Abstract
Background
The corpus striatum, comprised of the caudate, putamen, and globus pallidus, plays an important role in reward processing and may be involved in the pathophysiology of antisocial behavior. Few studies have explored whether differences are present in the striatum of antisocial individuals. Here we examine the structure of the striatum in relation to psychopathy.
Methods
Using a case-control design, we examined the volume of the striatum in psychopathic individuals compared to controls matched for age, sex, ethnicity, and substance dependence. Twenty-two psychopathic individuals assessed using the Psychopathy Checklist – Revised and twenty-two comparison subjects underwent structural magnetic resonance imaging (MRI). Volumes of the left and right lenticular nucleus (putamen and globus pallidus), caudate head, and caudate body were assessed and the psychopathic and control groups were compared.
Results
Psychopathic individuals showed a 9.6% increase in striatum volumes. Analyses of subfactors of psychopathy revealed that caudate body volumes were primarily associated with the interpersonal and affective features of psychopathy, while caudate head volumes were primarily associated with the impulsive, stimulation-seeking features.
Conclusions
These findings provide new evidence for differences in the striatum of psychopathic individuals. This structural difference may partially underlie the reward-seeking and decision-making deficits associated with psychopathy.
Keywords: psychopathy, striatum, structural MRI, antisocial, reward, imaging
INTRODUCTION
Brain imaging research is beginning to uncover significant neurobiological impairments in antisocial, violent, and psychopathic groups. Although the majority of these studies demonstrate reduced structure or functioning in antisocial groups (1–3), there is evidence that some brain regions may actually be larger (4), particularly regions outside of the cortex (5, 6). The corpus striatum, comprised of the striatum (caudate and putamen) and globus pallidus, is a subcortical region that has received surprisingly little attention in the study of psychopathy. The striatum has been linked to traits such as reward-seeking and impulsivity, which are prevalent in psychopathy (7). It also plays an important role in stimulus-reinforcement learning (8) which has been found to be impaired in psychopathy (9, 10).
To date, to our knowledge, there has been no research on the structure of the striatum in psychopathic individuals. In antisocial groups, brain imaging studies have produced mixed results regarding the structure and function of the striatum. Increases in volume of the striatum have been found in men with antisocial personality disorder (11), and increased functioning has been observed in violent, but not non-violent patients with alcoholism (12), and adolescents and adults with aggressive behavior (13). However, one study found reduced activity in the striatum in criminal psychopaths during an affective memory task (1). In healthy samples, individual differences in novelty-seeking have been associated with increased connectivity between the striatum and the hippocampus/amygdala; individual differences in reward dependence have been associated with increased connectivity between the striatum and prefrontal cortex (14). In contrast, reduced volume of the striatum has been observed in undergraduates scoring higher on a measure of reward sensitivity (15). Thus, the association between the striatum and antisocial or psychopathic traits remains unclear.
Several researchers have suggested that psychopathic individuals show increased sensitivity to reward and decreased sensitivity to punishment when both types of stimuli are available (16, 17). This hypothesis has been demonstrated empirically in several studies; individuals with psychopathic traits tend to focus on the prospect of reward despite signals of later punishment (9, 17). This is in turn thought to result in reward seeking behavior, impulsivity, and impairments in stimulus-reinforcement learning (17). One hypothesis is that differences in the structure or functioning of the striatum could contribute to the increased reward sensitivity observed in psychopathic individuals.
The key question in this study is whether psychopathic individuals demonstrate structural differences in the striatum compared to controls. Since striatal structure and function have previously been associated with age (18), gender (19), and substance dependence (20), we felt it important to match participants on these factors, in addition to ethnicity. Secondly, psychopathy consists of several features, including manipulativeness, deceitfulness, reduced emotional responsiveness, impulsivity, stimulation-seeking, and antisocial behavior. Previous studies of the striatum have primarily demonstrated associations with reward-seeking and impulsivity. A second goal of the study therefore was to determine whether structural differences in the striatum were associated with all aspects of psychopathy, or primarily features of reward-seeking and impulsivity.
METHOD
Participants
Given the higher prevalence rates of psychopathy in males than females (21, 22), the present study focused primarily on males to increase the likelihood of enrolling participants scoring high in psychopathy. Seventy-seven males and nine females were recruited from five temporary employment agencies (3). Participants were unselected, except for the following exclusion criteria: age younger than 21 years or older than 45 years, nonfluency in English, history of epilepsy, history of psychosis, claustrophobia, pacemaker, and metal implants. This community recruitment strategy is novel, but has the advantages that it samples individuals at high socioeconomic risk, with an eight-fold increase in the yield of those with psychopathy/antisocial personality (3). To maximize confidentiality and minimize denial of self-report crime, a certificate of confidentiality was obtained from the Secretary of Health and Human Service under section 303 (a) of the Public Health Act 42. University Institutional Review Board (IRB) approval was obtained. After complete description of the study to the subjects, written informed consent was obtained.
A cutoff score of 23 and above on the Psychopathy Checklist – Revised (PCL-R) (7) was used for membership into the psychopathic group. This cutoff score is lower than what is typically used in prison populations which employ extensive collateral information to supplement ratings. Although we have some collateral information, including official criminal records, it is likely that we are under-estimating scores in our community sample. Thus, while a higher cut-off score may be appropriate in prison samples, it may be less appropriate for community samples. Importantly, this cutoff score is consistent with our prior work in community samples (5, 6, 23) which have resulted in theoretically meaningful findings.
