Abstract
Despite the universality of tRNA modifications, some tRNAs lacking specific modifications are subject to degradation pathways, while other tRNAs lacking the same modifications are resistant. Here, we suggest a model in which some modifications have minor, possibly redundant, roles in specific tRNAs. This model is consistent with the low specificity of some modification enzymes. Limitations of this model include the limited assays and growth conditions on which these conclusions are based, as well as the high specificity exhibited by many modification enzymes with important roles in translation. The specificity of these enzymes is often enhanced by complex substrate recognition patterns and sub-cellular compartmentalization.
Keywords: tRNA, modifications, degradation, methylation, thiolation, editing
1. Introduction
tRNA modifications are universal. All characterized tRNA species bear numerous modifications of their bases and of their corresponding ribose moieties. Modifications are found on 11.9 % of the residues of the 561 sequenced tRNAs, with a median of 8 modifications per tRNA [1]. This data set includes tRNAs from a wide range of organisms, including archaea (59 tRNAs), eubacteria (135), fungi (65), animals (111) and plants (44), as well as from chloroplasts (35), mitochondria (95) and viruses (17). Furthermore, this data set includes tRNAs with each different amino acid acceptor and each different anticodon within each of the phylogenetic domains, and multiple tRNAs from several organisms. For example, in the yeast Saccharomyces cerevisiae, 16.4% of the residues of the 34 sequenced cytoplasmic tRNA species bear modifications, with a range from 7 to 17 modifications per tRNA, and 9.5 % of the residues of the 17 sequenced mitochondrial tRNAs bear modifications, with a range from 6 to 9 modifications per tRNA. Thus, these results imply the universal occurrence of tRNA modifications. In support of this claim, tRNA modifications are also conserved in the smallest free-living organisms [2], and in organisms living in both extremely cold and extremely hot environments, although modifications are much reduced in some organisms from cold environments [3,4].
Many modification enzymes act on multiple tRNA substrates, catalyzing the same modification at a particular position, or a defined set of positions, in different tRNA species of a single organism. This is illustrated by analysis of the modifications found in cytoplasmic tRNAs of the yeast Saccharomyces cerevisiae. In many cases one enzyme catalyzes all of the modifications of a particular type, whether the modification is only found at one position, or is found at multiple positions in the tRNA. Thus, for example, Trm11/Trm112 is responsible for each of the 20 known occurrences of m2G, which are all at position 10 [5], Trm6/Trm61 (also called Gcd10/Gcd14) is responsible for each of the 23 known occurrences of m1A, which are at position 58 [6,7], the Elp-Kti complex is responsible for each of the 11 occurrences of the cm5U moiety of mcm5U, ncm5U, ncm5Um, and mcm5s2U, which are found at position 34 [8-10] and Trm4 is responsible for all of the 30 known occurrences of m5C, which are found at positions 34, 40, 48, and 49 [11]. In other cases, a group of two or more enzymes catalyzes formation of the same modification, and each enzyme is responsible for the subset of modifications that occur at a particular position or set of positions. Thus, for example, Trm5 and Trm10 each catalyze formation of the subset of the 18 characterized m1G modifications that occur at G37 and G9 respectively [12,13], Dus1, Dus2, Dus3, and Dus4 each catalyze the subset of the 100 characterized dihydrouridine modifications that occur at positions 16 and 17, 20, 20a and 20b, and 47 respectively [14], and six pseudouridylases catalyze formation of the 102 characterized pseudouridine modifications, each acting at a subset of the 15 different positions with this modification [15-20].
Whereas many of the modifications around the anticodon have significant effects on translation or translation fidelity [10,21-24], a large body of physical evidence supports the claim that modifications are also important for the folding and stability of tRNAs. When compared to native modified tRNA, completely unmodified tRNA has a reduced Tm of ∼ 5°C, has reduced tertiary interactions at low Mg++ concentrations, and is more dynamic [25-32]. The stabilization effects of modifications are almost certainly due to body modifications (those that are in the central core of the tRNA and remote from the anticodon), since residues in the anticodon region do not interact with the main body of the tRNA.
Examination of individual modifications supports the claim that modifications have a role in stabilizing tRNA structure and/or folding. Thus T54 instead of U54 leads to a 6 °C increase in the Tm of E. coli tRNAfMet [33] and a 2 °C increase in the Tm of an otherwise unmodified T-stem-loop oligonucleotide [34], and each of T54, Ψ55 and m5C49 in an otherwise unmodified tRNAPhe 3′ half-molecule contribute significantly to the binding affinity for the unmodified 5′ half of the tRNA [35]. Furthermore, model studies demonstrate that both pseudouridine and 2′-O methylation have stabilizing effects on helices [36] [37-42], and that m1A9 promotes correct folding of human mitochondrial tRNALys [43].
However, it is not clear from these studies if individual modifications have quantitatively similar stabilizing or folding effects on all tRNAs bearing the corresponding modifications. We describe below in vivo evidence demonstrating that lack of certain specific modifications in the body of tRNA has major effects on the stability or function of only a small number of tRNA species with the corresponding modifications, and only minor effects on other tRNAs with the same modifications. We also summarize evidence for lack of specificity for at least some modification enzymes. These studies thus suggest that perhaps the modification of multiple species of tRNA by a particular enzyme occurs as a result of overlapping substrate specificity, and not necessarily because of equal evolutionary demand for these modifications in all tRNAs. Then we point out the caveats in these arguments, particularly, evidence suggesting that modifications with a role in translation generally have specific roles in each of their substrate tRNAs. We note, that in many of these cases, the modification activity itself is either highly specific or additional layers of control regulate substrate specificity.
