Abstract
The purpose of this study was to examine the differential effects of acute tryptophan (TRP) depletion vs. sham condition on plasma, cerebrospinal fluid (CSF) biochemical parameters, and mood in the following three subject groups: (1) nine antidepressant-free individuals with remitted depression, (2) eight paroxetine-treated individuals with recently remitted depression, and (3) seven healthy controls.
Plasma TRP decreased during TRP depletion and increased during sham condition (p<.01). CSF TRP and 5-hydroxyindoleacetic acid were lower during TRP depletion than sham condition (p<.01 each). During TRP depletion, CSF TRP correlated significantly with the plasma sum of large neutral amino acids (ΣLNAA) (R= −.52, p=.01), but did not significantly correlate with plasma TRP (R= .15, p= .52). The correlation between CSF TRP and ratio of TRP to ΣLNAA was (R= .41, p=.06) during TRP depletion, and (R= −.44, p=.04) during sham condition. A negative correlation trend was observed between CSF TRP levels and peak Hamilton Depression Rating Scale scores during TRP depletion in patients recovered from depression (R= −.45, p=.07), but not in healthy controls (R= −.01, p= .98). CSF Neuropeptide Y was higher during TRP depletion than sham condition (t= 1.75, p < .10).
These results illustrate the importance of assessing plasma ΣLNAA when using the TRP depletion paradigm. The use of a single CSF sampling technique although practical may result in data acquisition limitations.
Keywords: Monoamine-metabolites, Tryptophan-depletion, Cerebrospinal fluid (CSF), Neuropeptide Y, Sum of large neutral amino acids (ΣLNAA)
INTRODUCTION
The tryptophan (TRP) depletion paradigm continues to be a safe and effective tool to study the role of brain serotonin (5-HT) function in humans.
Ingestion of a 15-amino acid TRP-free drink (Young et al., 1989) induces hepatic protein synthesis and causes rapid depletion of (80% to 90%) plasma TRP in the anabolic process. The decrease in absolute TRP availability, along with an increase in levels of large neutral amino acids (LNAA) that compete with TRP for transport into the brain are believed to explain a rapid decrease brain TRP (Moja et al., 1989) and 5-HT synthesis (Nishizawa et al., 1997). It remains unclear whether TRP, the sum of LNAA (ΣLNAA) or the ratio of TRP to ΣLNAA (TRP/ΣLNAA) primarily determine TRP availability in the central nervous system (CNS).
Despite the consistent reduction in plasma TRP levels (Delgado et al., 1990, 1994; Moreno et al., 1999), the behavioral responses that follow TRP depletion in individuals with remitted depression have been quite variable. Cerebrospinal fluid (CSF) TRP and other CSF neurochemicals may more accurately reflect the changes in brain 5-HT activity than plasma TRP measurements, and may help explain the variability in behavioral response observed following TRP depletion. Carpenter and colleagues (1998) measured CSF levels of monoamine metabolites during TRP depletion in healthy young subjects by continuous sampling via an indwelling CSF catheter. CSF levels of TRP and 5-hydroxyindoleacetic acid (5-HIAA) were significantly reduced during TRP depletion. Moreno et al. (2000-a) replicated those findings in a small group of healthy male volunteers utilizing a single time point sampling technique. Salomon et al. (2003) reported a relationship between a CSF TRP threshold and depressive relapse during TRP depletion in subjects with depression who achieved remission following antidepressant treatment.
The present study intended to replicate and extend these findings by comparing CSF TRP and 5-HIAA concentrations after TRP depletion in subjects with remitted depression and in healthy volunteers. We also examined whether plasma TRP, ΣLNAA, or TRP/ΣLNAA correlate with CSF TRP, and CSF 5-HIAA. These data could provide important insights about the process by which TRP is transported into the CNS. Levels of other CSF neurochemicals were analyzed to explore their relationship to plasma amino acids, as well the anticipated depressive response. These CSF neurochemicals included: 3-methoxy-4-hydroxy-phenylglycol (MHPG), homovanillic acid (HVA), neuropeptide “Y” (NPY), and corticotrophin releasing hormone (CRH). CRH (Dinan, 1996; Pitchot et al., 2001) and NPY (Mathé et al., 1996; Mathé, 1999; Jiménez et al., 2001; Husum et al., 2003) have also been implicated in the pathophysiology of depression. Research has shown that NPY concentrations are reduced and CRH concentrations are increased in individuals with depression. In animal models of depression, NPY levels increase in response to electroconvulsive therapy, lithium, and lamotrigine (Jimenez-Vazquez et al., 2001). NPY and CRH are neuronally interconnected (Husum and Mathé, 2002) and have modulatory interactions with the monoamine systems. A study by Tyrka et al., (2004) reported a significant increase in CSF CRH during TRP depletion in the absence of significant mood changes. Given these findings we anticipated observing a serotonin mediated increase in CSF CRH levels and a decrease in CSF NPY levels during active depletion but not during sham depletion.