Twenty-two individuals met the criterion for membership into the psychopathic group. Individuals scoring in the bottom half (0–18) of the distribution of scores on the PCL-R were considered for the control group. This range was used due to the lower prevalence of substance / alcohol dependence in individuals scoring lower in psychopathy. Twenty-two participants matched on age, sex, ethnicity, and substance / alcohol dependence were selected for the control group. Thus, the final sample consisted of 22 psychopathic individuals (i.e. PCL-R scores 23–40, 20 males and 2 females, 54.5% white, mean age 31 years, and 77.3% with substance / alcohol dependence) and 22 controls (i.e., PCL-R scores 0–18, 20 males and 2 females, 54.5% white, mean age 31 years, and 77.3% with substance / alcohol dependence). The two groups differed on the number of convictions of the participants. For the low psychopathy group, 18 had no convictions, 3 had one or two convictions, none had between three and ten convictions, and 1 had more than ten convictions. For the high psychopathy group, 10 had no convictions, 6 had one or two convictions, 5 had between three and ten convictions, and 1 had more than ten convictions. The 2 (group) × 4 (conviction level) chi-square was significant (χ2 = 8.3, p = .04). The two groups did not differ on any Axis 1 disorders. No participants in either group had current or past psychotic, eating, or somatization disorders. Nine participants in the psychopathy group and eight participants in the control group had current or past mood disorders (χ2 = .096, p = .757). Three participants in each group had current or past anxiety disorders. None of the participants had past or present use of psychotropic medications.
Diagnostic, Cognitive, and Physical Assessment
Subtests of the Wechsler Adult Intelligence Scale – Revised (24) were used to estimate verbal, performance, and total IQ. Handedness was assessed using the abbreviated Oldfield Inventory (25). History of head injury was defined as head trauma resulting in hospitalization. Socioeconomic status was derived from a structured psychosocial interview with the participant (6). A physical examination was conducted to derive measures of height and weight.
Psychopathy was assessed using the Psychopathy Checklist – Revised (7) supplemented by five sources of collateral data. The PCL-R consists of 20 items and has been conceptualized in both two- and four-factor models. In the two-factor model, Factor 1 represents the interpersonal and affective characteristics (e.g., glibness, superficial charm, pathological lying, shallow affect, lack of guilt or remorse) and Factor 2 represents the antisocial traits and behaviors (e.g., impulsivity, stimulation seeking, juvenile delinquency). In the four-factor model, these two factors are further broken down – Facet 1 represents the interpersonal features, Facet 2 represents the affective features, Facet 3 represents the lifestyle features (e.g., irresponsibility, impulsivity), and Facet 4 represents antisocial behavior.
Ratings on the PCL-R were made by a Ph.D. clinical graduate student trained and supervised by one of the authors (A.R.). Internal reliability (Cronbach α) was .90. The five collateral sources for assessing psychopathy were (1) the Interpersonal Measure of Psychopathy (26), which provides an interviewer's ratings of interpersonal behaviors, has demonstrated construct validity with the Psychopathy Checklist – Revised in a prison sample, and has been validated for use with nonincarcerated samples (i.e., college students (26)); (2) self-reported crime as assessed by an adult extension (3) of the National Youth Survey self-report delinquency measure (27); (3) official criminal records; (4) data derived from, and behavioral observations made during, the Structured Clinical Interview for the DSM-IV mental disorders (SCID-I) (28) and (5) the SCID Axis II personality disorders (29). Both were administered by a clinical Ph.D. student who received training that included reliability checks with expert raters (30). It should be noted that because our sample was not incarcerated, the collateral information available was not as extensive as may be found in prison settings. As such, it is possible that PCL-R scores obtained in this sample might have been higher had such external information been available. Participants also completed self-report questionnaires to assess alcohol and substance dependence (3).
Magnetic Resonance Imaging
Imaging procedures have been previously reported (3). Briefly, structural MRI was conducted on a 1.5-T scanner (model S15/ACS; Phillips, Shelton, Conn). After an initial alignment sequence of 1 midsagittal and 4 parasagittal scans (spin-echo T1-weighted image acquisition: repetition time, 600 ms; echo time, 2ms) to identify the anterior – posterior commissure plane, 128 3-dimensional T1-weighted gradient-echo coronal images (repetition time, 34 ms; echo time, 12.4 ms; flip angle, 35°; overcontiguous slices, 1.7 mm; matrix, 256 × 256; and field of view, 23 cm) were taken directly orthogonal to the anterior – posterior commissure line.
Three-dimensional brain images were reconstructed using a SPARC workstation (Sun Microsystems Inc, Santa Clara, Calif) and semi-automated software (CAMRA S200 ALLEGRO; Cedar Software Corp, Mississauga, Ontario, Canada) for gray matter – white matter – cerebrospinal fluid segmentation. Tissue segmentation was performed using a thresholding algorithm, with the operator (masked to group membership) applying a cutoff value to the signal-intensity histogram to optimally differentiate white matter from gray matter, areas of which were defined using an automated seeding algorithm on each slice. Whole brain volume was defined as all cerebral gray and white matter excluding the ventricles, pons, and cerebellum.
