Abstract
Defects in pRb tumor suppressor pathway occur in ~50% of the deadly muscle-invasive urothelial carcinomas in humans and urothelial carcinoma is the most prevalent epithelial cancer in long-term survivors of hereditary retinoblastomas caused by loss-of-function RB1 mutations. Here we show that conditional inactivation of both RB1 alleles in mouse urothelium failed to accelerate urothelial proliferation. Instead, it profoundly activated the p53 pathway, leading to extensive apoptosis, and selectively induced pRb family member p107. Thus, pRb loss triggered multiple failsafe mechanisms whereby urothelial cells evade tumorigenesis. Additional loss of p53 in pRb-deficient urothelial cells removed these p53-dependent tumor barriers, resulting in late-onset hyperplasia, umbrella cell nuclear atypia and rare-occurring low-grade, superficial papillary bladder tumors, without eliciting invasive carcinomas. Importantly, mice deficient in both pRb and p53, but not those deficient in either protein alone, were highly susceptible to sub-threshold carcinogen exposure and developed invasive urothelial carcinomas that strongly resembled the human counterparts. The invasive lesions had a marked reduction of p107, but not p130, of the pRb family. Our data provide compelling evidence indicating that urothelium, one of the slowest cycling epithelia, is remarkably resistant to transformation by pRb or p53 deficiency; that concurrent loss of these two tumor suppressors is necessary but insufficient to initiate urothelial tumorigenesis along the invasive pathway; that p107 may play a critical role in suppressing invasive urothelial tumor formation; and that replacing/restoring the function of pRb, p107 or p53 could be explored as a potential therapeutic strategy to block urothelial tumor progression.
Keywords: bladder cancer, tumor suppressor gene, conditional knockout, tumor progression, uroplakin
INTRODUCTION
Urothelial carcinoma presents an interesting paradigm of tumor initiation and progression via divergent phenotypic and molecular pathways (1–6). About 70% of the carcinomas arise as low-grade, papillary tumors that are confined to the urothelial compartment. These tumors often occur at multiple loci in the bladder and, despite surgical removal and peri-operative chemotherapy, they recur time and again over the lifetime of the afflicted individuals. However, the chance for these tumors to advance to the muscle-invasive stage is relatively small and the 5-year survival rate approaches 95% (7, 8). The rest (~30%) of the urothelial carcinomas are high-grade and muscle-invasive at diagnosis. In spite of radical cystectomy in conjunction with debilitating chemotherapy and/or radiotherapy, over 50% of these tumors eventually spread to distant organs. The 5-year survival rate for patients with distant metastasis is only ~6% (9). Longitudinal studies indicate that most of the muscle-invasive urothelial carcinomas have no prior history of low-grade superficial papillary tumors and they may have arisen de novo or have derived from flat, high-grade carcinoma in situ (CIS) lesions (10, 11). Therefore, the two major urothelial carcinoma variants do not appear to represent a continuum of tumor progression from early to late stages, but rather they seem to result from distinct mechanisms of tumor initiation (1–6).
Emerging evidence from humans and animal models suggests that two distinct sets of genetic alterations drive urothelial tumorigenesis along divergent pathways. In human low-grade, non-invasive urothelial carcinomas, gain-of-function mutations of ras1 pathway components, particularly ras itself or its upstream-acting fibroblast growth factor receptor 3b (FGFR3b), are exceedingly common (4, 12). Mutations of these two genes seem always mutually exclusive (13), and together, they account for up to 90% of this urothelial tumor variant (2). Consistent with this, urothelium-specific expression of a constitutively active Ha-ras oncogene in transgenic mice elicits urothelial hyperplasia, approximately half of which evolves, over a 28-month period, to low-grade, superficial papillary carcinomas that bear strong resemblance to the human counterparts (14). Doubling the transgene dosage in the same transgenic line dramatically shortens the tumor latency, provoking early-onset urothelial carcinomas without triggering tumor invasion (15). Finally, human patients with Costello syndrome, which is caused by germline mutations in the Ha-ras gene, are prone to developing early-onset, low-grade, non-invasive urothelial carcinomas (16). Collectively, these data indicate that overactivation of the ras signaling pathway is a principal cause of low-grade, non-invasive urothelial carcinomas.
