Abstract
The cJun NH2-terminal kinase (JNK) signaling pathway has been implicated in the development of tumor necrosis factor (TNF) -dependent hepatitis. Indeed, JNK may play a critical role in hepatocytes during TNF-stimulated cell death in vivo. To test this hypothesis, we examined the phenotype of mice with compound disruption of the Jnk1 and Jnk2 genes. Mice with loss of JNK1/2 expression in hepatocytes exhibited no defects in the development of hepatitis compared with control mice. In contrast, mice with loss of JNK1/2 in the hematopoietic compartment exhibited a profound defect in hepatitis that was associated with markedly reduced expression of TNFα. Together, these data indicate that JNK is required for TNFα expression, but JNK is not required for TNFα-stimulated death of hepatocytes. Indeed, TNFα-induced similar hepatic damage in mice with hepatocyte-specific JNK1/2-deficiency and control mice. These observations confirm a role for JNK in the development of hepatitis, but identify hematopoietic cells as the site of the essential function of JNK.
Introduction
Tumor necrosis factor (TNF) is an inflammatory cytokine that exerts multiple biological activities on target tissues, including cell survival, cell proliferation, and cell death. The consequence of cellular exposure to TNF reflects the opposing actions of signaling pathways that are activated by cell surface TNF receptors, including NF-κB, MAP kinases, and caspases (Chen and Goeddel, 2002).
The type 1 TNF receptor (TNFR1) mediates signal transduction by recruiting proteins that assemble on the cytoplasmic tail of the receptor, including the scafffold protein TRADD, the protein kinase RIP1, and TRAF2/5 adapter proteins (Micheau and Tschopp, 2003). This complex signals the activation of NF-κB and MAP kinase pathways, is transiently assembled, and subsequently dissociates from the cytoplasmic tail of the receptor. A second complex is then formed by the TRADD scaffold protein together with RIP1, the adapter protein FADD, cFLIP, and pro-caspase 8 (Micheau and Tschopp, 2003). This complex activates caspase 8 and promotes apoptosis. The strength of caspase 8 signaling is regulated, in part, by the level of NF-κB-dependent expression of the pseudocaspase cFLIP that functions as an inhibitor of pro-caspase 8 activation (Thome and Tschopp, 2001). TNFα-induced caspase 8 activation can also be mediated after mitochondrial outer membrane permeabilization by Smac-induced degradation of cIAP1/2, CYLD-dependent release of RIP1 from the cytoplasmic tail of TNFR1, and the subsequent formation of a RIP1 complex with FADD and caspase 8 (Wang et al., 2008).
The type 2 TNF receptor (TNFR2) functions together with TNFR1, in part, by recruiting the E3 ligase c-IAP1 that can ubiquitinate and degrade c-IAP2 and the adapter protein TRAF2 (Chan and Lenardo, 2000; Conze et al., 2005; Fotin-Mleczek et al., 2002; Li et al., 2002; Wu et al., 2005). TNFR2 potentiates TNFR1-stimulated cell death by blocking TRAF2-mediated activation of anti-apoptotic signaling by NF-κB and MAP kinases. Indeed, cell surface TNFα, that binds both TNFR1 and TNFR2, is a more potent inducer of cell death than soluble TNFα, that preferentially binds TNFR1 (Eissner et al., 1995; Grell et al., 1995; Grell et al., 1998).
JNK represents one of the MAP kinases activated by TNFR1. The mechanism of JNK regulation is mediated by the TRAF2/5 adapter proteins that cause ubiquitin-dependent activation of the MAP kinase kinase kinase TAK1 that phosphorylates and activates the MAP kinase kinase MKK7 which, in turn, phosphorylates and activates JNK (Adhikari et al., 2007; Sato et al., 2005; Shim et al., 2005; Tournier et al., 2001). This TRAF-mediated activation of JNK is transient. A sustained phase of JNK activation, observed at later times, may be mediated by a TNF-induced complex of TRADD, RIP1, NOX1, and RAC1 (Kim et al., 2007) that causes accumulation of reactive oxygen species (ROS) and activation of the JNK pathway (Sakon et al., 2003) by increasing ASK1 activity (Tobiume et al., 2001) and decreasing phosphatase activity (Kamata et al., 2005). This second and sustained phase of TNF-stimulated JNK activation is negatively regulated by NF-κB (De Smaele et al., 2001; Reuther-Madrid et al., 2002; Tang et al., 2001) by suppression of ROS accumulation (Pham et al., 2004; Sakon et al., 2003).
The two phases of TNF-stimulated JNK activation appear to mediate different cellular responses (Ventura et al., 2006). The initial transient phase of TRAF-mediated JNK activation may signal cell survival (Lamb et al., 2003) and the later sustained phase of JNK activation may contribute to ROS-induced cell death (Ventura et al., 2004). The exact role of TNF-stimulated JNK in the regulation of cell survival and/or death has been controversial (Varfolomeev and Ashkenazi, 2004). Studies of cultured fibroblasts demonstrate that compound mutation of the Jnk1 and Jnk2 genes does not prevent TNF-stimulated apoptosis (Lamb et al., 2003), but JNK1/2-deficient fibroblasts do exhibit defects in the TNF-induced accumulation of cytotoxic ROS (Ventura et al., 2004). However, these studies employed artificial cell culture conditions (inhibition of transcription, translation, or NF-κB) to enable TNF-stimulated cell death. These procedures were necessary because treatment of wild-type fibroblasts with TNF alone does not cause cell death. This analysis illustrates the need for better methods to study TNF-induced cell death that are not dependent on the use of artificial cell culture conditions.
It has been reported that TNF-induced cell death can be studied using mouse models of hepatitis. For example, treatment of mice with the lectin ConA causes fulminant hepatitis in wild-type mice (Tiegs et al., 1992). Liver cell death in this model is TNF-dependent because ConA causes greatly reduced hepatitis in both Tnfr1−/− and Tnfr2−/− mice (Kusters et al., 1997; Maeda et al., 2003). Furthermore, ConA-induced hepatitis requires membrane bound TNFα, but not soluble TNFα (Kusters et al., 1997). This form of hepatitis has been reported to require JNK (Chang et al., 2006; Kamata et al., 2005; Maeda et al., 2003). The mechanism of JNK signaling may be mediated by phosphorylation and activation of the E3 ligase Itch that can ubiquinate and degrade the caspase 8 antagonist cFLIP (Chang et al., 2006). This function of JNK in TNF-stimulated cell death is consistent with several reports that have implicated an essential role of JNK in TNF-stimulated apoptosis of tumor cell lines (Deng et al., 2003; Verheij et al., 1996), fibroblasts (Dietrich et al., 2004; Liu et al., 2004), and hepatocytes (Chang et al., 2006; Kamata et al., 2005; Maeda et al., 2003; Wang et al., 2006), but is inconsistent with other reports that suggest a non-essential role of JNK in TNF-stimulated cell death (Hochedlinger et al., 2002; Lamb et al., 2003; Liedtke et al., 2002; Liu et al., 1996; Natoli et al., 1997).
The purpose of this study was to examine the role of JNK in TNF-mediated hepatitis using compound mutant mice with defects in both of the ubiquitously expressed genes that encode JNK isoforms. Our analysis demonstrates that JNK is essential for hepatitis. We show that JNK in hematopoietic cells is critically required for TNFα expression and that JNK is not required for TNFα-stimulated cell death during the development of hepatitis.
