Abstract
The epithelium of the gastrointestinal tract, which represents the greatest body surface area exposed to the outside environment, is confronted with a plethora of foreign and potentially harmful antigens. Consequently, the immune system of the gut faces the daunting task of distinguishing harmless dietary proteins and commensal bacteria from potentially dangerous pathogens, and of then responding accordingly. Mucosal T cells play a central role in maintaining barrier function and controlling the delicate balance between immune activation and immune tolerance. This review will focus on the unique features of mucosal T cell subsets that reside in the epithelium and lamina propria of the gut.
Keywords: Intestinal barrier, Intraepithelial lymphocytes, Lamina propria lymphocytes, CD8αα, Regulatory T cell
1. Introduction
The intestine represents a major immune organ with several specialized lymphoid structures and cell types and has been roughly divided into the inductive organized gut-associated lymphoid tissue (GALT) and effector sites. Organized GALT includes Peyer’s Patches (PP), isolated lymphoid follicles, the appendix, and the gut draining mesenteric lymph nodes (MLN), whereas effector cells accumulate in the lamina propria (LP) as lamina propria lymphocytes (LPL) and within the epithelium as intraepithelial-lymphocytes (IEL).
The challenging function of the gut immune system is to prevent penetration and spreading of commensals and pathogens while avoiding excessive or unnecessary immune responses. Several levels of protective barriers can be distinguished that act to avert microbial invasion of the host. The first line of passive defense attempts to prevent intact antigens and pathogens from entering the body and encountering the immune system (immune exclusion). The second immune barrier is formed by an active innate immune sensing system that provides a combination of maintaining homeostasis and initiating active pro-inflammatory immune responses to microbial invasion by immune surveillance at the mucosal surface. Finally, a highly developed adaptive immune system regulates the responses to antigens that have crossed the epithelial barrier.
The mucosal epithelium is formed by a single layer of tightly connected intestinal epithelial cells (IEC) and acts as a physical protective wall, separating luminal antigens from the underlying tissue compartments. The microvilli of the brush border and the tight junctions between the IEC are essential structural components in regulating permeability of the mucosal border. Specialized IEC, such as the paneth cells and associated innate immune cells also secrete several defensive compounds including mucins, proteolytic enzymes, nitric oxide, and anti-microbial peptides, both constitutively and in response to microbes [1]. In addition to these physical defense systems, antibody-secreting B cells play a key role in maintaining immunological quiescence (reviewed in Ref. [2]). At least 70% of all plasma cells are found in the gut LP and there is more IgA secreted than the total of all other Ig isotypes combined [3]. IgA class-switching is promoted by TGF-β, a cytokine that is abundantly present in the gut mucosa and the secretion of IgA is controlled by several factors including IL-6 and retinoic acid [4].Most plasma cells produce dimers of secretory IgA (sIgA) antibodies that are exported to the gut lumen and function by entrapping dietary antigens and micro-organisms in the mucus leading to their excretion and by preventing microbial components from attaching to the epithelium. In the gut wall, locally produced IgA can also interact with antigens that have reached the LP and the resulting immune complexes are either taken up by phagocytosis or transcytosed back to the lumen, again enforcing immune exclusion [5].
The barrier is not absolute however and under normal conditions there is extensive crosstalk between the luminal microbes and all arms of the mucosal immune system, coordinated by the IEC and dendritic cells (DC) as messengers. The importance of these interactions is mirrored by the fact that without bacterial colonization of the intestine the structure and function of the intestine itself, including the mucosal immune system, are highly impaired. The crosstalk between the outside environment and concealed immune system is executed via continuous sampling of luminal antigens. The so-called M cells, which are specialized enterocytes that are located in the follicle-associated epithelium, constantly transport intact antigens to the mucosal lymphoid tissue underneath for processing and antigen presentation [6]. Their primary function of trans-cellular endocytosis is facilitated by several distinctive morphological features including a reduced brush border, the absence of a thick glycocalyx, and lack of enzymatic activity. Inevitably perhaps, M cells are also used by many pathogens as a route of entry into the body [6]. Besides these “gateways to the mucosal immune system”, DC have been shown to take up apoptotic IEC and their contents (such as endocytosed antigens) in addition to direct sampling of luminal antigens across the mucosal epithelia without compromising the integrity of the barrier. These processes are mainly associated with promoting tolerance and immune suppression in order to prevent damage of the intestinal barrier and for maintenance of immune homeostasis. In addition, the controlled sampling may also allow for the generation of immune memory before invasion by the pathogen. Overall, the sampling of the gut content may be a mechanism to specifically “adapt” the mucosal immune system to the environment (and therefore also the pathogens likely to be encountered) by the individual.
In the event however that pathogens cross the barrier and gain uncontrolled access to the mucosal immune system, strong and protective immune responses may be initiated that eliminate infections before they become apparent or spread systemically. The rapid and effective protection is ensured by the presence of numerous DC, macrophages, and plasma cells as well as various subsets of effector T cells. Both the LP and the epithelial compartment contain large numbers of antigen-experienced T cells that play a crucial role in protection of the barrier and the host. These memory T cell subsets differ significantly from each other in their ontogeny, the type of antigens they recognize, the signals they received for their differentiation, and the specific effector and/or regulatory function they exert. In this review we will focus on the diverse subsets of mucosal T cells. We will address issues regarding their development, differentiation, function, and interaction with other cell types in the intestinal mucosa.
2. Mucosal T cell subsets
In the intestine, large populations of T cells reside in three main compartments; the organized GALT, the LP, and the epithelium. Whereas organized structures of the GALT, such as the MLN, contain naïve T cells, almost all T cells in the LP and epithelium display characteristics of an effector/memory phenotype. Although these frontline T cells are notoriously heterogeneous with regard to their phenotype and function, two major subsets can be distinguished based on T cell receptor (TCR) and co-receptor expression [7]. The first group, or “type a” cells, consists of TCRαβ+ MHC class II-restricted CD4+ and MHC class I-restricted CD8αβ+ lymphocytes that resemble conventional thymus-selected antigen-experienced T cells also found in the blood, spleen and other secondary lymphoid organs. After priming in response to their cognate antigen encountered in the periphery or at the local induction sites, these activated T cells migrate to the effector site of intestine, where some reside long-term as effector memory T cells. The second group, which is referred to as “type b” cells, express either TCRαβ or TCRγδ and they frequently express CD8αα molecules but lack expression of the typical TCR co-receptors CD4 or CD8αβ. The different mucosal T cell subtypes are present to a varying degree in different anatomical mucosal compartments (LP versus epithelium, small versus large intestine) and their distribution depends on age, strain, and housing conditions. However, in general the T cell component of the LP is largely composed of type a cells, whereas type b cells are far more prevalent in the mucosal epithelium (for an overview see Fig. 1). In the following sections we will discuss different aspects, including function, of the type a and b LPL and IEL.