EXPERIMENTAL PROCEDURES
Subjects
Twenty-four subjects were recruited through word of mouth and newspaper advertisements. Nine medication-free subjects who had been in remission from a Unipolar Major Depressive Episode (MDE) for at least three months but less than two years comprised the first group. Seven healthy control subjects who were age- and gender- matched to the above group were the second group. A third group (eight subjects) had achieved and maintained remission from a MDE for at least two weeks following paroxetine treatment. The sample consisted of 16 female and 8 male subjects between the ages of 23 and 60 years (mean 36.3 ± standard deviation [s.d.] 11.1).
All participants completed the Structured Clinical Interview for DSM-IV (SCID; Spitzer, 1987). The individuals with medication-free remitted depression were selected if they met Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) criteria for at least one prior MDE and had no current Axis I condition. Healthy subjects were selected if they did not meet lifetime criteria for DSM-IV Axis I conditions based on the SCID, and lacked history of mental illness in their first-degree relatives. The third group of subjects had recently achieved remission from a MDE after up to eight weeks of open label treatment with paroxetine at doses of 10 to 50 mg (mean 26.25 ± s.d. 15.06). Remission was defined as a post-treatment decrease in score ≥ 50% in the 25-item Hamilton Depression Rating Scale (HDRS) (Mazure et al., 1986) with a post treatment total score ≤ 9 maintained for two consecutive visits 2 weeks apart. Post-treatment scores ranged from 0-8 (mean 2.75 ± s.d. 2.6). For this group, the depletion procedures took place two to four weeks after reaching remission.
All subjects were free of general medical or neurological conditions based on a clinical history and review of systems, physical examination, electrocardiogram, routine blood tests, and urine drug screen. The University of Arizona Human Subjects Committee approved this study, and written informed consent was obtained after explaining the study in detail to each participant.
Procedure
Testing was performed in the outpatient Psychopharmacology Research Program at the University of Arizona Health Sciences Center and was conducted in a double blind, randomized, sham-controlled, crossover fashion. TRP depletion and sham condition were separated by one week, and each was administered across three days. On Day 1 of TRP depletion, subjects ingested a 102 gm TRP-free 15-amino acid mixture. On Day 1 of sham condition, subjects ingested an identical amino acid mixture but supplemented with 2.3 gm of TRP. See table 1 for the depletion procedure timeline.
Table 1.
Schedule for Depletion Sessions
| Day 1 | |
| 6:00 AM | Arrive at the Research Clinic (fasting after midnight). Behavioral ratings and plasma for TRP and LNAA levels (10 cc) |
| 6:15 AM | Ingestion of methionine, cysteine, and arginine capsules |
| 6:30 AM | Administration of amino acid drink. |
| 11:30 AM | Behavioral ratings, plasma for TRP levels (10 cc), vitals |
| 3:30 PM | Lumbar puncture forCSF acquisition (15 cc) |
| 4:00 PM | Dinner will be provided (Return to unrestricted food intake) |
| 5:30 PM | Behavioral ratings |
| 6:00 PM | After observation, in the absence of side effects, subjects were allowed to leave |
| Days 2 & 3 | |
| 8:00 AM | Behavioral ratings |
Throughout the day, subjects were asked to minimize activity and remain in a specialized procedure room that allowed for constant observation. Subjects were encouraged to read or listen to the radio. Although they were urged to maintain strict bed rest, they were allowed to get up only to use the restroom. They were instructed not to fall asleep, watch television, engage in extensive interactions, or become physically active. Subjects were allowed to drink water or fruit juice, but not to eat until after 4:30 PM. There were no dietary or activity restrictions after that time.
CSF samples for biochemical measurements were obtained by performing a standard lumbar puncture from the L3-L4 or L4-L5 interspace utilizing a fine-gauge spinal needle, while subjects were lying down on their side. Sampling occurred nine hours after ingestion of the drink (approximately 3:30 PM, which took place without unexpected delays) to coincide with the nadir of CSF 5-HIAA changes as reported by Carpenter et al. (1998). CSF was collected on ice as described by Virkunnenn et al. (1987). The first 1 cc was discarded, the following 12 cc were mixed thoroughly and frozen at −70 degrees Fahrenheit.
The time points selected for collection of plasma and CSF samples were based on the reported mean latency for nadirs of the most relevant biological indices (i.e., approximately 5 hours after ingestion of amino acid drink for plasma TRP and 9 hours after ingestion of amino acid drink for CSF 5-HIAA). This time selection was based in the speculation that plasma nadir at 5 hours may best predict the CSF nadir at 9 hours. Subjects went home about one hour after CSF collection and returned for observation and to complete behavioral rating scales for about one hour during the two subsequent mornings (Day-2, Day-3). Assessment scores from Day-2 were included in the data analyses because the latency for depressive responses during TRP depletion has been shown to vary significantly (Delgado et al., 1991 and 1999; Moreno et al., 1999). A second day of follow up (Day-3) was included in order to monitor the subject's return to baseline mood, but was not included in the data analysis as proposed a priori.
