Abstract
Cervical mucins are glycosylated proteins that form a protective cervical mucus. To understand the role of mucin glycans in Candida albicans infection, oligosaccharides from mouse cervical mucins were analyzed by liquid chromatography-mass spectrometry. Cervical mucins carry multiple α(1,2)fucosylated glycans, but α(1,2)fucosyltransferase Fut2-null mice are devoid of these epitopes. Epithelial cells in vaginal lavages from Fut2-null mice lacked Ulex europaeus agglutinin-1 (UEA-I) staining for α(1,2)fucosylated glycans. Hysterectomy to remove cervical mucus eliminated UEA-I and acid mucin staining in vaginal epithelial cells from wild type mice indicating the cervix as the source of UEA-I positive epithelial cells. To assess binding of α(1,2) fucosylated glycans on C. albicans infection, an in vitro adhesion assay was performed with vaginal epithelial cells from wild type and Fut2-null mice. Vaginal epithelial cells from Fut2-null mice were found to bind increased numbers of C. albicans compared to vaginal epithelial cells obtained from wild type mice. Hysterectomy lessened the difference between Fut2-null and wild type mice in binding of C. ablicans in vitro and susceptibility to experimental C. albicans vaginitis in vivo. We generated a recombinant fucosylated MUC1 glycanpolymer to test whether the relative protection of wild type mice compared to Fut2-null mice could be mimicked with exogenous mucin. While a small portion of the recombinant MUC1 epitopes displayed α(1,2)fucosylated glycans, the predominant epitopes were sialylated due to endogenous sialyltransferases in the cultured cells. Intravaginal instillation of recombinant MUC1 glycanpolymer partially reduced experimental yeast vaginitis suggesting that a large glycanpolymer, with different glycan epitopes, may affect fungal burden.
Keywords: fucosyltransferase, Candida albicans, Secretor gene, cervical mucins, hysterectomy, ABO/Lewis blood group
1 Introduction
Vulvovaginal candidiasis is a mucosal infection caused by opportunistic Candida species, typically Candida albicans. A polymorphism in approximately 20% of humans at the FUT2, or Secretor gene (OMIM 182100) abolishes α(1,2)fucosyltransferase activity (EC 2.4.1.69) in the Golgi apparatus of mucosal glandular cells 1. Loss of α(1,2) fucosylated glycans in the mucosal secretions of ‘nonsecretors’ is associated with a 2.4 to 4.4 fold increased relative risk for recurrent vaginitis by C. albicans accounting for an estimated 67% of the attributable risk in women with the nonsecretor phenotype 2,3. While current anti-fungal medications effectively target yeast metabolism for acute treatment, recurrent infections occur in 5-10% of reproductive age women with a mean time to recurrence of 4 to 10 months even under optimal management 4.
The immune response elicited during an episode of recurrent vulvovaginal candidiasis appears different from classical host-defense mechanism, as infection occurs despite normal Candida-specific Th1-type cell-mediated immunity 5,6 and T-cell immunodeficient knockout mice show no difference in susceptibility to vaginal candidiasis 7. These and other studies have led to a hypothesized major role for Candida-epithelial cell interactions in the mechanism of vaginal susceptibility vs. resistance 8. Due to the relative lack of cell-mediated immunity during recurrent vulvovaginal candidiasis, protection is believed to be acquired locally, possibly involving incompletely defined carbohydrate adhesion molecules 9,10 or epithelial cell mediated growth inhibition of Candida 11,12.
Several different fucosylated glycans have been implicated in mediating interactions between pathogens and host epithelial cells. Microbe adhesin molecules are lectin-like proteins that facilitate these interactions. Fucose-specific adhesins have been identified on germ tubes of C. albicans13. In vitro experiments using exogenous carbohydrates isolated from human breast milk 14 and antibodies against the H blood group antigen 15 demonstrate that C. albicans specifically bind α(1,2)fucosylated glycans. Many more adhesin molecules exist in the cell wall of Candida and the binding specificities of most have not been determined.
In our animal model of nonsecretors, Fut2-null mice, we reported an increased susceptibility to experimental vaginitis 16. Fut2 is expressed in secretory cells of uterine and endocervical glandular epithelium, but expression was not detected at the major site of Candidal adhesion and invasion, i.e. vaginal squamous epithelium 17. Despite this discrepancy, Ulex europaeus agglutinin I (UEA-I) lectin staining demonstrated the presence of α(1,2) fucosylated glycans at the apical surface and lumen of the vagina of wild type mice 16. In this study, we tested the hypothesis that secreted α(1,2) fucosylated mucins descend from the endocervix into the vagina, coat exposed epithelial cells with α(1,2) fucosylated glycans, and potentially alter microbe adhesion in vitro and fungal burden in vivo.
2 Materials and methods
2.1 Animals
Female C57BL/6J wild type (Jackson Laboratory, Bar Harbor, ME; stock no. 000664) and Fut2-null mutant mice 18 backcrossed for 12 generations to C57BL/6J (Jackson Laboratory stock no. 006262, designated B6.129P2-Fut2tm1Sdo), 8-10 weeks of age were maintained under Specific Pathogen Free conditions and handled according to institutionally approved guidelines. Mice were maintained in pseudoestrus using a 5 mg, 21 day controlled release 17β-estradiol pellet (Innovative Research of America, Sarasota, FL) 72 hours prior to subsequent experiments unless stated otherwise.
2.2 Histological staining
Vaginal washings were collected from female wild type and Fut2-null mice and processed for immunohistochemistry using either the α(1,2) fucose-specific lectin UEA-I conjugated to biotin (EY laboratories, Inc., San Mateo, CA), as previously described 18, or Alcian Blue, pH 2.5.
2.3 Preparation of cervical mucin oligosaccharides
Wild type and Fut2-null mice were euthanized 4 days after induction of pseudoestrus with 0.2mg/week estrogen injections. Cervical mucus samples were collected and stored at -80°C. Mucus from approximately 6 mice was pooled and dissolved in 150 μl ‘extrGuHCL’ (guanidinium chloride 6M/EDTA 5 mM/NaH2PO4 0.01M/ pH 6.7 adjusted with TRIS base). Following the addition of protease inhibitor (4 μl PMSF,100 mM in 2-propanol), the samples were homogenized with a mini mortar and stirred gently over night at 4°C, followed by reduction with dithiothreitol (25 mM final concentration) in 330 μl ‘redGuHCL’ (guanidinium chloride 6M/EDTA 5 mM/TRIS 0.1M/ pH 8.0). Alkylation with iodacetamide (63 mM final concentration) of the proteins was performed in dark conditions at 20°C over night. Samples were dialyzed against water over night (10000 MWCO, Slide-A-Lyzer, Pierce, Rockford, IL), followed by addition of 300 μl sample buffer (0.75 M TRIS-HCl pH 8.1, 2 % SDS, 0.01% bromophenol blue, 60% glycerol) before being concentrated down to 30-70 μl on 100 kD cut off membranes (Millipore, Billerica, MA). The samples were analyzed on SDS/PAGE composite gels19 and wet blotted to PVDF Immobilon PSQ membrane (Millipore) in transfer buffer (25 mM TRIS, 192 mM glycine, 0.04% SDS, 20% MeOH) for 4 hours at 400 mA. The blots were stained with Alcian Blue, visualized mucin bands excised, and the oligosaccharides released with reductive β-elimination 19.
