Abstract
Genetics aims to understand the relation between genotype and phenotype. However, because complete deletion of most yeast genes (~80%) has no obvious phenotypic consequence in rich medium, it is difficult to study their functions. To uncover phenotypes for this nonessential fraction of the genome, we performed 1144 chemical genomic assays on the yeast whole-genome heterozygous and homozygous deletion collections and quantified the growth fitness of each deletion strain in the presence of chemical or environmental stress conditions. We found that 97% of gene deletions exhibited a measurable growth phenotype, suggesting that nearly all genes are essential for optimal growth in at least one condition.
Small molecules are potent probes to help understand cellular physiology [for review, see (1)]. The emergent field of chemical genomics promises that, by understanding the relations between small molecules and genes on a systems level, we might understand genomic responses to small molecule perturbants. We show that the global response of all protein-coding gene deletions tested with a panel of several hundred perturbations yields insight into gene dispensability, multidrug resistance, and gene functions within the Saccharomyces cerevisiae cell.
The diploid yeast deletion collections comprise ~6000 heterozygous gene deletion strains and ~5000 homozygous gene deletion strains (~1000 genes are essential) (2, 3). We tested the growth responses of these cells to over 400 small molecules and diverse environmental stresses. Surveying a large swath of ecological space allowed us to identify genes required for growth in each tested condition. Essential genes are a potential source of new drug targets (4), whereas nonessential genes have been proposed to contribute to genetic robustness (via compensation by redundant pathways) (5, 6) or to be required for growth in particular conditions (7). Our results provide an experimental framework to test these hypotheses. We also identified previously unknown genes that function in multidrug resistance (MDR), that is, those genes required for growth in the presence of multiple drugs.
We screened small molecules from diverse sources and libraries, including drugs approved by the World Health Organization and the U.S. Food and Drug Administration, well-characterized chemical probes, and compounds with uncertain biological activity (tables S1 and S2). The structural diversity of these compounds is comparable to that of approved drugs (fig. S1). We also assayed the effects of various environmental treatments and stresses (for example, depletion of amino acids or vitamins). We performed 726 treatment experiments in each of the heterozygous deletion strains and 418 separate experiments in each of the homozygous strains, for a total of more than 6 million single-gene measurements. These sets include some repeated experiments in which drug dose or exposure time was varied. Collapsing such repeats yielded a total of 354 unique conditions for the heterozygous collection and 178 for the homozygous collection (124 of which were tested against both collections). A gene deletion strain was defined as sensitive to a treatment if it showed a growth defect in the treatment relative to its growth in control (no drug) conditions. We defined significant sensitivity and corrected for multiple comparisons by controlling the false discovery rate (FDR) to ~0.1 for genes exhibiting any phenotype (8)(figs. S2 and S3).
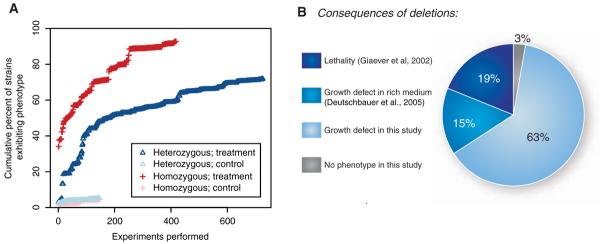
Previous studies revealed that 34% of homozygous deletion strains display a distinct phenotype (19% lethality and 15% fitness defect) when grown in rich medium (2, 3, 9). Three percent of heterozygous strains display a fitness defect (9). One interpretation of these observations is that the majority of the yeast genome is dispensable for growth. However, it is unlikely that yeast encounters such ideal conditions outside of the laboratory. In our experiments, nearly all of the deletion strains manifested a phenotype in one or more conditions (Fig. 1).
Fig. 1.
Fraction of genome required for optimal growth under experimental conditions. (A) Percent of gene deletion strains that exhibit significant sensitivity in at least one treatment as a function of number of experiments performed, ordered by date. We used a significance threshold (z score P < 1 × 10-5 and P < 1 × 10-6 for homozygous and heterozygous experiments, respectively) that limited the FDR of genes exhibiting any phenotype to ~0.1 (figs. S2 and S3). Treatment experiments measure the growth of the deletion strains in a drug or altered environmental conditions; control experiments measure growth of the same deletion strains in no-drug rich medium (8). The percent of strains exhibiting a phenotype begins at the percentage previously observed in rich medium (3% for heterozygotes and 34% for homozygotes). (B) Percentage of yeast genes with a phenotype under particular conditions: 19% are essential genes, 15% exhibited a growth defect as homozygous deletions in rich medium, and 63% exhibited a phenotype as either homozygous or heterozygous deletions under particular conditions in this study.