Volume measurements of the striatum were assessed using NIH Image software (31). All measures were obtained by manual tracings performed by raters who were blind to group membership. Volumes were calculated by summing the total area measurements across all slices and multiplying by slice thickness (1.7mm). No normalization techniques were used. Measurement of the striatum included the caudate, which was subdivided into the head and body, and the lenticular nucleus, which is the combined putamen and globus pallidus. Thus, six subregions of the striatum were assessed: left and right caudate head, left and right caudate body, and left and right lenticular nucleus. Landmarks for delineating the boundaries for the regions of the striatum were determined by referencing brain atlases (32, 33) and published MRI studies relevant to each region (34, 35). All volumetric measures were obtained in the coronal plane. Average reliability of volume measurements computed by intraclass correlation coefficients based on 10 randomly selected scans (raters masked to each other's ratings and to group membership) were .92 for the caudate and .69 for the lenticular nuclei.
Caudate
Bilateral caudate nucleus segmentation began in the first slice where it was clearly visible, which generally coincided with the genu of the corpus callosum (Figure 1). Segmentation was limited by the lateral ventricle medially and the internal capsule laterally. The posterior boundary of the caudate nucleus was defined as the slice before the first appearance of the splenium of the corpus callosum. Included in this measurement was most of the nucleus accumbens, which is ontogenetically and phylogenetically related to the caudate and cannot reliably be separated from it (35).
Figure 1.
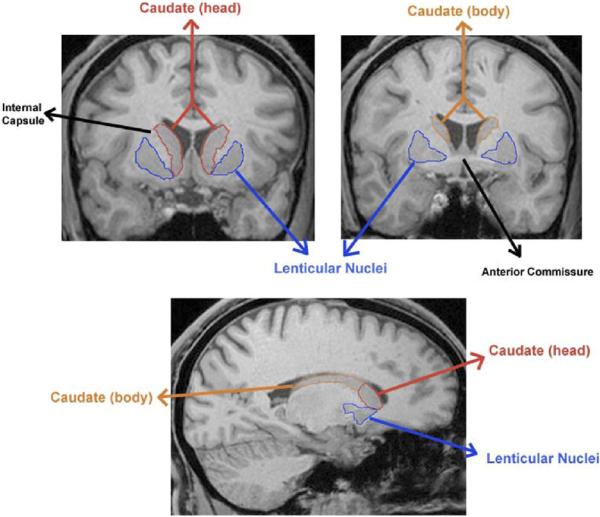
Top: Coronal slices of the striatum illustrating the delineation of the caudate and lenticular nuclei. Bottom: Sagittal slice depicting the distinction between the head and body of the caudate.
The caudate was subdivided into the head and the body based on the following landmarks: the anterior boundary of the head of the caudate was as defined above, while the posterior boundary was marked by the most prominent decussation of the anterior commissure, and generally corresponded with the last appearance of the nucleus accumbens. The anterior boundary of the body was the first slice following the posterior boundary of the head. The posterior boundary of the body was marked by the splenium of the corpus callosum (not inclusive).
Lenticular nuclei (putamen / globus pallidus)
Due to difficulty in reliably demarcating the globus pallidus from the putamen, these structures were combined, representing the lenticular nuclei (34). Segmentation began at the first appearance of the putamen, approximately 6.8 – 8.5 mm (4 or 5 slices) posterior to the anterior boundary of the caudate (Figure 1). Segmentation of the lenticular nuclei was limited by the internal capsule medially and external capsule laterally. Measurement proceeded in a posterior direction, encompassing the globus pallidus medially, and ended as the putamen assumed a slender, fusiform appearance approximately 50 mm posterior to the first slice (adapted from Hokama et al. 1995).
Statistical Analyses
Between-group t tests and χ2 tests were used to assess group differences on demographic, antisocial, cognitive, and MRI variables. All tests of significance are 2-tailed. Effect sizes were calculated using η2 (% variance accounted for) and Cohen's d. A univariate F test was used to determine whether the groups differed on overall striatal volume. To assess whether the combined subregions differed between groups, all striatal regions were entered into a multivariate analysis of variance (MANOVA). Follow-up univariate F tests were then conducted to assess which specific striatal regions differentiated the groups. Categorical group analyses were followed up with dimensional, multiple regression analyses conducted on the entire sample (N=88), controlling for age, sex, ethnicity, and substance and alcohol dependence. These analyses were used to explore the relationships between striatal volumes and the different factors/facets of psychopathy. In addition, due to previous findings linking the striatum to stimulation-seeking and impulsive features, Item 3 (Need for stimulation/proneness to boredom) and Item 14 (Impulsivity) were summed and examined as an index of these specific traits. We began by first looking at the relationship between total striatum volumes and the different factors/facets of psychopathy. If the relationship was significant or approaching significance, we performed additional, exploratory analyses of the the subregions of the striatum to determine whether the relationship with total volume was driven by a particular subregion.