2.4 LC-MS of oligosaccharides
Sample injection and LC was performed by using a PAL CTC autosampler (CTC Analytics AG, Switzerland) and a Agilent 1100 Series degasser and binary pump (Agilent Technologies, CA). The samples were resuspended in 15 μl of water and 2 μl were injected onto graphitized carbon columns (20 cm × 0.18 mm id) packed in-house with 5 μm Hypercarb particles (Thermo-Hypersil, Runcorn, UK). Oligosaccharides were eluted with a H2O/acetonitrile (ACN) gradient containing 8 mM NH4HCO3 (0-36% ACN 3-46 min, followed by a 10-minute wash step in 80% ACN). The flow rate was 2 μl/minute and maintained by splitting the liquid flow from the pump with a fused silica restrictor. MS was performed by using a Thermo LTQ linear ion trap mass spectrometer equipped with an Ion Max ion source (Thermo, San Jose, CA) with a 50 μm fused emitter operating in negative ion mode. The capillary temperature was 250°C, the capillary voltage was 26 V, and the electrospray voltage was 3.5 kV. For MS/MS experiments, the normalized collision energy 35%, with an activation time of 30 ms. MS was performed with four scan events: full scan with mass range m/z 380-2000, followed by successive MS/MS scans after collision induced fragmentation for the three most intense ions in each full scan.
2.5 In vitro epithelial cell adhesion assay
Wild type and Fut2-null female mice received either an abdominal ovariectomy (control surgery) or ovario-hysterectomy (including removal of the cervix) following a Jackson Laboratories protocol for these surgeries. Following a 1-2 week recovery, pseudoestrus was induced and maintained with 0.2mg/week intraperitoneal estrogen injections. Vaginal epithelial cells were freshly collected from control surgery and hysterectomized wild type and Fut2-null mice (n=5 per group) by lavage using approximately 100μl PBS per mouse. Cells were pooled, washed in PBS, counted and resuspended at a concentration of 8 × 104 cells ml-1. An in vitro filter adhesion assay described by Zhao and colleagues was used 20. C. albicans (3153A) grown to stationary phase in liquid salts-proline-biotin (SPB) medium supplemented with 12.5g/l glucose and 1g/l N-acetyl-D-glucosamine (GlcNAc) 21 for 2 days at 30°C in an orbital shaking incubator were quantified using a hemocytometer. For this assay, blastoconidia (2 × 106) were grown in 25 mL sterile Erlenmeyer flasks containing 4mL SPB medium supplemented with 1g/L GlcNAc and incubated at 37°C with 200 rpm shaking for 3h to promote germ tube formation. Vaginal epithelial cells (2 × 104, 250 μl) were added to each flask, incubated at 37°C with 200rpm shaking for 30 minutes to allow adhesion to occur. Cells were vacuum filtered across 12 mm pore size Nucleopore Track-Etch membrane filters (Whatman, NJ) and washed with 25 mL PBS. Filters were inverted onto glass microscope slides, dried and heat fixed. Slides were stained with crystal violet, washed with tap water and dried and the number of germ tubes adhering to 100 epithelial cells were counted. The experiment was performed in triplicate with similar results.
2.6 Inoculation of mice with experimental vaginal candidiasis
Female wild type and Fut2-null mice received either an abdominal ovariectomy or ovario-hysterectomy. Following a 2 week recovery, pseudoestrus was induced using a 5 mg, 21 day controlled release 17β-estradiol pellet (Innovative Research of America) and mice underwent experimental vaginal candidiasis as described previously 16. C. albicans (3153A), originally a clinical isolate now propagated in the laboratory, was grown to stationary phase in 1% phytone peptone (Becton Dickinson, Cockeysville, MD) supplemented with 0.1% glucose for 16-18h at 30°C in an orbital shaking incubator, washed in PBS and quantified using a hemocytometer. 4 days after inducing pseudoestrus, mice were intravaginally inoculated with 5 × 105 stationary-phase C. albicans (3153A) in 10 μl PBS. At 2 days post-inoculation mice were sacrificed, their lower reproductive tracts (vagina and cervix of ovariectomized mice, and vagina of hysterectomized mice) were removed en-bloc, weighed, homogenized in PBS, serially diluted and plated on Sabourand dextrose agar plates and incubated at 35°C for 48h, after which colony forming units (CFU) were determined.
2.7 Treatment of experimental vaginal candidiasis with recombinant MUC1
The human FUT2 cDNA1 was transferred to the pcDNA6 plasmid with a Blasticidin resistance using EcoRI/XbaI. The plasmid was transfected into CHO-K1 cells permanently expressing MUC1-IgG2a with 32 tandem repeats 22 using Lipofectamine 2000 (Invitrogen, Baltimore, MD). Stable clones were generated using 10 μg/mL Blasticidin and 250 μg/mL G418 (Invitrogen, Baltimore, MD). Clones (FUT2/MUC1-Ig(32TR)) were grown in T flasks in Iscove’s Modified Dulbecco’s Medium with 10% fetal bovine serum and single high-expressing clones were selected and adapted to grow in suspension in the protein-free medium ProCHO4-CDM (Lonza Biologicals) supplemented with ProHT supplement (Lonza Biologicals) in spinner flasks. A perfusion bioreactor culture of FUT2/MUC1-Ig(32TR) was performed in a 1 litre culture volume in ProCHO4-CDM with ProHT supplement, using the following set-points: pH=6.9, pO2=40% and T=37°C and a perfusion rate of 0.5-0.8 ml/min.
Wild type mice underwent abdominal ovariectomy and induced into pseudoestrus as described above after a 2 week recovery. After 4 days of pseudoestrus, mice were intravaginally inoculated with 5 × 105 stationary-phase C. albicans (3153A) which had been pre-incubated for 15 minutes with either PBS or 0.02mg of fucosylated Muc1 (FUT2/MUC1-Ig(32TR)) mucin (8 mice per group). At 3 days post-infection mice were sacrificed, the vagina and cervix removed en-bloc, weighed, homogenized in PBS, serially diluted and plated on Sabourand dextrose agar plates and incubated at 35°C for 48h, after which CFU were determined.
2.8 Statistics
Comparisons between groups in each experiment were made using two-way analysis of variance (ANOVA) using the program SPSS 13 (Chicago, Illinois). Statistical significance was defined as a P value of <0.05.
3 Results
3.1 Cervical mucins carry multiple α(1,2)fucosylated glycans, but Fut2-null mice are devoid of these epitopes
To understand the role of mucin glycans in relation to C. albicans infections, cervical mucins were isolated from wild type and Fut2-null mice and the O-linked oligosaccharides released and analyzed by mass spectrometry (liquid chromatography electrospray ionization mass spectrometry [LC-ESI-MS] and tandem mass spectrometry) 19,23,24. The sequences and/or monosaccharide composition of neutral and sialylated oligosaccharides from wild type and Fut2-null mice were identified. The core structures were mainly core 1 (Galβ1-3GalNAc) and core 2 (Galβ1-3(GlcNAcβ1-6)GalNAc). No sulfated oligosaccharides were detected. Deduced sequences and/or monosaccharide compositions corresponding to the fucose-containing oligosaccharides together with their relative abundance are listed in Table 1.
