Abstract
Movement disturbances are often overlooked consequences of chronic cocaine abuse. The purpose of this study was to systematically investigate sensorimotor performance in chronic cocaine users and characterize changes in brain activity among movement-related regions of interest (ROIs) in these users. Functional magnetic resonance imaging data were collected from fourteen chronic cocaine users and fifteen age and gender matched controls. All participants performed a sequential finger-tapping task with their dominant, right hand interleaved with blocks of rest. For each participant, percent signal change from rest was calculated for seven movement related ROIs in both the left and right hemisphere. Cocaine users had significantly longer reaction times and higher error rates than controls. Whereas the controls used a left-sided network of motor-related brain areas to perform the task, cocaine users activated a less lateralized pattern of brain activity. Users had significantly more activity in the ipsilateral (right) motor and premotor cortical areas, anterior cingulate cortex and the putamen than controls. These data demonstrate that, in addition to the cognitive and affective consequences of chronic cocaine abuse, there are also pronounced alterations in sensorimotor control in these individuals, which are associated with functional alterations throughout movement-related neural networks.
Keywords: motor control, substance abuse, neuroimaging, laterality, neural networks
1. Introduction
Disruptions of cognitive and affective processing associated with chronic cocaine exposure have been widely characterized in both human addicts and animal models. Behavioral consequences of cocaine use have been primarily attributed to alterations in the mesocorticolimbic dopamine system innervating ventral striatum and medial and orbitalfrontal cortex. Recent evidence, however, suggests that chronic cocaine use may lead to lasting effects in the nigrostriatal dopamine system as well, particularly the dorsal striatum (Letchworth et al., 2001; Nader et al., 2002; (Porrino et al., 2004; Volkow et al., 2006; Volkow et al., 2008; Wong et al., 2006). Despite the known involvement of the nigrostriatal dopamine system in motor control (Cenci, 2007; Goldstein et al., 1976), the potential influence of cocaine use on the motor system has rarely been considered.
There have been a number of clinical case studies that have reported extrapyramidal symptoms, including tics, dystonia, and dyskinesias in patients with a history of chronic stimulant abuse including amphetamine and cocaine (Bartzokis et al., 1999; Bauer, 1996b; Chouinard and Ford, 2000; Daniels et al., 1996; Pascual-Leone and Dhuna, 1990; Tanvetyanon et al., 2001). While abnormal motor control is typically reported within a week of the last use of cocaine (Rylander 1972, Pascual-Leone & Dhuna 1990, Daras et al 1994), in some individuals movement disorders were still present even after extended abstinence (Weiner et al., 2001). While many of the cocaine users in these studies also abused other drugs such as alcohol, Bauer et al (1996) found a greater incidence of tremor in cocaine-dependent patients than alcohol-dependent or polydrug abusers. Furthermore, the severity or the tremor was positively correlated with the number of self-reported uses of cocaine and negatively correlated with duration of abstinence (Bauer, 1996a). This persistence of the movement deficits suggests that prolonged cocaine exposure may have lasting effects on sensorimotor control systems. There have been other brief reports documenting dyskinesias in stimulant users that persist for one to 15 years after the patient’s last reported drug use (Lundh and Tunving, 1981; Pascual-Leone and Dhuna, 1990; Rylander, 1972; Thiel and Dressler, 1994). Given, the limited sample and retrospective nature of most of these studies, however, there is little consensus regarding the nature and severity of motor sequelae that occur in chronic cocaine users.
While sensorimotor control is modulated by dopamine in the nigrostriatal pathway, successful motor control relies on a distributed network of cortical and subcortical structures (Graybiel, 1991, 2004). Visuomotor integration tasks are highly lateralized and rely on activity in the contralateral primary motor cortex, dorsolateral prefrontal cortex, the supplementary motor area of the premotor cortex (Brodman Area 6), the anterior cingulate cortex, the caudate, thalamus, and the ipsilateral cerebellum (Foulkes and Miall, 2000; Inoue et al., 1998; Kawashima et al., 2000; Miall et al., 2000). These regions work together in temporally coordinated patterns to orchestrate smooth, purposeful movements (Pollok et al., 2005). Sequential finger-tapping tasks reveal sensorimotor deficits in several clinical populations with cortical-striatal pathology including, but not limited to, Parkinson’s disease (Mallol et al., 2007; Wu and Hallett, 2005), multiple sclerosis (Chipchase et al., 2003; Pelletier et al., 1993), Huntington’s chorea (Gavazzi et al., 2007), and Tourette’s syndrome (Biswal et al., 1998).
Functional activity in many of these same regions is also dysregulated in chronic cocaine users (Volkow et al., 1991; Kaufman et al., 2003). Active cocaine users have higher glucose metabolism in the basal ganglia and orbitofrontal cortex than matched controls (Volkow et al 1991). Furthermore, impaired response inhibition by cocaine users is associated with lower activity in the right supplementary motor area, and anterior cingulate cortex (Hester and Garavan, 2004). This study also noted elevated activity in the cerebellum, which may indicate functional compensation- a pattern that has also been observed in alcoholics (Desmond et al., 2003). Given that the primary motor cortex, supplementary motor area, anterior cingulate, caudate nucleus, putamen and cerebellum all contribute to ongoing motor control (Pollok et al., 2005; van Donkelaar et al., 2000), we hypothesized that disrupted activity in these regions would result in movement deficits in cocaine users.
The intent of this investigation was to determine if chronic, actively-using cocaine users have impaired motor control and/or altered brain function in movement-related regions of interest (ROIs). We aimed to test the hypotheses that a simple finger-sequencing task would highlight significant 1) behavioral and 2) neurofunctional impairments in movement related regions of interest in chronic, actively-using cocaine users relative to non-drug using controls. We further hypothesized that cocaine users would have disrupted functional lateralization during unimanual sequencing similar to that exhibited in other clinical populations. To achieve this, we used functional magnetic resonance imaging (fMRI) to assess blood oxygen level dependent (BOLD) activity throughout movement-related neural regions of interest as cocaine users and controls performed a basic finger-sequencing task.
2. Methods
2.1 Participants
Fifteen cocaine users and 14 control participants were recruited via local advertisements and word of mouth. Cocaine users were currently using cocaine on a weekly basis, not actively enrolled in a treatment program, and did not participate with the intent to be treated for cocaine dependence. All participants provided written informed consent to participate according to the procedures approved by the Wake Forest University School of Medicine Institutional Review Board. Participants were initially screened by telephone for eligibility. All participants had a psychiatric examination (Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (Ventura et al., 1998), completed a basic medical history inventory, completed a drug use survey and were administered an IQ test (Wechsler Abbreviated Scale of Intelligence; Wechsler 1999) at the time of screening. The data from the medical and psychiatric evaluations were used to establish eligibility for participation.