Measurements
Behavioral ratings at each time point included the HDRS, the Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1959) and the Symptom Checklist, a self rated scale of somatic symptoms which was utilized to measure the procedure tolerability (Woods et al., 1988). Experienced research assistants with established reliability on these scales performed the ratings. Subjects and raters remained blinded to the sequence of testing. Plasma TRP and LNAA were assayed by high performance liquid chromatography with electrochemical detection (HPLC-EC) (Gerhardt, 1986). CSF 5-HIAA and HVA were simultaneously quantified by mass fragmentography (Fri et al., 1974). CSF MHPG was measured by HPLC-EC (Krstulovic, 1984). CSF NPY and CRF were measured with sensitive and specific radioimmunoassay (RIA) as previously reported (Jiménez, 2001; Husum, 2002).
Data Analysis
Changes in plasma TRP levels were assessed with paired-samples t-test comparing 6:00 AM to 11:30 AM measurements. Changes in behavioral ratings (HDRS, HAM-A, and SC) were assessed by analysis of variance (ANOVA) in a repeated measures design. This allowed for an assessment of the main effect of time (6:00 AM, 11:30 AM, 5:30 PM, and Day 2), condition (active depletion vs. sham depletion), subject group (healthy controls, drug free in remission, and paroxetine treated subjects), as well as time × group, condition × group, time × condition, and time × condition × group interactions. Changes in CSF TRP, 5-HIAA, MHPG, HVA, CRH and NPY were assessed with paired sample t-test comparing amounts measured during TRP depletion to sham condition. ΣLNAA was determined by the addition of plasma levels of leucine, isoleucine, phenylalanine, tyrosine, and valine. Pearson's correlation coefficients were calculated to assess the relationship between plasma level of TRP and ΣLNAA, TRP/ΣLNAA (at five hours), and CSF TRP and 5-HIAA (at nine hours). Multiple linear regression coefficients were calculated to explore the relationship of plasma amino acids with other CSF neurochemicals, and CSF neurochemicals and maximum HDRS score following TRP depletion or sham condition. The “HDRS Maximum Score” as consistently reported in previous publications by the authors (Moreno et al., 1999, 2000-b, 2002) was selected from the highest HDRS score observed after baseline and up to 25 hours. Results were considered significant when p ≤ .05, and trends were reported when < .1. All tests were two-tailed. Data analysis and graphic presentation utilized SPSS version 16.0.
RESULTS
Baseline HDRS scores were as follows: drug-free remitted group (M = .89, SD = 1.16), healthy control group (M = .79, SD = .86), and paroxetine-treated remitted group (M = 3.32, SD = 3.00).
Depletion related side effects were similar in frequency and severity to those observed in previous reports. Six subjects (25%) complained of nausea, two of them (8%) experienced vomiting. In all cases nausea and vomiting resolved prior to lumbar puncture. Two of the subjects complained of headaches after the lumbar puncture. In one of the cases, the headache was mild and resolved promptly. In the other case, due to the severity and persistence or recurrence of the headache after 24 hours the subject required treatment with a blood patch, a procedure in which a small amount of the patient's blood is injected through a standard lumbar puncture procedure in order to correct the presumed leakage of CSF from the sampling site. This headache occurred following the sham condition and resolved without further complications. We were unable to obtain a CSF sample in one female subject during one of the test sessions. One female healthy control did not return for her second test session, and did not state her reason for discontinuation. All available data was analyzed.
Plasma Findings
During TRP depletion TRP levels decreased by an average of 89.1 % (range 79 to 94%) (t = 22.95, df = 20, p< 0.01), ΣLNAA levels increased by an average of 303% (range 157% to 479%) (t = −10.17, df = 20, p< 0.01), and TRP/ΣLNAA decreased by an average of 96.1% (range 90% to 99%; t = 15.91, df = 20, p< 0.01). During sham condition TRP levels increased by an average of 90.5 % (range −17% to +338%) (t = −7.64, df = 23, p< 0.01), ΣLNAA levels increased by an average of 269% (range 78% to 686%) (t = −7.57, df = 23, p< 0.01), and TRP/ΣLNAA decreased by an average of 25.31% (range +38 to −74%; t = 5.27, df = 23, p< 0.01). ΣLNAA level changes were very similar during TRP depletion and sham condition (difference of 13.6%, t = −.77, df = 20, p= 0.45). [See Table 2 for plasma levels].
Table 2.