Table 1.
Fucosylated oligosaccharides and nonfucosylated precursor ions detected in wild type and Fut2-null mice
| Relative abundanceb |
||||||
|---|---|---|---|---|---|---|
| Molecular mass | Ret.time (min) | Sequence/compositiona | No. of isoforms | Fuc epitope | Wild type | Fut2-null |
| 385 | 14.8 | Gal-3GalNAcol | 1 | + | ++++ | |
| 531 | 30.8 | Fuc-Gal-3GalNAcol | 1 | Fuc(α1-2)Gal- | + | - |
| 676 | 19.4 | Gal-3(NeuAc-6)GalNAcol | 1 | + | ++ | |
| 734 | 31.3 | Fuc-Gal-3(GlcNAc-6)GalNAcol | 1 | Fuc(α1-2)Gal- | + | - |
| 750 | 21.1 | Gal-3(Gal-GlcNAc-6)GalNAcol | 1 | + | ++ | |
| 750 | 22.4 | Gal-GlcNAc-Gal-3GalNAcol | 1 | + | +++ | |
| 822 | 36.6 | Fuc-Gal-3(NeuAc-6)GalNAcol | 1 | Fuc(α1-2)Gal- | ++ | - |
| 896 | 18.8 | Gal-3(Gal-(Fuc-)GlcNAc-6)GalNAcol | 1 | Fuc(α1-3/4)GlcNAc- | + | ++ |
| 896 | 22.9 | Gal-(Fuc-)GlcNAc-Gal-3GalNAcol | 1 | Fuc(α1-3/4)GlcNAc- | + | - |
| 896 | 26.0 | Gal-3(Fuc-Gal-4GlcNAc-6)GalNAcol | 1 | Fuc(α1-2)Gal- | + | - |
| 896 | 31.5 | Fuc-Gal-3(Gal-GlcNAc-6)GalNAcol | 1 | Fuc(α1-2)Gal- | + | - |
| 1042 | 32.7 | Fuc-Gal-3(Fuc-Gal-4GlcNAc-6)GalNAcol | 1 | Fuc(α1-2)Gal- | + | - |
| 1187 | 25.7 | Fuc-Gal-GlcNAc-Gal-3(NeuAc-6)GalNAcol | 1 | Fuc(α1-2)Gal- | + | - |
| 1187 | 30.4 | NeuAc-Gal-3(Fuc-Gal-4GlcNAc-6)GalNAcol | 1 | Fuc(α1-2)Gal- | + | - |
| 1187 | 36.4 | Fuc-Gal-3(NeuAc-Gal-GlcNAc-6)GalNAcol | 1 | Fuc(α1-2)Gal- | ++ | - |
| 1333 | 30.2 | NeuAc-Gal-3(Fuc-Gal-(Fuc-)GlcNAc-6)GalNAcol | 1 | Fuc(α1-3/4)GlcNAc- + Fuc(α1-2)Gal- | + | - |
| 749 | 21.9 | 2 Gal, GlcNAc, GalNAcol | 1 | + | - | |
| 1042 | 31.5 | 2 Fuc, 2 Gal, GlcNAc, GalNAcol | 1 | + | - | |
| 1187 | 21.9-26.3 | NeuAc, Fuc, 2 Gal, GlcNAc, GalNAcol | 3 | + | + | |
| 1478 | 27.9, 28.9 | 2 NeuAc, Fuc, 2 Gal, GlcNAc, GalNAcol | 2 | + | - | |
| 1553 | 27.5-33.2 | NeuAc, Fuc, 3 Gal, 2 GlcNAc, GalNAcol | 10 | ++ | - | |
| 1627 | 24.1-24.8 | 2 Fuc, 4 Gal, 3 GlcNAc, GalNAcol | 2 | - | ++ | |
| 1715 | 33.4-35.0 | NeuAc, Fuc, 4 Gal, 2 GlcNAc, GalNAcol | 3 | + | - | |
| 1773 | 23.2-23.9 | 2 Fuc, 4 Gal, 3 GlcNAc, GalNAcol | 2 | - | +++ | |
| 1844 | 30.7-36.1 | 2 NeuAc, Fuc, 3 Gal, 2 GlcNAc, GalNAcol | 5 | + | - | |
| 2209 | 33.4-38.0 | 2 NeuAc, Fuc, 4 Gal, 3 GlcNAc, GalNAcol | 6 | ++ | - | |
| 2355 | 35.3-42.1 | 2 NeuAc, 2 Fuc, 4 Gal, 3 GlcNAc, GalNAcol | 5 | ++++ | - | |
Compositions and sequences were elucidated by LC-ESI-MS and LC-ESI-MS/MS
The saccharides marked in bold are located on C-6 of GalNAcol. The following assumptions have been made based on previously published data confirming typical mucin monosaccharide components in mouse:39 all deoxyhexoses are fucose (Fuc); hexoses are galactose (Gal); N-acetylhexosamines are N-acetylglucosamines (GlcNAc), N-acetylhexosaminitols are N-acetylgalactosaminitols (GalNAcol); NeuAc are N-acetylneuraminic acid.
Measured as LC-MS chromatogram peak areas compared to the largest peak area of the oligosaccharides listed in the table. Oligosaccharides marked (–) were not detected in the sample.
In wild type mice, the most abundant fucose-containing epitope among the deduced sequences was the Fuc-Gal- epitope (blood group H-type epitope), an epitope suggested to be made by the Fut2 transferase. These were absent in the oligosaccharides obtained from Fut2-null mice. Oligosaccharides with the Fuc-GlcNAc- epitope, made by Lewis-type transferases, were found on both wild type and Fut2-null mice mucins. Sequences which are likely precursor oligosaccharides for the fucose-containing oligosaccharides were also detected. In particular, the oligosaccharide Galβ1-3GalNAcol, which is a precursor substrate for the Fut2 transferase to generate one of the most common fucosylated glycans, increased in relative abundance in the Fut2-null mouse. Several doubly charged ions corresponding to larger neutral and sialylated oligosaccharides (10-12 monosaccharide residues) were identified on mucins from both wild type and Fut2-null mice. These oligosaccharides separated into several peaks on the column, suggesting numerous glycoforms. For example octasaccharides with the molecular mass of 1553 Da where composed of at least ten different glycoforms (Table 1). Due to their large size, and the relative low abundance of each individual glycoform, complete sequences could not be interpreted. From these data, we conclude that the mucin molecules in Fut2-null mice lack α(1,2)fucosylated glycans resulting in a very different glycan profile compared to cervical mucins from wild type animals.