Results
Examination of hepatitis in mice deficient of JNK1 or JNK2
It has been reported that Jnk1−/− mice and Jnk2−/− mice exhibit a major defect in ConA-induced hepatitis (Maeda et al., 2003), although subsequent studies have reported a primary role played by JNK1 (Chang et al., 2006; Kamata et al., 2005). We tested the effect of ConA-induced hepatitis on wild-type, Jnk1−/−, and Jnk2−/− mice (Figure S1). ConA caused similar hepatic damage in wild-type and JNK knockout mice (Figures S1A & S2A). Serum transaminase activity, a hallmark of hepatic injury, was modestly reduced in both Jnk1−/− mice and Jnk2−/− mice compared to wild-type mice, but the differences were not statistically significant (Figure S1B). The effects of ConA on hepatic injury are thought to be mediated by induced cytokine expression. Similar amounts of serum cytokines were detected in the wild-type and knockout mice, although a selective loss of IL-1 expression was found in Jnk1−/− mice (Figure S1C). Together, these data suggest that JNK1 and JNK2 do not play a major role in ConA-stimulated hepatitis. Nevertheless, it was possible that JNK may play a role because studies using a different model of murine hepatitis (treatment with LPS) did show that both Jnk1−/− mice and Jnk2−/− mice exhibited a partial reduction in hepatic damage that was confirmed by histological analysis of liver sections (Figures S2B & S3A), measurement of serum transaminase activity (Figure S3B), and decreased expression of serum cytokines, including TNFα and IFNγ (Figure S3C).
Examination of hepatitis in compound mutant Jnk1−/− Jnk2−/− mice
Our analysis of hepatitis in Jnk1−/− mice and Jnk2−/− mice indicated that JNK1 and JNK2 may contribute to the disease process (Figures S1–S3). However, the roles of JNK1 and JNK2 are unclear because we found that Jnk1−/− mice and Jnk2−/− mice did not demonstrate defects in ConA-induced hepatitis (Figure S1). These observations suggested the possibility that JNK1 and JNK2 may play redundant roles during the development of hepatitis (Figure S4). To test this hypothesis, we examined the effect of ConA in mice with compound disruption of both the Jnk1 and Jnk2 genes. Since Jnk1−/− Jnk2−/− mice die during early embryonic development (Kuan et al., 1999), we employed a conditional gene ablation strategy.
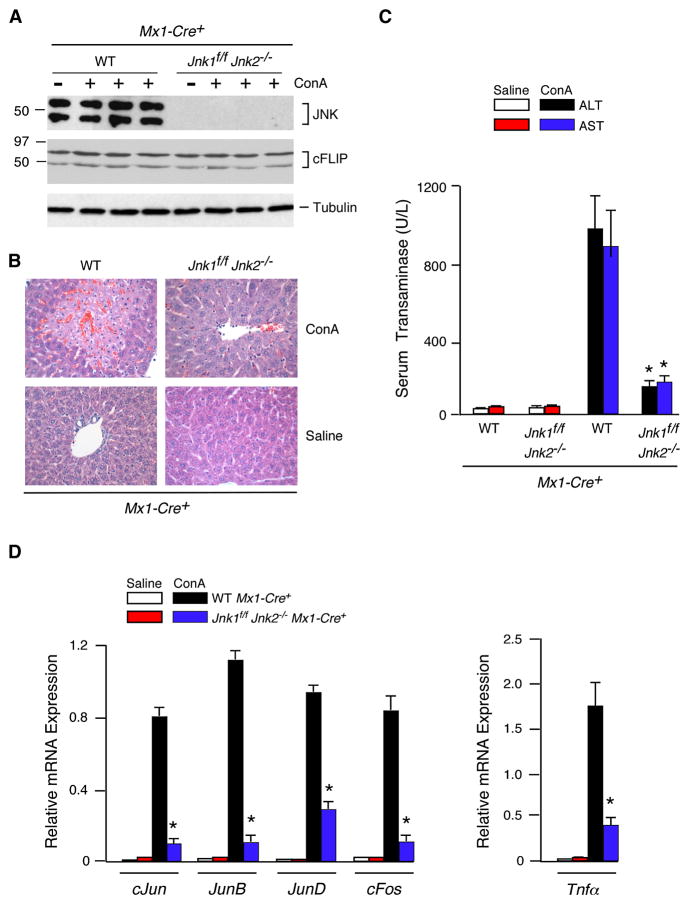
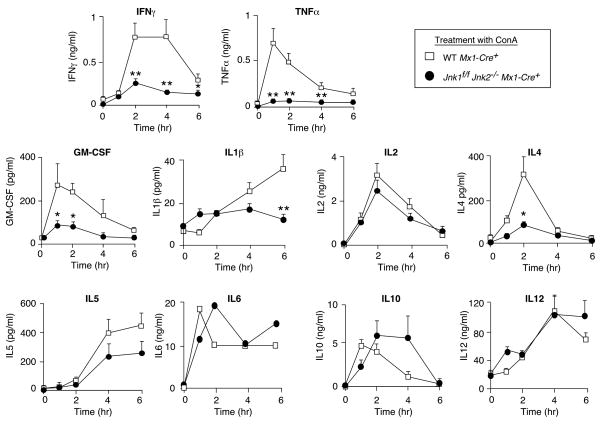
Efficient deletion of floxed alleles in both the liver and hematopoietic cells can be achieved in Mx1-Cre transgenic mice when these mice are treated with polyIC (Kuhn et al., 1995). We therefore treated Mx1-Cre+ (Control) mice and Mx1-Cre+ Jnk1f/f Jnk2−/− mice with polyIC (Figure 1). Deletion of floxed Jnk1 (Jnk1Δ/Δ) was confirmed by PCR analysis of genomic DNA. JNK protein was not detected in the liver of the compound mutant mice by immunoblot analysis (Figure 1A). Treatment of these mice with ConA demonstrated that the JNK1/2-deficient mice exhibited strong protection against hepatitis when examined by histological analysis of liver sections (Figure 1B & S2C) or measurement of serum transaminase activity (Figure 1C). The JNK1/2-deficient mice also exhibited reduced mortality compared with control mice in response to the ConA challenge (Figure S5A). Studies of hepatic gene expression demonstrated that the Jnk1Δ/Δ Jnk2−/− liver exhibited defects in the ConA-induced expression of several AP1-related genes (Figure 1D). This observation suggested that the Jnk1Δ/Δ Jnk2−/− liver may exhibit profound defects in AP1-dependent gene expression. Indeed, expression of the AP1 target gene Tnfα was profoundly reduced in the liver of ConA-treated JNK1/2-deficient mice (Figure 1D). This loss of TNFα expression was confirmed by measurement of the concentration of serum cytokines. ConA-induced the expression of many cytokines in the serum of control mice, but JNK1/2-deficient mice exhibited major defects in serum cytokine expression, including TNFα (Figure 2).
Figure 1. JNK-deficient mice are protected against ConA-induced hepatitis.
(A) PolyIC-treated control mice (Mx1-Cre+) and JNK-deficient mice (Jnk1f/f Jnk2−/− Mx1-Cre+) were aged 4 weeks and then treated intravenously (8 hrs) with ConA or solvent (saline). Liver extracts were examined by immunoblot analysis using antibodes to JNK1/2, cFLIP, and α-Tubulin.
(B) Representative H&E-stained liver sections prepared from control and JNK1/2-deficient mice treated (8 hrs) with ConA or solvent (saline) are presented. The amount of liver damage was quantitated (Figure S2C).