2. Evidence suggesting that some modifications may have quantitatively minor roles in some tRNAs
2.1. Lack of m7G and m5C leads to specific degradation of mature tRNAVal(AAC)
We have shown that yeast cells lacking m7G46 and m5C due to lack of Trm8 and Trm4 are temperature sensitive due to degradation and deacylation of mature tRNAVal(AAC) [44] by a rapid tRNA decay pathway that is mediated by the 5′-3′ exonucleases Rat1 and Xrn1, and by Met22 [45]. Two lines of evidence suggest that this degradation is specific for tRNAVal(AAC), and not other species lacking m7G46 and m5C. First, the temperature sensitive phenotype of trm8-Δ trm4-Δ mutants can be restored by introduction of multicopy plasmids expressing tRNAVal(AAC) [44], strongly suggesting that this tRNA is the only species that is adversely affected in the mutant. Second, whereas the levels of tRNAVal(AAC) are reduced to less than 20% of wild type levels in trm8-Δ trm4-Δ mutants at high temperature, the levels of a number of control tRNAs remain almost constant (Fig. 1), including all three tRNA species that, like tRNAVal(AAC), have both m7G46 and m5C49 (tRNAPhe [45], tRNAiMet [44] and tRNAVal(CAC) (J. Whipple and E. M. P., unpbulished), and the three other known tRNAs with m7G46 and m5C at other positions (tRNACys I.S. Chernyakov and E. M. P., unpublished), tRNALys(UUU) [44] and tRNAMet [44]).
Fig. 1.
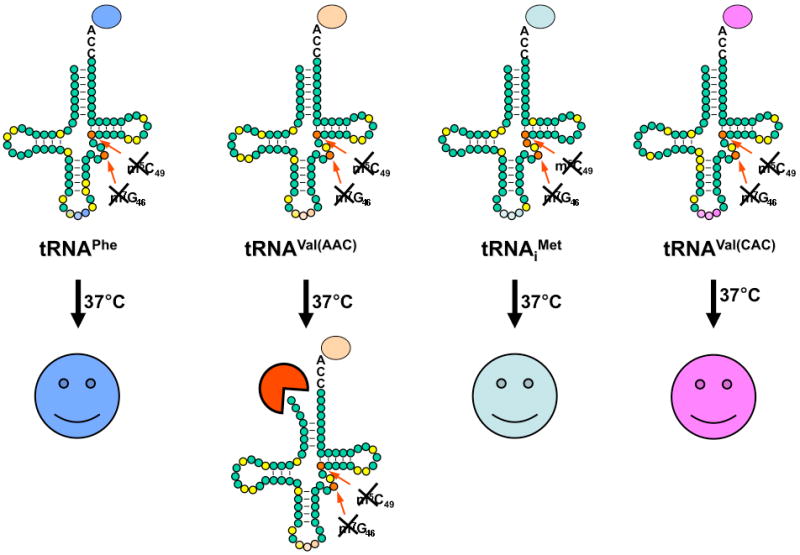
Illustration of the specificity of the rapid tRNA degradation pathway. The figure depicts four yeast tRNAs known to have m7G46 and m5C49, only one of which is a substrate for the rapid tRNA decay pathway in trm8-Δ trm4-Δ mutants, which lack these modifications. Mature tRNAVal(AAC) is rapidly degraded in trm8-Δ trm4-Δ mutants upon shift to 37 °C by the 5′-3′ exonucleases Rat1 or Xrn1, whereas tRNAPhe, tRNAVal(CAC), and tRNAiMet are resistant. Green circles represent each residue, yellow circles represent known modifications, orange circles represent sites of m7G and m5C modification, and the anticodon is colored to match its amino acid, indicated by an oval.
These results demonstrate clearly that the combined loss of m7G and m5C has only a minor effect on targeting other tRNAs for degradation by the RTD pathway, and suggest that perhaps these modifications have only minor stabilizing effects on the function of the resistant tRNAs. Presumably the modest but distinct role of m5C of tRNAPhe in stabilizing binding between the two halves of the tRNA [35] is not crucial in vivo under these conditions. Presumably also, the known tertiary interactions between m7G46 and G22 of the C13:G22 base pair of tRNAPhe are not grossly perturbed by loss of methylation at m7G, since these interactions involve donation of hydrogen bonds from the N1 and exocyclic N2 position of the G46 residue [46], which are available in the presence or absence of the methyl group.
2.2. Lack of ac4C12 and Um44 leads to specific degradation of mature tRNASer(CGA) and tRNASer(UGA)
We have also previously shown that yeast cells lacking ac4C12 and Um44 due to lack of Tan1 and Trm44 are temperature sensitive due to degradation of mature tRNASer(CGA) and tRNASer(UGA) by the RTD pathway [47]. Two lines of evidence suggest that this degradation is specific for these two tRNA species, and not the two other tRNASer species. First, overproduction of tRNASer(CGA) and tRNASer(UGA) restores healthy growth at high temperature. Second, there is no observed reduction in levels of tRNASer(IGA), the only other tRNA with both of these modifications, or of tRNASer(GCA), the only other tRNA likely to have these modifications [47]. In addition, tan1-Δ trm44-Δ strains have mildly reduced levels of tRNALeu(GAG) at high temperature, presumably due to the lack of ac4C12 since this tRNA does not have Um44; however, none of the other three tRNALeu species (which are the only other tRNA species with ac4C12) has reduced levels under these conditions.
2.3. Lack of m1A58 in tRNA appears to lead to specific degradation of pre-tRNAiMet in yeast
Anderson and co-workers have shown that pre-tRNAiMet lacking m1A58 is recognized by a nuclear surveillance system in a trm6ts mutant at non-permissive temperature, polyadenylated by Trf4 of the TRAMP complex, and degraded by Rrp6 and the nuclear exosome [6,48,49].