Plasma Amino Acid Levels During TRP Depletion and Sham Condition
| Plasma Levels | Active Depletion Mean ± S.D. |
Paired t-test p value 2-tailed |
Sham Condition Mean ± S.D. |
Paired t-test p value 2-tailed |
|---|---|---|---|---|
| Total TRP at 6:00 AM (μg/ml) | 11.21 ± 1.89 (N=21) | P<.01 | 11.66 ± 2.06 (N=24) | P<.01 |
| Total TRP at 11:30 AM (μg/ml) | 1.23 ± 0.36 (N=22) | 22.21 ± 7.53 (N=24) | ||
| ΣLNAA at 6:00 AM (μg/ml) | 1403.7 ± 260.4 (N=24) | P<.01 | 1501.8 ± 430.2 (N=24) | P<.01 |
| ΣLNAA at 11:30 AM (μ/ml) | 4249.4 ± 1326.3 (N=24) | 4007.4 ± 1672 (N=24) | ||
| TRP/ΣLNAA at 6:00 AM | .00828 ± .00227 (N=21) | P<.01 | .00814 ± .00193 (N=24) | P<.01 |
| TRP/ΣLNAA at 11:30 AM | .00032 ± .00015 (N=21) | .00608 ± .00195 (N=24) |
Abbreviations: N= number of subjects, TRP= tryptophan, Total TRP at 6:00AM= total tryptophan level obtained immediately prior to ingestion of the test drink, Total TRP at 11:30 AM= total tryptophan level obtained 5 hours after ingestion of the test drink, μg/ml= micrograms per milliliter, S.D.= standard deviation, ΣLNAA= sum of large neutral amino acids, TRP/ΣLNAA= Ratio of tryptophan to the sum of large neutral amino acids.
CSF Findings
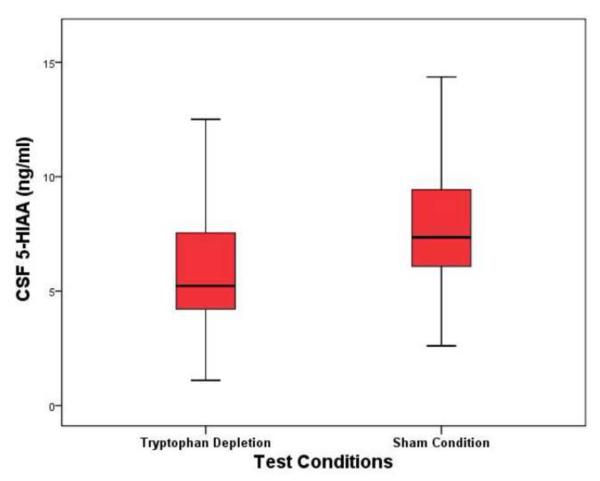
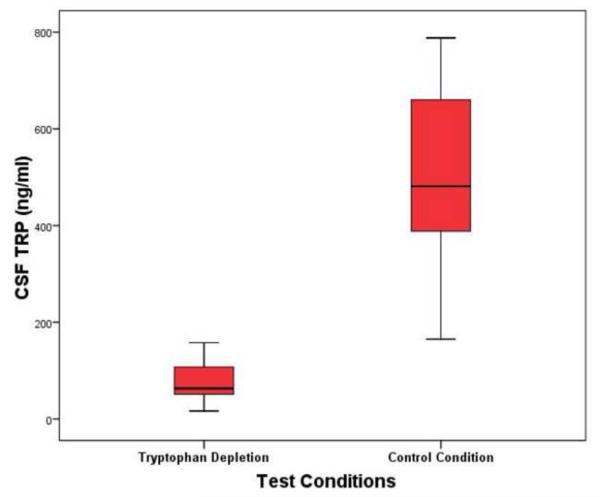
Mean CSF 5-HIAA concentration was significantly lower following active TRP depletion (values represent mean ± standard deviation) (6.34 ± 3.18 ng/ml) than following sham condition (8.36 ± 3.68 ng/ml), representing a mean 24.16% difference between conditions (t = −5.1, df = 20, p <0.01) [See Figure 1]. CSF TRP was significantly lower during TRP depletion (80.2 ± 47.29 ng/ml) than during sham condition (490.69 ± 174.85 ng/ml), representing a 73.66% difference between conditions (t = −13.51, df = 20, p <0.01) [See Figure 2]. CSF NPY was 4.23% higher during TRP depletion (54.39 ± 9.6 pmol/L) than during sham testing (52.18 ± 7.54 pmol/L) (t = 1.747, df = 19, p <0.10). There were no significant differences in CSF neurochemical findings between subject-groups, and there were no differences between TRP depletion and sham tests in CSF HVA, MHPG, and CRH (see table 3).
Figure 1. CSF 5-HIAA Concentrations at Nine Hours Following Active and Sham Tryptophan Depletion Protocol.
Abbreviations: CSF= cerebrospinal fluid, 5-HIAA= 5-hydroxyindoleacetic acid, ng/ml= nanograms per milliliter
Figure depicts: Mean, standard deviation, and confidence intervals.
Figure 2. CSF TRP Concentrations at Nine Hours Following Active and Sham Tryptophan Depletion Protocol.
Abbreviations: CSF= cerebrospinal fluid, TRP= tryptophan, ng/ml= nanograms per milliliter
Figure depicts: Mean, standard deviation, and confidence intervals.