All cocaine users met DSM-IV criteria for cocaine dependence and had used cocaine at least three times per week for a minimum of five years. At the time of the study all participants had positive urine drug screens for cocaine. Exclusionary criteria included a history of Axis-I psychiatric disorders, dependence on substances other than nicotine (and cocaine in the user group), head trauma, neurological disorders, systemic diseases that might affect the central nervous system, an intelligence quotient of less than 70, pharmaceutical treatment for any neuropsychiatric diagnosis for more than six months, and any psychotropic prescription medication use six months prior to testing. Control participants did not have a history of substance dependence other than nicotine and were chosen to match the cocaine user population on the basis of gender, race, right handedness (as determined through the Edinburgh Handedness Inventory) and education (Table 1).
Table 1.
Demographic and drug use history for participants
| Controls | Users | |
|---|---|---|
| Age | 31.1 ± 5.1 | 39.8 ± 3.7 |
| Females (percent) | 43 | 40 |
| Alcohol (AUDIT score) | 3.5 ± 3.9 | 8.1 ± 5.4 |
| Smokers (percent) | 29 | 93* |
| Smoking (pack years) | 187.5 ± 83.8 | 214.0 ± 150.0 |
| Depression (BDI) | 2.0 ± 2.9 | 22.3 ± 12.2* |
| Anxiety (Speilberger) | 25.3 ± 8.4 | 35.8 ± 8.5 |
| IQ | 100.7 ± 8.2 | 87.1 ± 10.5* |
| Age first cocaine use | - | 23.8 ± 7.9 |
| Total years of cocaine use | - | 16.3 ± 7.6 |
| Years at current level | - | 10.7 ± 7.7 |
Value are expressed as group means ± standard deviation
Significant differences between groups are indicated (p<0.05)
2.2 Procedure
On the scanning day, all participants arrived at the imaging center in the morning, approximately three hours prior to acquisition of their functional MRI scan. Urine samples were collected from all participants to test for pregnancy and drug use. Cocaine or its metabolites were found in all participants in the cocaine user group, indicating that they had used cocaine within the previous 72 hours. No user displayed any overt behavioral signs of cocaine intoxication or craving. During this prescan period the participants signed consent forms and completed a series of questionnaires including the Beck’s Depression Inventory and the Speilberger Test of Anxiety. Prior to task training, participants were given the opportunity for a smoke break to prevent the confounds of nicotine withdrawal.
2.3 Finger-Sequencing Task
We used a modified activation paradigm based on one that has been used previously to study patients with movement disorders (Sabatini et al., 2000) (Figure 1). For this basic visuo-motor integration task participants were required to watch a movie of right hand finger tapping movements on a monitor and mimic the ongoing actions with their own fingers. Their responses to the ongoing visual cues were recorded with an MR compatible response box placed under their right hand. Based on extensive pilot testing, designed to minimize errors of omission in cocaine users while maintaining attention in healthy controls, a 1 Hz tapping frequency (one finger movement per second) was used for this task. All participants were initially familiarized with the visuo-motor finger-sequencing task outside of the scanning environment. A maximum of three, one minute practice blocks were presented. All participants had to achieve 90% accuracy (as determined by number of button presses) during the practice session to continue with the study. After this accuracy rate was achieved, all participants performed another one minute practice block to minimize differences in time spent at maximal performance between the groups. During the practice session, all fourteen control participants reached criterion performance during the first minute block. Seven of the fifteen cocaine users reached criterion in the first minute block, whereas five required two practice blocks, and three users required three blocks.
Figure 1. Finger-tapping task.
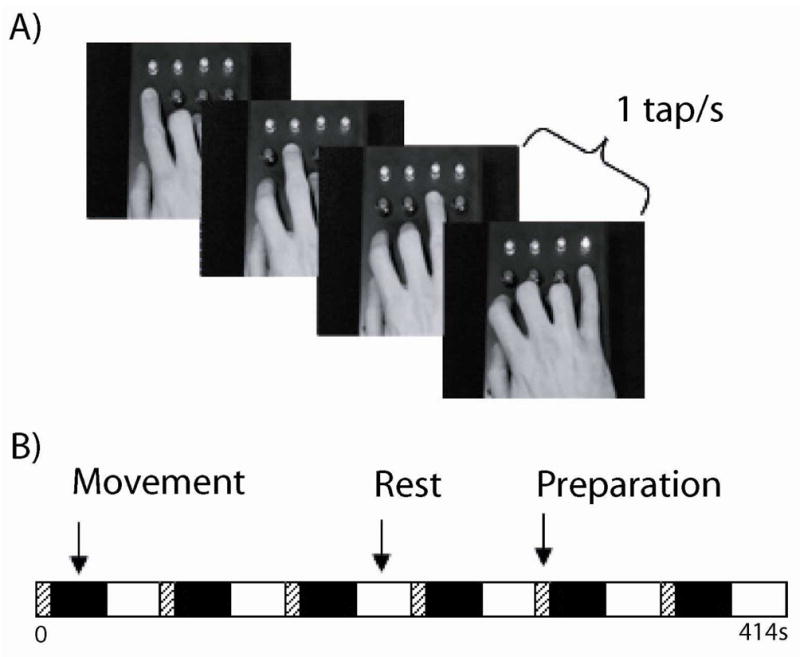
In the functional MRI scanner all participants were instructed to mimic movies of unpredictable finger-tapping sequences with their dominant right hand (A). These 30s task blocks were interleaved with 30s rest blocks and 9s motor preparation periods for each functional run (B). All participants performed two functional runs of the task.
In the MRI scanner the participants were able to see the movie of sequential finger-tapping movements via MR compatible goggles. Corrective lenses were inserted into the goggles if necessary. A brief test of visual acuity was performed via the response box to ensure that the participants could see the screen and were able to hit the appropriate keys on the response box. Each participant performed two runs of the task in the scanner. During the task participants were required to mimic the ongoing finger-tapping sequences with their dominant, right hand in 30s blocks interspersed with 30s rest periods. Following each rest block there was also a nine second preparation block in which the participant received a preparation cue which counted down the number of seconds remaining before the motor task began again. This preparation block was modeled separately in the statistical analysis and was used to eliminate the effects of attentional set-shifting to the visuo-motor performance data acquired in the task block.