Three lines of evidence suggest that the turnover of pre-tRNAiMet lacking m1A58 by this nuclear surveillance system is specific for tRNAiMet rather than the other species with m1A58. First, the normally essential Trm6/Trm61 m1A58 methyltransferase can be bypassed by overproduction of initiator tRNA (tRNAiMet) from a multicopy plasmid, demonstrating unequivocally that despite the occurrence of m1A in 23 characterized yeast tRNAs, the only essential m1A modification is that found on initiator tRNA [6]. Second, degradation of pre-tRNAiMet in a trm6ts mutant is specific, since steady state levels of several other tRNAs are unaffected under these conditions, including tRNAMet, which has m1A, tRNAHis, which does not have m1A, and tRNASer(CGA) and tRNAIle(UAU), which are not characterized [6]. Second, treatment of RNA from a trm6 mutant with the TRAMP complex and Rrp44 nuclease of the exosome, results in polyadenylation and partial degradation of tRNAiMet, but not of tRNATrp, tRNAPro(UGG), and tRNATyr, which have m1A, or tRNALeu(CAA) and tRNAGly(GCC), which lack m1A [50]. Thus, these results suggest that lack of m1A primarily affects tRNAiMet.
Anderson and co-workers [49] have speculated that one plausible explanation for the specificity of the nuclear surveillance system for pre-tRNAiMet lacking m1A58 is the unique T-loop structure of tRNAiMet, which is not found in elongator tRNAs, and involves hydrogen bonds between N6 and N7 of m1A58 with A54, and O2′ of m1A58 and A60 [51]. Lack of the methyl group of m1A58 may have only minor consequences on elongator tRNA species, which have a T54:A58 or a T54:m1A58 pair instead of the A54:m1A58 pair [46,52]. Nonetheless, lack of Trm6 and m1A58 in yeast is only partially overcome by overproduction of tRNAiMet, since the cells still show a growth phenotype and are temperature sensitive [6]. This suggests either that tRNAiMet lacking m1A58 is still poorly functional, or that one or more other species of tRNA is affected by lack of the m1A modification.
All three cases examined above document clear evidence that lack of modifications leads to degradation of specific tRNA species, through the action of quality control pathways that degrade hypomodified pre-tRNA by the nuclear surveillance system, or mature tRNA by the rapid tRNA decay pathway, with no reported observable effects on the levels of other tRNAs. These results imply that despite the substantial body of evidence that modifications can stabilize tRNA in vitro, the stabilizing effect of some of the modifications may be either too minor to measure in vivo for many tRNAs under conditions that have been tested, or redundant due to other stabilizing structural features of the tRNA.
Evidence in vitro also suggests that certain tRNA modification enzymes can lack specificity. Thus, yeast Pus1 protein presumably has low specificity because it is responsible for modification of tRNA substrates at a large number of positions (U1, U26, U27, U28, U32 U34, U35, U36, U65 and U67) [20] as well as for modification of U1RNA [53]. Similarly, Trm4 is responsible for m5C modification at C34, C40, C48 and C49 in numerous tRNAs [11], as well as for modification of C50 of tRNAHis when Thg1 is depleted [54]. These examples emphasize the rather low substrate specificity of some modification enzymes.
The in vivo and in vitro results described above suggest further that there may be some modifications of tRNAs that occur as an indirect consequence of the need to modify one or a few particular tRNAs for which the modification is crucial. According to this model, the modification of other tRNA species that occurs as a consequence of shared recognition features with the crucial tRNA substrates, may confer little or no beneficial (or deleterious) function to these other accidentally modified tRNAs.
3. Nonessential body modifications may have unappreciated roles
Despite the evidence described above suggesting the presence of some possibly ancillary or redundant modifications in tRNAs, it is important to recall that only a limited number of conditions have been tested for the function of modifications in vivo. Thus, although only specific tRNAs lacking particular modifications are targeted for degradation, that is not to say that modifications of the tRNA body only serve this one stabilizing role in the body and always act to prevent degradation by these two pathways. It is well known that modifications such as dihydrouridine can add to tRNA flexibility [3,55], that several modifications can affect the specificity of aminoacyl tRNA synthetases [56-58], that modifications can affect the folding of tRNA [43], and that modifications can affect the activity and targeting of endotoxins [59]. Thus, it seems plausible that other seemingly redundant modifications may exert their effects in one of these ways or in different ways on different tRNAs. It is also certainly possible that modifications might act by affecting other functions of tRNA, such as their interaction with cellular trafficking proteins, with translation elongation factors or with other components of the ribosome, or that modifications play different or unanticipated roles in response to different growth conditions, developmental states or stresses. These roles of modifications will undoubtedly emerge as increasingly sophisticated assays are used to probe function.
4. Some modifications likely have important roles in all modified tRNAs and are added in a highly specific manner
Several examples demonstrate that certain modification enzymes are exquisitely specific. Thus, for example, tRNAHis guanyltransferase is specific for its anticodon to ensure that only tRNAHis can obtain the extra G-1 residue that is used by HisRS to direct histidylation of the tRNA [54,60-62], t6A37 formation is directed specifically by U36 and A38 in the anticodon of tRNA in oocytes [63] and, as described above, a number of other modifications have well known specificity for residues around the anticodon of substrate tRNAs, and play important roles in decoding mRNAs and maintaining the reading frame during translation. In these cases the driving force for specificity is clearly translation.
Specificity is also often driven by exceedingly subtle architectural factors. Formation of a 2′-O-methylated U34 in tRNA[Ser]Sec in Xenopus oocytes clearly illustrates this point. There are two isoacceptors for this tRNA in vertebrates: one has the modified nucleotide mcm5U at the first position of the anticodon; and the second has the nucleotide mcm5Um. Notably, replacement of U55 (where Ψ is normally found) by G55 prevents ribose methylation at U34, preventing the formation of mcm5Um without affecting the levels of mcm5U. Biologically this difference is very relevant in that 2′-O-methylation of mcm5U at position 34 is enhanced in the presence of selenium and may have a role in the formation of selenoproteins. Given the role of Ψ55 in stabilizing tRNA tertiary structure, this suggests that structural changes caused by the lack of Ψ55 indeed affect anticodon methylation, creating connectivity between the two modified sites however distant they may be.
