginal Nurses Association of Canada; 1997. It takes a community. A Resource Manual for Community-based Prevention of Fetal Alcohol Syndrome and Fetal Alcohol Effects; pp. 33–5. [Google Scholar]
- 48.Identification of At-Risk Drinking and Intervention with Women of Childbearing Age: A Guide for Primary Care ProvidersNIH Publication No. 99-4368.<www.niaaa.nih.gov/publications/FASguides.htm> (Version current at February 18, 2002).
- 49.Alcohol and Child/Family Health conference proceedings. British Columbia: FAS Resource Society; 1988. [Google Scholar]
- 50.Ito S. Drug therapy for breastfeeding women. N Engl J Med. 2000;343:118. doi: 10.1056/NEJM200007133430208. [DOI] [PubMed] [Google Scholar]
- 51.Identification and Care of Fetal Alcohol Exposed Children: A Guide for Primary Care Providers, NIH Publication No. 99-4370. <www.niaaa.nih.gov/publications/FASguides.htm> (Version current at February 18, 2002).
- 52.Stromland K, Chen Y, Norberg T, Wennerstrom K, Michael G. Reference values of facial features in Scandinavian children measured with a range-camera technique. Scand J Plast Reconstr Surg Hand Surg. 1999;33:59–65. doi: 10.1080/02844319950159631. [DOI] [PubMed] [Google Scholar]
- 53.Brigance AH. Comprehensive Inventory of Basic Skills. North Billerica: Curriculum Associates Inc; 1983. [Google Scholar]
- 54.Ullmann RK, Sleator EK, Sprague RL. ACTeRs. Champaign: MetriTech Inc; 1991. [Google Scholar]
- 55.Fast DK, Conry J, Loock CA. Identifying fetal alcohol syndrome among youth in the criminal justice system. J Dev Behav Pediatr. 1999;20:370–2. doi: 10.1097/00004703-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 56.Streissguth AP, Barr HM, Kogan J, Bookstein FL. Understanding the occurrence of secondary disabilities in clients with Fetal Alcohol Syndrome (FAS) and Fetal Alcohol Effects Final report. Seattle: Fetal Alcohol and drug Unit; 1996. [Google Scholar]
- 57.Kleifeld J, Westcott S, editors. Fairbanks: University of Alaska Press; 1993. Fantastic Antone Succeeds. [Google Scholar]
- 58.Hinde J. Early intervention of alcohol affected children. In: Kleifeld J, Wescott S, editors. Fantastic Antone Succeeds. Fairbanks: University of Alaska Press; 1993. pp. 131–47. [Google Scholar]
- 59.Kvigne V, Struck J, Engelhart E, West T. Educational techniques for children with FAS/FAE FAS. In: Kleifeld J, Wetcott S, editors. Fantastic Antone Succeeds. Fairbanks: University of Alaska Press; 1993. pp. 323–39. [Google Scholar]
- 60.Levine MD. The Pediatric Early Elementary Examination (PEEX) Cambridge: Educators Publishing Service Inc; 1992. [Google Scholar]
- 61.Levine MD, Schneider EA. Pediatric Examination of Educational Readiness (PEER) Cambridge: Educators Publishing Service Inc; 1982. [Google Scholar]
- 62.Linkous LW. A reliability study of the Brigance CIBS; Annual Meeting of the Council for Exceptional Children; New Orleans. March 31 to April 4; 1986. [Google Scholar]