Abstract
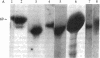
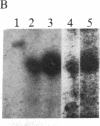
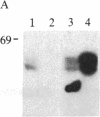
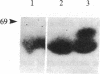
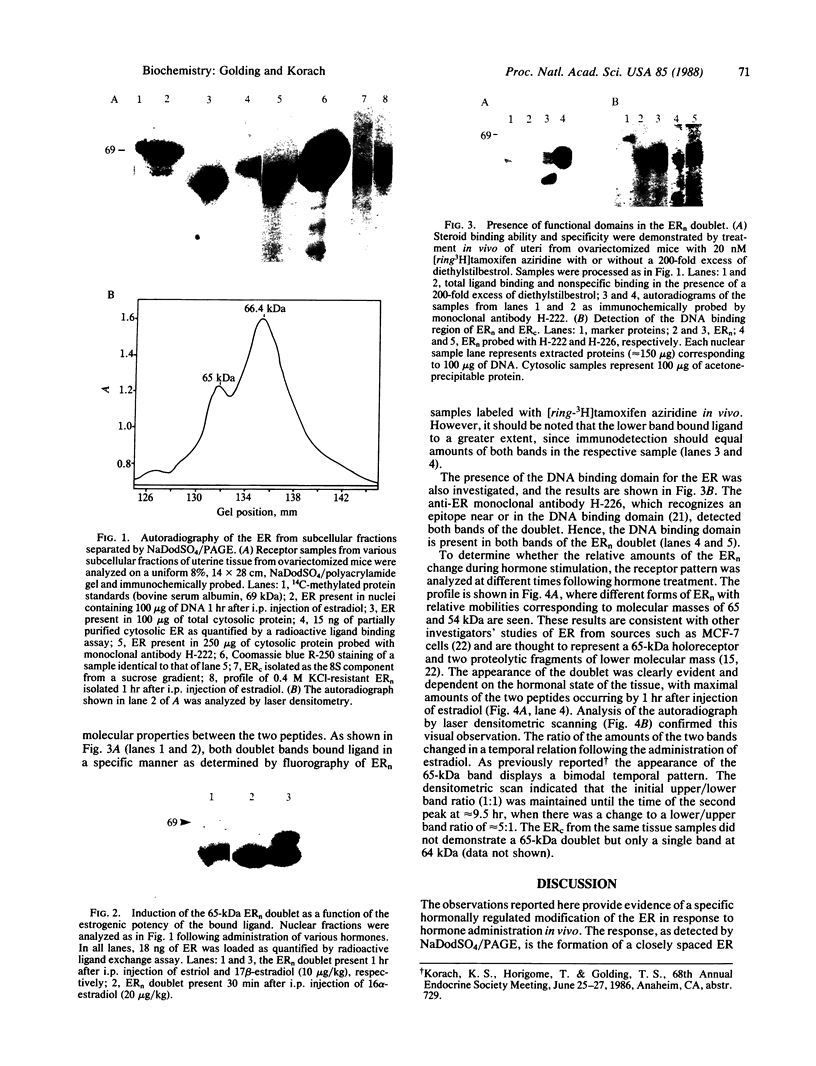
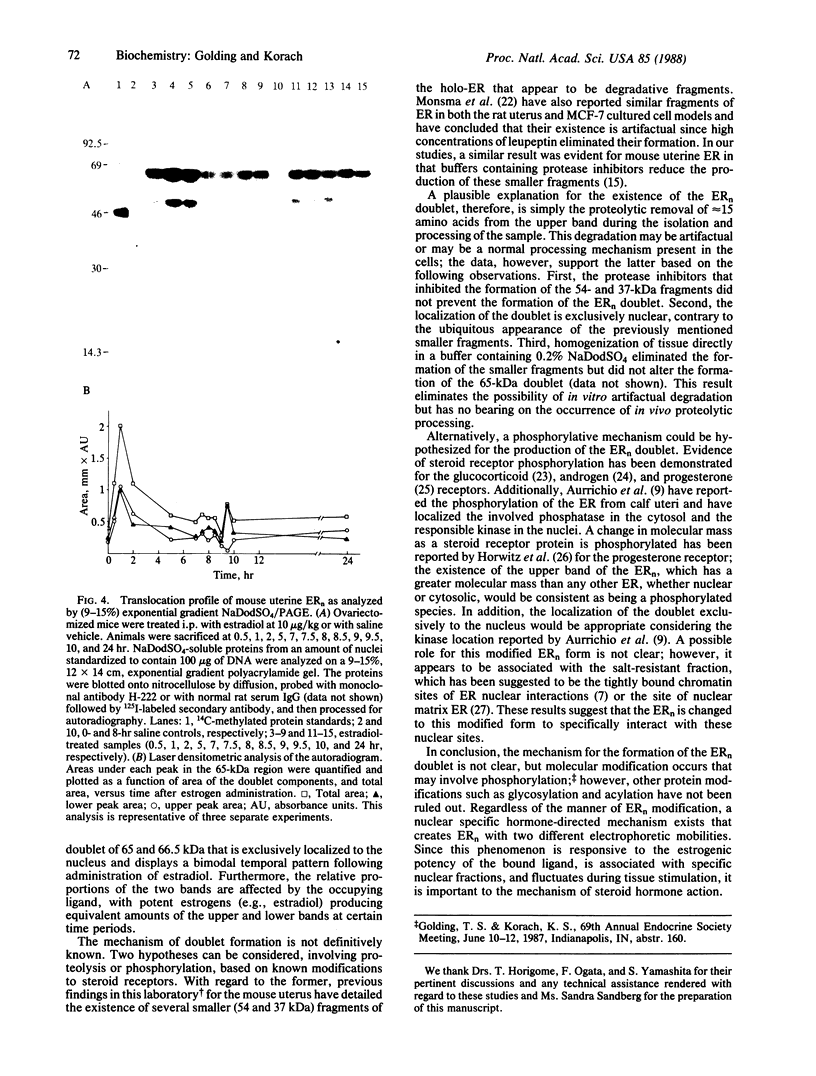
Holomeric estrogen receptor (ER) prepared from ovariectomized mouse uteri displays heterogeneous electrophoretic mobility when analyzed by NaDodSO4/PAGE. ER derived from nuclei (ERn) appears as a closely spaced doublet having apparent molecular masses of 66.4 and 65 kDa, while ER from the cytosolic compartment (ERc) has a single band of 65 kDa. Both partially purified ERc and the 8S form of unactivated ERc show only the 65-kDa band. The appearance of the ERn doublet is hormonally inducible, and the relative proportions of the two doublet bands are influenced by the type of hormone treatment, with weakly estrogenic compounds yielding the lower band as predominant while potent estrogens increase the proportion of the upper band. Steroid binding of the ERn doublet was determined by [3H]tamoxifen aziridine affinity labeling of both the 66.4- and the 65-kDa peptides; binding to the 65-kDa peptide was predominant. The ERn doublet displays a time dependency after estrogen administration with maximal amounts occurring in a bimodal fashion at 1 and 8 hr.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auricchio F., Migliaccio A., Castoria G., Rotondi A., Di Domenico M., Pagano M. Activation-inactivation of hormone binding sites of the oestradiol-17 beta receptor is a multiregulated process. J Steroid Biochem. 1986 Jan;24(1):39–43. doi: 10.1016/0022-4731(86)90029-4. [DOI] [PubMed] [Google Scholar]
- Barrack E. R., Hawkins E. F., Allen S. L., Hicks L. L., Coffey D. S. Concepts related to salt resistant estradiol receptors in rat uterine nuclei: nuclear matrix. Biochem Biophys Res Commun. 1977 Dec 7;79(3):829–836. doi: 10.1016/0006-291x(77)91186-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Peck E. J. Nuclear retention of receptor-oestrogen complex and nuclear acceptor sites. Nature. 1976 Apr 15;260(5552):635–637. doi: 10.1038/260635a0. [DOI] [PubMed] [Google Scholar]