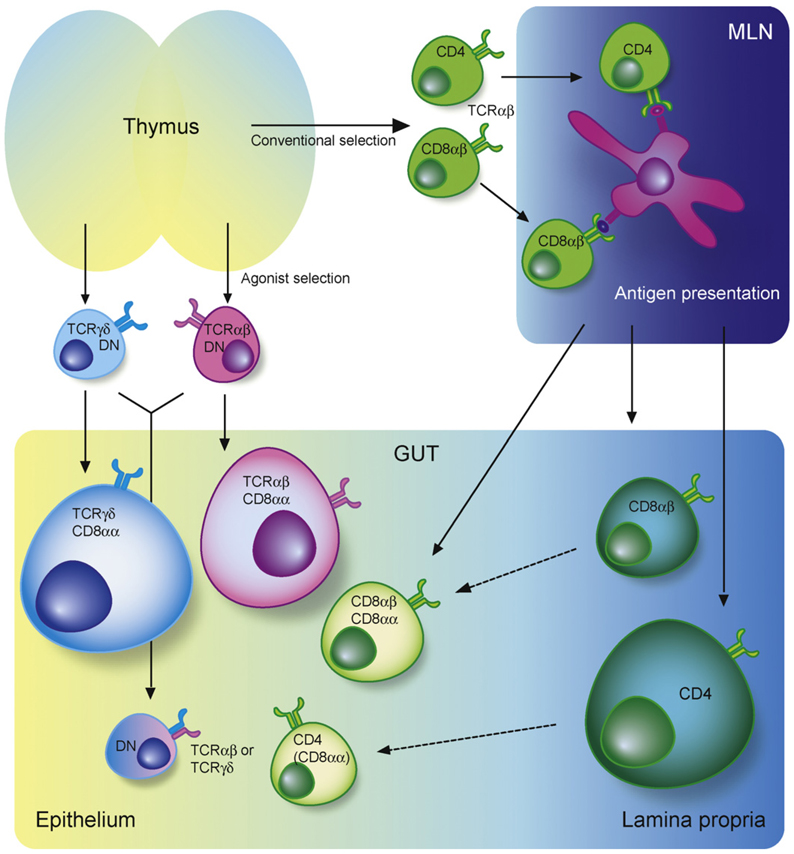
Fig. 1. Intestinal T cell subsets.
The lamina propria (LP) and epithelium of the intestine harbor diverse populations of T cells. Conventional or “type a” mucosal T cells that have matured in the thymus along the conventional selection pathway migrate, after antigen priming in the mesenteric lymph nodes (MLN), to mainly the LP but also the epithelium. Upon entry in the epithelium these cells often co-express CD8αα. Most intraepithelial lymphocytes (IEL) however belong to two subsets of unconventional or “type b” mucosal T cell populations: the TCRγδ+CD8αα+ IEL that are thymus derived and develop along the double-negative pathway and the TCRαβ+CD8αα+ IEL that have matured and differentiated in the thymus along the agonist-selection pathway. Both subsets migrate as antigen-experienced directly to the intestine where the majority of cells upregulate CD8αα, whereas some remain double-negative (DN).
3. IEL and LPL
Intraepithelial lymphocytes reside within the columnar epithelial layer. When they have been looked for, they have been found in all vertebrates that possess a thymus, in both small and large intestine. However, their frequencies vary along the gut and from species to species. In the mouse, it is estimated that there is approximately one IEL per 5–10 IEC in the small intestine versus one IEL per 40 IEC in the colon [8]. In the small intestine of mice a large population of IEL expresses TCRγδ, while the remaining population consists of TCRαβ+ IEL that are mainly TCRαβ+CD8αα+IEL, a subset of TCRαβ+CD8αβ+ and fewer TCRαβ+CD4+ T cells. In human the proportion of TCRγδ+ T cells in the small intestine is smaller (about 10%) but numbers greatly increase under certain allergic and/or inflammatory conditions such as celiac disease [9]. Conventional type a CD4+ IEL are more abundant in the large intestine (about 30% of total IEL). The different distributions of T cell subtypes between the small and large intestine may be a reflection of different roles of IEL in these two compartments, with regions of high bacterial load presumably demanding strong protective barrier function, whereas other relatively sterile, highly absorptive regions may require greater immune regulation in response to luminal (food) antigens and for the induction of oral tolerance.
T lymphocytes scattered throughout the LP consist of mainly type a CD4+ T helper (Th) cells and (less) cytotoxic CD8αβ+ T cells. Few non-conventional T cell subsets populate the LP but interesting subsets include invariant NKT [10] and mucosal-associated invariant T cells [11] that interact with the non-classical MHC molecules CD1d and MR1 respectively.
4. Type a mucosal T cells
Type a mucosal T cells are progeny of conventional naïve T cells and they have much in common with the antigen-induced memory cells in the periphery although they also display some distinct features. Type a cells gradually increase with age when more and more antigen-experienced T cells migrate and accumulate in the gut mucosa as long-lived memory cells. In contrast to type b mucosal T cells, type a cells are not confined to the epithelial compartment and they are abundant in the LP and the thoracic duct lymph as well [12]. Initial priming of many (but perhaps not all) type a precursor cells, takes place in the organized lymphoid structures of the GALT. Upon luminal antigen uptake, DC migrate to the T cell areas of the PP and MLN in a CCR7-dependent fashion where they initiate T cell activation. The activated T cells then home to the gut and their recruitment is regulated by distinct sets of adhesion molecules expressed by the T cells and their respective ligands on vascular endothelial cells (reviewed in Ref. [13]). Several adhesion receptors have been implicated in regulating T cell entry and localization within the intestinal epithelium. These receptor–ligand pairs include LFA-1 and ICAM-1, integrinα4β7, and MADCAM-1, the integrin αEβ7 (CD103) whose ligand E-cadherin is expressed on the basolateral surface of IEC and the chemokine CCR9, whose ligand CCL25 is constitutively expressed by small intestine IEC.
How are these gut homing T cells generated? The local mucosal lymphoid environment, rather than the nature of the antigen, plays a major role in imprinting homing properties of the primed T cells [14]. It was demonstrated that DC from PP and MLN have enhanced ability to generate gut-tropic T cells compared to splenic and peripheral lymph node DC. A seminal study by Iwata et al. further showed that this unique capacity of the mucosal DC was mediated through the selective release of the Vitamin A metabolite, retinoic acid (RA) by mucosal DC during priming [15]. RA induces α4β7 and CCR9 on activated CD4+ and CD8+ T cells and is pivotal for the imprinting of gut-homing cells as shown by the severe reduction in T cells in the small intestine of vitamin A deficient mice [15]. More recent studies suggest that the RA-producing gut IEC and MLN stromal cells may also contribute to the induction of gut homing by the mucosal DCs [16,17]. Expression of CD103 on IEL is initiated by TGF-β and the transcription factor Runx3 [18,19] and facilitated by signaling through CCR9 [20].