RESULTS
Striatum Structure
Groups were found to significantly differ on total striatum volume (F1,42 = 6.3, η2 = 0.13, p = .016) (Figure 2). An omnibus MANOVA on the six subregions of the striatum (left and right lenticular nucleus, caudate head, and caudate body) indicated an overall group difference (F6,37 = 2.7, η2 = .30; p<.03). Follow-up univariate F tests indicated that psychopathic individuals had increased left and right lenticular nuclei and a trend for a larger right caudate body (Table 2).
Figure 2.
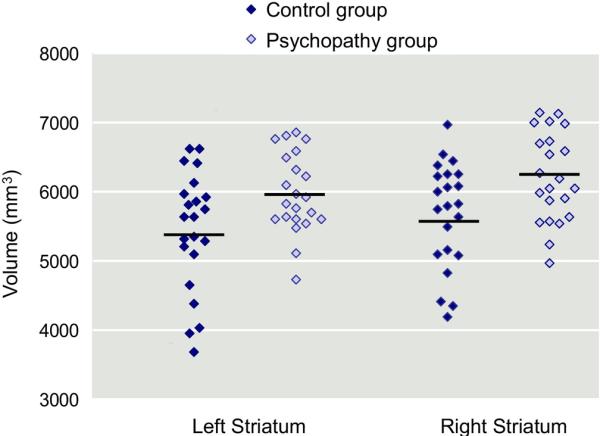
Scatterplots and means for volumes of the left and right striatum in the control (n=22) and psychopathic (n=22) groups.
Table 2.
Striatum Volume (mm3) in the Psychopathic and Control Groups
| Control Group (n=22) | Psychopathic Group (n=22) | F6,37 | P Value | η2 | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Total Striatum | 11116.4 | 1601.8 | 12184.3 | 1190.7 | 6.3 | .02 | .13 |
| Left Caudate Body | 739.6 | 258.5 | 825.1 | 226.4 | 1.4 | .25 | .03 |
| Right Caudate Body | 708.5 | 221.5 | 844.0 | 233.1 | 3.9 | .06 | .09 |
| Left Caudate Head | 1728.2 | 424.5 | 1890.4 | 317.7 | 2.1 | .16 | .05 |
| Right Caudate Head | 1869.6 | 487.1 | 1960.9 | 397.2 | 0.5 | .50 | .01 |
| Left Lenticular Nucleus | 2977.0 | 385.4 | 3258.1 | 378.7 | 6.0 | .02 | .12 |
| Right Lenticular Nucleus | 3093.6 | 333.3 | 3405.8 | 344.0 | 9.2 | .004 | .18 |
Relationships between Striatal Measures and Factors of Psychopathy
To examine the relationship between striatal regions and different factors of psychopathy, multiple regressions were conducted on the entire sample. Age, sex, ethnicity, and substance and alcohol dependence were included as covariates in all regression analyses (Table 3). Total striatum volume was associated with all of the facets of psychopathy except for Facet 4, which approached significance (p<.10). The caudate body was primarily associated with Factor 1 and its Interpersonal and Affective subfacets. The head of the caudate was primarily associated with the Lifestyle subfacet of psychopathy, particularly with the impulsive and stimulation-seeking items. The lenticular nucleus demonstrated relationships with both factors of psychopathy, and with the affective, impulsive/stimulation-seeking, and antisocial features; thus it did not appear to demonstrate specific relationships to one particular subfactor.
Table 3.
Regression Analyses Demonstrating Relationships between Psychopathy Factors and Striatal Regions (n=88)
| Striatum Measures |
|||||||
|---|---|---|---|---|---|---|---|
| Total Striatum | Caudate Body |
Caudate Head |
Lenticular Nucleus |
||||
| Left | Right | Left | Right | Left | Right | ||
| Total Psychopathy | .23* | .14 | .21* | .15 | .08 | .22* | .17† |
| Factor 1 Affective-Interpersonal | .27* | .23* | .28** | .12 | .10 | .23* | .20† |
| Facet 1 (Interpersonal) | .22* | .21* | .29** | .11 | .08 | .17 | .12 |
| Facet 2 (Affective) | .27* | .21* | .22* | .11 | .09 | .25* | .23* |
| Factor 2 Impulsive Antisocial | .22* | .12 | .18† | .13 | .07 | .21* | .20* |
| Facet 3 (Lifestyle) | .23* | .08 | .13 | .22* | .18† | .19† | .12 |
| Impulsivity / Stimulation-Seeking | .26** | .02 | .12 | .30** | .22* | .20* | .12 |
| Facet 4 (Antisocial) | .16† | .13 | .18† | .03 | .03 | .19† | .23* |
p<.10
p<.05
p<.01
Note: Summary of estimates from multiple regression models predicting striatum region volumes from psychopathy scores, age, sex, ethnicity, and substance and alcohol dependence. Numbers indicate standardized beta (β). Positive β indicates higher volumes for individuals higher in psychopathy. Exploratory analyses of the subregions of the striatum were performed to further probe the relationships between the psychopathy factors and total striatum volumes. Results were not corrected for multiple comparisons and should be considered preliminary.