In the experiments using homozygous deletion strains, only 416 strains (containing deletions of 7% of the genome) did not manifest a phenotype that was different from the no-drug control phenotype (hereafter “exhibited no phenotype”). Because more than half of these 7% of the genome are either of unknown function or designated as dubious (10), it is likely the majority do not encode proteins. Of the heterozygous deletion strain experiments, 2049 (34% of the genome) exhibited no phenotype. Nearly a third of these are either of unknown function or designated as dubious (10) and are also unlikely to be protein-coding. Only 205 strains (3%) failed to exhibit a phenotype under either homozygous or heterozygous conditions. Thus, considering all ~6000 yeast genes, 97% manifested a significant fitness defect when deleted. In a control analysis of rich-medium experiments, only about 10% of strains manifested a false positive fitness defect [FDR of ~0.1 (Fig. 1A and figs. S2 and S3)]. We further tested the small set of 205 deletion strains that showed no growth defect: 62 deletions do not appear to encode proteins (10), and 155 are expressed at low levels or not at all (11). This gene set was not enriched for duplicated genes (12), as might have been predicted by the redundancy hypothesis (5-7), nor were duplicated gene deletion strains sensitive in a greater or fewer number of conditions than nonduplicated strains on average (fig. S5).
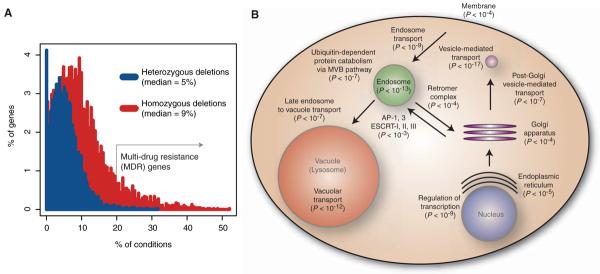
Certain deletion strains were sensitive to multiple drug treatments (Fig. 2A) (13-15), and therefore the corresponding deleted genes appear to be required for resistance to diverse perturbations. We therefore refer to them as multidrug resistance (MDR) genes, with “multiple” defined as greater than 20% of all unique treatments. We predicted that MDR genes in our data would include well-characterized pleiotropic efflux pumps such as PDR5 (human homolog is MDR1) and their regulators such as the transcription factor PDR1 (16). These transporters do score as MDR in our assay, but several hundred additional genes behaved similarly: In total, 51 genes were defined as MDR as heterozygous deletion strains, and 510 genes were defined as MDR as homozygous deletion strains (Fig. 2A). These genes are highly enriched for certain Gene Ontology functions (17), particularly endosome transport, vacuolar degradation, and transcription; together these cellular processes compose a coherent system (Fig. 2B). This coordinated system of endocytosis and vacuolar or lysosomal degradation, conserved from yeast to humans, is a mechanism whereby the cell regulates transmembrane proteins, including the transporters PDR5, FUR4, and TAT1 and signaling receptors (18). Subsets of these highly conserved pathways have been previously associated with MDR in yeast (13-15, 19) These results are consistent with recent findings in mammalian cells that MDR is correlated with changes in intracellular trafficking (20, 21), although the exact contribution of these pathways to drug resistance is not known (22-25). The most frequently observed MDR gene was IRS4, and although it has not previously been associated with any drug response, we found that this gene conferred resistance to 55% of the unique compounds. IRS4 regulates phosphoinositides (PIs), well-conserved second messengers that regulate vesicular trafficking, membrane transporters, and membrane lipid composition (18). These functions largely encompassed the remaining MDR genes' functions (Fig. 2B), suggesting that IRS4 signaling may coordinate MDR-specific pathways. To address this, we examined the MDR genes that conferred resistance to the same compounds as IRS4; these genes were enriched for vesicle transport function (hypergeometric P < 1 × 10-20), whereas those genes not related to IRS4 were not enriched. Additional interesting MDR genes included nine genes involved in aromatic amino acid biosynthesis (TRP1, TRP2, TRP3, TRP4, TRP5, ARO1, ARO2, ARO7, and TKL1) and GCN4, a master transcriptional regulator of amino acid biosynthesis (15). The sensitivity profiles of these strains were nearly identical to one another, and the strains were sensitive to nearly 30% of all tested compounds. Lastly, although transcription factors (TFs) are underrepresented in the set of essential genes in rich media (~3% of TFs are essential) (26), we found that 16 TFs (~10%) conferred MDR. Our compendium of conditions under which TFs are required for growth suggests experimental conditions to better characterize these proteins.
Fig. 2.