3.2 Epithelial cells in vaginal washings from hysterectomized mice lack acid mucins and α(1,2)fucosylated glycans
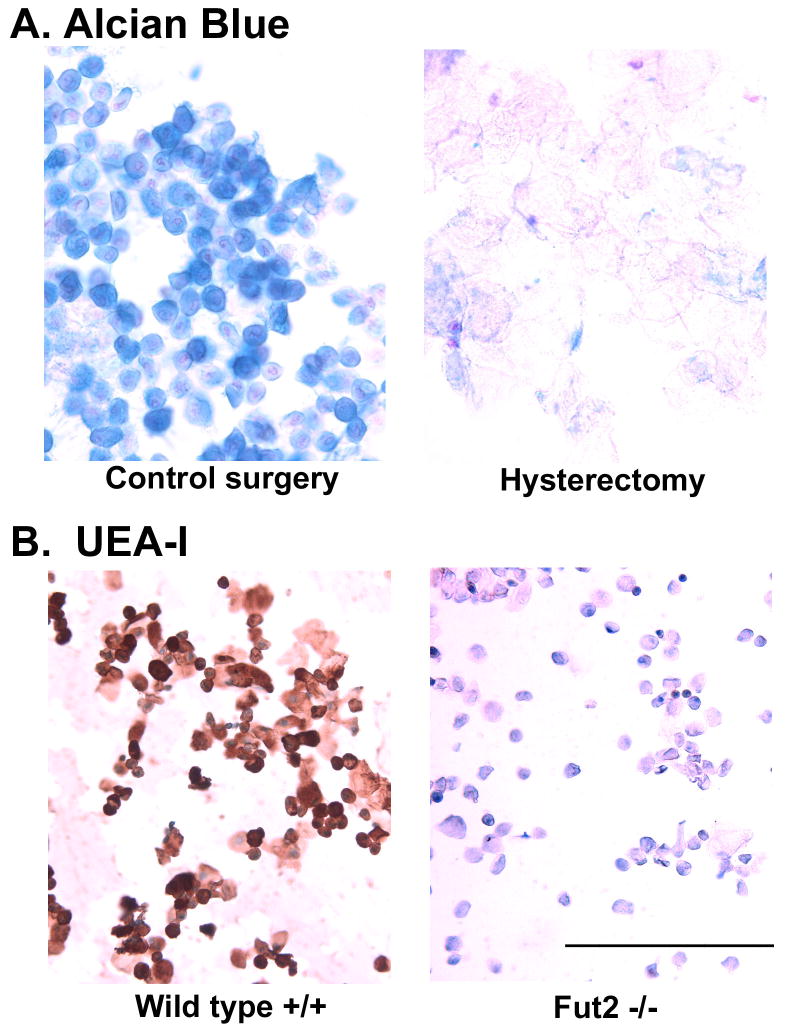
Using lectin histochemistry on tissue sections, we previously observed UEA-I staining associated with endocervical glands and ectocervical squamous cells 16. In the present study, we postulated that removal of the cervix by hysterectomy would allow us to separate the effects of the cervix from vaginal effects on fungal burden. In these experiments, groups of wild type and Fut2-null mice underwent either control surgery (ovariectomy alone) or ovario-hysterectomy (ovariectomy and total hysterectomy) to remove the entire cervix along with uterus. To determine the length of time necessary for cervical cells and secretions to clear from the lower reproductive tract, vaginal lavage was performed every other day post-surgery and the cells obtained processed for cytologic analysis. By 9 days post-surgery, Alcian blue pH 2.5 staining for acid mucins was absent in vaginal lavages from both surgical groups. Beginning on day 10, a strong pseudoestrus was then induced with estradiol subcutaneous pellets. The cytological appearance of epithelial cells obtaining by vaginal lavage showed a range of sizes from round, relatively small squamous cells to large, flat squamous cells (not shown). Very few leukocytes were seen in vaginal lavages indicating a satisfactory pseudo-estrus effect. On day 14, Alcian blue staining of epithelial cells from vaginal lavages was observed only in wild type mice with the cervix/uterus still present (control surgery) (Fig. 1A). Alcian blue staining in wild type mice was most intense on round, relatively small squamous cells, compared to large squamous cells. Alcian blue-positive cells were absent from the washings of hysterectomized mice (Fig. 1A). Washings from hysterectomized mice were found to contain only large, flat squamous cells with no Alcian blue staining consistent with the interpretation that epithelial cells associated with the cervix or near the cervix are the source of the Alcian blue-positive squamous cells. Similar results on Alcian blue staining were obtained by hysterectomy in Fut2-null mice (supplemental Fig. 1).
Fig. 1.
Vaginal epithelial cells obtained by vaginal lavage from wild type and Fut2-null mice stained with UEA-I and Alcian blue pH 2.5. Wild type and Fut2-null mice underwent either control surgery (ovariectomy alone) or hysterectomy (ovariectomy and total hysterectomy) to remove the entire cervix along with uterus. (A) Intense Alcian blue pH 2.5 mucin staining was present on epithelial cells after control surgery, but absent in vaginal epithelial cells from hysterectomized mice. (B) Specific UEA-I lectin staining was associated with vaginal epithelial cells from wild type mice, but absent in washings Fut2-null mice. Bar = 100μm. (see Supplemental Fig. 1 for positive and negative controls in all groups).
In parallel cytology slides, vaginal lavages from control surgery and hysterectomy surgery mice were also stained with UEA-I for α(1,2)fucosylated glycans. After control surgery, wild type mice expressed UEA-I positive glycans associated with squamous epithelial cells while Fut2-null mice did not (Fig. 1B). UEA-I staining was heterogeneous with the most intensely stained cells being round and relatively small, whereas larger epithelial cells showed almost no UEA-I staining. In hysterectomized mice, neither wild type nor Fut2-null mice showed UEA-I positive staining (supplemental Fig. 1). Taken together, these data show that hysterectomy removes Alcian blue-positive and UEA-I positive cells from the vagina, and the most likely source of Alcian blue-positive and UEA-I positive epithelial cells is the cervix.
3.3 Increased binding of C. albicans to epithelial cells from vaginal lavages of Fut2-null mice
Groups of wild type and Fut2-null mice underwent either control surgery (ovariectomy alone) or ovario-hysterectomy (ovariectomy and total hysterectomy) as described above. After a post-operative recovery period of two weeks, pseudoestrus was induced with estrogen pellet. Using a previously published in vitro adhesion assay 20, vaginal squamous cells were collected by lavage and incubated with germinating C. albicans. The in vitro adhesion assay chosen utilizes germinating C. albicans since this stage of growth is the most clinically relevant. Epithelial cells were incubated with germinating C. albicans for 30 min, vacuum filtered across 12 micrometer pore size filters, and extrensively washed. Following staining with crystal violet, the number of yeast organisms adherent to 100 squamous cells were counted by light microscopy. Germinating C. albicans showed greatest adherence to squamous cells isolated from Fut2-null mice with an intact cervix. These had a mean of 9 yeast bound per epithelial cell, compared with means around 4 yeast bound per epithelial cell from the other groups (P=<0.01, Fig. 2). Removal of the cervix by hysterectomy from wild type mice did not alter the total number of C. albicans binding to vaginal squamous cells.