Potential Confounds
Because the psychopathic group had significantly higher weight than controls (Table 1), this variable was entered as a covariate in a MANOVA containing the six measures. The main effect for group remained significant (F6,42 = 2.8, P<.022). The two groups demonstrated trend-level differences in performance IQ, height, and socioeconomic status, so these variables were also entered together with weight in the MANOVA. Again, the main effect of group on striatal measures remained significant (F6,42 = 2.5, P<.043).
Table 1.
Comparisons between the Control and Psychopathic Groups on Demographic and Psychiatric Measures
| Control Group (n=22) | Psychopathic Group (n=22) | Statistic | P Value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Case-control Matched Variables | ||||||
| Age | 31.0 | 6.6 | 31.1 | 6.9 | t= .02 | .98 |
| Sex, % male | 90.9 | 90.9 | χ2= .00 | 1.00 | ||
| Ethnicity, % white | 54.5 | 54.5 | χ2= .00 | 1.00 | ||
| Substance / alcohol dependence, % | 77.3 | 77.3 | χ2= .00 | 1.00 | ||
| Alcohol, % | 68.1 | 68.1 | χ2= .00 | 1.00 | ||
| Sedative, % | 9.0 | 9.0 | χ2= .00 | 1.00 | ||
| Cannabis, % | 45.4 | 54.5 | χ2= .21 | .65 | ||
| Stimulants, % | 18.2 | 13.6 | χ2= .17 | .68 | ||
| Opiates, % | 13.6 | 4.5 | χ2= 1.10 | .29 | ||
| Cocaine, % | 27.2 | 36.4 | χ2= .42 | .52 | ||
| Hallucinogens/PCP, % | 22.7 | 22.7 | χ2= .00 | 1.00 | ||
| Psychopathy Scores | ||||||
| Total | 12.9 | 3.5 | 27.2 | 4.1 | t= 12.5 | <.001 |
| Factor 1 Affective-Interpersonal | 3.2 | 2.4 | 10.3 | 2.7 | t= 9.2 | <.001 |
| Factor 2 Impulsive Antisocial | 6.6 | 2.2 | 12.1 | 2.9 | t= 7.0 | <.001 |
| Facet 1 (Interpersonal) | 1.9 | 1.6 | 5.2 | 1.4 | t= 7.5 | <.001 |
| Facet 2 (Affective) | 1.4 | 1.2 | 5.1 | 1.8 | t= 8.1 | <.001 |
| Facet 3 (Lifestyle) | 4.0 | 1.7 | 7.0 | 2.0 | t= 5.4 | <.001 |
| Facet 4 (Antisocial) | 3.6 | 1.9 | 6.8 | 1.9 | t= 5.6 | <.001 |
| Verbal IQ | 100.4 | 18.0 | 100.2 | 12.5 | t= .04 | .97 |
| Performance IQ | 104.8 | 21.3 | 95.9 | 14.4 | t= 1.6 | .11 |
| Total IQ | 113.3 | 29.8 | 107.5 | 20.3 | t= .77 | .49 |
| Handedness score | 36.1 | 8.1 | 35.4 | 9.2 | t= .25 | .80 |
| Height | 175.3 | 9.1 | 179.3 | 9.6 | t= 1.4 | .15 |
| Weight | 76.4 | 13.0 | 83.7 | 12.0 | t= 1.9 | .05 |
| Whole Brain Volume | 1183.5 | 101.8 | 1182.4 | 815.4 | t= .04 | .97 |
| History of Head Injury, % | 47.6 | 45.5 | χ2= .02 | .89 | ||
| Socioeconomic Status | 36.9 | 11.6 | 31.9 | 8.9 | t= 1.7 | .12 |
DISCUSSION
To our knowledge, this study establishes for the first time the existence of a structural difference in the striatum of psychopathic individuals. This group had a 9.6% increase in the volume of the striatum compared with controls, a large effect size corresponding to d = .76. Within the subregions, the left and right lenticular nuclei were 9.4% and 10.0% larger, respectively. Dimensional, regression analyses of the subfactors of psychopathy revealed that volumes of the lenticular nuclei were associated with several aspects of psychopathy. In contrast, the caudate body was primarily associated with the interpersonal and affective features of psychopathy, and the caudate head was primarily associated with the impulsive and antisocial features. These volume differences in the striatum could not be attributed to age, sex, ethnicity, substance or alcohol abuse, whole brain volume, or socioeconomic status. Findings implicate the striatum as a region that may be partially involved in the pathophysiology of psychopathy.
The present finding of increased volume is not likely to be attributable to environmental insults such as head injury, which tend to result in volumetric reductions, or to substance and alcohol dependence, which was held constant. This raises the important question of how increased striatal volume may develop. One possibility is that increased volume of the striatum in psychopathic individuals is due to neurodevelopmental pathology, which may include genetic or environmental (e.g., prenatal) influences. Results from recent imaging genetics studies suggest there may be a genetic basis to reward-related striatum activity that may increase the risk for impulsivity and reward dependency, such as that observed in psychopathy. Genetic polymorphisms associated with increased dopamine release in the striatum has been found to account for 9–12% of between-subject variability in reward-related striatum activity (36). A polymorphism in the human fatty acid amid hydrolase (FAAH) gene has also been found to account for increases in reward-related ventral striatum activity (37). Interestingly, this polymorphism was also associated with reduced threat-related amygdala activity, making it a particularly interesting mechanism to be examined in future studies of psychopathy.