(C) Serum transaminase activity in control and JNK1/2-deficient mice treated (8 hrs) with ConA or solvent (saline) was measured (mean ± SD; n = 6). Statistically significant differences between wild-type and JNK1/2-deficient mice are indicated (*, P < 0.01).
(D) RNA was isolated from the liver of control and JNK1/2-deficient mice treated (8 hrs) with ConA or solvent (saline). The expression of mRNA was measured by quantitative RT-PCR assays. The mRNA expression was normalized to the amount of Gapdh mRNA and presented as the mean ± SD (n = 6). Statistically significant differences between wild-type and JNK1/2-deficient mice are indicated (*, P < 0.01).
Figure 2. JNK-deficient mice exhibit defects in the expression of serum cytokines.
PolyIC-treated control mice (Mx1-Cre+) and JNK-deficient mice (Jnk1f/f Jnk2−/− Mx1-Cre+) were aged 4 weeks and then treated intravenously with ConA. The amount of serum cytokines post-treatment with ConA was measured by ELISA (mean ± SD; n = 7). Statistically significant differences between wild-type and JNK1/2-deficient mice are indicated (*, P < 0.05; **, P < 0.01).
The protection of Mx1-Cre+ Jnk1f/f Jnk2−/− mice against hepatitis (Figures 1 & S2) was confirmed by studies using the LPS model of hepatitis. JNK1/2-deficiency prevented LPS-induced hepatic damage detected by histological analysis of liver sections (Figures S2D & S6A), and measurement of serum transaminase activity (Figure S6B). Decreased expression of AP1-related genes and Tnfα mRNA in the liver (Figure S6D) and decreased amounts of serum TNFα were also detected in the JNK1/2-deficient mice (Figure S6E). The JNK1/2-deficient mice also exhibited reduced mortality compared with control mice in the response to LPS challenge (Figure S7A). Together, these data indicate that compound deficiency of both JNK1 and JNK2 caused strong protection against two different models of hepatitis that are induced by treatment of mice with ConA or LPS, respectively.
JNK-deficiency in hepatocytes does not protect mice against hepatitis
The observation that compound mutation of Jnk1 and Jnk2 causes protection against both ConA-and LPS- induced hepatitis (Figures 1, S2 & S6) suggested that JNK1 and JNK2 may play redundant roles during progression of the disease process. It has been proposed that these models of hepatitis are mediated by TNFα-induced and JNK-mediated phosphorylation and activation of the E3 ligase Itch and the subsequent ubiquitin-mediated degradation of the caspase 8 antagonist cFLIP in hepatocytes (Chang et al., 2006). To test this hypothesis, we examined cFLIP in control and JNK1/2-deficient liver of mice treated with ConA; no changes in cFLIP expression were detected (Figure 1A). In contrast, treatment with LPS did cause cFLIP degradation, but this degradation of cFLIP was JNK-independent (Figure S6C). The observation that LPS, but not ConA, caused cFLIP degradation may reflect the more potent effects of LPS on hepatitis compared with ConA. Together, these observations indicated that JNK-mediated cFLIP degradation is not required for murine models of hepatitis caused by ConA or LPS.
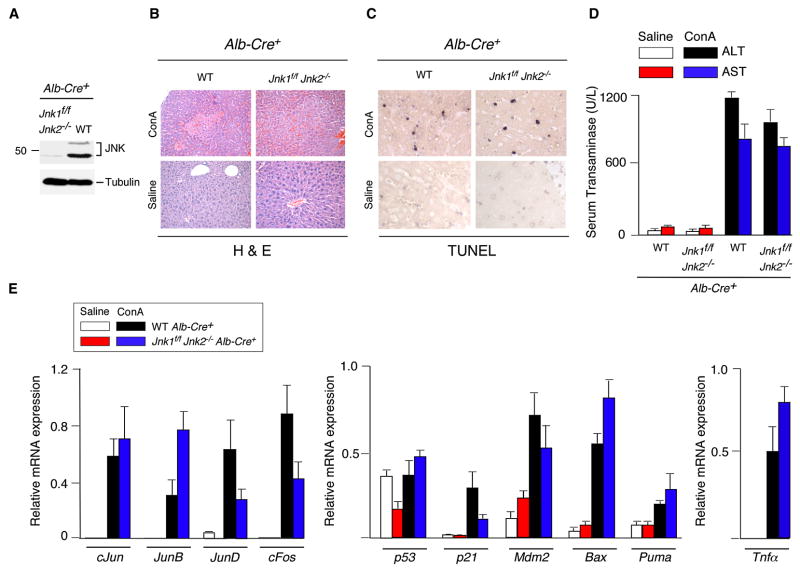
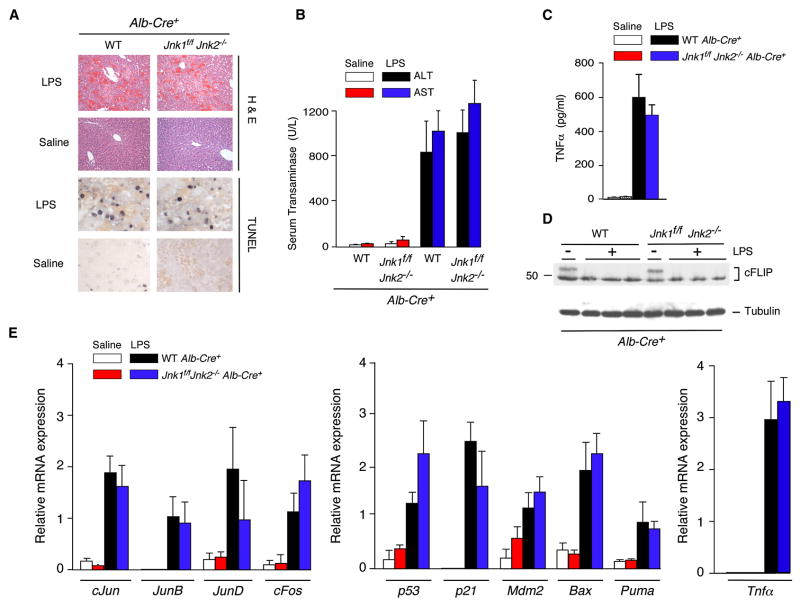
To test the role of JNK in hepatocytes, we examined the effect of hepatocyte-specific JNK-deficiency using Alb-Cre transgenic mice. Immunoblot analysis of liver extracts prepared from control (Alb-Cre+) mice and JNK-deficient (Alb-Cre+ Jnk1f/f Jnk2−/−) mice demonstrated that very low amounts of JNK were detected in JNK-deficient liver (Figure 3A). The presence of low levels of JNK in the Alb-Cre deleted mice is consistent with the finding that the Alb-Cre transgene is selectively expressed in hepatocytes, but not in other liver cell-types, including stellate cells, endothelial cells, NK cells, NKT cells, and Kupffer cells (Postic et al., 1999). Control studies demonstrated that hepatocyte-specific JNK1/2-deficiency caused markedly reduced hepatic JNK activity (monitored be measurement of phospho-Ser73 cJun) in mice treated with ConA or LPS (Figures 4B & S8B).
Figure 3. JNK-deficiency in hepatocytes does not protect mice against ConA-induced hepatitis.
(A) Liver extracts prepared from control mice (Alb-Cre+) and mice with hepatocyte-specific JNK-deficiency (Jnk1f/f Jnk2−/− Alb-Cre+) were examined by immunoblot analysis using antibodies to JNK1/2 and α-Tubulin.