Genes required for optimal growth in multiple conditions. (A) The percent of deletion strains inhibited by the given percent of unique conditions at P < 0.01 (z score). Most strains are perturbed by multiple distinct conditions. Genes whose deletion strains showed sensitivity to at least 20% of the unique small molecules are defined as MDR genes. (B) Enriched functions of the homozygous MDR genes. Shown are the important GO biological processes, molecular functions, and cellular components, the hypergeometric enrichment P value, and the locations of the processes in the cell. AP, adaptor protein; ESCRT, endosomal sorting complex required for transport.
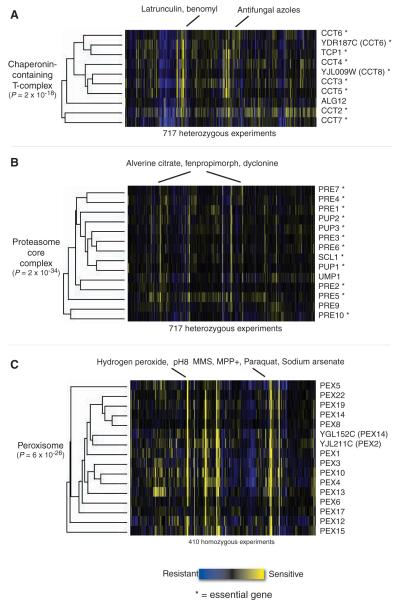
We defined the co-fitness between any two genes as the correlation of their fitness profiles across all experiments (19). We clustered genes by this metric and found that those that are co-fit often clustered by known function, having shared Gene Ontology biological processes and molecular functions (17). Three examples of functionenriched clusters were extracted from genomewide clustering, and they serve to validate co-fitness as an informative metric that provides biological insight into the function of these genes. One heterozygous cluster (Fig. 3A) comprised all eight chaperonin-containing T (CCT) complex genes: TCP1, CCT2, CCT3, CCT4, CCT5, CCT6 (and its overlapping gene YDR187C), CCT7, and YJL009W, which overlaps CCT8. The CCT cytoskeletal-folding complex is essential and conserved between yeast and human. These strains were especially sensitive to the cytoskeletal poisons latrunculin and benomyl, illustrating cases where drugs and gene deletions act synergistically in an essential pathway to produce a phenotype. Another heterozygous deletion strain cluster included 13 of the 14 proteasome core complex genes and UMP1, a chaperone required for maturation of that complex (Fig. 3B). These strains were sensitive to alverine citrate, fenpropimorph, dyclonine, and dihydromotuporamine C, all of which share a nitrogen-containing structural motif and may target the lipid synthesis pathway (4). This observation suggests a possible relation between lipid synthesis and proteasomemediated degradation. The cluster of homozygous strains shown in Fig. 3C contains 15 genes encoding components of the peroxisome, an organelle conserved from yeast to human that is responsible for breakdown of peroxides and other metabolites (27). Not surprisingly, the deletion strains were sensitive to hydrogen peroxide, presumably because the defective peroxisome could not properly metabolize it. The peroxisome requires a low pH, and the deletion strains were also sensitive to high pH. There is also growing evidence for peroxisomal involvement in managing oxidative stress (27); interestingly, the deletion strains were sensitive in the presence of chemicals that induce oxidative stress, such as MPP+ (1-methyl-4-phenylpyridinium), paraquat, and sodium arsenate.
Fig. 3.
Gene clusters with similar co-fitness profiles and similar biological functions, extracted from twoway hierarchical clustering on the complete data set (using all genes and all experiments). Each cell in the matrix is a sensitivity score of the deletion strain in the experiment: yellow indicates that the strain was sensitive; blue indicates resistance. Essential genes are marked with asterisks, and open reading frames (ORFs) that overlap a dubious ORF are in parentheses. (A) CCT complex genes, mediators of cytoskeletal assembly, cluster as heterozygous deletion strains primarily because of sensitivity to the cytoskeletal disrupting agents latrunculin and benomyl. (B) Genes of the proteasome core complex cluster in the heterozygous deletion assays because of sensitivity to three structurally similar compounds that target the lipid synthesis pathway. (C) Peroxisomal genes clustered as homozygous deletions because of sensitivity to hydrogen peroxide, high pH, and oxidative stress-inducing conditions.
Our finding that nearly all genes in yeast are required for wild-type growth in at least one experimental condition addresses the debate concerning the purpose of nonessential genes. The definition of essentiality is controversial, but under laboratory conditions over 80% of the genome is dispensable for life. We have found conditions that render the remaining genes essential for optimal growth in some condition and show that genetic redundancy is therefore limited in providing tolerance of the tested conditions (5-7). The chemical-genetic interactions defined here are complementary to genetic interactions (28) and should improve the resolution of genetic and chemical-genetic interaction maps, with applications such as predicting drug activities and synergies (29, 30).