Whereas the regulation of T cell migration to the small intestine is well described, the mechanisms underlying recruitment of T cells to the large intestine remain poorly defined. Although colon-tropism is dependent on α4β7 (but not CCR9) [21], RA seems neither necessary nor sufficient to induce T cell migration to the large intestine [21,22].
5. Type a IEL function and regulation
In the small intestine type a IEL are mostly CD8αβ+TCRαβ+ memory cells that show cytolytic effector function upon antigen challenge. Compared to central memory T cells in the spleen, these effector memory T cells can be rapidly activated and they may provide initial immediate cytotoxic responses to local infection [23] (Fig. 2). Their TCR repertoire is more restricted than that of peripheral memory CD8αβ+ T cells suggesting that repeated re-stimulation in the intestine may lead to TCR focusing or that a TCR-dependent mechanism drives the selective migration and/or differentiation of mucosal CD8αβ effector memory T cells [24]. While antigen stimulation can drive their proliferation, cytokines such as IL-7 and IL-15 mediate their slow turnover in the absence of TCR triggering [25].
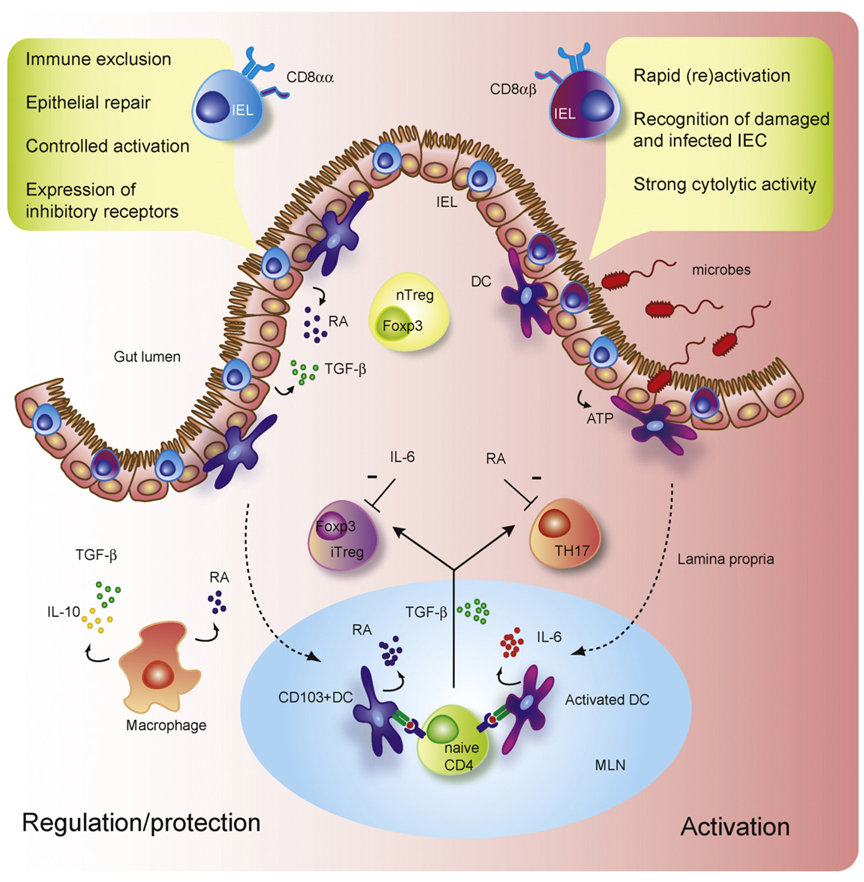
Fig. 2. Mucosal T cell regulation and activation.
The functionally diverse T cell populations of the intestine that are shaped by the gut environment are important players in sustaining the delicate immune balance between activation and regulation. The CD8αα+ intraepithelial cells (IEL) of the intestine play a crucial role in protection of the mucosal barrier. They are involved in maintaining and restoring barrier homeostasis by stimulating intestinal epithelial cell (IEC) turnover. Upon pathogen entry rapid activation and high cytolytic activity of the CD8αβ+ IEL contribute to the prevention of pathogen spreading by killing infected IEC. The activation of IEL is highly controlled through the expression of inhibitory receptors that may alter the threshold for activation. Under the influence of IEC and IEL, lamina propria (LP) dendritic cells (DC) acquire ability to produce retinoic acid (RA) thereby inducing gut-homing receptors during CD4 T cell priming in the mesenteric lymph nodes (MLN). Additionally, gut-derived CD103+ DC and LP macrophages favor the conversion of Foxp3+ induced regulatory T cells (iTreg) in a RA, TGF-β, and IL-10 (in the case of LP macrophages)-mediated fashion, whereas activated DC promote the differentiation of IL-17-producing Th17 cells via a combination of IL-6 and TGF-β. This pro- and anti-inflammatory immune deviation of iTreg and Th17 is reciprocally controlled by RA and IL-6. Finally, the LP is also home to agonist-selected Foxp3-expressing naturally occurring regulatory T cells (nTreg).
After activation and migration to the intestine some CD8αβ+ effector T cells reside as effector memory IEL and they can be rapidly activated in response to local re-infections (reviewed in Ref. [26]). Transfer of antigen-specific type a CD8αβ+ IEL to infected hosts has demonstrated indeed that these memory T cells play an important role in providing immune protection to several infections including LCMV [27], rotavirus [28], Toxoplasma gondii [29], and Giardia lamblia [30].
Although most memory CD8αβ+ IEL retain an activated phenotype (e.g. CD69 expression and maintenance of a heightened cytolitic state) that enables them to rapidly respond to antigen re-exposure, they also display reduced ability to proliferate or produce inflammatory cytokines [31]. These unique features might have evolved in order to maintain the balance of immunological protection without compromising organ integrity. In this respect, most type a CD8αβ+ IEL co-express CD8αα. In contrast to CD8αβ however, which functions as a TCR co-receptor to enhance TCR signals, CD8αα functions as a co-repressor of TCR signaling [24]. We showed before that the transient expression of CD8αα during primary activation rescues the cells from activation induced cell death (AICD) and allows for their further differentiation to memory T cells [32]. CD8αα is readily re-induced on migrating effector cells in the gut environment and it is possible that constitutive expression of CD8αα on these CD8αβ+ IEL increases the threshold for TCR activation to prevent aberrant activation of these effector type T cells. In addition, CD8αα may interact with its ligand TL expressed on IEC to control homeostatic proliferation [33], survival and T cell activation [31,34]. Another example of how the tissue environment may influence local T cell responses is the expression of the P2X7 purinoreceptor by IEL. It was recently shown that CD8αβ+ IEL selectively express high levels of P2X7, probably induced by RA in the gut environment [35]. Expression of P2X7 was associated with increased ATP-induced apoptosis and in P2XY-deficient mice enhanced intestinal, but not spleen CD8 T responses, were observed following Listeria monocytogenes infection [35].