(B,C) Control mice (Alb-Cre+) and JNK-deficient mice (Jnk1f/f Jnk2−/− Alb-Cre+) were treated intravenously (8 hrs) with ConA or solvent (saline). Representative H&E-stained (B) and TUNEL- stained (C) liver sections prepared from control and JNK1/2-deficient mice are presented.
(D) Serum transaminase activity in control and JNK-deficient mice after treatment (8 hrs) with ConA or solvent (saline) was measured (mean ± SD; n = 6). No statistically significant differences between wild-type and JNK1/2-deficient mice were detected.
(E) RNA was isolated from the liver of control and JNK-deficient mice after treatment (8 hrs) with ConA or solvent (saline). The expression of mRNA was measured by quantitative RT-PCR assays (Taqman©). The mRNA expression was normalized to the amount of Gapdh mRNA and presented as the mean ± SD (n = 6). No statistically significant differences between wild-type and JNK1/2-deficient mice were detected.
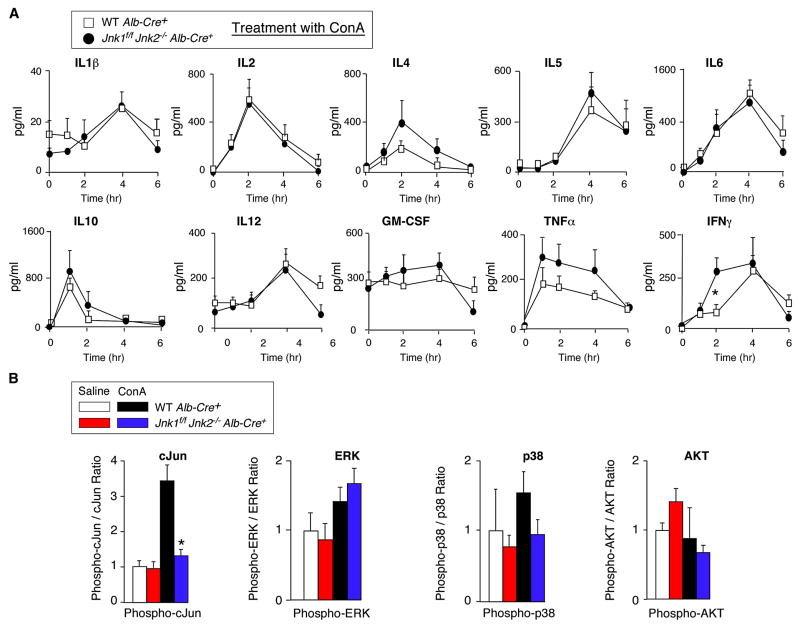
Figure 4. Effect of hepatocyte-specific JNK-deficiency on serum cytokine expression.
(A) Control mice (Alb-Cre+) and mice with hepatocyte-specific JNK-deficiency (Jnk1f/f Jnk2−/−Alb-Cre+) were treated intravenously with ConA. The amount of serum cytokines (IL1, IL2, IL4, IL5, IL6, IL10, IL12, GM-CSF, TNFα, and IFNγ) post-treatment with ConA was measured by ELISA (mean ± SD; n = 8). Statistically significant differences between wild-type and JNK1/2-deficient mice are indicated (*, P < 0.05).
(B) The amount of total and phospho- cJun, ERK, p38 MAPK, and AKT in liver extracts at 8 hrs post-treatment of mice with ConA or saline was measured by multiplexed ELISA (mean ± SD; n = 5). Statistically significant differences between wild-type and JNK1/2-deficient mice are indicated (*, P < 0.01).
Treatment with ConA caused similar hepatic damage in control and hepatocyte-specific JNK1/2-deficient mice (Figures 3B & S2C). No differences in TUNEL staining of liver sections of the control and JNK1/2-deficient mice were detected (Figure 3C). Similarly, no significant difference in serum transaminase activity between the control and JNK1/2-deficient mice was observed (Figure 3D). The JNK1/2-deficient mice and control mice also exhibited similar mortality in response to the ConA challenge (Figure S5B). Gene expression analysis in JNK1/2-deficient mice did not indicate a consistent loss of AP1-related gene expression, p53 pathway-related gene expression, or Tnfα mRNA expression in the liver compared to control mice (Figure 3E). Measurement of serum cytokines demonstrated that JNK1/2-deficiency in hepatocytes did not cause decreased expression of TNFα or other cytokines examined in ConA-treated mice (Figure 4A).
Our analysis of ConA-treated mice demonstrated that JNK expression in hepatocytes is not required for the development of hepatitis (Figures 3, S2C & S5B). This conclusion was confirmed by studies of LPS-induced hepatitis (Figures 5 & S7A). LPS caused similar hepatic damage in control and hepatocyte-specific JNK1/2-deficient mice that was detected by histological analysis of liver sections (Figure 5A), TUNEL labeling of apoptotic cells in liver sections (Figure 5A), and serum transaminase activity (Figure 5B). Furthermore, JNK1/2-deficiency in hepatocytes did not cause changes in the LPS-induced expression of AP1-related genes, p53 pathway-related genes, or Tnfα mRNA in liver (Figure 5E). LPS also caused a similar increase in the serum concentration of TNFα in mice with JNK1/2-deficient hepatocytes and control mice (Figure 5C & S8A). Moreover, cFLIP expression in LPS-treated mice was not affected by hepatocyte-specific deficiency of JNK1/2 (Figure 5D).
Figure 5. JNK-deficiency in hepatocytes does not protect mice against LPS-induced hepatitis.
(A) Control mice (Alb-Cre+) and mice with hepatocyte-specific JNK-deficiency (Jnk1f/f Jnk2−/−Alb-Cre+) were treated intravenously (8 hrs) with LPS plus GalN or solvent (saline). Representative H&E- and TUNEL- stained liver sections prepared from control and JNK-deficient mice are presented.
(B) Serum transaminase activity in control and JNK1/2-deficient mice after treatment (8 hrs) with LPS/GalN or solvent (saline) was measured (mean ± SD; n = 6). No statistically significant differences between wild-type and JNK1/2-deficient mice were detected.
(C) The concentration of TNFα in the serum of control mice (Alb-Cre+) and JNK1/2-deficient mice (Jnk1f/f Jnk2−/− Alb-Cre+) was measured after treatment (1 hr) with LPS/GalN or solvent (saline) by ELISA (mean ± SD; n = 6). No statistically significant differences between wild-type and JNK1/2-deficient mice were detected.
(D) Liver extracts prepared from control mice (Alb-Cre+) and JNK1/2-deficient mice (Jnk1f/f Jnk2−/− Alb-Cre+) were examined by immunoblot analysis using antibodies to cFLIP and α-Tubulin.
(E) RNA was isolated from the liver of control and JNK-deficient mice after treatment (8 hrs) with LPS/GalN or solvent (saline). The expression of mRNA was measured by quantitative RT-PCR assays. The mRNA expression was normalized to the amount of Gapdh mRNA and presented as the mean ± SD (n = 6). No statistically significant differences between wild-type and JNK1/2-deficient mice were detected.
These data demonstrate that there is no critical role of JNK in hepatocytes for the development of hepatitis, including treatment with ConA or LPS (Figures 3, 5 & S2C,D). This finding contrasts with conclusions drawn from previous studies that have suggested an essential role of JNK in hepatocytes for ConA-induced hepatitis (Chang et al., 2006; Kamata et al., 2005; Maeda et al., 2003).