- Dougherty J. J., Puri R. K., Toft D. O. Phosphorylation in vivo of chicken oviduct progesterone receptor. J Biol Chem. 1982 Dec 10;257(23):14226–14230. [PubMed] [Google Scholar]
- Garcia T., Jung-Testas I., Baulieu E. E. Tightly bound nuclear progesterone receptor is not phosphorylated in primary chick oviduct cultures. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7573–7577. doi: 10.1073/pnas.83.20.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Dastidar P., Coty W. A., Griest R. E., Woo D. D., Fox C. F. Progesterone receptor subunits are high-affinity substrates for phosphorylation by epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1654–1658. doi: 10.1073/pnas.81.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goueli S. A., Holtzman J. L., Ahmed K. Phosphorylation of the androgen receptor by a nuclear cAMP-independent protein kinase. Biochem Biophys Res Commun. 1984 Sep 17;123(2):778–784. doi: 10.1016/0006-291x(84)90297-3. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Gilna P., Waterfield M., Baker A., Hort Y., Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986 Mar 7;231(4742):1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Grody W. W., Schrader W. T., O'Malley B. W. Activation, transformation, and subunit structure of steroid hormone receptors. Endocr Rev. 1982 Spring;3(2):141–163. doi: 10.1210/edrv-3-2-141. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Francis M. D., Wei L. L. Hormone-dependent covalent modification and processing of human progesterone receptors in the nucleus. DNA. 1985 Dec;4(6):451–460. doi: 10.1089/dna.1985.4.451. [DOI] [PubMed] [Google Scholar]
- Korach K. S. Estrogen action in the mouse uterus: characterization of the cytosol and nuclear receptor systems. Endocrinology. 1979 May;104(5):1324–1332. doi: 10.1210/endo-104-5-1324. [DOI] [PubMed] [Google Scholar]