Although most type a IEL are CD8αβ+ CTL, CD4+ T cells are also present within the epithelium, especially in the colon however they are more prevalent among the LPL.
6. Type a LPL function and regulation
All classical CD4+ Th subsets can be found in the intestinal LP including the Th1 subset that drives cell-mediated immune responses associated with intracellular infection and cytotoxicity and the Th2 subset that is involved in IgE production, clearance of helminth infection, and allergic sensitization. However, in the past few years it has become apparent that the LP is also home to (1) a Th cell population that constantly produces pro-inflammatory cytokines such as IL-17A, IL-22, and IL-17F, the so-called Th17 cells and (2) high percentages of Foxp3-expressing regulatory T cells (Treg) (Fig. 2).
Foxp3+ Treg are known to play an important role in intestinal homeostasis and one of the most pronounced features of the immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, caused by a mutation in the FOXP3 gene, is intestinal inflammation [36]. Furthermore, it was demonstrated that transfer of Treg can inhibit and even cure colonic inflammation in a naïve T cell transfer colitis model [37]. Mechanisms of action by Treg include production of regulatory cytokines (IL-10 and TGF-β) and expression of inhibitory receptors such as CTLA-4 [38]. Both thymus-derived natural Treg and peripherally induced Treg preferentially accumulate in the gut. The mechanism of induced Treg differentiation in response to peripherally encountered antigens may be of particular benefit to the intestine, where the immune system has to deal with an enormous load of innocuous foreign antigens derived from the food and the flora. The environment of the gut is indeed thought to favor Treg conversion and CD103+ DC that are enriched in the LP and MLN are highly effective in inducing Foxp3+ Treg in an antigen-specific manner [39,40]. This induction is depending on TGF-β which is abundantly present in the intestine. Although TGF-β, along with its unique ability to induce Foxp3+ Treg, has been mainly associated with immune suppression, a key study from Veldhoen et al. showed that TGF-β in the context of the pro-inflammatory cytokine IL-6, supports de novo differentiation of pro-inflammatory effector Th17 cells [41]. In the gut, IL-17 producing cells play an important role in the host defense against extracellular bacterial and fungal pathogens [42]. Interestingly, under steady-state conditions “naturally occurring” Th17 cells are also (selectively) enriched in the LP of the gut, demonstrating that the mutually exclusive pathways of Treg and Th17 development co-exist in, and probably contribute to, intestinal immune homeostasis. We recently identified RA as a key regulator in controlling the pro- and anti-inflammatory TGF-β-driven immune balance [43]. In addition to promoting induced-Treg conversion [39,43], RA is also capable of inhibiting the TGF-β/IL-6-driven induction of Th17 cells [43]. Distinct intestinal APC subsets that have the capacity to release RA during priming play a prominent role in this immune deviation. CD103+ DC from the LP and MLN are most efficient in generating Treg (compared to CD103− and splenic DC) and the induction of Treg can be blocked by inhibition of the retinal-converting enzyme retinal dehydrogenase (Aldh), which is highly expressed in this DC subset. In contrast, a population of CD11chiCD11bhi LP DC has been shown to promote Th17 differentiation [44] and the presence of the TLR5 ligand flagellin further increased this ability [45]. Finally, LP resident macrophages seem to favor Foxp3+ Treg differentiation via amechanism depending on IL-10, RA and exogenous TGF-β [44].
The preferential accumulation of both Treg and Th17 cells in the LP of the intestine seems to be at least in part regulated by commensal bacteria. The presence of commensal DNA was shown to limit the conversion of Treg in a TLR9-dependent fashion [46]. TLR9-deficient mice displayed increased frequencies of Foxp3+ Treg and reduced IL-17 producing cells in the LP. In contrast, it has also been reported that commensals can induce Treg, which protects the host from pathogen-induced inflammatory responses [47]. In line with this, induction of Treg has been suggested as one of the mechanisms of action of probiotics.
Data from several papers indicate that commensal bacteria are required for IL-17 production by intestinal type a cells, since germ-free mice contain virtually no Th17 cells in the LP [48–50]. Surprisingly, the “spontaneous” production of IL-17 in the LP was independent of TLR (MyD88 or Trif) signaling [49,50]. It was suggested that ATP produced by commensals can drive Th17 differentiation by stimulating a subset of LP DCs to produce TGF-β, IL-6, and IL-23 [50].
7. Type b mucosal T cells
Most type b mucosal T cells reside in the epithelial compartment of the small intestine and express either TCRαβ or TCRγδ. Although these type b TCRγδ+ and TCR αβ+ IEL are clearly different from one another, they share many “unconventional” characteristics that distinguish them from type a IEL [51]. Type b IEL contain a large number of “self”-reactive T cells and in addition to their activated phenotype they also typically express CD8αα homodimers in the absence of the TCR co-receptors CD4 or CD8αβ and they lack expression of some “typical” T cell markers including CD2, CD28, and Thy-1. They are already present at birth and remain the dominant IEL population in young animals. In older mice type b IEL are gradually replaced by an expanding pool of type a effector/memory T cells. Nonetheless, abundant type b IEL were detected when wild mice were sampled. Although some IEL can be detected at fetal stages, the major colonization of the gut epithelium by (mostly type b) IEL takes place perinatally. Two important events shortly after birth have a huge impact on IEL numbers: first the microbial colonization of the gut immediately after birth and second the changes in antigen load and composition occurring at weaning [52]. These events are associated with waves of IEL seeding. The importance of exogenous antigen challenge in the development of TCRαβ+ IEL is demonstrated by the fact that in germ-free mice IEL numbers are greatly reduced and skewed toward γδ T cells. However, early migration of tybe b IEL to the intestine seems, in contrast to type a IEL, to be independent of antigen-stimulation but crucially depending on a CCR9/CCL25- and α4β7-integrin-mediated pathway [53]. In addition, whereas conventional type a IEL trafficking crucially depends on sphingosine-1 phosphate (S1P), S1P does not play a role in the migration pathways of type b IEL [54].
Even though it is clear that CD8αα+ IEL do not follow the pathway of conventionally selected thymocytes, their precise ontogeny, intermediate stages of development, as well as the sites where they develop have long been a matter of debate (reviewed in Ref. [55]). CD8αα+ IEL were initially classified as thymus-independent T cells, however more recent studies showed that under normal conditions all gut IEL, including the type b IEL, are of thymic origin.