Table 3.
CSF Neurochemicals During TRP Depletion and Sham Condition
| CSF Neurochemicals |
Active Depletion Mean ± S.D. |
Sham Condition Mean ± S.D. |
T-test “p” 2-tailed |
|---|---|---|---|
| TRP ng/ml | 80.2 ± 47.29 (N=23) |
490.69 ± 174.85 (N=22) |
<01 |
| 5-HIAA ng/ml | 6.34 ± 3.18 (N=23) |
8.36 ± 3.68 (N=22) |
<01 |
| MHPG ng/ml | 10.86 ± 5.77 (N=23) |
11.74 ± 6.22 (N=22) |
.34 |
| HVA ng/ml | 28.66 ± 17.45 (N=23) |
27.21 ± 15.86 (N=22) |
.58 |
| NPY pmol/L | 54.39 ± 9.6 (N=22) |
52.18 ± 7.54 (N=21) |
<.10 |
| CRH pmol/L | 8543.6 ± 2515.82 (N=22) |
8844.6 ± 2124.8 (N=21) |
.47 |
Abbreviations: CSF= cerebrospinal fluid, N= number of subjects, TRP= tryptophan, ng/ml= nanograms per milliliter, pmol/L = picomoles per liter, S.D.= standard deviation. 5-HIAA= 5-hydroxyindoleacetic acid, MHPG= 3-methoxy-4-hydroxy-phenylglycol, HVA= homovanillic acid, NPY= Neuropeptide Y, CRH= Corticotrophin releasing hormone.
Relationship of Plasma amino acids and CSF neurochemicals
A number of significant correlations between plasma TRP, ΣLNAA levels, TRP/ΣLNAA and CSF neurochemicals were observed. Of note, during TRP depletion, CSF TRP levels were more highly correlated with plasma ΣLNAA levels and TRP/ΣLNAA than with plasma TRP levels. TRP/ ΣLNAA correlated significantly with CSF TRP levels during sham condition. Additionally, CSF 5-HIAA correlated strongly with CSF HVA during both test conditions. (Pertinent findings are reported in table 4).
Table 4.
Selected Correlations Between Biochemical Parameters During TRP Depletion and Sham Condition
| Biochemical Parameters |
Active Depletion Pearson's R (p value) |
Sham Condition Pearson's R (p value) |
|---|---|---|
| Plasma (T+5hr) TRP & CSF TRP | .15 (.52) N=21 | .08 (.74) N=22 |
| Plasma (T+5hr) TRP & CSF 5-HIAA | .05 (.82) N=21 | .39 (.07) N=22 |
| Plasma (T+5hr) ΣLNAA & CSF TRP | −.52 (.01) N=21 | .34 (.12) N=22 |
| Plasma (T+5hr) ΣLNAA & CSF 5-HIAA | .30 (.19) N=21 | .15 (.49) N=22 |
| Plasma (T+5hr) TRP/ΣLNAA & CSF TRP | .41 (.06) N=21 | −.44 (.04) N=22 |
| Plasma (T+5hr) TRP/ΣLNAA & CSF 5-HIAA | −.11 (.62) N=21 | .03 (.91) N=22 |
| Plasma (T+5hr) TRP & CSF NPY | .05 (.08) N=20 | .32 (.15) N=21 |
| Plasma (T+5hr) ΣLNAA & CSF MHPG | .68 (<.01) N=21 | −.10 (.64) N=22 |
| CSF TRP & CSF 5-HIAA | −.35 (.11) N=23 | −.05 (.84) N=22 |
| CSF 5-HIAA & CSF NPY | .24 (.28) N=22 | .46 (.03) N=21 |
| CSF 5-HIAA & CSF MHPG | .36 (.09) N=23 | .13 (.57) N=22 |
| CSF 5-HIAA & CSF HVA | .69 (<.01) N=23 | .55 (<.01) N=22 |
| CSF HVA & CSF NPY | .32 (.18) N=22 | .38 (.09) N=21 |
Abbreviations: N= number of subjects, T+5hr = 5 hours after ingestion of amino acid mixture, CSF = cerebrospinal fluid, TRP = tryptophan, ΣLNAA = sum of large neutral amino acids, TRP/ΣLNAA = Ratio of tryptophan to the sum of large neutral amino acids, 5-HIAA = 5-hydroxyindoleacetic acid, MHPG = 3-methoxy-4-hydroxy-phenylglycol, HVA = homovanillic acid, NPY = Neuropeptide Y, CRH = Corticotrophin releasing hormone.
Behavioral Response
ANOVA of HDRS scores did not show statistical significant main effects of time (F = 1.66, df = 3, p >0.1), condition (F = 0.05, df = 1, p > 0.1), or any significant interaction effects. Similarly, HAMA and SC scores showed no significant main or interaction effects. There were no sex differences in CSF TRP and CSF5-HIAA for any of the groups.