JNK is required for TNFα expression, but is not required for TNFα-induced hepatic damage
The requirement of JNK for hepatocyte death during the development of hepatitis (Figures 1 & S6) is not mediated by the function of JNK in hepatocytes (Figures 3–5). These data suggest that JNK may play a non-cell autonomous essential role. We considered several possible mechanisms. The simplest possibility was a requirement of JNK for ConA- and LPS- induced expression of inflammatory cytokines by cells that are not hepatocytes. This is consistent with the finding that Mx1-Cre+ JNK1/2-deficient mice, that are protected against ConA- and LPS-induced hepatitis (Figures 1, S2C,D & S6), exhibit defects in the expression of inflammatory cytokines, including TNFα (Figures 1D, 2 & S6D,E). In contrast, hepatocyte-specific JNK1/2-deficiency did not protect against hepatitis and did not cause marked changes in inflammatory cytokine expression (Figures 4A, 5C,E & S8A).
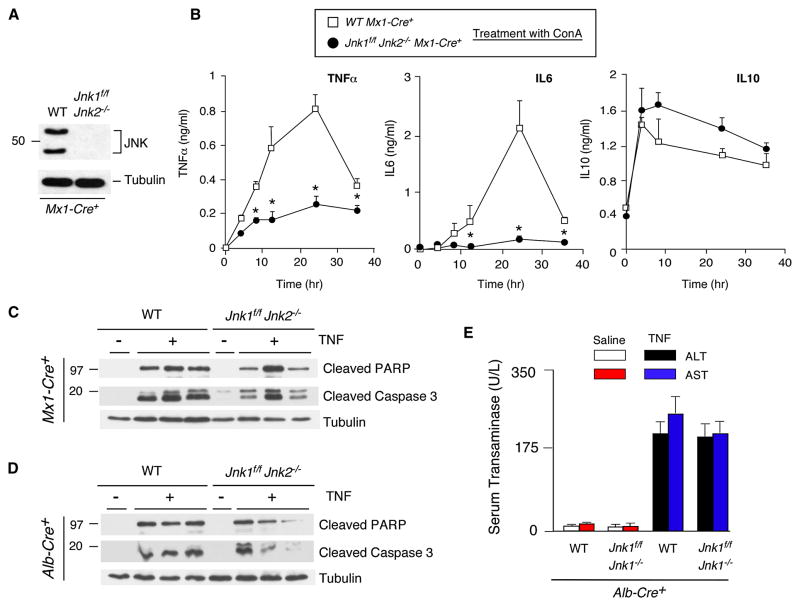
To test whether JNK is required for inflammatory cytokine expression, we prepared in vitro primary cultures of bone marrow-derived macrophages from polyIC-treated control (Mx1-Cre) and JNK1/2-deficient mice (Mx1-Cre+ Jnk1f/f Jnk2−/−). No JNK was detected in the JNK1/2-deficient macrophages by immunoblot analysis (Figure 6A). Treatment with ConA demonstrated increased concentrations of cytokines (TNFα and IL6) in the culture medium of control macrophages (Figure 6B). The amount of these cytokines in the culture medium of JNK1/2-deficient macrophages was markedly suppressed (Figure 6B). In contrast, JNK1/2-deficiency did not alter the expression of IL10 (Figure 6B). Similarly, JNK1/2-deficient T cells exhibited defects in ConA-stimulated expression of TNFα (Figure S4). Together, these data demonstrate that JNK1/2 is required for the expression of the inflammatory cytokine TNFα.
Figure 6. JNK-deficiency inhibits TNFα expression, but not TNFα-induced liver damage.
(A,B) Bone marrow-derived macrophages prepared from polyIC-treated control mice (Mx1-Cre+) and JNK-deficient mice (Jnk1f/f Jnk2−/− Mx1-Cre+) were examined by immunoblot analysis using antibodies to JNK1/2 and α-Tubulin. The macrophages were treated with ConA and cytokines in the culture medium were measured by ELISA (mean ± SD; n = 7). Statistically significant differences between wild-type and JNK1/2-deficient mice are indicated (*, P < 0.01).
(C,D) PolyIC-treated control mice (Mx1-Cre+) and JNK-deficient mice (Jnk1f/f Jnk2−/− Mx1-Cre+) (C) or control mice (Alb-Cre+) and mice with hepatocyte-specific JNK-deficiency (Jnk1f/f Jnk2−/−Alb-Cre+) (D) were treated intravenously (8 hrs) with TNFα plus GalN or solvent (saline). Liver extracts were examined by immunoblot analysis using antibodies to capsase-cleaved PARP, cleaved caspase-3, and α-Tubulin.
(E) Serum transaminase activity in control and JNK1/2-deficient mice after treatment (8 hrs) with LPS/GalN or solvent (saline) was measured (mean ± SD; n = 6). No statistically significant differences between wild-type and JNK1/2-deficient mice were detected.
If the essential role of JNK in hepatitis (Figures 1 & S6) reflects a requirement of JNK for the expression of inflammatory cytokines (Figures 2, 6B & S4), it would be predicted that the defect in hepatitis caused by JNK-deficiency would be complemented by treatment of mice with TNFα. We detected no significant difference between control and JNK-deficient mice in TNFα-induced serum aminotransferase activity (Figure 6E) and mortality (Figure S7B). Immunoblot analysis demonstrated that TNFα caused similar caspase 3 activation and PARP cleavage in the liver of control mice, Mx1-Cre+ JNK1/2-deficient mice, and Alb-Cre+ JNK1/2-deficient mice (Figure 6C,D). These data confirm that the essential role of JNK1/2 for hepatitis is mediated by a requirement of JNK1/2 for inflammatory cytokine expression, including TNFα, and does not reflect a role for JNK in TNFα-stimulated hepatocyte death.
The pro-death role of inflammatory cytokines in hepatitis is opposed by factors that sustain cell survival. One example is the role of IL22 to activate the Stat3 and AKT signaling pathways in hepatocytes. Indeed, loss of IL22 expression increases ConA-induced hepatitis (Zenewicz et al., 2007). Altered expression of IL22 might therefore contribute to the effects of JNK1/2-deficiency on ConA-induced hepatitis. However, no significant difference in IL22 expression was detected between control and JNK1/2-deficient mice (Figure S9). Together, these data indicate that IL22 does not mediate the effects of JNK1/2-deficiency on hepatitis.