There is increasing evidence from several sources that both γδ and αβ type b IEL are at last in part functionally programmed during thymic differentiation. Moreover, there are data strongly supporting the hypothesis that TCRαβ+CD8αα+ IEL precursors undergo a unique self-antigen-dependent agonist selection process. In the thymus, conventional T cells are selected based on an intermediate, positive signal through their TCRαβ, which allows further maturation (positive selection), whereas cells that have a too strong affinity for self-antigens are clonally deleted (negative selection). Elimination of auto-reactive T cell by negative selection is believed to represent one of the main mechanisms for the establishment of central tolerance. However, recent evidence suggests that alternative pathways exist that specifically select for auto-reactive T cells (agonist selection) [56–58]. Like CD4+CD25+ regulatory T cells and NKT cells, TCRαβ+CD8αα+ IEL precursor cells preferentially accumulate under conditions that lead to the deletion of conventional TCRαβ+ T cells [56,59,60]. Consistent with this, “forbidden” TCR combinations can be found within the TCRαβ+CD8αα+ IEL repertoire that are normally purged from the thymic repertoire by stromal presentation of “super-antigens” [61]. Thymic selection of TCRαβ+CD8αα+ IEL precursor cells seems to be most effective in the presence of high-affinity self-antigens [58,60,62] and the selection process drives their initial functional and phenotypic differentiation [58]. Agonist selected TCRαβ+CD8αα+ IEL precursor cells exit the thymus as TCRαβ+CD4−CD8− double negative (DN), and they migrate directly to the intestine in a S1P independent fashion. Further maturation, including the induction of CD8αα, occurs extra-thymically in the IL-15-rich environment of the gut [63].
Normal CD8αα+ IEL homeostasis requires several cytokines and whereas IL-7 is indispensable for the development of TCRγδ+ IEL, in mice lacking IL-15, both CD8αα+ TCRαβ+ and TCRγδ+ IEL are strongly reduced. In a recent paper it was demonstrated that IL-15 does not affect IEL development in the thymus however, but mainly controls homeostasis of both TCRγδ+ and TCRαβ+ CD8αα+ IEL in the intestine [64]. Furthermore, it was shown that toll-like receptor (TLR) signaling via MyD88, induces IL-15 production by IEC and plays a crucial role in the maintenance of TCRγδ+ and TCRαβ+ CD8αα+ IEL [65]. This suggests that crosstalk between commensal bacteria and the epithelium is also important for type b IEL homeostasis.
8. Type b IEL function and regulation
The unique localization of IEL is associated with an unusual T cell repertoire and antigen-specificity. The TCRαβ repertoire of type b is oligoclonal [66,67] and contains numerous self-reactive TCR [61]. Results showing that the TCR repertoire of TCRαβ+CD8αα+ IEL from the fetal intestine or from littermates in the same cage or from germ-free mice showed the same degree of random oligo-clonality suggest that the microflora is not responsible for the antigen specificity of the repertoire of these cells [67,68]. However, TCRαβ+CD8αα+ IEL numbers are highly reduced in germ-free mice and after initial seeding of these cells in the intestine, the microflora is thought to induce initial oligoclonal expansion and continuous renewal. The TCRγδ+ T subset is the only intestinal IEL population that is present under germ-free conditions and has been reported to display a restricted TCR repertoire in mice [69].
TCRαβ+CD8αα+ IEL are enriched for self-antigens [61], which would be consistent with the proposal that they are agonist-selected during ontogeny. However, both identity and nature of the possible auto-antigens for type b IEL are unknown and research is complicated by the fact that TCRαβ+CD8αα+ IEL may be reactive with either classical class I molecules, non-classical class I molecules, or even class II molecules [56]. TCRαβ+CD8αα+ IEL are drastically reduced in β2-microglobulin (β2m)-deficient mice [70], but they are present in mice lacking transporter associated with antigen-processing (TAP) [71] or classical MHC class I molecules [72]. Therefore, is has been hypothesized that one or more β2m-dependent, TAP-independent non-classical MHC class 1 molecules are responsible for their generation and/or maintenance. Proposed candidates include Qa-2 [73] and thymus leukemia antigen (TL), both abundantly expressed by IEC, although an interaction between these molecules and the TCRαβ of CD8αα+ IEL has never been shown. Nevertheless, CD8αα does bind with high affinity to TL and this interaction does not lead to activation, but has been shown to restrain proliferation and modulate reactivity of CD8αα IEL [24,34,74].
Although ligand recognition by TCRγδ+ IEL also remains elusive, data suggest that most γδ T cells are activated upon TCR- or natural killer receptor (NK)-mediated recognition of a restricted set of conserved yet poorly defined endogenous stress determinants [75]. In some cases, they can recognize unprocessed antigens directly. In humans, the (Vδ1γ) TCR and activating NK-receptor NKG2D expressed on γδ IEL both directly recognize the highly polymorphic class I-like molecules MICA and MICB that are induced on IEC upon stress [76]. Activation of the γδ T cell by this interaction may lead to fast cytolysis of the stressed (e.g. damaged or infected) IEC thereby hampering systemic dissemination of pathogens. In a recent study by Jensen et al. it was shown that antigen-expression in the thymus followed by thymic selection has little influence on antigen specificity of a subpopulation of γδ T cells [77]. This finding suggests that TCRγδ recognition may also include pathogen-derived or stress-induced self-antigens that may not be expressed in the thymus.
One of the most prominent features of type b IEL is their “activated yet resting” phenotype. Although they are cytolytic upon isolation from the intestinal epithelium in the absence of overt stimulation they do not seem to behave as activated T cells in other ways. They have a limited capacity to proliferate and their presence often correlates with immune quiescence instead of productive immunity. This dual identity was confirmed by serial anaylsis of gene expression (SAGE) and subsequent gene microarray data showing thatCD8αα+ IEL express high levels of particular effector molecules including Granzyme, Fas ligand, RANTES, and CD69, whereas common cytokines such as IFN-γ, IL-2 and IL-4, and cytokine-receptors (IL-2Rα, IL12-R) were underrepresented [78,79]. Furthermore in addition to CD8αα, they also highly express other molecules associated with immune regulation including LAG-3, CTLA-4, PD-1, TGF-β, and the inhibitory NK receptors 2B4, Ly-49 A, E, and G [78]. Together these findings imply a continuous balancing act for type b IEL between an activation and hyporesponsive state and although these partially activated cells may gain a full activation status upon the appropriate signal, their main function may not entail destructive effector immunity.
What is then the physiological function of these cytotoxic unconventional T cells residing at the front line of the intestinal mucosa? How to they behave and survive in the harsh environment of the gut? Are they effector or regulatory cells, warriors or peacekeepers, front runners or bystanders, or all of them in one? Very few answers to these questions are available and addressing function and behavior has been hampered by the unavailability of defined antigen-specific type b IEL clones and of mice exclusively lacking type b IEL. However, one of the major known functions of IEL, particularly of TCRγδ+ IEL, is the control of IEC homeostasis. Under homeostatic conditions TCRγδ+ IEL regulate the continuous turnover of IEC [80,81]. In addition, γδ T cells can enhance epithelial cell growth in vitro through secretion of keratinocyte growth factor [82], which indicates that they may be involved in epithelial damage repair elicited by inflammation. In line with this, TCRδ−/− mice show increased susceptibility to DSS- and hapten-induced colitis [83,84].