2.4 Functional MRI acquisition
Images were acquired on a 1.5T General Electric scanner with a birdcage-type standard quadrature head coil and an advanced nuclear magnetic resonance echoplanar system. The head was positioned along the canthomeatal line. Foam padding was used to limit head motion. High-resolution T1-weighted anatomical images (3D SPGR, TR=14 ms, TE=7700 ms, voxel dimensions 1.0×1.0×1.0 mm, 176×256 voxels, 160 slices) were acquired for co-registration and normalization of functional images. A total of 144 coplanar functional images were acquired using a gradient echoplanar sequence (TR=3000 ms, TE=40 ms, voxel dimensions 3.0×3.0×3.0 mm, imaging matrix 64×64 voxels). The first two radio frequency excitations were performed prior to image acquisition to achieve steady-state transverse relaxation. The scanning planes were oriented parallel to the anterior commissure–posterior commissure line and extended from the superior extent of motor cortex to the base of the cerebellum. The first six of the 144 volumes of data were aquired before the task began to allow time for the signal to reach an equilibrium state before any stimulation onset.
2.5 Functional MRI preprocessing
All analyses were performed using custom scripts written in MATLAB 7.0 (Mathworks, Natick, MA). Spatial preprocessing was performed with standard parametric mapping techniques (SPM5, London, UK (Ashburner and Friston, 2005)) and region of interest timecourse extraction was performed using MarsBaR 0.41 (Brett et al., 2002). The data were corrected for acquisition time (slice timing), realigned to the first volume (motion correction), normalized into a standardized neuroanatomical space (Montreal Neurological Institute (MNI) brain template), and smoothed using a Gaussian kernel of 8 mm for the group analysis to reduce the variance due to anatomical variability. Analyses of time data series were performed individually using a boxcar model convolved with the canonical hemodynamic response function. Statistical contrast maps were made for each individual comparing brain activity associated with performing the task to that during periods of rest. These data were modeled across all participants in order to obtain a voxel-based representation of brain areas being used to perform the task for each group.
A region of interest analysis approach was used to probe alterations in the amplitude of activity within a network of regions known to be modulated by finger-sequencing tasks. Fourteen anatomically defined ROIs, representing both the left and right hemispheres of seven cortical and subcortical regions within the motor network were extracted from an MNI based atlas (WFU Pick Atlas, Wake Forest University) (Maldjian et al., 2003). Regions chosen for analysis included primary motor cortex (Motor), supplementary motor area (SMA), anterior cingulate cortex (ACC), caudate (Cau), putamen (Put), thalamus (Thal) and the cerebellum (Cereb).
2.6 Statistical Analysis
Motor performance measures (reaction time & error rate) were analyzed between groups by means of t-tests for independent samples with post-hoc Bonferroni correction for multiple comparisons. Errors were divided into two categories: commission errors (pressing the button for the wrong finger) and omission errors (not pressing a button in the allotted period of time).
Two primary measures of functional activity were calculated for each region of interest in this study: percent signal change from rest and a laterality index. For each of these dependent measures a multivariate analysis of variance determined whether there was an overall group difference (a main effect of group) and whether any group differences were independent of the regions of interest within the motor network (a group × region interaction) (p<0.05). Furthermore, multiple post-hoc independent sample t-tests were used to determine which regions of interest were significantly different (p<0.05, corrected).
Percent signal change from rest
The magnitude of motor related activity was measured by calculating the percent signal change from rest for each ROI. A vector of the average BOLD signal within each ROI was extracted for the full timecourse of the task. Percent signal change from rest was calculated for each individual by comparing the BOLD signal during movement blocks to rest blocks.
Laterality
For each pair of left and right ROIs a Laterality Index (Cramer et al., 1999) was calculated for each subject (100 * (L − R)/(L + R)), where L and R represent the mean percent signal change from rest in the left and right hemisphere respectively. A positive LI indicates lateralization to the left hemisphere, whereas a negative LI indicates right hemisphere lateralization. Mean LIs were calculated for each structure and compared across groups using multivariate analyses of variance (group × hemisphere × structure).
3. Results
Active cocaine users reported a mean (± SD) duration of 10.7 (±7.7) years of continuous use at their current pattern (days per month and amount used per occasion). The mean total years of cocaine use was 16.3 (±7.6) and ranged from 11–25 years. All participants reported smoking as their primary route of administration. Mean (± SD) age of first use was 23.8 (± 7.9) years of age (range 14–39). Fourteen of the participants were current tobacco smokers (1 pack/day or less) and all reported alcohol use in the past month. None were dependent on alcohol as assessed by psychiatric evaluation as well as the Alcohol Use Disorders Identification Test (AUDIT). Although no participants met dependence criteria for any illegal drugs other than cocaine, all participants reported some lifetime use of other illegal substances including marijuana (15 of 15), methamphetamine (2 of 15), LSD (4 of 15), heroin (2 of 15) and ecstasy (2 of 15).
All control participants had no history of use of illegal substances (other than marijuana, four of 14 participants with less than 50 total lifetime uses more than two years previously). Four were current tobacco smokers and all reported alcohol use in the past month. There were significant differences in IQ (t= 2.31, p < .05), rates of cigarette smoking (U′=173.00, p<0.01), and depression scores (Beck’s Depression Inventory scores Controls = 2.0± 2.9, Users = 22.3±12.2; t = 6.061, p<0.001). The extent to which these variables contributed to the effects of interest were determined through post-hoc analyses of covariance as described below. There was no significant difference in AUDIT score (Controls = 3.5±3.9, Users = 8.1±5.4) or Speilberger’s Anxiety Score (Controls = 25.3±8.4, Users = 35.8±8.5).
3.1 Motor performance
Multiple analyses of variance with reaction time and error rates modeled as dependent variables, revealed a significant overall difference in motor performance between the groups. This difference in overall performance remained after co-varying for IQ, BDI score and cigarette smoking (F(2,87)=3.59, p<0.05). Response latency (the time between seeing the finger movement and performing an action) was significantly longer in active cocaine users (1141.12 ± 30.96 ms) than controls (518 ± 7.51 ms) (t(27)= 73.24, p<0.0001) (Figure 2A). The within-group variance was small and the range of values among cocaine users (974.1–1432.1) did not overlap with the range of values for the controls (487.8–567.8). Relative to controls, active cocaine users made significantly more errors of commission (9.64 ± 3.16 range 1–47, vs. 0.27 ± 0.13, range 0–2; t = 3.23, p<0.01), and errors of omission (8.08 ± 2.84, range 0–28, vs. 1.27 ± 0.89, range 0–12; t = 2.42, p<0.05) (Figure 2B). There no significant difference in response latency or the number of errors made in the first task block compared to the last task block.