JNK is required for TNF expression by hematopoietic cells
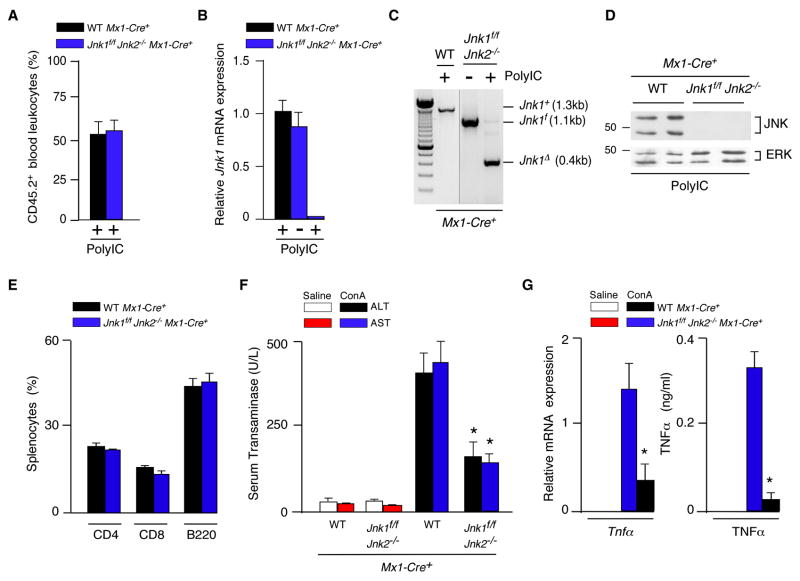
Hematopoietic cells represent one source of the inflammatory cytokines that may cause hepatitis following treatment with ConA or LPS (Dong et al., 2007). To test the role of hematopoietic cells, we constructed radiation chimeras by transplantation of control (Mx1-Cre+) and JNK1/2-deficient (Mx1-Cre+ Jnk1f/f Jnk2−/−) bone marrow from polyIC-treated donor mice into lethally irradiated congenic recipient mice. Efficient reconstitution of B6.SJL (CD45.1) mice with Jnk1+/+ Jnk2+/+ or Jnk1Δ/ΔJnk2−/− bone marrow from C57BL/6J (CD45.2) mice was confirmed by staining peripheral blood leukocytes with antibodies to CD45.1/CD45.2 and analysis by flow cytometry. Indeed, competitive repopulation assays demonstrated that mice, reconstituted with an equal number of wild-type B6.SJL plus Jnk1Δ/ΔJnk2−/− or Jnk1+/+ Jnk2+/+ C57BL/6J bone marrow cells, displayed similar numbers of CD45.1 and CD45.2 peripheral blood leukocytes at 2 months post-transplantation (Figure 7A). Together, these data demonstrate that JNK1 and JNK2 are not essential for the re-population of the hematopoietic compartment following bone marrow transplantation.
Figure 7. JNK-deficiency in hematopoietic cells protects mice against hepatitis.
(A) Competitive bone marrow transplantation assays were performed using lethally-irradiated wild-type B6.SJL mice transplanted with an equal number of bone marrow cells isolated from polyIC-treated (4 weeks) control B6.SJL (CD45.1) mice plus Jnk1f/f Jnk2−/− Mx1-Cre+ or wild-type Mx1-Cre+ C57BL/6J (CD45.2) mice. Peripheral blood leukocytes were stained with antibodies to CD45.1 and CD45.2 at 6 months post-transplantation by flow cytometry (mean ± SD; n = 3).
(B–E) Lethally-irradiated wild-type mice were transplanted with bone marrow from polyIC-treated control mice (Mx1-Cre+) or JNK1/2-deficient mice (Jnk1f/f Jnk2−/− Mx1-Cre+) mice. Peripheral blood leukocytes were isolated and Jnk1 mRNA was examined by quantitative Taqman© RT-PCR analysis (B) and genomic DNA was genotyped by PCR analysis using amplimers to detect the Jnk1+, Jnk1f, and Jnk1Δ alleles (C). Splenocytes were examined by immunoblot analysis using antibodies to JNK1/2 and ERK1/2 (D) and sub-populations of splenocytes were examined by flow cytomtetry (E).
(F) Mice at 6 months post-transplantation were treated intravenously (8 hrs) with ConA or solvent (saline) and serum transaminase activity (ALT and AST) was measured (mean ± SD; n = 6). Statistically significant differences between mice transplanted with control and JNK1/2-deficient bone marrow are indicated (*, P < 0.01).
(G) Mice at 6 months post-transplantation were treated intravenously with ConA or solvent (saline). The expression of Gapdh, and Tnfα mRNA in the liver was measured by quantitative RT-PCR assays (Taqman©) at 8 hr. post-injection (left panel). The mRNA expression in each sample was normalized to the amount of Gapdh mRNA and presented as the mean ± SD (n = 6). The concentration of TNFα in the blood was measured at 1 hr. post-injection by ELISA and is presented as the mean ± SD (n = 7) (right panel). Statistically significant differences between mice transplanted with control and JNK1/2-deficient bone marrow are indicated (*, P < 0.01).
Radiation chimeras with Jnk1+/+ Jnk2+/+ or Jnk1Δ/ΔJnk2−/− hematopoietic cells in the blood were identified by quantitative RT-PCR analysis of Jnk1 mRNA expression (Figure 7B), by PCR analysis of genomic DNA (Figure 7C), and by immunoblot analysis using a JNK antibody (Figure 7D). Flow cytometry analysis of splenocytes demonstrated similar numbers of CD4 T cells, CD8 T cells, and B cells in mice reconstituted with control and JNK1/2-deficient bone marrow (Figure 7E).
Long-lived resident hematopoietic cells in the liver are slowly replaced following bone marrow transplantation; we therefore examined mice at 6 months post-transplantation (Alves-Guerra et al., 2003). Treatment of the radiation chimeras transplanted with Jnk1+/+ Jnk2+/+ bone marrow demonstrated that treatment with ConA caused hepatic damage, including increased amounts of serum transaminase activity (Figure 7F). In contrast, radiation chimeras transplanted with Jnk1Δ/ΔJnk2−/− bone marrow were protected against hepatic damage caused by ConA, including reduced amounts of serum transaminase activity (Figure 7F), and decreased amounts of Tnfα mRNA in the liver and TNFα protein expression in blood (Figure 7G). Together, these data confirm that JNK1/2 in hematopoietic cells is required for the development of hepatitis.
Discussion
The results of our study demonstrate that JNK is essential for the development of hepatitis in mouse models. This finding is important because of the implication that JNK may be a useful target for drug therapy for some forms of hepatitis in humans. Indeed, drugs with specificity for JNK are currently under clinical development (Manning and Davis, 2003). However, an unexpected finding of this study was that JNK appears to play no role in hepatocytes, but JNK is essential for TNFα expression by hematopoietic cells. Measurement of TNFα may therefore serve as a relevant biomarker that may be useful for monitoring the efficacy of JNK inhibition in protocols using JNK inhibitors in humans.
JNK is not essential for TNF-stimulated cell death of hepatocytes
It has previously been reported that JNK is required for TNFα-stimulated cell death. Thus, partial JNK-deficiency caused by disruption of the Jnk1 or Jnk2 genes has been reported to reduce TNF-stimulated apoptosis by unknown mechanisms (Dietrich et al., 2004; Kamata et al., 2005; Liu et al., 2004; Maeda et al., 2003; Wang et al., 2006). Furthermore, partial JNK-deficiency has been reported to cause reduced JNK-mediated phosphorylation of the E3 ligase Itch, failure to ubiquitinate and degrade the caspase 8 antagonist cFLIP, and protection against TNF-stimulated cell death in the ConA model of murine hepatitis (Chang et al., 2006). However, it is difficult to interpret the results of these studies because it is established that partial JNK-deficiency results in adaptation and a gain-of-function phenotype caused by the remaining JNK isoforms (Jaeschke et al., 2006).
Here we report the analysis of compound mutation of the two ubiquitously expressed genes that encode JNK. The absence of a requirement of JNK for TNFα-stimulated hepatocyte apoptosis is consistent with our previous analysis of TNFα-stimulated JNK1/2-deficient primary MEF (Lamb et al., 2003) and is also consistent with our finding that apoptosis caused by a different death receptor ligand (FasL) is also JNK-independent (Tournier et al., 2000).