An important question concerns how increased striatum volumes may predispose to psychopathy. First, results are congruent with data supporting the involvement of the striatum in reward sensitivity, which facilitates stimulation-seeking behavior, persistence in repeating actions related to rewards, and enhanced learning from rewarding signals (14, 38). Psychopathic individuals demonstrate increased stimulation-seeking (7), maladaptive response perseveration (39), and superior performance on tasks involving rewards only (40). Second, activity in the striatum has been associated with individual differences in impulsivity, as indicated by a preference for immediate over delayed rewards (41); psychopathic individuals are described as impulsive (7) and have been found to have deficits in delaying rewards (40). Third, the striatum is part of a neural circuitry involved in stimulus-reinforcement learning – the process of learning appropriate behaviors for given situations through reward and punishment information (8). Abnormalities in the striatum may disrupt this process. For example, a recent study found reduced striatum activity during threat of electric shocks in violent individuals with antisocial personality disorder (APD) (42). Impaired stimulus-reinforcement learning is theorized to be associated with poor decision-making, impaired socialization, and diminished empathy-based learning following cues of distress in others (43). Empirical studies have repeatedly confirmed that psychopathic individuals have deficits in stimulus-reinforcement learning (9, 10). Furthermore, the striatum is densely connected to the amygdala and ventromedial prefrontal cortex (44) which are necessary for stimulus-reinforcement learning and have been implicated in the related deficits observed in psychopathy (2, 43). The present results suggest that differences in the striatum may further contribute to these previously observed deficits in stimulus-reinforcement learning.
Heightened sensitivity to reward may be a source of motivation for criminal behavior; indeed, approximately 45% of psychopaths have been found to be motivated by material gain in their crimes (45). Community individuals scoring higher in psychopathy have also indicated a willingness to violate moral principles in exchange for a lesser amount of money than low-scoring individuals (46), demonstrating a moral flexibility in the presence of reward. Furthermore, adolescents with aggressive conduct disorder have shown increased activity in the striatum when viewing images of others in pain (47); it is suggested that aggressive individuals may enjoy seeing their victims in pain and, because of diminished amygdala / ventromedial prefrontal cortex connectivity, may not effectively regulate positively reinforced aggressive behavior. Together, these studies suggest that reward sensitivity, in combination with disinhibition, may play a large role in motivating the antisocial behavior observed in psychopathy.
The differences in the striatum observed in the present study were not exclusively associated with impulsive, stimulation-seeking traits, but demonstrated approximately equal relationships to all of the factors of psychopathy, although relationships with Factor 4 were marginally significant. This suggests that volume increases cannot be attributed solely to impulsive, stimulation-seeking traits, but are related to the broader construct of psychopathy. Further exploratory analyses of the relationship between the factors of psychopathy and the subregions of the striatum revealed that the head and body of the caudate demonstrated differential associations with the subfactors of psychopathy. The body was primarily associated with the interpersonal and affective features of psychopathy whereas the head was primarily associated with the impulsive and antisocial features. The head and body of the caudate have been found to be involved in functionally dissociable neural circuits (48), although further research will be needed to elucidate these differential relationships with respect to psychopathy. Activation specifically in the caudate body has been observed during deception (49, 50) and may be involved in inhibiting truthful responses (50). Although speculative, increased volume of the caudate body in individuals scoring higher on the interpersonal and affective features may reflect an enhanced ability to deceive others. In contrast, the head of the caudate may be more involved in responding to rewarding feedback (48) which may in turn lead to impulsive, stimulation-seeking traits. Increased caudate functioning has been observed in children with psychopathic traits during punished reversal errors, which is suggested to reflect impairment in processing reinforcement information (2). It should be noted that we did not correct for multiple comparisons in the correlations involving the subregions of the striatum, so these results should be regarded as preliminary.
It is important to be cautious in interpreting the findings of increased striatal volumes observed in the present study. First, although greater volume of a brain region is commonly interpreted as an indication of better functioning (11) and vice versa (23), increased volume may also reflect a lack of synaptic pruning during development, a process by which unnecessary connections are eliminated to increase the efficiency of other connections; thus, it is possible that increased volume could indicate poorer functioning (11). This interpretation would be consistent with previous findings of reduced striatum activity in criminal psychopaths (1) and violent individuals with APD (42). Second, it is unclear whether increased volume affects excitatory or inhibitory connections. Third, only an association has been shown between striatal volume and psychopathy, so causality cannot be assumed; structural differences in the striatum are more likely to contribute to a wider network of brain systems that motivate and regulate behavior. Finally, the volumetric difference observed in the present study is a phenotypical variable that is not, per se, a sign of abnormality.
Limitations of this study include the fact that samples were predominantly male, so findings cannot be generalized to psychopathic women. Group sizes were modest, although dimensional analyses on the larger sample indicated that the key results are reliable. Volumetric measurements were obtained from manual tracings, which may be more subjective than other methods. The putamen and globus pallidus were measured as one structure (lenticular nucleus), so differential relationships could not be assessed. Finally, we lack evidence from a behavioral measure that could potentially confirm or disconfirm the hypothesized link between striatal volumes and specific traits of psychopathy.