Figure 2. Task Performance.
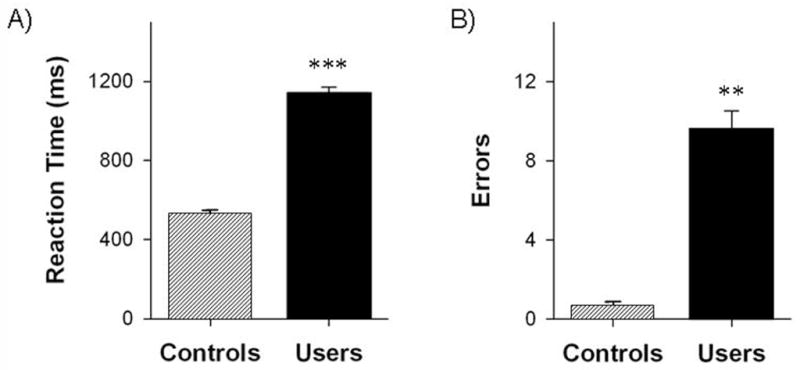
Mean (with standard error) reaction time (a) and number of errors (b) on the finger-sequencing task for cocaine users (solid) and matched control participants (lined). * p<0.05, ** p<0.01, ***p<0.00001
3.2 Task-related brain activity
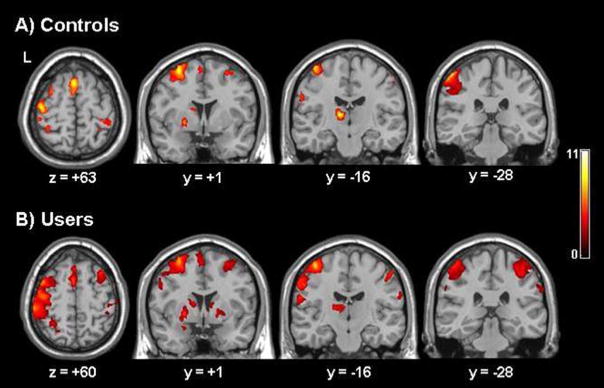
Voxel-based analyses revealed that controls had significant clusters of activity in movement-related brain regions throughout the left hemisphere during periods of finger-sequencing relative to the rest (Figure 3). Within the left hemisphere, there were significant clusters of BOLD signal increases for the control group in the left thalamus, precentral gyrus (Brodmann Area (BA) 4), postcentral gyrus (BA 1,2,3,5), medial premotor cortex (BA 6), visual association areas of the middle temporal lobe (BA 21, 37), superior frontal gyrus (BA 9), caudate, putamen, and cerebellum (voxel threshold: p<0.005, uncorrected; cluster threshold p<0.05, corrected). There was also a significant increase in BOLD signal in the right cerebellum, middle temporal lobe (BA 21, 37), thalamus, middle frontal gyrus (BA 10,46), superior parietal lobe (BA 7), and postcentral gyrus (BA 1,2,3). A region of interest analysis was employed to further characterize these data.
Figure 3. Distribution of brain activity during right-hand finger tapping.
Voxel-based maps of significant brain activity for controls (A) and chronic cocaine users (B). To extend the data analysis beyond motor-related regions of interest, contrast maps were made for each individual comparing brain activity associated with performing the task to periods of rest. These data were modeled across all subjects in order to get a voxel-based representation of brain areas being used to perform the task for each group. To determine the areas significantly activated during the task relative to rest for each subject a minimum cluster size was set at 25 voxels with a threshold of statistical significance was set at p < 0.005 for uncorrected values.
Controls
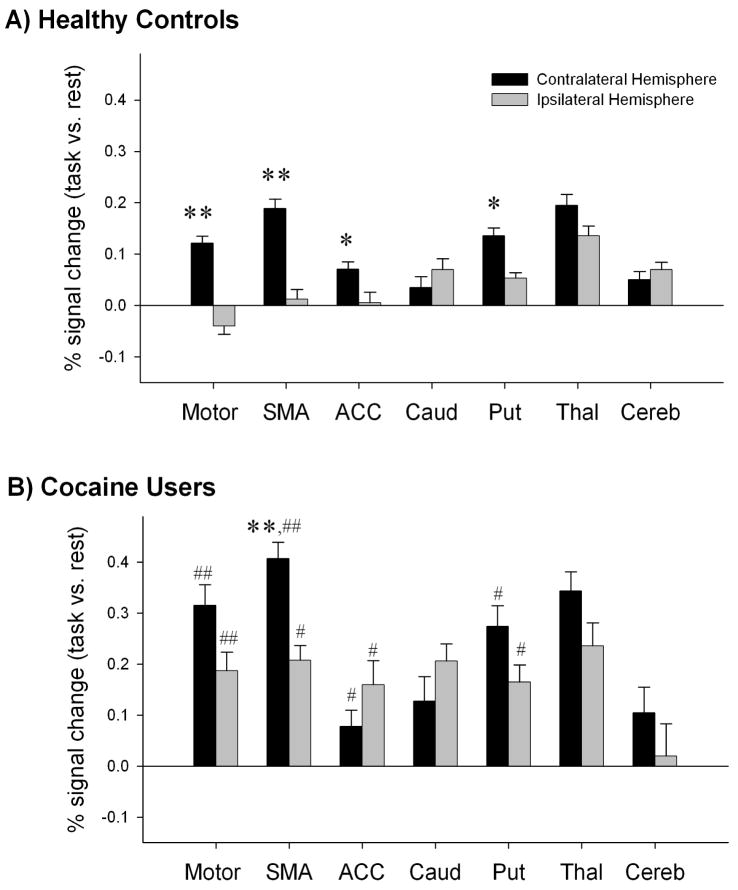
A three-way analysis of variance with repeated measures (signal magnitude: rest vs. task × ROI × hemisphere) revealed a significant interaction among all three factors (F(13,210)= 2.87, p <0.01). Planned comparisons of signal magnitude revealed significant activation in left primary motor cortex (t(13)=5.823, p<0.0001), left supplementary motor area (t(13)=13.54, p< 0.0001), left putamen (t(13)= 8.48, p<0.0001) and left (t(13)= 12.05, p< 0.0001) and right (t(13)=8.38, p< 0.0001) thalamus of controls during the motor task relative to rest. Consistent with the voxel-based analysis, finger- tapping elicited activation in a largely contralateral network of cortico-striatal-thalamic brain regions. Within controls there was significantly more activity in the contalateral (left) primary motor cortex (t(13)= 6.05, p< 0.0001), supplementary motor area (t(13)= 5.55, p< 0.0001), anterior cingulate cortex (t(13)= 3.335, p< 0.01), and putamen (t(13)= 4.90, p< 0.0001) than the ipsilateral analogues of the same regions (Figure 4A).