Another example of a modification that depends on another modification occurs in mitochondrial tRNATrp of T. brucei. This tRNA undergoes mitochondrial thiolation and contains 2-thiouridine (s2U) at an unusual position, U33 of the anticodon loop (Fig. 2). This modification is commonly found at U34 (the first position of the anticodon) in tRNAGlu, tRNAGln and tRNALys in bacteria and eukarya [64,65]. tRNATrp also undergoes C to U editing at the first position of the anticodon; however, tRNATrp is not 100% edited and both UCA and CCA anticodon-containing isoacceptors co-exist in mitochondria. These two tRNAs are then presumably dedicated to the decoding of the UGA and UGG codons in mitochondria. This has raised the question about the relationship between C to U editing and the unusual thiolation at position 33 (Fig. 2). A recent report showed that if s2U levels decrease, the levels of tRNATrp that undergo C to U editing (a specialized form of postranscriptional modification) go up to nearly 100% [66]. In this latter case, thiolation serves as a negative determinant for C to U editing and helps keep the ratios of edited/unedited tRNAs in check, perhaps suggesting some biological role for both forms of the tRNA.
Fig. 2.
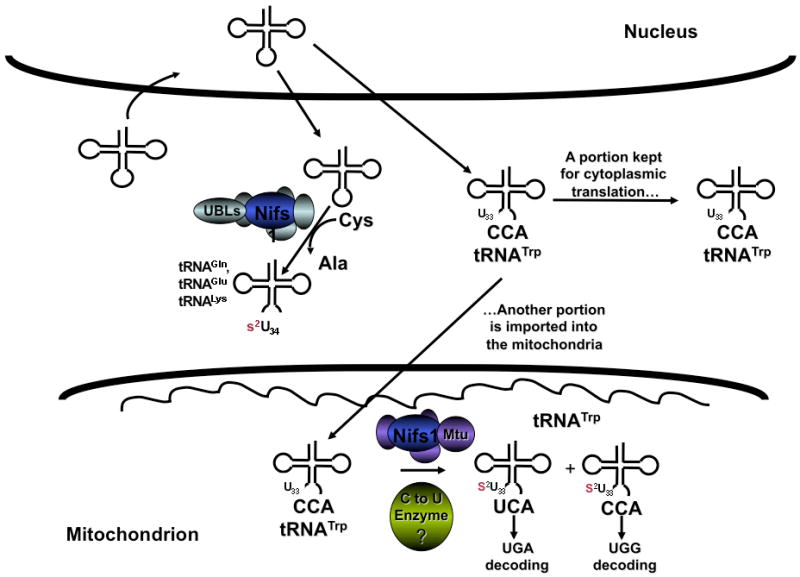
The role of intracellular localization on tRNA modification specificity. In eukaryotes there are three locations where tRNAs can be modified: the nucleus, cytoplasm and organelles (chloroplasts and mitochondria). Highlighted here are two examples where localization may impact tRNA modification. In the case of cytoplasmic thiolation, only tRNAGln, tRNAGlu and tRNALys with a U at position 34 are substrates for thiolation. In some cases like the example of tRNATrp in trypanosomes, thiolation occurs at an unusual position (U33) following import into the mitochondria. Thus tRNATrp transits through the nucleus and is only a thiolation substrate for the mitochondrial enzymes. Likewise, this tRNA only undergoes C to U editing following mitochondrial import, suggesting the requirement for mitochondria-specific modifications for editing, and highlighting the possible interrelation among different modifications in the same substrate. Nifs refers to the universally conserved desulfurase involved in tRNA thiolation in all organisms. UBLs are the ubiquitin-like factors involved in cytoplasmic thiolation. Mtu is the mitochondrial homolog of the bacterial mnmA, responsible for transferring the sulfur to tRNAs. The question mark denotes the fact that the C to U editing enzyme still remains unknown.
Yet another way that specificity is controlled in vivo is by compartmentalization. In principle, the modification content of a given tRNA can be affected either by the localization of modification enzymes in specific compartments, or by the localization of the tRNA. Some modification enzymes are imported into the nucleus following their synthesis in the cytoplasm [67], some are strictly cytoplasmic [68], and others are imported into the mitochondria [69] [70,71]. This trafficking creates a situation in which a particular enzyme, because of its intracellular localization, may never encounter a particular substrate. Although the discovery of retrograde tRNA nuclear import implies that tRNAs that escape to the cytoplasm without certain modifications can still in principle be imported back into the nucleus to obtain these modifications [72-74], it is still true that different nuclear-cytoplasmic trafficking patterns can preclude the encounter between tRNA and its substrate. In addition, tRNAs that are synthesized or imported into mitochondria are confined to this compartment, and are subject to modification only by enzymes that can be imported there.
One well-studied example of modification specificity apparently conferred by mitochondrial location is the C to U editing of the T. brucei tRNATrp species discussed above. This editing is confined to mitochondrial tRNATrp and as far as we know does not affect cytoplasmic tRNATrp or any other tRNA species in either compartment. This finding suggests strongly that editing is driven by location of the editing enzyme, as well as by tRNATrp specificity.
A second example of modification specificity apparently conferred by mitochondrial location is the formation of s2U at U33 of this same T. brucei tRNATrp. Although both cytoplasm and mitochondria share a need for the desulfurase Nifs1 (the eukaryotic homolog of bacterial iscS) [66,75-77] to initiate the sulfur transfer reaction, the cytoplasmic thiolation pathway differs from that in mitochondria (and bacteria) [10,78,79] (Fig. 2). In the cytoplasm, Nifs1 transfers the sulfur group from cysteine to a series of ubiquitin-like proteins (UBLs) [78,80], and finally to the tRNA. In mitochondria, the route to tRNA thiolation is less clear but appears to involve Nifs1 and Mtu1 (the eukaryotic homolog to mnmA) [80]. Since there are no tRNA genes in the T. brucei mitochondrial genome, tRNATrp is transcribed in the nucleus and transits through the cytoplasm where a portion of it is maintained in the cytoplasm for translation of nucleus-encoded mRNAs, and another portion is imported into the mitochondria. Surprisingly tRNATrp is not thiolated in the cytoplasm but receives the unusual U33 thiolation following mitochondrial import (Fig. 2). This result suggests strongly that specificity for s2U33 modification of tRNATrp derives from the mitochondrial location of the U33 thiolation machinery, thereby preventing modification of cytoplasmic tRNATrp, whereas the cytoplasmic thiolation system is specific for U34 modification of cytoplasmic tRNAGln, tRNAGlu and tRNALys [66,75-77].