The present study represents a beginning step in determining whether differences in the striatum may be a contributing factor in the development of psychopathy. Important questions for future research include how the striatum may interact with other brain regions to produce psychopathy, as well as how genetic and developmental influences may alter the striatum in ways that may increase risk for psychopathic traits. Future research to place this specific structural brain difference within the functional context of more widespread frontolimbic circuits could help to confirm and extend the present findings.
Acknowledgements
This study was supported by Research Scientist Development Award K02 MH01114-01, Independent Scientist Award K02 MH01114-08, and grant 5 R03 MH50940-02 from the National Institute of Mental Health, Bethesda, Md (Dr. Raine), and by the Wacker Foundation, Dallas, Tex (Dr. Raine).
Footnotes
Financial Disclosures The authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- 2.Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, Kosson DS, Chen G, Towbin KE, Leibenluft E, Pine D, Blair RJ. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008;65:586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- 4.Tiihonen J, Rossi R, Laakso MP, Hodgins S, Testa C, Perez J, Repo-Tiihonen E, Vaurio O, Soininen H, Aronen HJ, Kononen M, Thompson P, Frisoni GB. Brain anatomy of persistent violent offenders: More rather than less. Psychiatry Research: Neuroimaging. 2008;163:201–212. doi: 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Bihrle S, Lacasse L, Colletti P. Hippocampal Structural Asymmetry in Unsuccessful Psychopaths. Biol Psychiatry. 2004;55:185–191. doi: 10.1016/s0006-3223(03)00727-3. [DOI] [PubMed] [Google Scholar]
- 6.Raine A, Lencz T, Taylor K, Hellige JB, Bihrle S, Lacasse L, Lee M, Ishikawa SS, Colletti P. Corpus Callosum Abnormalities in Psychopathic Antisocial Individuals. Arch Gen Psychiatry. 2003;60:1134–1142. doi: 10.1001/archpsyc.60.11.1134. [DOI] [PubMed] [Google Scholar]
- 7.Hare RD. Manual for the Hare Psychopathy Checklist-Revised. Multi-Health Systems; Toronto: 1991. [Google Scholar]
- 8.Haruno M, Kuroda T, Doya K, Toyama K, Kimura M, Samejima K, Imamizu H, Kawato M. A neural correlate of reward-based behavioral learning in caudate nucleus: a functional magnetic resonance imaging study of a stochastic decision task. Journal of Neuroscience. 1994;24:1660–1665. doi: 10.1523/JNEUROSCI.3417-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. J Abnorm Psychol. 1986;95:252–256. [PubMed] [Google Scholar]
- 10.Blair RJ, Mitchell DGV, Leonard A, Budhani S, Peschardt KS, Newman C. Passive avoidance learning in individuals with psychopathy: modulation by reward but not by punishment. Pers Individ Dif. 2004;37(6):1179–1192. [Google Scholar]
- 11.Barkataki I, Kumari V, Das M, Taylor P, Sharma T. Volumetric structural brain abnormalities in men with schizophrenia or antisocial personality disorder. Behavioral Brain Research. 2006;15:239–247. doi: 10.1016/j.bbr.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Tiihonen J, Kuikka J, Bergstrom K, Hakola P, Karhu J, Ryynanen OP, Fohr J. Altered striatal dopamine re-uptake site densities in habitually violent and non-violent alcoholics. Nature Medicine. 1995;1:654–657. doi: 10.1038/nm0795-654. [DOI] [PubMed] [Google Scholar]
- 13.Amen DG, Stubblefield M, Carmichael B, Thisted R. Brain SPECT findings and aggressiveness. Ann Clin Psychiatry. 1996;8:129–137. doi: 10.3109/10401239609147750. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MX, Schoene-Bake J-C, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nature Neuroscience. 2009;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- 15.Barros-Loscertales A, Meseguer V, Sanjuan A, Belloch V, Parcet MA, Torrubia R, Avila C. Striatum gray matter reduction in males with an overactive behavioral activation system. European Journal of Neuroscience. 2006;24:2071–2074. doi: 10.1111/j.1460-9568.2006.05084.x. [DOI] [PubMed] [Google Scholar]
- 16.van Honk J, Schutter DJLG. Unmasking feigned sanity: A neurobiological model of emotion processing in primary psychopathy. Cognit Neuropsyhiatry. 2006;11(3):285–306. doi: 10.1080/13546800500233728. [DOI] [PubMed] [Google Scholar]
- 17.Scerbo AS, Raine A, O'Brien M, Chan CJ, Rhee C, Smiley N. Reward dominance and passive avoidance learning in adolescent psychopaths. J Abnorm Child Psychol. 1990;18:451–463. doi: 10.1007/BF00917646. [DOI] [PubMed] [Google Scholar]
- 18.Gunning-Dixon F, Head D, McQuain J, Acker JD, Raz N. Differential aging of the human striatum: A prospective MR imaging study. Am J Neuroradiol. 1998;19:1501–1507. [PMC free article] [PubMed] [Google Scholar]
- 19.Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci. 2001;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- 21.Forth AE, Brown SL, Hart SD, Hare RD. The assessment of psychopathy in male and female noncriminals: Reliability and validity. Pers Individ Dif. 1996;20:531–543. [Google Scholar]