Relationship of HDRS and CSF TRP
During TRP depletion, Pearson's correlation of maximum HDRS score and CSF TRP had a trend of significance in subjects in remission from depression (R = −.45, N = 17, p =.07); but not in healthy controls (R = −.01, N = 5, p =.98).
DISCUSSION
The observed changes in CSF TRP and 5-HIAA are consistent with data from previous depletion studies (Carpenter et al., 1998; Williams et al., 1999; Young et al., 1989; Moreno et al., 2000; and Salomon et al., 2003), and are extended by the inclusion of individuals with remitted depression both medication free and paroxetine treated, in addition to healthy controls. Additionally, this study examined the relationship of plasma TRP and ΣLNAA to CSF TRP and other neurochemicals. Interestingly, CSF TRP had higher correlations with plasma ΣLNAA and TRP/ΣLNAA than with plasma TRP. These data suggest that during TRP depletion, the availability of LNAA transporters in the blood brain barrier has a larger impact than plasma TRP levels in the CNS availability of TRP.
The following important limitations should be noted: a) given the use of a single sampling technique, CSF values recorded for TRP depletion and sham condition can only be assessed relative to each other. It is therefore not possible to determine to what extent the differences observed between the two experimental conditions represents changes in TRP depletion, control condition, or both; b) although the time selection for CSF sampling coincides with the mean latency for nadir of CSF 5-HIAA in a previous study (Carpenter et al., 1998), given the individual variability of timing for nadir, sampling at one standard time point may have missed the absolute nadir for subjects; c) other CSF neurochemicals measured in this study are likely to have different time progressions to either nadir or peaks, and a one time cross sectional sample further limits our ability to detect possible effects; d) a lumbar puncture can be variably uncomfortable, painful, and anxiety provoking, potentially altering the concentrations of CSF neurochemicals; e) the depressive responses in this study were modest which may have limited the study's ability to detect correlations between plasma or CSF-biochemical variables with mood ratings.
Cautioning our interpretation based on the above limitations, the study findings are potentially contributory and relevant to a better understanding of the mechanism of action of TRP depletion paradigm studies. Another interesting issue relates to the reported relationship between CSF TRP and HDRS scores in remitted subjects. In contrast to Salomon et al. (2003) our data failed to observe an absolute CSF TRP threshold below which all subjects experienced a categorically depressive response. Rather than disproving the concept of a threshold, given the single CSF sampling concerns and limited power of the present study, our findings support the concept of a continuous inverse relationship between CSF-TRP and depressive ratings during TRP depletion in patients who are in remission from a major depressive episode whether drug-free or treated with an SSRI.
ΣLNAA levels during sham testing correlated with CSF MHPG levels. Given that MHPG readily crosses the blood brain barrier it is generally understood that CSF MHPG reflects peripheral levels rather than CNS catecholamine activity during TRP depletion. This observation may be explained by the fact that the LNAA tyrosine and phenylalanine, which are ingested as part of the TRP depletion and sham conditions, are precursors for the synthesis of norepinephrine and dopamine which in turn metabolize to MHPG and HVA respectively. The reported strong correlation of CSF 5HIAA and HVA observed during both experimental conditions is consistent with multiple similar reports as discussed by Agren et al. (1986).
The neurotrophic factor NPY was slightly higher during active depletion compared to sham condition. Given that NPY has been reported to be lower during depression and higher after successful treatments; it may be speculated that the observed CSF elevation could presumably represent a compensatory mechanism in response to the stress of this challenge paradigm, similar to the “compensatory” increases in plasma Brain Derived Neurotrophic Factor (BDNF) previously reported during TRP depletion (Neumeister et al., 2005). A lack of changes in CSF CRH levels was observed when an increase was predicted, this is inconsistent with the reported increase in CSF-CRH at 8 hours after TRP depletion (Tyrka et al. 2004).
Summary
Using a single CSF sampling technique, this study compared behavioral ratings, plasma and CSF neurochemical changes in subjects in remission from a MDE and healthy controls that underwent TRP depletion. CSF TRP and 5-HIAA differences between TRP depletion and sham condition are consistent with data from previous studies which utilized continuous CSF sampling methods. Data from this study support the role of LNAA transport capacity, rather than absolute TRP levels, in the decrease of CNS TRP observed during TRP depletion. Observed behavioral effects were modest which limited assessment of relationships between neurochemical and behavioral findings. In spite of this, there was a statistical trend suggesting a negative correlation between CSF TRP and maximum HDRS score during depletion in subjects who are in remission from a MDE.
ACKNOWLEDGEMENTS
Supported by The National Institute of Mental Health RO3 MH57364-01A1, the Arizona Hispanic Center of Excellence, and the University of Arizona Health Sciences Center Dean's Physician Scientist Career Development Award to Dr. Moreno.
Supported by the Swedish Medical Research Council Grant 10414 to Dr. Mathé.
We acknowledge Jeri Lizabeth Fackelman, Ph.D. for her editorial contribution to the manuscript.