Although type b IEL have minimal pathogen-specificity they may allow for immune protection and prevention of microbial invasion by elimination of infected IEC through direct recognition of self-antigen and stress-induced receptors. Accordingly, data from an intracellular Eimeria vermiformis infectious model suggest that IEL are involved in killing infected IEC while sustaining barrier function through the expression of junctional molecules and the production of TGF-β [85]. Furthermore, γδ IEL have been shown to restrict epithelial transmigration of Toxoplasma and Salmonella [86] by transforming tight junctions, thereby maintaining epithelial barrier function. γδ IEL have also been demonstrated to enhance protective immunity of pathogen-specific CD8αβ+ T cells in Toxoplasma gondii infection [29] and stimulate innate immunity by rapidly producing IL-17 upon bacterial infection [87]. However, in general the absence of type b IEL does not seem to significantly compromise protective immunity against a range of pathogens [88]. Alternatively, type b IEL have been implicated as immune regulators in several infectious and inflammatory models. In the case of infection with the IEC-specific protozoa E. vermiformis, TCRδ−/− mice were able to effectively clear the infection but also displayed exaggerated intestinal damage, apparently due to a failure to regulate the (consequences of) the TCRαβ+ T cell response [89]. In an elegant study by Saurer et al. the impact of a potent LCMV infection on the potentially self-reactivity TCRαβ+CD8αα+ IEL was assessed [90]. They demonstrated by using a double transgenic mice expressing both LCMV epitope-specific TCR (under control of the MHC class I promoter) and the cognate antigen transgene that upon LCMV infection, TCRαβ+CD8αα+ IEL display signs of virus-induced activation. However, unlike the conventional virus-specific CD8αβ+ T cells, the responding self-reactive CD8αα+ IEL did not induce antigen-cytotoxicity nor did they promote an inflammatory response. Furthermore, it was also shown that TCRαβ+CD8αα+ IEL could prevent inflammation in the CD4+CD45RBhi T cell transfer model of colitis [91]. Together these findings are in support of an immune regulatory role for type b IEL in the intestinal mucosa.
Although we are still far from fully understanding the physiological function of type b IEL subsets, they seem to be principally involved in (self-reactivity-induced) immune regulation and maintenance of gut immune homeostasis as has been hypothesized more than 30 years ago [92] (Fig. 2).
9. Concluding remarks
The immune system of the gut constantly faces a delicate balancing act between fighting pathogenic intruders on the one hand, and preventing excessive immune responses to harmless antigens and commensals on the other hand. This dilemma seems to be reflected in the functionally diverse mucosal T cell pallet. At the frontline of the intestinal barrier, cytotoxic effector memory cells and pro-inflammatory Th subsets provide rapid protective immunity, whereas Treg and unique self-reactive IEL maintain barrier integrity and immune homeostasis and control excessive inflammation. The differentiation, activation, and function of these intestinal T cell subsets are tightly regulated by interaction with other cell types and soluble factors in the gut environment. However, we are only starting to understand the mechanisms involved in the development and functions of this complex and sophisticated network of mucosal T cells and their cross-talk with other cells and the exterior. Further knowledge on these key players of mucosal immunology may provide novel strategies for the development of mucosal vaccines and new therapeutic avenues for the treatment of inflammatory bowel disease, celiac disease and food allergy.
Acknowledgements
This work was supported by a Ter Meulen Fonds fellowship from the Royal Netherlands Academy of Arts and Sciences (FW) and by Grant RO1AI050265 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institute of Allergy and Infectious Diseases or the National Institutes of Health. This is manuscript 1103 from the La Jolla Institute for Allergy and Immunology.
References
- 1.Muller CA, Autenrieth IB, Peschel A. Innate defenses of the intestinal epithelial barrier. Cell Mol Life Sci. 2005;62:1297–1307. doi: 10.1007/s00018-005-5034-2. [DOI] [PubMed] [Google Scholar]
- 2.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 4.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 5.van Egmond M, Damen CA, van Spriel AB, Vidarsson G, van Garderen E, van de Winkel JG. IgA and the IgA Fc receptor. Trends Immunol. 2001;22:205–211. doi: 10.1016/s1471-4906(01)01873-7. [DOI] [PubMed] [Google Scholar]
- 6.Corr SC, Gahan CC, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol. 2008;52:2–12. doi: 10.1111/j.1574-695X.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- 7.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the third way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 8.Beagley KW, Fujihashi K, Lagoo AS, Lagoo-Deenadaylan S, Black CA, Murray AM, et al. Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. J Immunol. 1995;154:5611–5619. [PubMed] [Google Scholar]
- 9.Spencer J, Isaacson PG, MacDonald TT, Thomas AJ, Walker-Smith JA. Gamma/delta T cells and the diagnosis of coeliac disease. Clin Exp Immunol. 1991;85:109–113. doi: 10.1111/j.1365-2249.1991.tb05690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dieren JM, van der Woude CJ, Kuipers EJ, Escher JC, Samsom JN, Blumberg RS, et al. Roles of CD1d-restricted NKT cells in the intestine. Inflamm Bowel Dis. 2007;13:1146–1152. doi: 10.1002/ibd.20164. [DOI] [PubMed] [Google Scholar]
- 11.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 12.Arstila T, Arstila TP, Calbo S, Selz F, Malassis-Seris M, Vassalli P, et al. Identical T cell clones are located within the mouse gut epithelium and lamina propia and circulate in the thoracic duct lymph. J Exp Med. 2000;191:823–834. doi: 10.1084/jem.191.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson-Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol Rev. 2007;215:226–242. doi: 10.1111/j.1600-065X.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–2490. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edele F, Molenaar R, Gutle D, Dudda JC, Jakob T, Homey B, et al. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol. 2008;181:3745–3749. doi: 10.4049/jimmunol.181.6.3745. [DOI] [PubMed] [Google Scholar]
- 18.El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, et al. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grueter B, Petter M, Egawa T, Laule-Kilian K, Aldrian CJ, Wuerch A, et al. Runx3 regulates integrin alpha E/CD103 and CD4 expression during development of CD4−/CD8+ T cells. J Immunol. 2005;175:1694–1705. doi: 10.4049/jimmunol.175.3.1694. [DOI] [PubMed] [Google Scholar]