- 22.Hare RD. Hare Psychopathy Checklist-Revised (PCL-R) 2nd Edition Multi-Health Systems, Inc.; Toronto: 2003. [Google Scholar]
- 23.Yang Y, Raine A, Lencz T, Bihrle S, Lacasse L, Colletti P. Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biol Psychiatry. 2005;15(57):1103–1108. doi: 10.1016/j.biopsych.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. Wechsler Adult Intelligence Scale - Revised. Psychological Corp; San Antonio: 1981. [Google Scholar]
- 25.Bryden MP. Measuring handedness with questionnaires. Neuropsychologia. 1977;15:617–624. doi: 10.1016/0028-3932(77)90067-7. [DOI] [PubMed] [Google Scholar]
- 26.Kosson DS, Steuwerald BL, Forth AE, Kirkhart KJ. A new method for assessing the interpersonal behavior of psychopathic individuals: Preliminary validation studies. Psychological Assessment. 1997;9:89–101. [Google Scholar]
- 27.Elliot DS, Ageton SS, Huizinga D, Knowles BA, Canter RJ. The prevalence and incidence of delinquent behavior. Behavioral Research Institute; Boulder, CO: 1983. pp. 1976–1980. [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. User's Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders: SCID-I Clinician Version. American Psychiatric Press; Washington, DC: 1996. [Google Scholar]
- 29.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- 30.Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 31.Rasband WS, Bright DS. NIH Image: A public domain image processing program for the Macintosh. Microbeam Analysis Society Journal. 1995;4:137–149. [Google Scholar]
- 32.De Armond SJ, Fusco MM, Dewey MM. Structure of the Human Brain: A Photographic Atlas. Oxford University Press; New York: 1989. [Google Scholar]
- 33.Haines DE. Neuroanatomy: an atlas of structures, sections, and systems. Williams & Wilkins; USA: 1995. [Google Scholar]
- 34.Corson P, Nopoulos P, Miller DD, Arndt S, Andreasen NC. Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry. 1999;156:1200–1204. doi: 10.1176/ajp.156.8.1200. [DOI] [PubMed] [Google Scholar]
- 35.Hokama H, Shenton ME, Nestor PG, Kikinis R, Levitt JJ, Metcalf D, Wible CG, O'Donnell BF, Jolesz FA, McCarley RW. Caudate, putamen, and globus pallidus volume in schizophrenia. Psychiatry Research: Neuroimaging. 1995;61:209–229. doi: 10.1016/0925-4927(95)02729-h. [DOI] [PubMed] [Google Scholar]
- 36.Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2007;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent effects of genetic variation in endocannabinoid signaling on human threat and reward-related brain function. Biol Psychiatry. doi: 10.1016/j.biopsych.2008.10.047. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Doherty J. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Newman JP, Patterson CM, Kosson DS. Response Perseveration in Psychopaths. J Abnorm Psychol. 1987;96(2):145–148. doi: 10.1037//0021-843x.96.2.145. [DOI] [PubMed] [Google Scholar]
- 40.Newman JP, Kosson DS, Patterson CM. Delay of gratification in psychopathic and nonpychopathic offenders. Journal of Abnormal Psychology. 1992;101(4):630–636. doi: 10.1037//0021-843x.101.4.630. [DOI] [PubMed] [Google Scholar]
- 41.Hariri AR, Brown SM, Williamson DE, Flory JD, deWit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral straital activity. Journal of Neuroscience. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumari V, Das M, Taylor PJ, Barkataki I, Andrew C, Sumich A, Williams SCR, ffytche DH. Neural and behavioral responses to threat in men with a history of serious violence and schizophrenia or antisocial personality disorder. Schizophrenia Research. 2009;110:47–58. doi: 10.1016/j.schres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Blair RJ. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci. 2007;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 45.Williamson S, Hare RD, Wong S. Violence: Criminal psychopaths and their victims. Canadian Journal of Behavioral Science. 1987;19:454–462. [Google Scholar]
- 46.Glenn AL, Iyer R, Graham J, Koleva S, Haidt J. Are all types of morality compromised in psychopathy? J Personal Disord. doi: 10.1521/pedi.2009.23.4.384. in press. [DOI] [PubMed] [Google Scholar]
- 47.Decety J, Michalska KJ, Akitsuki Y, Lahey BB. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biological Psychology. doi: 10.1016/j.biopsycho.2008.09.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seger CA, Cincotta CM. The roles of the caudate nucleus in human classification learning. Journal of Neuroscience. 2005;25:2941–2951. doi: 10.1523/JNEUROSCI.3401-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohamed FB, Faro SH, Gordon NJ, Platek SM, Ahmad H, Williams JM. Brain mapping of deception and truth telling about an ecologically valid situation: Functional MR imaging and polygraph investigation--initial experience. Radiology. 2006;238:679–688. doi: 10.1148/radiol.2382050237. [DOI] [PubMed] [Google Scholar]
- 50.Lee TMC, Liu HL, Tan LH, Chan CCH, Mahankali S, Feng CM, Hou JW, Fox PT, Gao JH. Lie detection by functional magnetic resonance imaging. Hum Brain Mapping. 2002;15:157–164. doi: 10.1002/hbm.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]