ROLE OF THE FUNDING SOURCES
Supported by The National Institute of Mental Health RO3 MH57364-01A1 for funding the study portion that included medication free subjects and healthy control groups.
The University of Arizona Health Sciences Center Dean's Physician Scientist Career Development Award to Dr. Moreno to assist with study portion that included the paroxetine treatment group.
The Arizona Hispanic Center of Excellence for logistic support.
The Swedish Medical Research Council Grant 10414 for supporting the efforts of Dr. A. Mathé.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
STATEMENT OF INTEREST
None
REFERENCES
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. Journal of Endocrinology. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Agren H, Mefford IN, Rudorfer MV, Linnoila M, Potter WZ. Interacting neurotransmitter systems. A non-experimental approach to the 5HIAA-HVA correlation in human CSF. J Psychiatr Res. 1986;20(3):175–93. doi: 10.1016/0022-3956(86)90002-6. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Anderson GM, Pelton GH, Gudin JA, Kirwin PD, Price LH, Heninger GR, McDougle CJ. Tryptophan Depletion During Continuous CSF Sampling in Healthy Human Subjects. Neuropsychopharmacology. 1998;19:26–35. doi: 10.1016/S0893-133X(97)00198-X. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action: Reversal of antidepressant induced remission by rapid depletion of plasma tryptophan. Arch. Gen. Psychiatry. 1990;47:411–418. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Price LH, Aghajanian GK, Miller HM, Salomon RM, Heninger GR, Charney DS. Serotonin and the neurobiology of depression: Effects of tryptophan depletion in drug-free depressed patients. Arch. Gen. Psychiatry. 1994;51:865–874. doi: 10.1001/archpsyc.1994.03950110025005. [DOI] [PubMed] [Google Scholar]
- Dinan TG. Serotonin and the regulation of hypothalamic-pituitary-adrenal axis function. Life Sci. 1996;58:1683–1694. doi: 10.1016/0024-3205(96)00066-5. [DOI] [PubMed] [Google Scholar]
- Fri CG, Winsel FA, Sedval G. Simultaneous quantification of homovanillic acid and 5-hydroxyindoleacetic acid in cerebrospinal fluid by mass fragmentography. Life Sci. 1974;14:2469–2480. doi: 10.1016/0024-3205(74)90143-x. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Drebing CJ, Fredman R. The simultaneous determination of free Homovanillic acid, (3-methoxy-4-hydrxyphenyl) glycol, and vanilmandelic acid in human plasma by high-performance liquid chromatography coupled with dual-electrode coulometric electrochemical detection. Anal. Chem. 1986;58(13):2879–2883. doi: 10.1021/ac00126a067. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32:51–53. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Husum H, Mathé AA. Early life stress changes concentrations of Neuropeptide Y and Corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology. 2002;27:756–764. doi: 10.1016/S0893-133X(02)00363-9. [DOI] [PubMed] [Google Scholar]
- Husum H, Van Kammen D, Termeer E, Bolwig T, Mathé AA. Topiramate normalizes hippocampal NPY-LI in Flinders Sensitive Line “Depressed “rats and upregulates NPY, galanin, and CRH in the hypothalamus. Implications for mood-stabilizing and weight –loss inducing effects. Neuropsychopharmacology. 2003;28:1292–1299. doi: 10.1038/sj.npp.1300178. [DOI] [PubMed] [Google Scholar]
- Jiménez-Vasquez PA, Mathé AA, Thomas JD, Riley EP, Ehlers CL. Early maternal separation alters neuropeptide Y concentrations in selected brain regions in adult rats. Dev. Brain Res. 2001;131:149–152. doi: 10.1016/s0165-3806(01)00264-4. [DOI] [PubMed] [Google Scholar]
- Kopin IJ, Jimerson DC, Markey SP, Ebert MH, Polinsky RJ. Disposition and metabolism of MHPG in humans: Application to studies in depression. Pharmacopsychiatry. 1984;17:3–8. doi: 10.1055/s-2007-1017399. [DOI] [PubMed] [Google Scholar]
- Krstulovic AM, Friedman MJ, Colin H, Guiochon G, Gaspar M, Pajer KA. Analytical methodology for assays of serum tryptophan metabolites in control subjects and newly abstinent alcoholics: preliminary investigation by liquid chromatography with amperometric detection. J. Chromatogr. 1984;297:271–281. doi: 10.1016/s0021-9673(01)89048-8. [DOI] [PubMed] [Google Scholar]
- Mathé AA. Lerer B, Kellner C, editors. Neuropeptides and Electroconvulsive Treatment. J. ECT. 1999;15:60–75. [PubMed] [Google Scholar]