5. Summary and Concluding Remarks
The picture that emerges from this discussion is the intriguing possibility that modifications may not necessarily have the same beneficial effect on all tRNAs. Two lines of evidence are cited above to support this claim. First, we summarized findings supporting the view that although some modifications may stabilize specific tRNA species from degradation by either the nuclear surveillance or the rapid decay pathway, several tRNAs with the same modifications are largely resistant to these pathways. Second, we summarized evidence that some modifications in the body of the tRNA, are catalyzed by enzymes with low specificity. We have also made three arguments that each modification might be important for each tRNA. First, we pointed out that only a limited number of growth conditions and assays have been tested, and suggested that new roles of modifications would be uncovered as more sophisticated assays are used to explore the effects of modifications on tRNA charging, folding, and flexibility, as well as on other aspects of translation and toxin defense. Second, we highlighted the high specificity of tRNA modification enzymes that act near the anticodon, or that otherwise affect translation by affecting charging fidelity. Third, we provided support for increased levels of specificity of particular modification enzymes acting around the anticodon, due to subtle and complex modes of substrate recognition. We also described how intracellular compartmentalization of tRNAs and modification enzymes may affect substrate availability and indirectly influence specificity.
We emphasize that it is not that surprising that modifications are not equally useful for all tRNAs. Since as argued above, the substrate specificity of at least some tRNA modification enzymes is necessarily relaxed to accommodate their disparate targets, it seems plausible that particular modifications on some tRNA species are not as important as the same modification on other tRNA species. It is also conceivable that some modifications serve no actual role on certain tRNAs. Thus, while the driving force for maintaining the modification enzymes is their important role on specific tRNAs, we suggest that the seemingly redundant modification of other tRNA species by these enzymes can occur because of overlapping substrate specificity, and the benign effects of these additional modifications on the tRNA species that receive them.
Acknowledgments
We are grateful to E. Grayhack for advice on the manuscript. Research in the authors' laboratories is supported by grants GM 52347 to E.M.P and GM084065 to J.D.A. from the National Institutes of Health and by an MCB0620707 grant from the National Science Foundation to J.D.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sprinzl M, Vassilenko KS. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 2005;33:D139–40. doi: 10.1093/nar/gki012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraser CM, et al. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 3.Dalluge JJ, Hamamoto T, Horikoshi K, Morita RY, Stetter KO, McCloskey JA. Posttranscriptional modification of tRNA in psychrophilic bacteria. J Bacteriol. 1997;179:1918–23. doi: 10.1128/jb.179.6.1918-1923.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noon KR, Guymon R, Crain PF, McCloskey JA, Thomm M, Lim J, Cavicchioli R. Influence of temperature on tRNA modification in archaea: Methanococcoides burtonii (optimum growth temperature [Topt], 23 degrees C) and Stetteria hydrogenophila (Topt, 95 degrees C) J Bacteriol. 2003;185:5483–90. doi: 10.1128/JB.185.18.5483-5490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purushothaman SK, Bujnicki JM, Grosjean H, Lapeyre B. Trm11p and Trm112p are both required for the formation of 2-methylguanosine at position 10 in yeast tRNA. Mol Cell Biol. 2005;25:4359–70. doi: 10.1128/MCB.25.11.4359-4370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson J, et al. The essential Gcd10p-Gcd14p nuclear complex is required for 1- methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 1998;12:3650–62. doi: 10.1101/gad.12.23.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson J, Phan L, Hinnebusch AG. The Gcd10p/Gcd14p complex is the essential two-subunit tRNA(1- methyladenosine) methyltransferase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2000;97:5173–8. doi: 10.1073/pnas.090102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang B, Johansson MJ, Bystrom AS. An early step in wobble uridine tRNA modification requires the Elongator complex. Rna. 2005;11:424–36. doi: 10.1261/rna.7247705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esberg A, Huang B, Johansson MJ, Bystrom AS. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol Cell. 2006;24:139–48. doi: 10.1016/j.molcel.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Johansson MJ, Esberg A, Huang B, Bjork GR, Bystrom AS. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol Cell Biol. 2008;28:3301–12. doi: 10.1128/MCB.01542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motorin Y, Grosjean H. Multisite-specific tRNA:m5C-methyltransferase (Trm4) in yeast Saccharomyces cerevisiae: identification of the gene and substrate specificity of the enzyme. Rna. 1999;5:1105–18. doi: 10.1017/s1355838299982201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjork GR, Jacobsson K, Nilsson K, Johansson MJ, Bystrom AS, Persson OP. A primordial tRNA modification required for the evolution of life? Embo J. 2001;20:231–9. doi: 10.1093/emboj/20.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackman JE, Montange RK, Malik HS, Phizicky EM. Identification of the yeast gene encoding the tRNA m1G methyltransferase responsible for modification at position 9. Rna. 2003;9:574–85. doi: 10.1261/rna.5070303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing F, Hiley SL, Hughes TR, Phizicky EM. The specificities of four yeast dihydrouridine synthases for cytoplasmic tRNAs. J Biol Chem. 2004;279:17850–60. doi: 10.1074/jbc.M401221200. [DOI] [PubMed] [Google Scholar]