JNK is required for TNFα expression
The expression of TNFα is regulated at multple steps, including Tnfα gene transcription, Tnfα mRNA stability, and Tnfα mRNA translation (Han et al., 1990; Tsai et al., 2000). The ERK and p38 MAP pathways are implicated in the regulation of Tnfα mRNA stability and translation (Dumitru et al., 2000; Hitti et al., 2006) and it is established that JNK plays an important role in Tnfα gene transcription (Ventura et al., 2003). The Tnfα gene contains JNK-responsive promoter elements that are required for normal Tnfα gene expression, including a divergent AP1 site that selectively binds heterodimeric complexes of ATF2 and cJun (Tsai et al., 2000). JNK can phosphorylate ATF2 and cJun within the NH2-terminal transcription activation domain leading to increased transcription activity (Davis, 2000). Indeed, JNK1/2-deficient fibroblasts exhibit a severe defect in Tnfα mRNA expression (Ventura et al., 2003) and JNK1/2-deficient macrophages and T cells express profoundly reduced amounts of TNFα in the culture medium (Figure 6B & S4).
Role of JNK in immune cells in the liver
JNK is required for the production of TNFα by hematopoietic cells for the development of hepatitis. Since membrane bound TNFα, but not soluble TNFα, is required for ConA-induced hepatitis (Kusters et al., 1997), it is likely that the hematopoietic cells that are the source of JNK-dependent TNFα expression include resident inflammatory cells in the liver (e.g. Kupffer cells and NKT cells).
The liver represents the major reservoir of NKT cells in the body and these cells are critically important for ConA-induced hepatitis (Kaneko et al., 2000; Takeda et al., 2000; Tiegs et al., 1992). Indeed, NKT cell-mediated expression of TNFα, IFNγ, and IL4 have been implicated in ConA-induced hepatitis (Kusters et al., 1996; Kusters et al., 1997; Tagawa et al., 1998). Interestingly, JNK-deficiency causes major defects in the ConA-induced expression of these cytokines in serum (Figure 2). Infiltration of the liver by neutrophils and eosinophils following ConA treatment (Bonder et al., 2004; Hatada et al., 2005; Jaruga et al., 2003; Louis et al., 2002) and the production of superoxide by Kupffer cells (Morita et al., 2003) may also contribute to hepatitis development. Nevertheless, TNFα expression by NKT cells is critically required for ConA-induced hepatitis. Thus, ConA causes greatly reduced hepatitis in Tnfα−/− mice, Tnfr1−/− mice, and Tnfr2−/− mice (Kusters et al., 1997; Maeda et al., 2003).
Conclusions
The results of this study demonstrate that JNK plays a critical role in mouse models of TNFα-dependent hepatitis. It is possible that under some specific conditions, JNK may influence cFLIP degradation (Chang et al., 2006) or ROS production (Ventura et al., 2004) during TNFα-stimulated cell death. However, JNK is not required for TNFα-mediated death of hepatocytes in models of fulminant hepatitis. In contrast, JNK is essential for the expression of TNFα by hematopoietic cells that promote hepatitis.
EXPERIMENTAL PROCEDURES
Mice
We have previously described Jnk1−/− mice (Dong et al., 1998), Jnk2−/− mice (Yang et al., 1998), and Jnk1f/f mice (Das et al., 2007). The JNK knockout mice were maintained on a C57BL/6J mouse strain background (back-crossed 10 generations). Alb-Cre mice (Postic et al., 1999), Mx1-Cre mice (Kuhn et al., 1995), B6.SJL mice, and C57BL/6J mice were obtained from The Jackson Labs. The mice were housed in a facility accredited by the American Association for Laboratory Animal Care (AALAC). Deletion of floxed alleles in Mx1-Cre mice was performed by treatment of 4 week old mice with 20 μg/g polyinosinic-polycytidylic acid (polyIC) (Mikkola et al., 2003) followed by recovery (4 weeks). Genomic DNA was examined using PCR amplimers (5′-CTCAGGAAGAAAGGGCTTATTTC-3′ and 5′-GAACCACTGTTCCAATTTCCATCC-3′) to distinguish between the wild-type (Jnk1+), floxed (Jnk1f), and deleted (Jnk1Δ) alleles (Das et al., 2007). Radiation chimeras using congenic C57BL/6J and B6.SJL mice were generated by exposure of recipient mice to two doses of ionizing radiation (525 Gy) and reconstitution of the mice with 107 donor bone marrow cells by injection into the tail vein. We examined three models of murine hepatitis using intravenous injection of: a) 25 mg/kg ConA (Sigma); b) 35 μg/kg E. coli 0111:B lipopolysacchride (LPS) (Sigma) plus 1g/kg N-acetyl-galactosamine (GalN) (Sigma); and c) 20 μg/kg TNFα (R&D Systems) plus 1g/kg GalN. The animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Massachusetts Medical School.
Serum analysis
Alanine transaminase (ALT) and aspartate aminotrasferase (AST) activity in serum was measured using the ALT and AST Reagent kit (Pointe Scientific) with a Tecan Sapphire microplate reader (Tecan Trading AG). The serum concentration of cytokines was measured by multiplexed ELISA using a Luminex 200 instrument (Millipore).
Biochemical analysis
Immunoblot analysis was performed by probing with antibodies to ERK1/2 (Santa Cruz), JNK1/2 (Pharmingen), cFLIP (Alexis), cleaved PARP, cleaved caspase 3 (Cell Signaling), and α-Tubulin (Sigma). The amount of total and phospho- JNK1/2, cJun, ERK1/2, p38 MAPK, and AKT in tissue extacts was measured by multiplexed ELISA using a Luminex 200 instrument (Millipore). The expression of mRNA was examined by quantitative RT-PCR analysis using a 7500 Fast Real Time PCR machine. Taqman© assays (Applied Biosystems).
Supplementary Material
Acknowledgments
We thank Tamera Barrett, Vicky Benoit, Linda Lesco, Jian-Hua Liu, and Judith Reilly for expert technical assistance, and Kathy Gemme for administrative assistance. These studies were supported by a grant from the National Institutes of Health. Core facilities used by these studies were supported by the NIDDK Diabetes and Endocrinology Center (P30 DK 32520) at the University of Massachusetts. R.A.F. and R.J.D. are investigators of the Howard Hughes Medical Institute.