- 20.Ericsson A, Svensson M, Arya A, Agace WW. CCL25/CCR9 promotes the induction and function of CD103 on intestinal intraepithelial lymphocytes. Eur J Immunol. 2004;34:2720–2729. doi: 10.1002/eji.200425125. [DOI] [PubMed] [Google Scholar]
- 21.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 22.McDermott MR, Mark DA, Befus AD, Baliga BS, Suskind RM, Bienenstock J. Impaired intestinal localization of mesenteric lymphoblasts associated with vitamin A deficiency and protein-calorie malnutrition. Immunology. 1982;45:1–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Masopust D, Jiang J, Shen H, Lefrancois L. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J Immunol. 2001;166:2348–2356. doi: 10.4049/jimmunol.166.4.2348. [DOI] [PubMed] [Google Scholar]
- 24.Cheroutre H, Lambolez F. Doubting the TCR coreceptor function of CD8alphaalpha. Immunity. 2008;28:149–159. doi: 10.1016/j.immuni.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 26.Cheroutre H, Madakamutil L. Mucosal effector memory T cells: the other side of the coin. Cell Mol Life Sci. 2005;62:2853–2866. doi: 10.1007/s00018-005-5232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller S, Buhler-Jungo M, Mueller C. Intestinal intraepithelial lymphocytes exert potent protective cytotoxic activity during an acute virus infection. J Immunol. 2000;164:1986–1994. doi: 10.4049/jimmunol.164.4.1986. [DOI] [PubMed] [Google Scholar]
- 28.Dharakul T, Labbe M, Cohen J, Bellamy AR, Street JE, Mackow ER, et al. Immunization with baculovirus-expressed recombinant rotavirus proteins VP1, VP4, VP6, and VP7 induces CD8+ T lymphocytes that mediate clearance of chronic rotavirus infection in SCID mice. J Virol. 1991;65:5928–5932. doi: 10.1128/jvi.65.11.5928-5932.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lepage AC, Buzoni-Gatel D, Bout DT, Kasper LH. Gut-derived intraepithelial lymphocytes induce long term immunity against Toxoplasma gondii. J Immunol. 1998;161:4902–4908. [PubMed] [Google Scholar]
- 30.Kanwar SS, Ganguly NK, Walia BN, Mahajan RC. Direct and antibody dependent cell mediated cytotoxicity against Giardia lamblia by splenic and intestinal lymphoid cells in mice. Gut. 1986;27:73–77. doi: 10.1136/gut.27.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 32.Madakamutil LT, Christen U, Lena CJ, Wang-Zhu Y, Attinger A, Sundarrajan M, et al. CD8alphaalpha-mediated survival and differentiation of CD8 memory T cell precursors. Science. 2004;304:590–593. doi: 10.1126/science.1092316. [DOI] [PubMed] [Google Scholar]
- 33.Hershberg R, Eghtesady P, Sydora B, Brorson K, Cheroutre H, Modlin R, et al. Expression of the thymus leukemia antigen in mouse intestinal epithelium. Proc Natl Acad Sci USA. 1990;87:9727–9731. doi: 10.1073/pnas.87.24.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, et al. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science. 2001;294:1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 35.Heiss K, Janner N, Mahnss B, Schumacher V, Koch-Nolte F, Haag F, et al. High sensitivity of intestinal CD8+ T cells to nucleotides indicates P2X7 as a regulator for intestinal T cell responses. J Immunol. 2008;181:3861–3869. doi: 10.4049/jimmunol.181.6.3861. [DOI] [PubMed] [Google Scholar]
- 36.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, et al. Characterization of Foxp3 + CD4 + CD25+ and IL-10-secreting CD4 + CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–185. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 44.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 45.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 46.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Mahony C, Scully P, O’Mahony D, Murphy S, O’Brien F, Lyons A, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4:e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niess JH, Leithauser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180:559–568. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- 49.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 51.Pennington DJ, Silva-Santos B, Shires J, Theodoridis E, Pollitt C, Wise EL, et al. The inter-relatedness and interdependence of mouse T cell receptor gammadelta+ and alphabeta+ cells. Nat Immunol. 2003;4:991–998. doi: 10.1038/ni979. [DOI] [PubMed] [Google Scholar]
- 52.Helgeland L, Brandtzaeg P, Rolstad B, Vaage JT. Sequential development of intraepithelial gamma delta and alpha beta T lymphocytes expressing CD8 alpha beta in neonatal rat intestine: requirement for the thymus. Immunology. 1997;92:447–456. doi: 10.1046/j.1365-2567.1997.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staton TL, Habtezion A, Winslow MM, Sato T, Love PE, Butcher EC. CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nat Immunol. 2006;7:482–488. doi: 10.1038/ni1319. [DOI] [PubMed] [Google Scholar]
- 54.Kunisawa J, Kurashima Y, Higuchi M, Gohda M, Ishikawa I, Ogahara I, et al. Sphingosine 1-phosphate dependence in the regulation of lymphocyte trafficking to the gut epithelium. J Exp Med. 2007;204:2335–2348. doi: 10.1084/jem.20062446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheroutre H, Lambolez F. The thymus chapter in the life of gut-specific intra epithelial lymphocytes. Curr Opin Immunol. 2008;20:185–191. doi: 10.1016/j.coi.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, et al. Precursors of functional MHC class I- or class II-restricted CD8alphaalpha(+) T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16:355–364. doi: 10.1016/s1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- 57.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4 + CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 58.Yamagata T, Mathis D, Benoist C. Self-reactivity in thymic double-positive cells commits cells to a CD8 alpha alpha lineage with characteristics of innate immune cells. Nat Immunol. 2004;5:597–605. doi: 10.1038/ni1070. [DOI] [PubMed] [Google Scholar]
- 59.Cruz D, Sydora BC, Hetzel K, Yakoub G, Kronenberg M, Cheroutre H. An opposite pattern of selection of a single T cell antigen receptor in the thymus and among intraepithelial lymphocytes. J Exp Med. 1998;188:255–265. doi: 10.1084/jem.188.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guehler SR, Bluestone JA, Barrett TA. Immune deviation of 2C transgenic intraepithelial lymphocytes in antigen-bearing hosts. J Exp Med. 1996;184:493–503. doi: 10.1084/jem.184.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rocha B, Vassalli P, Guy-Grand D. The. V beta repertoire of mouse gut homodimeric alpha CD8+ intraepithelial T cell receptor alpha/beta + lymphocytes reveals a major extrathymic pathway of T cell differentiation. J Exp Med. 1991;173:483–486. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levelt CN, de Jong YP, Mizoguchi E, O’Farrelly C, Bhan AK, Tonegawa S, et al. High-and low-affinity single-peptide/MHC ligands have distinct effects on the development of mucosal CD8alphaalpha and CD8alphabeta T lymphocytes. Proc Natl Acad Sci USA. 1999;96:5628–5633. doi: 10.1073/pnas.96.10.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gangadharan D, Cheroutre H. The CD8 isoform CD8alphaalpha is not a functional homologue of the TCR co-receptor CD8alphabeta. Curr Opin Immunol. 2004;16:264–270. doi: 10.1016/j.coi.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Lai YG, Hou MS, Hsu YW, Chang CL, Liou YH, Tsai MH, et al. IL-15 does not affect IEL development in the thymus but regulates homeostasis of putative precursors and mature CD8 alpha alpha + IELs in the intestine. J Immunol. 2008;180:3757–3765. doi: 10.4049/jimmunol.180.6.3757. [DOI] [PubMed] [Google Scholar]