Figure 4. Activity in motor related regions during right-hand finger tapping.
Percent signal change in the contralateral hemisphere (black) and the ipsilateral hemisphere (gray) of controls (a) and cocaine users (b). Significance is indicated for brain regions that differ significantly between hemispheres within each group (* p<0.01, ** p<0.001) and within hemisphere between groups (# p<0.01, ## p<0.001).
Cocaine users: a significant three-way interaction among factors (F(14,196)=4.93, p <0.01) was also found in cocaine users. Planned comparisons of signal magnitude revealed significant activation in both left and right primary motor cortex (t(13)=13.73, p<0.0001; t(13)=8.27, p<0.0001), left and right supplementary motor area (t(13)= 33.81, p< 0.0001; t(13)=17.02, p<0.0001), right caudate (t(13)=10.10, p<0.0001), left and right putamen (t(13)=16.77, p<0.0001; t(13)=10.99, p<0.0001), and left and right thalamus (t(13)= 34.19, p<0.0001; t(13)= 23.18, p<0.0001). Like controls, cocaine users activated cortico-striatal-thalamic regions for this task. In contrast to controls, however, there was largely bilateral activation across this network in cocaine users. Within users the contralateral supplementary motor area was the only region significantly more active than its ipsilateral analogue (t(13)= 7.3, p< 0.0001) (Figure 4B).
Between-group comparisons revealed that cocaine users had significantly higher percent signal changes than controls in the left and right primary motor cortex (M1) (t(27)= 3.93, p < 0.0001; t(27) = 4.58, p<0.0001), left and right supplementary motor area (t(27)= 4.35, p<0.0001; t(27)= 3.58, p< 0.005), left and right anterior cingulate (t(27)= 3.12, p< 0.01 ; t(27)= 2.66, p < 0.05), and left and right putamen (t(27)= 3.03, p <0.01; t(27)= 2.45, p<0.05). The group differences in percent signal change from rest in the 14 ROIs remained significant after covarying for IQ, BDI score, and smoking rates (F(13,376)=2.43, p<0.05).
3.4 Laterality of brain activation
To further address the issue of laterality, a laterality index (LI) of each ROI for cocaine users and controls was calculated in accordance with previous methods (Cramer et al., 1999). As anticipated based on the voxel-based data, controls had significantly higher BOLD signal in the left primary motor cortex, supplementary motor area, anterior cingulate cortex, putamen, and thalamus relative to the right hemisphere. In contrast, the caudate and the cerebellum are lateralized toward the right hemisphere (Figure 5). In users however, LIs were generally lower than those of controls. Analysis of variance across regions of interest revealed a significant difference in laterality indices between the groups (F(6,22)=29.32, p <0.0001). This difference remained significant after covarying for IQ, BDI score, and smoking rates (F(6,22)=22.78, p <0.0001). Specifically, the control group was significantly more lateralized to the left (as indicated by a positive LI) than users in the primary motor cortex (t(27) = 3.059, p= 0.005) and the anterior cingulate cortex (t(27)= 2.865, p= 0.008).
Figure 5. Degree of functional laterality for motor-related regions of interest.
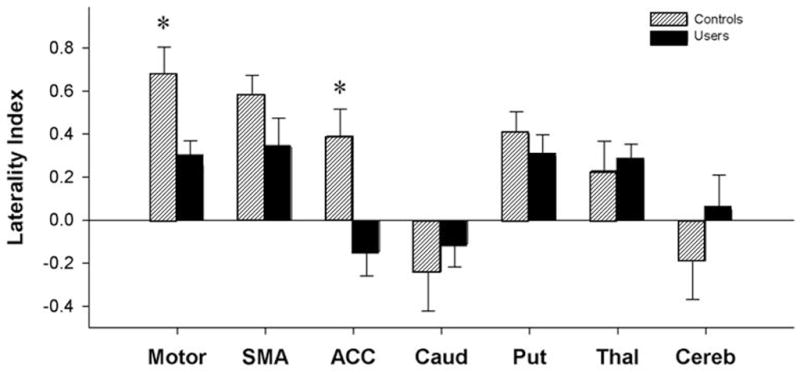
The laterality index (LI) for each region of interest is plotted for both controls (lined) and cocaine users (solid). A positive LI means that activity is lateralized to the left hemisphere, while a negative LI indicates right sided laterality. Brain regions with significant between group differences are indicated with asterisks (* p<0.05).
4. Discussion
The current study demonstrates that cocaine users have significant sensorimotor impairments accompanied by abnormal functional brain activity in cortical and subcortical areas that subserve motor control. Specifically, cocaine users have a less lateralized pattern of brain activity in motor-related brain regions than matched controls during a simple movement task. These data extend findings from prior reports which have documented motor abnormalities in stimulant users (Lundh and Tunving, 1981; Pascual-Leone and Dhuna, 1990; Rylander, 1972). Furthermore, similar loss of functional laterality in cortical motor areas has been observed in several other neurologic diseases, thus suggesting that cocaine use is associated with functional dysregulation throughout movement-related neural networks.
The slower reaction times and higher error rates among cocaine users observed in this study are consistent with several previous clinical case studies of chronic users. Bauer et al (1996a) documented a resting hand tremor in cocaine-dependent patients that remained intact after three months of abstinence (Bauer, 1996b). Similar to the current study, Bauer found that cocaine-dependent individuals exhibited slower reaction times than controls during several sustained- and divided-attention tasks. Multiple studies of cognitive functioning in cocaine users have assessed reaction time as a secondary outcome measure. While several of these studies demonstrate slower reaction times in cocaine users (Bolla et al., 2004; Bolla et al., 1999; Roberts and Bauer, 1993), other studies do not reveal differences (Di Sclafani et al., 2002; Gooding et al., 2008). This discrepancy may be due to an interaction between cognitive demands of a task and sensorimotor performance (Schall and Thompson, 1999). In contrast to prior studies, however, the current investigation was explicitly designed to investigate motor performance as a primary outcome using a task with minimal cognitive load.