Fig. 2.
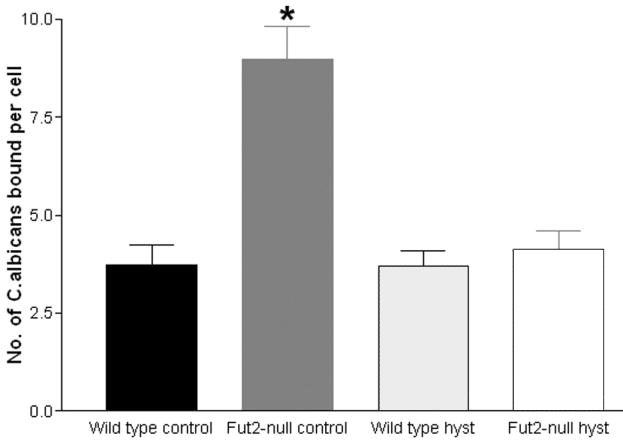
Analysis of adherence of vaginal epithelial cells to C. albicans in an in vitro adhesion assay. Following estrogen treatment, vaginal epithelial cells from wild type and Fut2-null mice after control surgery or hysterectomy surgery (n=5) were incubated with C. albicans in an in vitro adhesion assay. Error bars show S.E.M. The experiment was performed three times with similar results (a representative fig. is presented) and analyzed using two-way ANOVA. Statistically significant difference (p=<0.01) indicated by *.
3.4 Hysterectomy reduces susceptibility of Fut2-null mice to experimental vaginal candidiasis
In comparison to the in vitro adhesion data in the previous experiment, we wanted to determine the effect of removal of the cervix on fungal burden in vivo. Groups of wild type and Fut2-null mice underwent either control surgery (removal of ovaries) or ovario-hysterectomy surgery (ovariectomy and total hysterectomy) as described above. After a post-operative recovery period of two weeks, pseudoestrus was induced with estrogen and C. albicans inoculated vaginally. In our initial experiments, the time course for fungal burden was monitored on a daily basis using vaginal swabs. We observed peak fungal burden at days 2-3 post-inoculation (data not shown). In subsequent experiments, mice were euthanized 2 days post-inoculation and fungal burden quantified by removing the entire vagina, homogenizing, and measuring CFU of C. albicans per g of vaginal tissue. Consistent with our previously reported increased susceptibility of Fut2-null mice to experimental vaginal candidiasis 16, Fut2-null mice showed a 3-fold increase in susceptibility to C. albicans compared to wild type mice after control surgery (p=< 0.05) (Fig. 3). Unexpectedly, the increased susceptibility of Fut2-null mice compared to wild type mice was eliminated by hysterectomy (Fig. 3). While the mechanism of the increased susceptibility of Fut2-null mice is not known, we hypothesis that C. albicans binds more strongly to non-α(1,2)fucosylated glycans on epithelial cells associated with the cervix, consistent with the interpretation of the in vitro cell binding experiments. Hysterectomy with removal of the cervix was observed to reduce this susceptibility normalizing the difference between wild type and Fut2-null mice, but the precise mechanisms are not know, nor the glycan structures that specifically binds C. albicans known.
Fig. 3.
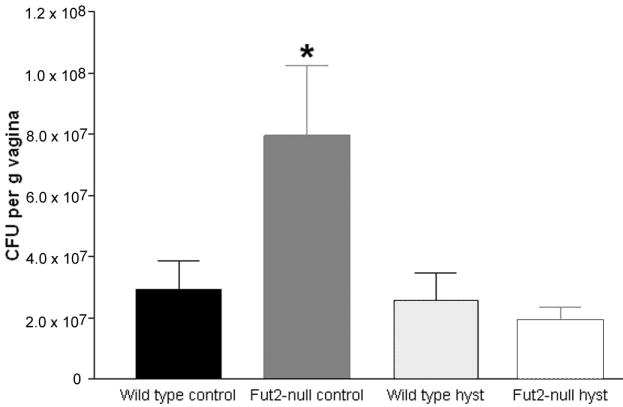
Quantification of colonization of C. albicans in wild type and Fut2-null mice after control surgery or hysterectomy surgery. Following surgery and estrogen treatment, wild type and Fut2-null mice (n= 11-13 per group) were inoculated vaginally with C. albicans to induced experimental vaginal candidiasis. After 2 days, mice were euthanized and the vagina was removed, homogenized, and fungal burden determined. Error bars show S.E.M. The experiment was performed in triplicate with similar results. For statistical analysis, the three trials were combined and subjected to two-way ANOVA. Statistically significant differences (p=<0.05) are indicated by *.
3.5 Generation and analysis of recombinant MUC1 glycanpolymer
The relative protection of wild type mice compared to Fut2-null mice to experimental yeast vaginitis may be due to the secretion of cervical mucins. To test this hypothesis, we administered exogenous mucins displaying α(1,2)fucosylated glycans. We generated a recombinant MUC1 glycanpolymer by transfecting human FUT2 cDNA into CHO-K1 cells (which lack a functional Fut2 gene) permanently expressing the extracellular MUC1 with 32 tandem repeats fused to mouse-IgG2a 22. A perfusion bioreactor culture of FUT2/MUC1-Ig(32TR) cells were performed and the resulting recombinant MUC1 glycanpolymer purified and analyzed for carbohydrate composition (Table 2). Just as for the cervical mucins (Table 1), the NeuAca2-6GalNAc substructure was relatively abundant. However, in contrast to the cervical mucins where the core 1 Galb1-3GalNAc was largely fucosylated, the recombinant mucin was largely sialylated. The recombinant MUC1 mucin was determined to contain 4% Fuc-2Gal-3GalNAc oligosaccharides (Table 2). The activity of the introduced FUT2 transferase was thus not sufficient to override the endogenous sialyltransferases in CHO cells to make a mucin more similar to the mouse cervical mucins 25. The recombinant MUC1 mucin thus displayed a smaller proportion of α(1,2)fucosylated glycans relative the cervical mucins.
Table 2.
Relative amounts of O-glycans on a recombinant Fut2 MUC1 tandem repeat (32 repeats) fused with mouse IgG Fc
| Structures | m/z | Corrected Areaa | Rel. abundance % |
|---|---|---|---|
| Cho/MUC1/Fut2 | |||
| Gal-3GalNAcol | 385 | 2678 | 0.3 |
| Fuc-Gal-3GalNAcol | 531 | 45669 | 4.3 |
| Gal-3(NeuAc-6)GalNAcol | 676a | 1975 | 0.2 |
| NeuAc-3Gal-3GalNAcol | 676b | 737228 | 69 |
| NeuAc-3Gal-3(NeuAc-6)GalNAcol | 967 | 284894 | 27 |
Correction factors from 40.