- 15.Ansmant I, Motorin Y, Massenet S, Grosjean H, Branlant C. Identification and characterization of the tRNA:Psi 31-synthase (Pus6p) of Saccharomyces cerevisiae. J Biol Chem. 2001;276:34934–40. doi: 10.1074/jbc.M103131200. [DOI] [PubMed] [Google Scholar]
- 16.Becker HF, Motorin Y, Planta RJ, Grosjean H. The yeast gene YNL292w encodes a pseudouridine synthase (Pus4) catalyzing the formation of psi55 in both mitochondrial and cytoplasmic tRNAs. Nucleic Acids Res. 1997;25:4493–9. doi: 10.1093/nar/25.22.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behm-Ansmant I, Grosjean H, Massenet S, Motorin Y, Branlant C. Pseudouridylation at position 32 of mitochondrial and cytoplasmic tRNAs requires two distinct enzymes in Saccharomyces cerevisiae. J Biol Chem. 2004;279:52998–3006. doi: 10.1074/jbc.M409581200. [DOI] [PubMed] [Google Scholar]
- 18.Behm-Ansmant I, Urban A, Ma X, Yu YT, Motorin Y, Branlant C. The Saccharomyces cerevisiae U2 snRNA:pseudouridine-synthase Pus7p is a novel multisite-multisubstrate RNA:Psi-synthase also acting on tRNAs. RNA. 2003;9:1371–82. doi: 10.1261/rna.5520403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecointe F, Simos G, Sauer A, Hurt EC, Motorin Y, Grosjean H. Characterization of yeast protein Deg1 as pseudouridine synthase (Pus3) catalyzing the formation of psi 38 and psi 39 in tRNA anticodon loop. Journal of Biological Chemistry. 1998;273:1316–23. doi: 10.1074/jbc.273.3.1316. [DOI] [PubMed] [Google Scholar]
- 20.Motorin Y, Keith G, Simon C, Foiret D, Simos G, Hurt E, Grosjean H. The yeast tRNA:pseudouridine synthase Pus1p displays a multisite substrate specificity. Rna. 1998;4:856–69. doi: 10.1017/s1355838298980396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbonavicius J, Qian Q, Durand JM, Hagervall TG, Bjork GR. Improvement of reading frame maintenance is a common function for several tRNA modifications. Embo J. 2001;20:4863–73. doi: 10.1093/emboj/20.17.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy FVt, Ramakrishnan V. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat Struct Mol Biol. 2004;11:1251–2. doi: 10.1038/nsmb866. [DOI] [PubMed] [Google Scholar]
- 23.Murphy FVt, Ramakrishnan V, Malkiewicz A, Agris PF. The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat Struct Mol Biol. 2004;11:1186–91. doi: 10.1038/nsmb861. [DOI] [PubMed] [Google Scholar]
- 24.Agris PF, Vendeix FA, Graham WD. tRNA's wobble decoding of the genome: 40 years of modification. J Mol Biol. 2007;366:1–13. doi: 10.1016/j.jmb.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 25.Sampson JR, Uhlenbeck OC. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci U S A. 1988;85:1033–7. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall KB, Sampson JR, Uhlenbeck OC, Redfield AG. Structure of an unmodified tRNA molecule. Biochemistry. 1989;28:5794–801. doi: 10.1021/bi00440a014. [DOI] [PubMed] [Google Scholar]
- 27.Perret V, Garcia A, Puglisi J, Grosjean H, Ebel JP, Florentz C, Giege R. Conformation in solution of yeast tRNA(Asp) transcripts deprived of modified nucleotides. Biochimie. 1990;72:735–43. doi: 10.1016/0300-9084(90)90158-d. [DOI] [PubMed] [Google Scholar]
- 28.Derrick WB, Horowitz J. Probing structural differences between native and in vitro transcribed Escherichia coli valine transfer RNA: evidence for stable base modification-dependent conformers. Nucleic Acids Research. 1993;21:4948–53. doi: 10.1093/nar/21.21.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yue D, Kintanar A, Horowitz J. Nucleoside modifications stabilize Mg2+binding in Escherichia coli tRNA(Val): an imino proton NMR investigation. Biochemistry. 1994;33:8905–11. doi: 10.1021/bi00196a007. [DOI] [PubMed] [Google Scholar]
- 30.Maglott EJ, Deo SS, Przykorska A, Glick GD. Conformational transitions of an unmodified tRNA: implications for RNA folding. Biochemistry. 1998;37:16349–59. doi: 10.1021/bi981722u. [DOI] [PubMed] [Google Scholar]
- 31.Serebrov V, Vassilenko K, Kholod N, Gross HJ, Kisselev L. Mg2+binding and structural stability of mature and in vitro synthesized unmodified Escherichia coli tRNAPhe. Nucleic Acids Res. 1998;26:2723–8. doi: 10.1093/nar/26.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vermeulen A, McCallum SA, Pardi A. Comparison of the global structure and dynamics of native and unmodified tRNAval. Biochemistry. 2005;44:6024–33. doi: 10.1021/bi0473399. [DOI] [PubMed] [Google Scholar]
- 33.Davanloo P, Sprinzl M, Watanabe K, Albani M, Kersten H. Role of ribothymidine in the thermal stability of transfer RNA as monitored by proton magnetic resonance. Nucleic Acids Res. 1979;6:1571–81. doi: 10.1093/nar/6.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sengupta R, Vainauskas S, Yarian C, Sochacka E, Malkiewicz A, Guenther RH, Koshlap KM, Agris PF. Modified constructs of the tRNA TPsiC domain to probe substrate conformational requirements of m(1)A(58) and m(5)U(54) tRNA methyltransferases. Nucleic Acids Res. 2000;28:1374–80. doi: 10.1093/nar/28.6.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nobles KN, Yarian CS, Liu G, Guenther RH, Agris PF. Highly conserved modified nucleosides influence Mg2+-dependent tRNA folding. Nucleic Acids Res. 2002;30:4751–60. doi: 10.1093/nar/gkf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawai G, et al. Conformational rigidity of specific pyrimidine residues in tRNA arises from posttranscriptional modifications that enhance steric interaction between the base and the 2′-hydroxyl group. Biochemistry. 1992;31:1040–6. doi: 10.1021/bi00119a012. [DOI] [PubMed] [Google Scholar]