In addition to the slower reaction times, cocaine users made 10% more errors than controls throughout the task. As a matter of perspective, a similar study in Parkinson’s disease patients revealed that patients made 21% more errors on a novel finger sequencing task than matched controls but that there was no difference in the error rate after practice (Wu and Hallett, 2005). This suggests that while the behavioral deficit in these users is not as large as Parkinson’s disease, they are unable to enhance their performance with extended practice. The robust differences between the populations on these basic motor metrics underscore the importance of considering sensorimotor processing deficits when interpreting performance data from other cognitive studies in cocaine users.
Sensorimotor impairments among chronic cocaine users were associated with loss of functional laterality within the sensorimotor regions. Healthy controls recruit a largely left-sided network of cortical and subcortical regions to perform a sequential finger tapping task with their right hand (Boecker et al., 1994; Colebatch et al., 1991; Rijntjes et al., 1999). In the current study, controls activate significantly greater contralateral (left) than the ipsilateral motor cortex (Cramer et al., 1999; Rao et al., 1993; Verstynen et al., 2005), supplementary motor area (Babiloni et al., 2003; Rogers et al., 2004), and putamen (Winstein et al., 1997). Activecocaine users, however, exhibited a far less lateralized pattern of activity within motor cortical regions. This observation is further supported by the laterality analysis which revealed that cocaine users lack the typical left-sided laterality (positive laterality index) in cortical motor areas that was present in controls.
The loss of functional laterality during performance of a motor task is observed in several other neurologic conditions, including multiple sclerosis, ischemic stroke and advanced aging. Multiple sclerosis is a chronic condition associated with diffuse, widespread neurologic damage, and progressive motor impairment. During the active phase of the disease, the motor disability is proportional to activity in the ipsilateral premotor and primary motor cortex (Lee et al., 2000; Reddy et al., 2002) as well as the correlation of the left and right primary motor cortices (Rocca et al., 2007). Motor disability in multiple sclerosis is associated with a progressive loss of motor network laterality. The loss of laterality in these patients may reflect loss of typical inhibitory tone in the ipsilateral motor cortex or degradation of callosal white matter (Reddy et al., 2002). Though this functional and structural link has not been directly tested in cocaine users, prior reports have documented decreased white matter density integrity along the corpus callosum of cocaine users (Moeller et al., 2005) which is typically involved in transcallosal inhibition of the ipsilateral motor cortex.
Although a loss of laterality can correlate with disease severity, as in multiple sclerosis, it may also indicate compensatory neural adaptation to injury. Adaptive reorganization is observed, for example, during recovery from an ischemic stroke (Cramer, 1999; Marshall et al., 2000; Nelles et al., 1999; Pineiro et al., 2001; Weiller et al., 1992). Right middle cerebral artery stroke is typically associated with damage to the right primary motor cortex and ensuing paralysis of the left hand. In the months following the ischemic stroke, recovery of the affected hand correlates with activity in both the ipsilateral and contralateral motor cortex (Cramer, 2004). A longitudinal study by Kim et al (2006), however, demonstrated that 6 months after a stroke, patients regained the typical cortical laterality during unilateral movement (Kim et al., 2006). Loss of laterality, therefore, is adaptive in the initial stages of stroke recovery, but long-term recovery is associated with a rebalancing of activity to the contralateral side. While a chronic cocaine use has been associated with cerebrovasculitis (Kaye & Fainstat 1987, Fredericks et al 1991, Strickland et al 1993) and ischemic stroke (Levine & Welch 1988, Daras et al 1994) there was no evidence of prior focal ischemic damage in the cocaine users presented here. As the present group of cocaine users in the current study had been using cocaine for at least 10 years with no reported change in their drug taking behavior in the last 7 years, it is unlikely that the loss of laterality seen here reflects short-term adaptive plasticity as is seen in stroke patients.
It is possible, however, that the loss of laterality in cocaine users represents a long-term adaptive process, as in advanced aging. Several studies have demonstrated that otherwise healthy older adults rely on both their contralateral and ipsilateral primary motor cortex to perform a finger-sequencing task (Hutchinson et al., 2002, Mattay et al., 2002, Ward & Frackowiak 2003). Naccarato et al. (2006) recently demonstrated performance on a finger-thumb apposition task was positively correlated with the BOLD signal change in the ipsilateral primary motor cortex of older adults (Naccarato et al., 2006). They conclude that in aging, increased activity in both the ipsilateral and contralateral motor cortex, is a compensatory strategy to maintain behavioral performance. Although the cocaine users in the current study do not perform as well as healthy aging populations, the elevated activity in the left and right corticies in the cocaine users may represent adaptation to the decreased cortical tissue density observed in chronic cocaine users (Franklin et al., 2002; Matochik et al., 2003). Though alcohol use was not significantly greater in the cocaine users than controls, it is useful to note that compensatory increases in cortical and cerebellar activity have also been observed in heavy alcohol users (Desmond et al., 2003).
The results of the current investigation largely support and extend prior studies, but there are a few limitations which must be considered when interpreting the results. As with many human drug abuse investigations, it is difficult to isolate a socially and demographically matched control group that does not have a history of drug abuse. While the participants were matched for age, handedness, and education, the cocaine users were more likely to be smokers and had higher scores on a depression inventory, and lower IQ than controls. To address the impact of these variables, as well as current drug use status on motor performance and BOLD signal change, post-hoc analyses of covariance were performed. These analyses did not reveal any significant relationships among the covariates, task performance, or brain activation.
To avoid any deleterious effects of nicotine withdrawal on motor performance, all participants were given the opportunity to smoke upon request up to an hour prior to image acquisition (Wang et al., 2007; Xu et al., 2007). Although there is some evidence that IQ affects motor performance (Sen et al., 1983), IQ score was not correlated with performance measures or percent signal change in any regions, further limiting the possibility that varying IQ scores are a significant confound in the results. The elevated depressive symptoms in cocaine addicts, relative to controls, cannot be ruled out as a contributing factor to their sensorimotor performance impairments (Goodwin, 1997). Individuals with major depressive disorder often display psychomotor slowing and an inability to perform sustained attention tasks (Pardo et al., 2006; Weiland-Fiedler et al., 2004). These cognitive deficits in patient with depression are accompanied by decreases in frontal cortical BOLD signal (Wang et al., 2008). Given that the cocaine users have a significant increase in the spatial extent of activity in both the left and right hemispheres during motor task performance, the depressive symptoms alone are unlikely to be the source of the neurofunctional alterations seen in these users.