To test the recombinant MUC1 glycanpolymer as a potential therapeutic treatment for yeast vaginitis, we “treated” mice in the experimental yeast vaginitis model with intravaginal instillation of recombinant MUC1 glycanpolymer. Ovariectomized wild type mice underwent pseudoestrus and intravaginal inoculation with C. albicans (3153A) which had been pre-incubated for 15 minutes with either PBS or 0.02 mg of recombinant MUC1. For 2 days mice underwent daily intravaginal instillation with PBS or recombinant MUC1. Mice were euthanized at 3 days post-inoculation and fungal burden determined by excising the entire vagina and quantifying CFU per g of vaginal weight (Fig. 4). A trend to reduced fungal burden in the recombinant MUC1 group was observed although this failed to reach statistical significance. This suggests that a large glycanpolymer may affect fungal burden but the possibility remains that mucins could affect positively or negatively multiple steps in adhesion and/or proliferation of C. albicans.
Fig. 4.
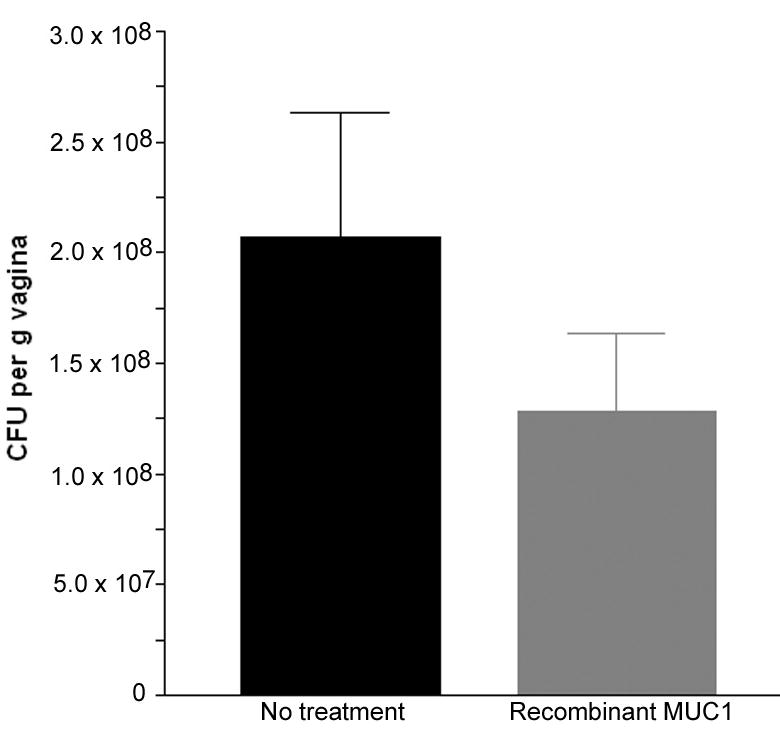
Treatment of experimental vaginal candidiasis with fucosylated MUC1. Control surgery wild type mice underwent experimental vaginal candidiasis. C. albicans aliquots were pre-incubated with either PBS or fucosylated extracellular MUC1 fused to mouse IgG2aFc (CHOMUC1 FUT2) prior to inoculation (8 mice per group). Once a day for the next two days, an additional intravaginal treatment was performed with PBS or fucosylated MUC1. Mice were euthanized at 3 days post-inoculation and fungal burden determined. Error bars show S.E.M. (no statistically significant difference).
4 Discussion
Small numbers of C. albicans are normally found as part of the human vaginal flora and is tolerated by the vaginal mucosal immune system 26. Perturbations in the vaginal flora due to pH and other factors allow this opportunistic organism to multiply leading to symptomatic infection 8. A woman’s hormonal changes during the ovulatory cycle have profound effects on the potential for infection. At a molecular level, estrogen is required for candidal proliferation and adhesion in vivo, reduces epithelial-cell-mediated anti-candidal activity, and causes a decrease in immunoglobulins in vaginal secretions 27-29. We postulate that estrogen-regulated Fut2 is involved in host-microbe interaction with C. albicans since: 1) Epidemiological evidence indicates that ABO/Lewis histo-blood group non-secretors are more susceptible to recurrent vulvovaginal candidiaiss 2,3; 2) Fut2-null mice display an increased susceptibility to experimental vaginal candidiasis 16; and 3) Fucose has been implicated in C. albicans adhesion from various in vitro studies 13-15.
Previously we have shown cell-specific localization of Fut2 within secretory glands of the endocervix. This finding is consistent with its predicted role in fucosylating glycans, principally mucins, within mucosal secretions. Since Fut2 expression was not detected in vaginal squamous epithelial cells while UEA-I lectin staining was found within the vaginal lumen and on exfoliated epithelial cells of wild type mice, we proposed that UEA-I reactivity was due to the presence of α(1,2)fucosylated glycans secreted from the endocervix 16. In this study, staining of vaginal washings from estrogen-treated hysterectomized wild type and Fut2-null mice with Alcian blue pH 2.5 and UEA-I supports this hypothesis. We showed that cervical mucins carry a number of glycans with the Fucα1-2Gal epitope as made by the Fut2 enzyme. This epitope is attached to GalNAc-Ser/Thr, is the most abundant substructure, and as expected is absent in Fut2-null mice. In humans, fucosylated blood group antigen 30 and mucin expression 31 within cervical mucus varies during the ovulatory cycle.
Removal of the cervix and uterus from Fut2-null mice was found to decrease the adhesion of germinating C. albicans to isolated vaginal epithelial cells in vitro. In testing the overall effect of removal of cervical secretions on fungal burden in vivo by surgically removing the cervix and uterus, we found that hysterectomy lessened the difference between wild type and Fut2-null mice. Removal of the cervix and uterus likely did more than remove mucins, it also removed other cervical mucus components that may promote yeast growth making the vaginal environment less conducive to experimental vaginitis. Since all mice underwent oophorectomy followed by pseudoestrus with exogenous estrogen, we attempted to minimize differences due to hormone levels.
The relationship of cervical mucus changes during the ovulatory cycle to infection by micro-organisms is complex since cervical mucus provides a chemical, as well as physical, barrier to infection and absence of these factors may promote the opportunity for microbes to proliferate 32,33. Under estrogen control, cervical mucins act as a hydrated scaffold for spermatozoa only around the time of ovulation while at other times preventing passage of spermatozoa or ascending infection. For Neisseria gonorrhoeae, ascending infection is highest during the early proliferative phase of the menstrual cycle and is less prevalent among women who use oral contraceptives 34. In contrast, C. albicans infection is more common in the luteal phase of the menstrual cycle, with use of oral contraceptive and hormone therapy, and under non-hormonal factors such as antibiotic use, uncontrolled diabetes mellitus, and HIV infection 35. Hence, it is possible that one set of cervical secretions allow for growth/proliferation of fungus while another differentially affects adhesion.