- 65.Yu Q, Tang C, Xun S, Yajima T, Takeda K, Yoshikai Y. MyD88-dependent signaling for IL-15 production plays an important role in maintenance of CD8 alpha alpha TCR alpha beta and TCR gamma delta intestinal intraepithelial lymphocytes. J Immunol. 2006;176:6180–6185. doi: 10.4049/jimmunol.176.10.6180. [DOI] [PubMed] [Google Scholar]
- 66.Regnault A, Cumano A, Vassalli P, Guy-Grand D, Kourilsky P. Oligoclonal repertoire of the CD8 alpha alpha and the CD8 alpha beta TCR-alpha/beta murine intestinal intraepithelial T lymphocytes: evidence for the random emergence of T cells. J Exp Med. 1994;180:1345–1358. doi: 10.1084/jem.180.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Regnault A, Levraud JP, Lim A, Six A, Moreau C, Cumano A, et al. The expansion and selection of T cell receptor alpha beta intestinal intraepithelial T cell clones. Eur J Immunol. 1996;26:914–921. doi: 10.1002/eji.1830260429. [DOI] [PubMed] [Google Scholar]
- 68.Koningsberger JC, Chott A, Logtenberg T, Wiegman LJ, Blumberg RS, van Bergr Henegouwen GP, et al. TCR expression in human fetal intestine and identification of an early T cell receptor beta-chain transcript. J Immunol. 1997;159:1775–1782. [PubMed] [Google Scholar]
- 69.Takagaki Y, DeCloux A, Bonneville M, Tonegawa S. Diversity of gamma delta T-cell receptors on murine intestinal intra-epithelial lymphocytes. Nature. 1989;339:712–714. doi: 10.1038/339712a0. [DOI] [PubMed] [Google Scholar]
- 70.Neuhaus O, Emoto M, Blum C, Yamamoto S, Kaufmann SH. Control of thymus-independent intestinal intraepithelial lymphocytes by beta 2-microglobulin. Eur J Immunol. 1995;25:2332–2339. doi: 10.1002/eji.1830250832. [DOI] [PubMed] [Google Scholar]
- 71.Sydora BC, Brossay L, Hagenbaugh A, Kronenberg M, Cheroutre H. TAP-independent selection of CD8+ intestinal intraepithelial lymphocytes. J Immunol. 1996;156:4209–4216. [PubMed] [Google Scholar]
- 72.Gapin L, Cheroutre H, Kronenberg M. Cutting edge: TCR alpha beta+ CD8 alpha alpha + T cells are found in intestinal intraepithelial lymphocytes of mice that lack classical MHC class I molecules. J Immunol. 1999;163:4100–4104. [PubMed] [Google Scholar]
- 73.Das G, Gould DS, Augustine MM, Fragoso G, Sciutto E, Stroynowski I, et al. Qa-2-dependent selection of CD8alpha/alpha T cell receptor alpha/beta(+) cells in murine intestinal intraepithelial lymphocytes. J Exp Med. 2000;192:1521–1528. doi: 10.1084/jem.192.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olivares-Villagomez D, Mendez-Fernandez YV, Parekh VV, Lalani S, Vincent TL, Cheroutre H, et al. Thymus leukemia antigen controls intraepithelial lymphocyte function and inflammatory bowel disease. Proc Natl Acad Sci USA. 2008;105:17931–17936. doi: 10.1073/pnas.0808242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thedrez A, Sabourin C, Gertner J, Devilder MC, Allain-Maillet S, Fournie JJ, et al. Self/non-self discrimination by human gammadelta T cells: simple solutions for a complex issue? Immunol Rev. 2007;215:123–135. doi: 10.1111/j.1600-065X.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 76.Shao L, Kamalu O, Mayer L. Non-classical MHC class I molecules on intestinal epithelial cells: mediators of mucosal crosstalk. Immunol Rev. 2005;206:160–176. doi: 10.1111/j.0105-2896.2005.00295.x. [DOI] [PubMed] [Google Scholar]
- 77.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Denning TL, Granger SW, Mucida D, Graddy R, Leclercq G, Zhang W, et al. Mouse TCRalphabeta + CD8alphaalpha intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J Immunol. 2007;178:4230–4239. doi: 10.4049/jimmunol.178.7.4230. [DOI] [PubMed] [Google Scholar]
- 79.Shires J, Theodoridis E, Hayday AC. Biological insights into TCRgammadelta+ and TCRalphabeta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15:419–434. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 80.Komano H, Fujiura Y, Kawaguchi M, Matsumoto S, Hashimoto Y, Obana S, et al. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 1995;92:6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guy-Grand D, DiSanto JP, Henchoz P, Malassis-Seris M, Vassalli P. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-gamma TNF) in the induction of epithelial cell death and renewal. Eur J Immunol. 1998;28:730–744. doi: 10.1002/(SICI)1521-4141(199802)28:02<730::AID-IMMU730>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 82.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Inagaki-Ohara K, Chinen T, Matsuzaki G, Sasaki A, Sakamoto Y, Hiromatsu K, et al. Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. J Immunol. 2004;173:1390–1398. doi: 10.4049/jimmunol.173.2.1390. [DOI] [PubMed] [Google Scholar]
- 85.Inagaki-Ohara K, Dewi FN, Hisaeda H, Smith AL, Jimi F, Miyahira M, et al. Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection. Infect Immun. 2006;74:5292–5301. doi: 10.1128/IAI.02024-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dalton JE, Cruickshank SM, Egan CE, Mears R, Newton DJ, Andrew EM, et al. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology. 2006;131:818–829. doi: 10.1053/j.gastro.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. Gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 89.Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, et al. T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA. 1996;93:11774–11779. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saurer L, Seibold I, Rihs S, Vallan C, Dumrese T, Mueller C. Virus-induced activation of self-specific TCR alpha beta CD8 alpha alpha intraepithelial lymphocytes does not abolish their self-tolerance in the intestine. J Immunol. 2004;172:4176–4183. doi: 10.4049/jimmunol.172.7.4176. [DOI] [PubMed] [Google Scholar]
- 91.Poussier P, Ning T, Banerjee D, Julius M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J Exp Med. 2002;195:1491–1497. doi: 10.1084/jem.20011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferguson A. Intraepithelial lymphocytes of the small intestine. Gut. 1977;18:921–937. doi: 10.1136/gut.18.11.921. [DOI] [PMC free article] [PubMed] [Google Scholar]