Finally, all cocaine users in the current study had positive urine screens for cocaine at the time of scanning. However, there was no evidence of intoxication present in any of the participants. Furthermore, recent work by Goldstein and colleagues (Woicik et. al., 2009) reported that users with positive urines were less impaired on multiple cognitive performance measures than users with negative urine drug screens. Signs of withdrawal were not observed at any time during the study, although the potential interaction between craving and task performance cannot be completely ruled out (Garavan and Hester 2009). Testing of an additional group of participants is necessary to fully evaluate the role of current cocaine use and craving on these tasks.
In summary, this investigation confirms prior reports of sensorimotor processing deficits in cocaine users and demonstrates that these deficits are associated with dysfunctional activity throughout motor-related neural networks. Specifically, cocaine users have less lateralized activity in cortical motor areas relative to controls. This may either be compensatory, as in stroke recovery, or indicate a loss of cortical inhibition, as in multiple sclerosis patients. The incorporation of sensorimotor deficits to the complement of executive and affective impairments in cocaine users further supports the idea that cocaine abuse and dependence may be a whole brain disease, and is not limited to striatal dysfunction.
Table 2.
Spatial extent and magnitude of BOLD signal change in sensorimotor regions of interest
| Controls | Users | ||||
|---|---|---|---|---|---|
| voxels | maximum | voxels | maximum | ||
| Left | Motor Cortex | 763 | 9.19 | 957 | 8.20 |
| Left | Supplementary Motor Area | 181 | 7.63 | 228 | 5.94 |
| Left | Anterior Cingulate Cortex | 44 | 4.40 | 214 | 5.14 |
| Left | Caudate | 35 | 5.40 | 69 | 5.00 |
| Left | Putamen | 145 | 7.51 | 105 | 5.95 |
| Left | Thalamus | 165 | 9.21 | 307 | 5.40 |
| Left | Cerebellum | 364 | 7.39 | 379 | 5.72 |
| Right | Motor Cortex | 24 | 4.69 | 464 | 2.47 |
| Right | Supplementary Motor Area | 46 | 4.90 | 177 | 5.10 |
| Right | Anterior Cingulate Cortex | 10 | 4.61 | 12 | 4.55 |
| Right | Caudate | 37 | 4.20 | 66 | 5.05 |
| Right | Putamen | 94 | 6.27 | 145 | 5.82 |
| Right | Thalamus | 122 | 6.37 | 138 | 5.72 |
| Right | Cerebellum | 753 | 8.90 | 932 | 5.75 |
The number of voxels that exceeded the p<0.005 threshold (voxels) and the maximum t-value during motor task performance relative to rest are shown for each group.
Acknowledgments
This work was supported by the National Institute of Drug Abuse grants DA021456 (CAH), DA020074 (LJP), and DA06634 (LJP). The authors thank TJRB and HRS for their comments on this manuscript and Marla Torrence for her assistance in recruitment and processing of the participants.
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Carducci F, Del Gratta C, Demartin M, Romani GL, Babiloni F, Rossini PM. Hemispherical asymmetry in human SMA during voluntary simple unilateral movements. An fMRI study. Cortex. 2003;39:293–305. doi: 10.1016/s0010-9452(08)70110-2. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Resting hand tremor in abstinent cocaine-dependent, alcohol-dependent, and polydrug-dependent patients. Alcohol Clin Exp Res. 1996;20:1196–1201. doi: 10.1111/j.1530-0277.1996.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Biswal B, Ulmer JL, Krippendorf RL, Harsch HH, Daniels DL, Hyde JS, Haughton VM. Abnormal cerebral activation associated with a motor task in Tourette syndrome. AJNR Am J Neuroradiol. 1998;19:1509–1512. [PMC free article] [PubMed] [Google Scholar]
- Boecker H, Kleinschmidt A, Requardt M, Hanicke W, Merboldt KD, Frahm J. Functional cooperativity of human cortical motor areas during self-paced simple finger movements. A high-resolution MRI study. Brain. 1994;117 (Pt 6):1231–1239. doi: 10.1093/brain/117.6.1231. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1999;11:361–369. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Cenci MA. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 2007;30:236–243. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Chipchase SY, Lincoln NB, Radford KA. Measuring fatigue in people with multiple sclerosis. Disabil Rehabil. 2003;25:778–784. doi: 10.1080/0963828031000093477. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Deiber MP, Passingham RE, Friston KJ, Frackowiak RS. Regional cerebral blood flow during voluntary arm and hand movements in human subjects. J Neurophysiol. 1991;65:1392–1401. doi: 10.1152/jn.1991.65.6.1392. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Stroke recovery. Lessons from functional MR imaging and other methods of human brain mapping. Phys Med Rehabil Clin N Am. 1999;10:875–886. ix. [PubMed] [Google Scholar]
- Cramer SC. Changes in motor system function and recovery after stroke. Restor Neurol Neurosci. 2004;22:231–238. [PubMed] [Google Scholar]
- Cramer SC, Finklestein SP, Schaechter JD, Bush G, Rosen BR. Activation of distinct motor cortex regions during ipsilateral and contralateral finger movements. J Neurophysiol. 1999;81:383–387. doi: 10.1152/jn.1999.81.1.383. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes AJ, Miall RC. Adaptation to visual feedback delays in a human manual tracking task. Exp Brain Res. 2000;131:101–110. doi: 10.1007/s002219900286. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Gavazzi C, Nave RD, Petralli R, Rocca MA, Guerrini L, Tessa C, Diciotti S, Filippi M, Piacentini S, Mascalchi M. Combining functional and structural brain magnetic resonance imaging in Huntington disease. J Comput Assist Tomogr. 2007;31:574–580. doi: 10.1097/01.rct.0000284390.53202.2e. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Anagnoste B, Battista AF, Ogawa M. Monkeys with nigrostriatal lesions effects of monoaminergic drugs. Pharmacol Ther [B] 1976;2:97–103. doi: 10.1016/0306-039x(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Burroughs S, Boutros NN. Attentional deficits in cocaine-dependent patients: converging behavioral and electrophysiological evidence. Psychiatry Res. 2008;160:145–154. doi: 10.1016/j.psychres.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM. Neuropsychological and neuroimaging evidence for the involvement of the frontal lobes in depression. J Psychopharmacol. 1997;11:115–122. doi: 10.1177/026988119701100204. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Basal ganglia--input, neural activity, and relation to the cortex. Curr Opin Neurobiol. 1991;1:644–651. doi: 10.1016/s0959-4388(05)80043-1. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Network-level neuroplasticity in cortico-basal ganglia pathways. Parkinsonism Relat Disord. 2004;10:293–296. doi: 10.1016/j.parkreldis.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Kawashima R, Satoh K, Kinomura S, Goto R, Koyama M, Sugiura M, Ito M, Fukuda H. PET study of pointing with visual feedback of moving hands. J Neurophysiol. 1998;79:117–125. doi: 10.1152/jn.1998.79.1.117. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Tajima N, Yoshida H, Okita K, Sasaki T, Schormann T, Ogawa A, Fukuda H, Zilles K. The effect of verbal feedback on motor learning--a PET study. Positron emission tomography. Neuroimage. 2000;12:698–706. doi: 10.1006/nimg.2000.0643. [DOI] [PubMed] [Google Scholar]