Footnotes
Supplemental data include Supplemental Experimental Procedures, nine Supplemental Figures, Supplemental References and can be found with the article on-line at http://cell.com/cgi/content/full/***.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- Alves-Guerra MC, Rousset S, Pecqueur C, Mallat Z, Blanc J, Tedgui A, Bouillaud F, Cassard-Doulcier AM, Ricquier D, Miroux B. Bone marrow transplantation reveals the in vivo expression of the mitochondrial uncoupling protein 2 in immune and nonimmune cells during inflammation. J Biol Chem. 2003;278:42307–42312. doi: 10.1074/jbc.M306951200. [DOI] [PubMed] [Google Scholar]
- Bonder CS, Ajuebor MN, Zbytnuik LD, Kubes P, Swain MG. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J Immunol. 2004;172:45–53. doi: 10.4049/jimmunol.172.1.45. [DOI] [PubMed] [Google Scholar]
- Chan FK, Lenardo MJ. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur J Immunol. 2000;30:652–660. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- Conze DB, Albert L, Ferrick DA, Goeddel DV, Yeh WC, Mak T, Ashwell JD. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol Cell Biol. 2005;25:3348–3356. doi: 10.1128/MCB.25.8.3348-3356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Jiang F, Sluss HK, Zhang C, Shokat KM, Flavell RA, Davis RJ. Suppression of p53-dependent senescence by the JNK signal transduction pathway. Proc Natl Acad Sci U S A. 2007;104:15759–15764. doi: 10.1073/pnas.0707782104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Dietrich N, Thastrup J, Holmberg C, Gyrd-Hansen M, Fehrenbacher N, Lademann U, Lerdrup M, Herdegen T, Jaattela M, Kallunki T. JNK2 mediates TNF-induced cell death in mouse embryonic fibroblasts via regulation of both caspase and cathepsin protease pathways. Cell Death Differ. 2004;11:301–313. doi: 10.1038/sj.cdd.4401353. [DOI] [PubMed] [Google Scholar]
- Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- Dong Z, Wei H, Sun R, Tian Z. The roles of innate immune cells in liver injury and regeneration. Cell Mol Immunol. 2007;4:241–252. [PubMed] [Google Scholar]
- Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Eissner G, Kohlhuber F, Grell M, Ueffing M, Scheurich P, Hieke A, Multhoff G, Bornkamm GW, Holler E. Critical involvement of transmembrane tumor necrosis factor-alpha in endothelial programmed cell death mediated by ionizing radiation and bacterial endotoxin. Blood. 1995;86:4184–4193. [PubMed] [Google Scholar]
- Fotin-Mleczek M, Henkler F, Samel D, Reichwein M, Hausser A, Parmryd I, Scheurich P, Schmid JA, Wajant H. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J Cell Sci. 2002;115:2757–2770. doi: 10.1242/jcs.115.13.2757. [DOI] [PubMed] [Google Scholar]
- Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95:570–575. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Brown T, Beutler B. Endotoxin-responsive sequences control cachectin/tumor necrosis factor biosynthesis at the translational level. J Exp Med. 1990;171:465–475. doi: 10.1084/jem.171.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatada S, Ohta T, Shiratsuchi Y, Hatano M, Kobayashi Y. A novel accessory role of neutrophils in concanavalin A-induced hepatitis. Cell Immunol. 2005;233:23–29. doi: 10.1016/j.cellimm.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol. 2006;26:2399–2407. doi: 10.1128/MCB.26.6.2399-2407.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K, Wagner EF, Sabapathy K. Differential effects of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene. 2002;21:2441–2445. doi: 10.1038/sj.onc.1205348. [DOI] [PubMed] [Google Scholar]
- Jaeschke A, Karasarides M, Ventura JJ, Ehrhardt A, Zhang C, Flavell RA, Shokat KM, Davis RJ. JNK2 is a positive regulator of the cJun transcription factor. Mol Cell. 2006;23:899–911. doi: 10.1016/j.molcel.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Jaruga B, Hong F, Sun R, Radaeva S, Gao B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J Immunol. 2003;171:3233–3244. doi: 10.4049/jimmunol.171.6.3233. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, Taniguchi M. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Kusters S, Gantner F, Kunstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- Kusters S, Tiegs G, Alexopoulou L, Pasparakis M, Douni E, Kunstle G, Bluethmann H, Wendel A, Pfizenmaier K, Kollias G, Grell M. In vivo evidence for a functional role of both tumor necrosis factor (TNF) receptors and transmembrane TNF in experimental hepatitis. Eur J Immunol. 1997;27:2870–2875. doi: 10.1002/eji.1830271119. [DOI] [PubMed] [Google Scholar]
- Lamb JA, Ventura JJ, Hess P, Flavell RA, Davis RJ. JunD mediates survival signaling by the JNK signal transduction pathway. Mol Cell. 2003;11:1479–1489. doi: 10.1016/s1097-2765(03)00203-x. [DOI] [PubMed] [Google Scholar]
- Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–347. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- Liedtke C, Plumpe J, Kubicka S, Bradham CA, Manns MP, Brenner DA, Trautwein C. Jun kinase modulates tumor necrosis factor-dependent apoptosis in liver cells. Hepatology. 2002;36:315–325. doi: 10.1053/jhep.2002.34615. [DOI] [PubMed] [Google Scholar]
- Liu J, Minemoto Y, Lin A. c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for tumor necrosis factor alpha-induced c-Jun kinase activation and apoptosis. Mol Cell Biol. 2004;24:10844–10856. doi: 10.1128/MCB.24.24.10844-10856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- Louis H, Le Moine A, Flamand V, Nagy N, Quertinmont E, Paulart F, Abramowicz D, Le Moine O, Goldman M, Deviere J. Critical role of interleukin 5 and eosinophils in concanavalin A-induced hepatitis in mice. Gastroenterology. 2002;122:2001–2010. doi: 10.1053/gast.2002.33620. [DOI] [PubMed] [Google Scholar]
- Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003;19:725–737. doi: 10.1016/s1074-7613(03)00301-7. [DOI] [PubMed] [Google Scholar]
- Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Mikkola HK, Klintman J, Yang H, Hock H, Schlaeger TM, Fujiwara Y, Orkin SH. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature. 2003;421:547–551. doi: 10.1038/nature01345. [DOI] [PubMed] [Google Scholar]
- Morita A, Itoh Y, Toyama T, Fujii H, Nishioji K, Kirishima T, Makiyama A, Yamauchi N, Okanoue T. Activated Kupffer cells play an important role in intra-hepatic Th1-associated necro-inflammation in Concanavalin A-induced hepatic injury in mice. Hepatol Res. 2003;27:143–150. doi: 10.1016/s1386-6346(03)00206-7. [DOI] [PubMed] [Google Scholar]
- Natoli G, Costanzo A, Ianni A, Templeton DJ, Woodgett JR, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- Reuther-Madrid JY, Kashatus D, Chen S, Li X, Westwick J, Davis RJ, Earp HS, Wang CY, Baldwin AS., Jr The p65/RelA subunit of NF-kappaB suppresses the sustained, antiapoptotic activity of Jun kinase induced by tumor necrosis factor. Mol Cell Biol. 2002;22:8175–8183. doi: 10.1128/MCB.22.23.8175-8183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. Embo J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa Y, Kakuta S, Iwakura Y. Involvement of Fas/Fas ligand system-mediated apoptosis in the development of concanavalin A-induced hepatitis. Eur J Immunol. 1998;28:4105–4113. doi: 10.1002/(SICI)1521-4141(199812)28:12<4105::AID-IMMU4105>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- Thome M, Tschopp J. Regulation of lymphocyte proliferation and death by FLIP. Nat Rev Immunol. 2001;1:50–58. doi: 10.1038/35095508. [DOI] [PubMed] [Google Scholar]
- Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419–1426. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai EY, Falvo JV, Tsytsykova AV, Barczak AK, Reimold AM, Glimcher LH, Fenton MJ, Gordon DC, Dunn IF, Goldfeld AE. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Mol Cell Biol. 2000;20:6084–6094. doi: 10.1128/mcb.20.16.6084-6094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- Ventura JJ, Kennedy NJ, Lamb JA, Flavell RA, Davis RJ. c-Jun NH(2)-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol Cell Biol. 2003;23:2871–2882. doi: 10.1128/MCB.23.8.2871-2882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr, Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18:2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura JJ, Hubner A, Zhang C, Flavell RA, Shokat KM, Davis RJ. Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell. 2006;21:701–710. doi: 10.1016/j.molcel.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CJ, Conze DB, Li X, Ying SX, Hanover JA, Ashwell JD. TNF-alpha induced c-IAP1/TRAF2 complex translocation to a Ubc6-containing compartment and TRAF2 ubiquitination. Embo J. 2005;24:1886–1898. doi: 10.1038/sj.emboj.7600649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DD, Conze D, Whitmarsh AJ, Barrett T, Davis RJ, Rincon M, Flavell RA. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity. 1998;9:575–585. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.