Results from this study suggest that it is the epithelial cells from cervix that carries fucosylated glycans, but it is not known if this is due to their endogenous expression or the binding of fucosylated glycoconjugates. In vaginal cells collected by lavage, the binding of germinating C. albicans in vitro differs between wild type and Fut2-null mice. Future experiments may further examine the mechanism by exposing vaginal cells from wild type mice to cervical secretions from Fut2-null mice and measuring C. albicans binding in vitro. and testing the effects of recombinant mucins on binding and proliferation of C. albicans. It is possible that soluble mucins and other components from cervix secretions bind C. albicans separate from the binding to the epithelial cells. For instance, the major mucin secreted from cervix, fucosylated Muc5b 23, may bind C. albicans and compete with the binding to the vaginal cells. Furthermore, vaginal cells collected by lavage may not represent the binding properties of epithelial cells of the vaginal epithelium. Instead, exfoliated epithelial cells may act as a sink that are trapping C. albicans and slowly removing them from the vagina.
There is limited clinical information on whether removal of the cervix affects susceptibility to yeast vaginitis in humans. One epidemiological study suggests hysterectomized patients harbor more aggressive strains of Candida because they are less likely to respond to one course of therapy compared to non-hysterectomized patients 36. However, this is a limited study that did measure additional factors, e.g., use of estrogen, type of hysterectomy, or vaginal pH, which is more acidic in hysterectomized women because of absence of endocervical secretions 37,38. Further research is required to assess whether leaving the cervix in place with a supracervical hysterectomy compared to a total hysterectomy would affect the risk of recurrent yeast vaginitis.
Supplementary Material
Vaginal epithelial cells obtained by vaginal lavage from wild type and Fut2-null mice stained with UEA-I and Alcian blue pH 2.5 with control panels shown. Wild type and Fut2-null mice underwent either control surgery (ovariectomy alone) or hysterectomy (ovariectomy and total hysterectomy) to remove the entire cervix along with uterus. Intense Alcian blue pH 2.5 mucin staining (top panel) was present in washings from ovariectomized control wild-type (A) and Fut2-null mice (C) but absent in washings from hysterectomized wild type (B) and Fut2-null mice (D). Duplicate slides stained with UEA-I (bottom panel) show specific brown lectin staining associated with vaginal epithelial cells from pseudoestrus ovariectomized wild type mice (E) but absent in washings from pseudoestrus ovariectomized Fut2-null (G), hysterectomized wild type (F) and Fut2-null (H) mice. Bar = 100μm.
Acknowledgments
We thank Paul Fidel for the gift of C. albicans strain 3153A, the personnel of the Cancer Center Research Histology and Immunoperoxidase Core at the University of Michigan for histological processing, Janet Hoff at the Center for Integrative Genomics at the University of Michigan for performing abdominal oophorectomy, Brady West at the Centre for Statistical Consultation and Research of the University of Michigan, the Mammalian Protein Expression Core Facility, and Dr. Hasse Karlsson for assistance with the LC-MS analysis and spectra deduction. This work was supported by grants from the University of Michigan Biomedical Research Council and Horace H. Rackham School of Graduate Studies, the Swedish Research Council (equipment and grant no. 7461), and the IngaBritt and Arne Lundberg Foundation.
Abbreviations
- CFU
Colony forming unit
- Fut2
a(1,2)fucosyltransferase “Secretor” gene
- UEA-I
Ulex europaeus agglutinin I
Footnotes
Electronic Supplementary Material The online version of this article contains supplementary material, which is available to authorized users.
References
- 1.Kelly RJ, Rouquier S, Giorgi D, Lennon GG, Lowe JB. Sequence and expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995;270:4640–4649. doi: 10.1074/jbc.270.9.4640. [DOI] [PubMed] [Google Scholar]
- 2.Chaim W, Foxman B, Sobel JD. Association of recurrent vaginal candidiasis and secretory ABO and Lewis phenotype. J Infect Dis. 1997;176:828–830. doi: 10.1086/517314. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni DG, Venkatesh D. Non-secretor status; a predisposing factor for vaginal candidiasis. Indian J Physiol Pharmacol. 2004;48:225–229. [PubMed] [Google Scholar]
- 4.Sobel JD, Wiesenfeld HC, Martens M, Danna P, Hooton TM, Rompalo A, Sperling M, Livengood C, 3rd, Horowitz B, Von Thron J, Edwards L, Panzer H, Chu TC. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876–883. doi: 10.1056/NEJMoa033114. [DOI] [PubMed] [Google Scholar]
- 5.Fidel PL., Jr Immunity to Candida. Oral Dis. 2002;8(Suppl 2):69–75. doi: 10.1034/j.1601-0825.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- 6.Nawrot U, Grzybek-Hryncewicz K, Zielska U, Czarny A, Podwinska J. The study of cell-mediated immune response in recurrent vulvovaginal candidiasis. FEMS Immunol Med Microbiol. 2000;29:89–94. doi: 10.1111/j.1574-695X.2000.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 7.Wormley FL, Jr, Cutright J, Fidel PL., Jr Multiple experimental designs to evaluate the role of T-cell-mediated immunity against experimental vaginal Candida albicans infection. Med Mycol. 2003;41:401–409. doi: 10.1080/3693780310001597683. [DOI] [PubMed] [Google Scholar]
- 8.Fidel PL., Jr History and update on host defense against vaginal candidiasis. Am J Reprod Immunol. 2007;57:2–12. doi: 10.1111/j.1600-0897.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 9.Barousse MM, Steele C, Dunlap K, Espinosa T, Boikov D, Sobel JD, Fidel PL., Jr Growth inhibition of Candida albicans by human vaginal epithelial cells. J Infect Dis. 2001;184:1489–1493. doi: 10.1086/324532. [DOI] [PubMed] [Google Scholar]
- 10.Steele C, Leigh J, Swoboda R, Ozenci H, Fidel PL., Jr Potential role for a carbohydrate moiety in anti-Candida activity of human oral epithelial cells. Infect Immun. 2001;69:7091–7099. doi: 10.1128/IAI.69.11.7091-7099.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barousse MM, Espinosa T, Dunlap K, Fidel PL., Jr Vaginal epithelial cell anti-Candida albicans activity is associated with protection against symptomatic vaginal candidiasis. Infect Immun. 2005;73:7765–7767. doi: 10.1128/IAI.73.11.7765-7767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yano J, Lilly EA, Steele C, Fortenberry D, Fidel PL., Jr Oral and vaginal epithelial cell anti-Candida activity is acid labile and does not require live epithelial cells. Oral Microbiol Immunol. 2005;20:199–205. doi: 10.1111/j.1399-302X.2005.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vardar-Unlu G, McSharry C, Douglas LJ. Fucose-specific adhesins on germ tubes of Candida albicans. FEMS Immunol Med Microbiol. 1998;20:55–67. doi: 10.1111/j.1574-695X.1998.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 14.Brassart D, Woltz A, Golliard M, Neeser JR. In vitro inhibition of adhesion of Candida albicans clinical isolates to human buccal epithelial cells by Fuc alpha 1----2Gal beta-bearing complex carbohydrates. Infect Immun. 