- Kim YH, You SH, Kwon YH, Hallett M, Kim JH, Jang SH. Longitudinal fMRI study for locomotor recovery in patients with stroke. Neurology. 2006;67:330–333. doi: 10.1212/01.wnl.0000225178.85833.0d. [DOI] [PubMed] [Google Scholar]
- Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, Wudel J, Pal PK, de la Fuente-Fernandez R, Calne DB, Stoessl AJ. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol. 2000;47:493–503. [PubMed] [Google Scholar]
- Lundh H, Tunving K. An extrapyramidal choreiform syndrome caused by amphetamine addiction. J Neurol Neurosurg Psychiatry. 1981;44:728–730. doi: 10.1136/jnnp.44.8.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mallol R, Barros-Loscertales A, Lopez M, Belloch V, Parcet MA, Avila C. Compensatory cortical mechanisms in Parkinson’s disease evidenced with fMRI during the performance of pre-learned sequential movements. Brain Res. 2007;1147:265–271. doi: 10.1016/j.brainres.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–661. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Miall RC, Imamizu H, Miyauchi S. Activation of the cerebellum in coordinated eye and hand tracking movements: an fMRI study. Exp Brain Res. 2000;135:22–33. doi: 10.1007/s002210000491. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron JC. Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? An fMRI study. Neuroimage. 2006;32:1250–1256. doi: 10.1016/j.neuroimage.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Nelles G, Spiekermann G, Jueptner M, Leonhardt G, Muller S, Gerhard H, Diener HC. Reorganization of sensory and motor systems in hemiplegic stroke patients. A positron emission tomography study. Stroke. 1999;30:1510–1516. doi: 10.1161/01.str.30.8.1510. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Humes SW, MIP Neurocognitive dysfunction in antidepressant-free, non-elderly patients with unipolar depression: alerting and covert orienting of visuospatial attention. J Affect Disord. 2006;92:71–78. doi: 10.1016/j.jad.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Dhuna A. Cocaine-associated multifocal tics. Neurology. 1990;40:999–1000. doi: 10.1212/wnl.40.6.999. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Habib M, Lyon-Caen O, Salamon G, Poncet M, Khalil R. Functional and magnetic resonance imaging correlates of callosal involvement in multiple sclerosis. Arch Neurol. 1993;50:1077–1082. doi: 10.1001/archneur.1993.00540100066018. [DOI] [PubMed] [Google Scholar]
- Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Functional MRI detects posterior shifts in primary sensorimotor cortex activation after stroke: evidence of local adaptive reorganization? Stroke. 2001;32:1134–1139. doi: 10.1161/01.str.32.5.1134. [DOI] [PubMed] [Google Scholar]
- Pollok B, Gross J, Muller K, Aschersleben G, Schnitzler A. The cerebral oscillatory network associated with auditorily paced finger movements. Neuroimage. 2005;24:646–655. doi: 10.1016/j.neuroimage.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD, et al. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–2318. doi: 10.1212/wnl.43.11.2311. [DOI] [PubMed] [Google Scholar]
- Reddy H, Narayanan S, Woolrich M, Mitsumori T, Lapierre Y, Arnold DL, Matthews PM. Functional brain reorganization for hand movement in patients with multiple sclerosis: defining distinct effects of injury and disability. Brain. 2002;125:2646–2657. doi: 10.1093/brain/awf283. [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Dettmers C, Buchel C, Kiebel S, Frackowiak RS, Weiller C. A blueprint for movement: functional and anatomical representations in the human motor system. J Neurosci. 1999;19:8043–8048. doi: 10.1523/JNEUROSCI.19-18-08043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LA, Bauer LO. Reaction time during cocaine versus alcohol withdrawal: longitudinal measures of visual and auditory suppression. Psychiatry Res. 1993;46:229–237. doi: 10.1016/0165-1781(93)90091-t. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Pagani E, Absinta M, Valsasina P, Falini A, Scotti G, Comi G, Filippi M. Altered functional and structural connectivities in patients with MS: a 3-T study. Neurology. 2007;69:2136–2145. doi: 10.1212/01.wnl.0000295504.92020.ca. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Carew JD, Meyerand ME. Hemispheric asymmetry in supplementary motor area connectivity during unilateral finger movements. Neuroimage. 2004;22:855–859. doi: 10.1016/j.neuroimage.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Rylander G. Psychoses and the punding and choreiform syndromes in addiction to central stimulant drugs. Psychiatr Neurol Neurochir. 1972;75:203–212. [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain. 2000;123 (Pt 2):394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Sen A, Jensen AR, Sen AK, Arora I. Correlation between reaction time and intelligence in psychometrically similar groups in America and India. Appl Res Ment Retard. 1983;4:139–152. doi: 10.1016/0270-3092(83)90006-1. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Stein JF, Passingham RE, Miall RC. Temporary inactivation in the primate motor thalamus during visually triggered and internally generated limb movements. J Neurophysiol. 2000;83:2780–2790. doi: 10.1152/jn.2000.83.5.2780. [DOI] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol. 2005;93:1209–1222. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, Krishnan RR, McCarthy G. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008;163:143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, Bonne O, Charney DS, Neumeister A. Evidence for continuing neuropsychological impairments in depression. J Affect Disord. 2004;82:253–258. doi: 10.1016/j.jad.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31:463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- Weiner WJ, Rabinstein A, Levin B, Weiner C, Shulman LM. Cocaine-induced persistent dyskinesias. Neurology. 2001;56:964–965. doi: 10.1212/wnl.56.7.964. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Grafton ST, Pohl PS. Motor task difficulty and brain activity: investigation of goal-directed reciprocal aiming using positron emission tomography. J Neurophysiol. 1997;77:1581–1594. doi: 10.1152/jn.1997.77.3.1581. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasic JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Simon S, Jarvik M, Olmstead R, Brody AL, Ernst M, London ED. Effect of cigarette smoking on prefrontal cortical function in nondeprived smokers performing the Stroop Task. 2007 doi: 10.1038/sj.npp.1301272. [DOI] [PMC free article] [PubMed] [Google Scholar]