- Kurl R. N., Jacob S. T. Phosphorylation of purified glucocorticoid receptor from rat liver by an endogenous protein kinase. Biochem Biophys Res Commun. 1984 Mar 15;119(2):700–705. doi: 10.1016/s0006-291x(84)80307-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., McCarty K. S., Jr, McCarty K. S., Sr Electrophoretic characterization of purified bovine, porcine, murine, rat, and human uterine estrogen receptors. J Biol Chem. 1985 Feb 25;260(4):2515–2526. [PubMed] [Google Scholar]
- Migliaccio A., Lastoria S., Moncharmont B., Rotondi A., Auricchio F. Phosphorylation of calf uterus 17 beta-estradiol receptor by endogenous Ca2+-stimulated kinase activating the hormone binding of the receptor. Biochem Biophys Res Commun. 1982 Dec 15;109(3):1002–1010. doi: 10.1016/0006-291x(82)92039-3. [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Rotondi A., Auricchio F. Calmodulin-stimulated phosphorylation of 17 beta-estradiol receptor on tyrosine. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5921–5925. doi: 10.1073/pnas.81.19.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma F. J., Jr, Katzenellenbogen B. S., Miller M. A., Ziegler Y. S., Katzenellenbogen J. A. Characterization of the estrogen receptor and its dynamics in MCF-7 human breast cancer cells using a covalently attaching antiestrogen. Endocrinology. 1984 Jul;115(1):143–153. doi: 10.1210/endo-115-1-143. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Puca G. A., Nola E., Sica V., Bresciani F. Estrogen-binding proteins of calf uterus. Partial purification and preliminary characterization of two cytoplasmic proteins. Biochemistry. 1971 Sep 28;10(20):3769–3780. doi: 10.1021/bi00796a020. [DOI] [PubMed] [Google Scholar]
- Skafar D. F., Notides A. C. Modulation of the estrogen receptor's affinity for DNA by estradiol. J Biol Chem. 1985 Oct 5;260(22):12208–12213. [PubMed] [Google Scholar]
- Walter P., Green S., Greene G., Krust A., Bornert J. M., Jeltsch J. M., Staub A., Jensen E., Scrace G., Waterfield M. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichman B. M., Notides A. C. Estradiol-binding kinetics of the activated and nonactivated estrogen receptor. J Biol Chem. 1977 Dec 25;252(24):8856–8862. [PubMed] [Google Scholar]