1991;59:1605–1613. doi: 10.1128/iai.59.5.1605-1613.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron BJ, Douglas LJ. Blood group glycolipids as epithelial cell receptors for Candida albicans. Infect Immun. 1996;64:891–896. doi: 10.1128/iai.64.3.891-896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurd EA, Domino SE. Increased susceptibility of secretor factor gene Fut2-null mice to experimental vaginal candidiasis. Infect Immun. 2004;72:4279–4281. doi: 10.1128/IAI.72.7.4279-4281.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domino SE, Hurd EA. LacZ expression in Fut2-LacZ reporter mice reveals estrogen-regulated endocervical glandular expression during estrous cycle, hormone replacement, and pregnancy. Glycobiology. 2004;14:169–175. doi: 10.1093/glycob/cwh019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domino SE, Zhang L, Gillespie PJ, Saunders TL, Lowe JB. Deficiency of reproductive tract alpha(1,2)fucosylated glycans and normal fertility in mice with targeted deletions of the FUT1 or FUT2 alpha(1,2)fucosyltransferase locus. Mol Cell Biol. 2001;21:8336–8345. doi: 10.1128/MCB.21.24.8336-8345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz BL, Packer NH, Karlsson NG. Small-scale analysis of O-linked oligosaccharides from glycoproteins and mucins separated by gel electrophoresis. Anal Chem. 2002;74:6088–6097. doi: 10.1021/ac025890a. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X, Oh SH, Cheng G, Green CB, Nuessen JA, Yeater K, Leng RP, Brown AJ, Hoyer LL. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology. 2004;150:2415–2428. doi: 10.1099/mic.0.26943-0. [DOI] [PubMed] [Google Scholar]
- 21.Casanova M, Cervera AM, Gozalbo D, Martinez JP. Hemin induces germ tube formation in Candida albicans. Infect Immun. 1997;65:4360–4364. doi: 10.1128/iai.65.10.4360-4364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Backstrom M, Link T, Olson FJ, Karlsson H, Graham R, Picco G, Burchell J, Taylor-Papadimitriou J, Noll T, Hansson GC. Recombinant MUC1 mucin with a breast cancer-like O-glycosylation produced in large amounts in Chinese-hamster ovary cells. Biochem J. 2003;376:677–686. doi: 10.1042/BJ20031130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersch-Bjorkman Y, Thomsson KA, Holmen Larsson JM, Ekerhovd E, Hansson GC. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol Cell Proteomics. 2007;6:708–716. doi: 10.1074/mcp.M600439-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Thomsson KA, Schulz BL, Packer NH, Karlsson NG. MUC5B glycosylation in human saliva reflects blood group and secretor status. Glycobiology. 2005;15:791–804. doi: 10.1093/glycob/cwi059. [DOI] [PubMed] [Google Scholar]
- 25.Domino SE, Zhang L, Lowe JB. Molecular cloning, genomic mapping, and expression of two secretor blood group alpha(1,2)fucosyltransferase genes differentially regulated in mouse uterine epithelium and gastrointestinal tract. J Biol Chem. 2001;276:23748–23756. doi: 10.1074/jbc.M100735200. [DOI] [PubMed] [Google Scholar]
- 26.Fidel PL, Jr, Sobel JD. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335–348. doi: 10.1128/cmr.9.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fidel PL, Jr, Cutright J, Steele C. Effects of reproductive hormones on experimental vaginal candidiasis. Infect Immun. 2000;68:651–657. doi: 10.1128/iai.68.2.651-657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parr MB, Parr EL. Mucosal immunity in the female and male reproductive tracts. In: Ogra PL, Mestecky JJ, Lamm ME, editors. Handbook of mucosal immunity. San Diego, Calif: Academic Press; 1994. pp. 677–689. [Google Scholar]
- 29.Zhang X, Essmann M, Burt ET, Larsen B. Estrogen effects on Candida albicans: a potential virulence-regulating mechanism. J Infect Dis. 2000;181:1441–1446. doi: 10.1086/315406. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffer AJ, Navas EL, Venegas MF, Anderson BE, Kanerva C, Chmiel JS, Duncan JL. Variation of blood group antigen expression on vaginal cells and mucus in secretor and nonsecretor women. J Urol. 1994;152:859–864. doi: 10.1016/s0022-5347(17)32591-0. [DOI] [PubMed] [Google Scholar]
- 31.Gipson IK. Mucins of the human endocervix. Front Biosci. 2001;6:D1245–1255. doi: 10.2741/gipson. [DOI] [PubMed] [Google Scholar]
- 32.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002;187:137–144. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 33.Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, Ponci F, De Grandi P. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J Natl Cancer Inst. 2003;95:1128–1137. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- 34.Nowicki S, Tassell AH, Nowicki B. Susceptibility to gonococcal infection during the menstrual cycle. Jama. 2000;283:1291–1292. doi: 10.1001/jama.283.10.1291. [DOI] [PubMed] [Google Scholar]
- 35.Sobel JD. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis. 1992;14(Suppl 1):S148–153. doi: 10.1093/clinids/14.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 36.Ventolini G, Baggish MS. Post-menopausal recurrent vaginal candidiasis: effect of hysterectomy on response to treatment, type of colonization and recurrence rates post-treatment. Maturitas. 2005;51:294–298. doi: 10.1016/j.maturitas.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Murta EF, Filho AC, Barcelos AC. Relation between vaginal and endocervical pH in pre- and post-menopausal women. Arch Gynecol Obstet. 2005a;272:211–213. doi: 10.1007/s00404-005-0740-4. [DOI] [PubMed] [Google Scholar]
- 38.Murta EF, Silva AO, Silva EA, Adad SJ. Frequency of infectious agents for vaginitis in non- and hysterectomized women. Arch Gynecol Obstet. 2005b;273:152–156. doi: 10.1007/s00404-005-0023-0. [DOI] [PubMed] [Google Scholar]
- 39.Thomsson KA, Hinojosa-Kurtzberg M, Axelsson KA, Domino SE, Lowe JB, Gendler SJ, Hansson GC. Intestinal mucins from cystic fibrosis mice show increased fucosylation due to an induced Fucalpha1-2 glycosyltransferase. Biochem J. 2002;367:609–616. doi: 10.1042/BJ20020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson FJ, Backstrom M, Karlsson H, Burchell J, Hansson GC. A MUC1 tandem repeat reporter protein produced in CHO-K1 cells has sialylated core 1 O-glycans and becomes more densely glycosylated if coexpressed with polypeptide-GalNAc-T4 transferase. Glycobiology. 2005;15:177–191. doi: 10.1093/glycob/cwh158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vaginal epithelial cells obtained by vaginal lavage from wild type and Fut2-null mice stained with UEA-I and Alcian blue pH 2.5 with control panels shown. Wild type and Fut2-null mice underwent either control surgery (ovariectomy alone) or hysterectomy (ovariectomy and total hysterectomy) to remove the entire cervix along with uterus. Intense Alcian blue pH 2.5 mucin staining (top panel) was present in washings from ovariectomized control wild-type (A) and Fut2-null mice (C) but absent in washings from hysterectomized wild type (B) and Fut2-null mice (D). Duplicate slides stained with UEA-I (bottom panel) show specific brown lectin staining associated with vaginal epithelial cells from pseudoestrus ovariectomized wild type mice (E) but absent in washings from pseudoestrus ovariectomized Fut2-null (G), hysterectomized wild type (F) and Fut2-null (H) mice. Bar = 100μm.