Acknowledgments
We thank J. Rine, C. Boone, K. Kuchler, E. Ericson, and members of the Giaever and Nislow labs for comments. Supported by grants from the National Human Genome Research Institute (to R.W.D, C.N., and G.G.), the NSF (to M.E.H. and D.K.), the Canadian Institute for Health Research (no. 81340 to G.G. and C.N.), and the Canadian Research Chair in Genomics (to G.G.).
Footnotes
Supporting Online Material www.sciencemag.org/cgi/content/full/320/5874/362/DC1
References and Notes
- 1.Schreiber SL. Nat. Chem. Biol. 2005;1:64. doi: 10.1038/nchembio0705-64. [DOI] [PubMed] [Google Scholar]
- 2.Winzeler EA, et al. Science. 1999;285:901. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 3.Giaever G, et al. Nature. 2002;418:387. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 4.Giaever G, et al. Proc. Natl. Acad. Sci. U.S.A. 2004;101:793. [Google Scholar]
- 5.Gu Z, et al. Nature. 2003;421:63. [Google Scholar]
- 6.Deutscher D, Meilijson I, Kupiec M, Ruppin E. Nat. Genet. 2006;38:993. doi: 10.1038/ng1856. [DOI] [PubMed] [Google Scholar]
- 7.Papp B, Pal C, Hurst LD. Nature. 2004;429:661. doi: 10.1038/nature02636. [DOI] [PubMed] [Google Scholar]
- 8. Materials and methods are available as supporting material on Science Online and at http://chemogenomics. stanford.edu/supplements/global.
- 9.Deutschbauer AM, et al. Genetics. 2005;169:1915. doi: 10.1534/genetics.104.036871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. www.yeastgenome.org/
- 11.David L, et al. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5320. [Google Scholar]
- 12.Kellis M, Birren BW, Lander ES. Nature. 2004;428:617. doi: 10.1038/nature02424. [DOI] [PubMed] [Google Scholar]
- 13.Parsons AB, et al. Nat. Biotechnol. 2004;22:62. doi: 10.1038/nbt919. [DOI] [PubMed] [Google Scholar]
- 14.Parsons AB, et al. Cell. 2006;126:611. doi: 10.1016/j.cell.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 15.Fry RC, Begley TJ, Samson LD. Annu. Rev. Microbiol. 2005;59:357. doi: 10.1146/annurev.micro.59.031805.133658. [DOI] [PubMed] [Google Scholar]
- 16.Jungwirth H, Kuchler K. FEBS Lett. 2006;580:1131. doi: 10.1016/j.febslet.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 17.Boyle EI, et al. Bioinformatics. 2004;20:3710. doi: 10.1093/bioinformatics/bth456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katzmann DJ, Odorizzi G, Emr SD. Nat. Rev. Mol. Cell Biol. 2002;3:893. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 19.Dudley AM, Janse DM, Tanay A, Shamir R, McDonald Church G. Mol. Systems Biol. 2005;1 doi: 10.1038/msb4100004. www.nature.com/msb/journal/v1/n1/full/msb4100004.html. [DOI] [PMC free article] [PubMed]
- 20.Liang XJ, Mukherjee S, Shen DW, Maxfield FR, Gottesman MM. Cancer Res. 2006;66:2346. doi: 10.1158/0008-5472.CAN-05-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajagopal A, Simon SM. Mol. Biol. Cell. 2003;14:3389. doi: 10.1091/mbc.E02-11-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagshaw RD, Mahuran DJ, Callahan JW. Mol. Cell. Proteomics. 2005;4:133. doi: 10.1074/mcp.M400128-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Gong Y, Duvvuri M, Krise JP. J. Biol. Chem. 2003;278:50234. doi: 10.1074/jbc.M306606200. [DOI] [PubMed] [Google Scholar]
- 24.Egner R, Kuchler K. FEBS Lett. 1996;378:177. doi: 10.1016/0014-5793(95)01450-0. [DOI] [PubMed] [Google Scholar]
- 25.Ferte J. Eur. J. Biochem. 2000;267:277. doi: 10.1046/j.1432-1327.2000.01046.x. [DOI] [PubMed] [Google Scholar]
- 26.Chua G, Robinson MD, Morris Q, Hughes TR. Curr. Opin. Microbiol. 2004;7:638. doi: 10.1016/j.mib.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Schrader M, Fahimi HD. Histochem. Cell Biol. 2004;122:383. doi: 10.1007/s00418-004-0673-1. [DOI] [PubMed] [Google Scholar]
- 28.Tong AHY, et al. Science. 2004;303:808. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- 29.Sharom JR, Bellows DS, Tyers M. Curr. Opin. Chem. Biol. 2004;8:81. doi: 10.1016/j.cbpa.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Blower PE, et al. Pharmacogenomics J. 2002;2:259. doi: 10.1038/sj.tpj.6500116. [DOI] [PubMed] [Google Scholar]