- Mathé AA, Rudorfer MV, Stenfors C, Manji HK, Potter WC, Theodorsson E. Effects of electroconvulsive treatment on somatostatin, neuropeptide Y, endothelin and neurokinin A concentrations in cerebrospinal fluid of depressed patients. Depression. 1996;3:250. [Google Scholar]
- Mazure CM, Nelson JC, Price LH. Reliability and validity of the symptoms of major depressive illness. Arch. Gen. Psychiatry. 1986;43:451–456. doi: 10.1001/archpsyc.1986.01800050053006. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ. The role of corticotropin releasing factor in depressive illness: A critical review. Neurosci. Biobehav. Rev. 1998;22:635–651. doi: 10.1016/s0149-7634(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Moja EA, Cipollo P, Castoldi D, Tofanetti O. Dose-response decrease in plasma tryptophan and in brain tryptophan and serotonin after tryptophan-free amino acid mixtures in rats. Life Sci. 1989;44:971–976. doi: 10.1016/0024-3205(89)90497-9. [DOI] [PubMed] [Google Scholar]
- Moreno FA, Gelenberg AJ, Heninger GR, Potter RL, McKnight K, Allen J, Phillips AP, Delgado PL. Tryptophan depletion and depressive vulnerability. Biol. Psychiatry. 1999;46:498–505. doi: 10.1016/s0006-3223(99)00095-5. [DOI] [PubMed] [Google Scholar]
- Moreno FA, McGavin C, Malan TP, Jr., Gelenberg AJ, Heninger GR, Mathé AA, Delgado PL. Tryptophan depletion selectively reduces CSF 5-HT metabolites in healthy young men: results from a single lumbar puncture sampling technique. IJNP. 2000a;3:277–283. doi: 10.1017/S1461145700002133. [DOI] [PubMed] [Google Scholar]
- Moreno FA, Heninger GR, McGahuey CA, Delgado PL. Tryptophan Depletion and Risk of Depression Relapse: A prospective study of TRP depletion as a potential predictor of depressive episodes. J Biol Psychiatry. 2000b;48(4):327–329. doi: 10.1016/s0006-3223(00)00893-3. [DOI] [PubMed] [Google Scholar]
- Moreno FA, Rowe DC, Kaiser B, Chase D, Michaels T, Gelernter J, Delgado PL. Association between a Serotonin Transporter Promoter Region Polymorphism and Mood Response during Tryptophan Depletion. Molecular Psychiatry. 2002;7(2):213–216. doi: 10.1038/sj.mp.4000962. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlöv E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of corticotropin releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Yuan P, Young TA, Luckenbaugh DA, Charney DS, Manji H. Effects of tryptophan depletion on serum levels of brain derived neurotrophic factor in unmedicated patients with remitted depression and healthy subjects. Am. J. Psychiatry. 2005;162:805–807. doi: 10.1176/appi.ajp.162.4.805. [DOI] [PubMed] [Google Scholar]
- Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksec M. Differences between males and females in rates of serotonin synthesis in human brain. Proc. Natl. Acad. Sci. USA. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchot W, Herrera C, Ansseau M. HPA axis dysfunction in major depression: Relationship to 5-HT (1A) receptor activity. Neuropsychobiology. 2001;44:74–77. doi: 10.1159/000054919. [DOI] [PubMed] [Google Scholar]
- Salomon RM, Kennedy JS, Johnson BW, Schmidt DE, Kwentus J, Gwirtsman HE, Ebert MH. Association of critical CSF tryptophan threshold level with depressive relapse. Neuropsychopharmacology. 2003;28(5):956–960. doi: 10.1038/sj.npp.1300098. [DOI] [PubMed] [Google Scholar]
- Spitzer RL. Structured Clinical Interview for DSM III-R. Biometrics Research Department, New York State Psychiatric Institute; New York: 1987. [Google Scholar]
- Tyrka AR, Carpenter LL, McDougle CJ, Kirwin PD, Owens MJ, Nemeroff CB, Strong DR, Price LH. Increased cerebrospinal fluid corticotrophin releasing factor concentrations during tryptophan depletion in healthy adults. Biol. Psychiatry. 2004;7:531–534. doi: 10.1016/j.biopsych.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Williams WA, Shoaf SE, Hommer D, Rawlings R, Linnoila MI. Effects of Acute Tryptophan Depletion on Plasma and Cerobrospinal Fluid Tryptophan and 5-hydroxyindoleacetic acid in Normal Volunteers. J. Neurochem. 1999;72(4):1641–1647. doi: 10.1046/j.1471-4159.1999.721641.x. [DOI] [PubMed] [Google Scholar]
- Woods SW, Charney DS, Goodman WK, Heninger GR. Carbon dioxide induced anxiety. Arch. Gen. Psychiatry. 1988;45:43–52. doi: 10.1001/archpsyc.1988.01800250051007. [DOI] [PubMed] [Google Scholar]
- Young SN, Ervin FR, Pihl RO, Finn P. Biochemical aspects of tryptophan depletion in primates. Psychopharmacology. 1989;98(4):508–511. doi: 10.1007/BF00441950. [DOI] [PubMed] [Google Scholar]