- 37.Drake AF, Mason SF, Trim AR. Optical studies of the base-stacking properties of 2′-O-methylated dinucleoside monophosphates. J Mol Biol. 1974;86:727–39. doi: 10.1016/0022-2836(74)90349-0. [DOI] [PubMed] [Google Scholar]
- 38.Zmudzka B, Bollum FJ, Shugar D. Polydeoxyribouridylic acid and its complexes with polyribo- and deoxyriboadenylic acids. J Mol Biol. 1969;46:169–83. doi: 10.1016/0022-2836(69)90064-3. [DOI] [PubMed] [Google Scholar]
- 39.Durant PC, Davis DR. Stabilization of the anticodon stem-loop of tRNALys,3 by an A+-C base-pair and by pseudouridine. J Mol Biol. 1999;285:115–31. doi: 10.1006/jmbi.1998.2297. [DOI] [PubMed] [Google Scholar]
- 40.Yarian CS, et al. Structural and functional roles of the N1- and N3-protons of psi at tRNA's position 39. Nucleic Acids Res. 1999;27:3543–9. doi: 10.1093/nar/27.17.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newby MI, Greenbaum NL. A conserved pseudouridine modification in eukaryotic U2 snRNA induces a change in branch-site architecture. Rna. 2001;7:833–45. doi: 10.1017/s1355838201002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newby MI, Greenbaum NL. Sculpting of the spliceosomal branch site recognition motif by a conserved pseudouridine. Nat Struct Biol. 2002;9:958–65. doi: 10.1038/nsb873. [DOI] [PubMed] [Google Scholar]
- 43.Helm M, Giege R, Florentz C. A Watson-Crick base-pair-disrupting methyl group (m1A9) is sufficient for cloverleaf folding of human mitochondrial tRNALys. Biochemistry. 1999;38:13338–46. doi: 10.1021/bi991061g. [DOI] [PubMed] [Google Scholar]
- 44.Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell. 2006;21:87–96. doi: 10.1016/j.molcel.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 45.Chernyakov I, Whipple JM, Kotelawala L, Grayhack EJ, Phizicky EM. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev. 2008;22:1369–80. doi: 10.1101/gad.1654308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim SH, Suddath FL, Quigley GJ, McPherson A, Sussman JL, Wang AH, Seeman NC, Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974;185:435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- 47.Kotelawala L, Grayhack EJ, Phizicky EM. Identification of yeast tRNA Um44 2′-O-methyltransferase (Trm44) and demonstration of a Trm44 role in sustaining levels of specific tRNASer species. RNA. 2008;14:158–169. doi: 10.1261/rna.811008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadaba S, Krueger A, Trice T, Krecic AM, Hinnebusch AG, Anderson J. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev. 2004;18:1227–40. doi: 10.1101/gad.1183804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kadaba S, Wang X, Anderson JT. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. Rna. 2006;12:508–21. doi: 10.1261/rna.2305406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider C, Anderson JT, Tollervey D. The exosome subunit Rrp44 plays a direct role in RNA substrate recognition. Mol Cell. 2007;27:324–31. doi: 10.1016/j.molcel.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basavappa R, Sigler PB. The 3 A crystal structure of yeast initiator tRNA: functional implications in initiator/elongator discrimination. EMBO Journal. 1991;10:3105–11. doi: 10.1002/j.1460-2075.1991.tb07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westhof E, Dumas P, Moras D, Romby P. Crystallographic refinement of yeast aspartic acid transfer RNA Yeast tRNAAsp tertiary structure in solution and areas of interaction of the tRNA with aspartyl-tRNA synthetase. A comparative study of the yeast phenylalanine system by phosphate alkylation experiments with ethylnitrosourea. Journal of Molecular Biology. 1985;184:119–45. doi: 10.1016/0022-2836(85)90294-3. [DOI] [PubMed] [Google Scholar]
- 53.Massenet S, Motorin Y, Lafontaine DL, Hurt EC, Grosjean H, Branlant C. Pseudouridine mapping in the Saccharomyces cerevisiae spliceosomal U small nuclear RNAs (snRNAs) reveals that pseudouridine synthase pus1p exhibits a dual substrate specificity for U2 snRNA and tRNA. Mol Cell Biol. 1999;19:2142–54. doi: 10.1128/mcb.19.3.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu W, Hurto RL, Hopper AK, Grayhack EJ, Phizicky EM. Depletion of Saccharomyces cerevisiae tRNA(His) guanylyltransferase Thg1p leads to uncharged tRNAHis with additional m(5)C. Mol Cell Biol. 2005;25:8191–201. doi: 10.1128/MCB.25.18.8191-8201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalluge JJ, Hashizume T, Sopchik AE, McCloskey JA, Davis DR. Conformational flexibility in RNA: the role of dihydrouridine. Nucleic Acids Res. 1996;24:1073–9. doi: 10.1093/nar/24.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Putz J, Florentz C, Benseler F, Giege R. A single methyl group prevents the mischarging of a tRNA. Nat Struct Biol. 1994;1:580–2. doi: 10.1038/nsb0994-580. [DOI] [PubMed] [Google Scholar]
- 57.Muramatsu T, Nishikawa K, Nemoto F, Kuchino Y, Nishimura S, Miyazawa T, Yokoyama S. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature. 1988;336:179–81. doi: 10.1038/336179a0. [DOI] [PubMed] [Google Scholar]
- 58.Senger B, Auxilien S, Englisch U, Cramer F, Fasiolo F. The modified wobble base inosine in yeast tRNAIle is a positive determinant for aminoacylation by isoleucyl-tRNA synthetase. Biochemistry. 1997;36:8269–75. doi: 10.1021/bi970206l. [DOI] [PubMed] [Google Scholar]
- 59.Lu J, Huang B, Esberg A, Johansson MJ, Bystrom AS. The Kluyveromyces lactis gamma-toxin targets tRNA anticodons. Rna. 2005;11:1648–54. doi: 10.1261/rna.2172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nameki N, Asahara H, Shimizu M, Okada N, Himeno H. Identity elements of Saccharomyces cerevisiae tRNA(His) Nucleic Acids Res. 1995;23:389–94. doi: 10.1093/nar/23.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rudinger J, Florentz C, Giege R. Histidylation by yeast HisRS of tRNA or tRNA-like structure relies on residues -1 and 73 but is dependent on the RNA context. Nucleic Acids Res. 1994;22:5031–7. doi: 10.1093/nar/22.23.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackman JE, Phizicky EM. tRNAHis guanylyltransferase adds G-1 to the 5′ end of tRNAHis by recognition of the anticodon, one of several features unexpectedly shared with tRNA synthetases. Rna. 2006;12:1007–14. doi: 10.1261/rna.54706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morin A, Auxilien S, Senger B, Tewari R, Grosjean H. Structural requirements for enzymatic formation of threonylcarbamoyladenosine (t6A) in tRNA: an in vivo study with Xenopus laevis oocytes. Rna. 1998;4:24–37. [PMC free article] [PubMed] [Google Scholar]
- 64.Agris PF, Soll D, Seno T. Biological function of 2-thiouridine in Escherichia coli glutamic acid transfer ribonucleic acid. Biochemistry. 1973;12:4331–7. doi: 10.1021/bi00746a005. [DOI] [PubMed] [Google Scholar]
- 65.Seno T, Agris PF, Soll D. Involvement of the anticodon region of Escherichia coli tRNAGln and tRNAGlu in the specific interaction with cognate aminoacyl-tRNA synthetase. Alteration of the 2-thiouridine derivatives located in the anticodon of the tRNAs by BrCN or sulfur deprivation. Biochim Biophys Acta. 1974;349:328–38. doi: 10.1016/0005-2787(74)90120-8. [DOI] [PubMed] [Google Scholar]
- 66.Wohlgamuth-Benedum JM, Rubio MA, Paris Z, Long S, Poliak P, Lukes J, Alfonzo JD. Thiolation controls cytoplasmic tRNA stability and acts as a negative determinant for tRNA editing in mitochondria. J Biol Chem. 2009;284:23947–53. doi: 10.1074/jbc.M109.029421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grosshans H, Lecointe F, Grosjean H, Hurt E, Simos G. Pus1p-dependent tRNA pseudouridinylation becomes essential when tRNA biogenesis is compromised in yeast. J Biol Chem. 2001;276:46333–9. doi: 10.1074/jbc.M107141200. [DOI] [PubMed] [Google Scholar]
- 68.Gaston KW, Rubio MA, Spears JL, Pastar I, Papavasiliou FN, Alfonzo JD. C to U editing at position 32 of the anticodon loop precedes tRNA 5′ leader removal in trypanosomatids. Nucleic Acids Res. 2007;35:6740–9. doi: 10.1093/nar/gkm745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li JM, Hopper AK, Martin NC. N2,N2-dimethylguanosine-specific tRNA methyltransferase contains both nuclear and mitochondrial targeting signals in Saccharomyces cerevisiae. Journal of Cell Biology. 1989;109:1411–9. doi: 10.1083/jcb.109.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin NC, Hopper AK. How single genes provide tRNA processing enzymes to mitochondria, nuclei and the cytosol. Biochimie. 1994;76:1161–7. doi: 10.1016/0300-9084(94)90045-0. [Review] [28 refs] [DOI] [PubMed] [Google Scholar]
- 71.Slusher LB, Gillman EC, Martin NC, Hopper AK. mRna leader length and initiation codon context determine alternative Aug selection for the yeast gene Mod5. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:9789–93. doi: 10.1073/pnas.88.21.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hopper AK, Shaheen HH. A decade of surprises for tRNA nuclear-cytoplasmic dynamics. Trends Cell Biol. 2008;18:98–104. doi: 10.1016/j.tcb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Shaheen HH, Horetsky RL, Kimball SR, Murthi A, Jefferson LS, Hopper AK. Retrograde nuclear accumulation of cytoplasmic tRNA in rat hepatoma cells in response to amino acid deprivation. Proc Natl Acad Sci U S A. 2007;104:8845–50. doi: 10.1073/pnas.0700765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takano A, Endo T, Yoshihisa T. tRNA Actively Shuttles Between the Nucleus and Cytosol in Yeast. Science. 2005;309:140–142. doi: 10.1126/science.1113346. [DOI] [PubMed] [Google Scholar]
- 75.Kamenski P, Kolesnikova O, Jubenot V, Entelis N, Krasheninnikov IA, Martin RP, Tarassov I. Evidence for an adaptation mechanism of mitochondrial translation via tRNA import from the cytosol. Mol Cell. 2007;26:625–37. doi: 10.1016/j.molcel.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 76.Nakai Y, Umeda N, Suzuki T, Nakai M, Hayashi H, Watanabe K, Kagamiyama H. Yeast Nfs1p is involved in thio-modification of both mitochondrial and cytoplasmic tRNAs. J Biol Chem. 2004;279:12363–8. doi: 10.1074/jbc.M312448200. [DOI] [PubMed] [Google Scholar]
- 77.Umeda N, Suzuki T, Yukawa M, Ohya Y, Shindo H, Watanabe K, Suzuki T. Mitochondria-specific RNA-modifying enzymes responsible for the biosynthesis of the wobble base in mitochondrial tRNAs. Implications for the molecular pathogenesis of human mitochondrial diseases. J Biol Chem. 2005;280:1613–24. doi: 10.1074/jbc.M409306200. [DOI] [PubMed] [Google Scholar]
- 78.Leidel S, et al. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature. 2009;458:228–32. doi: 10.1038/nature07643. [DOI] [PubMed] [Google Scholar]
- 79.Schlieker CD, Van der Veen AG, Damon JR, Spooner E, Ploegh HL. A functional proteomics approach links the ubiquitin-related modifier Urm1 to a tRNA modification pathway. Proc Natl Acad Sci U S A. 2008;105:18255–60. doi: 10.1073/pnas.0808756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Noma A, Sakaguchi Y, Suzuki T. Mechanistic characterization of the sulfur-relay system for eukaryotic 2-thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Res. 2009;37:1335–52. doi: 10.1093/nar/gkn1023. [DOI] [PMC free article] [PubMed] [Google Scholar]