Much less is known about what triggers the muscle-invasive urothelial carcinomas despite their high mortality rate. Among the numerous genomic, genetic and epigenetic alterations, those affecting RB1 and p53 tumor suppressor genes are by far the most common (17). While inactivating mutations of RB1 are rare, reduced or loss of RB1 expression accounts for 40–50% of the invasive carcinomas, and is strongly associated with poor clinical outcome. Interestingly, long-term survivors of hereditary retinoblastomas that harbor RB1 mutations were highly susceptible to developing high-grade urothelial carcinomas (18). As for p53, its loss-of-function mutations occur in up to 60% of the muscle-invasive urothelial carcinomas (19), and are often associated with disease progression (20). Furthermore, pRb and p53 abnormalities coexist in 40–50% of the muscle-invasive urothelial carcinomas, and together, they predict a more aggressive tumor behavior and poorer patient survival than carcinomas bearing abnormalities in only one gene (21–23). Therefore, defects in pRb and p53 have been synonymous with, and have been speculated to play crucial roles in, the invasive urothelial tumorigenesis.
Despite the strong clinical correlation, little experimental evidence exists to either prove or refute the presumed importance of pRb and/or p53 deficiency in invasive urothelial tumorigenesis. Mice with RB1 ablated in all tissues die embryonically (24), thus precluding studies on whether RB1 deficiency is tumorigenic for urothelium. The lethality also makes it impossible to decipher how other members of pRb (pocket) family including p107 (RB2) and p130 (RB3) respond to pRb loss in the urothelium. Mice globally deficient for p53 survive to term, but they succumb to thymic lymphomas and soft tissue sarcomas 3–7 months of age, when urothelial cells remain normal (25). Because of these constraints, it has been impossible to discern whether urothelial defects of pRb or p53 are tumor-initiating or are merely tumor-promoting. It is also unclear whether these two genetic defects intersect at certain point of the multistage urothelial tumorigenesis. Although urothelial expression of an SV40 large T antigen in transgenic mice elicited urothelial carcinoma in situ and invasive carcinomas (26, 27), it cannot be ruled out that this was due to the broad effects of this oncogene on inactivating not only pRb and p53, but also pRb family members p107 and p130 (28).
To address these issues, we ablated RB1 and p53 genes alone or in a combination in mouse urothelia, taking advantage of our urothelium-specific knockout system and the availability of loxP-flanked (“floxed”) RB1 and p53 transgenic mice (29, 30). We studied the urothelial responses to the loss of these tumor suppressors and the tumorigenic potential of these genetic defects under normal conditions and carcinogenic stress. Our findings shed light on the combinatorial factors necessary for driving the invasive urothelial tumorigenesis and the cell type specificity and context dependence of tumor suppressor deficiency.
MATERIALS AND METHODS
Generation and Characterization of Conditional Knockout Mice
UPII-Cre transgenic mice that expressed Cre recombinase in urothelium-specific manner (31) were crossed with “floxed” RB1 mice where exon 19 was flanked with two loxP sites (29), and additional crosses produced homozygous mice for both UPII-Cre and “floxed” RB1 alleles. UPII-Cre mice were also crossed with “floxed” p53 mice where exons 5 and 6 were flanked by two loxP sites (30). Further crosses produced homozygous mice for both UPII-Cre and “floxed” p53 alleles. The two double transgenics were inter-crossed to produce homozygous mice for all three alleles (UPII-Cre, “floxed” p53 and “floxed” RB1). Genotyping of UPII-Cre transgene was done with Southern blotting and that for “floxed” RB1 and p53 alleles with PCR (29, 30). Four groups of mice were used: (1) UPII-Cre only mice; (2) Cre/“floxed” RB1 (RB1−/−); (3) UPII-Cre/“floxed” p53 (p53−/−); and (4) UPII-Cre/“floxed” p53/“floxed” RB1 (RB1−/−/p53−/−). Cre-mediated recombination of the “floxed” genes was assessed on DNA and RNA levels using PCR. All animal experiments were conducted in accordance with regulations for the humane use of animals for scientific research and under active protocols approved by institutional animal care and use committee.
In situ Hybridization
Site-specific probes were used to determine the truncation of RB1 and/or p53 in urothelium. Briefly, RT-PCR amplified the cDNA corresponding to exon 19 of the RB1 gene or cDNA corresponding to exons 5 and 6 of the p53 gene. The cDNAs were transcribed into anti-sense and sense cRNAs in the presence of digoxigenin-conjugated UTP. Mouse bladders were fixed in 4% paraformaldehyde and embedded in paraffin. Four micron-thick sections were digested in protease K for 15 min, incubated with the probes for 16 hours, reacted with anti-digoxigenin antibody conjugated with alkaline phosphatase and developed in a nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate solution (Hoffmann-La Roche Inc., Nutley, NJ).
Quantitative Real-time PCR
The expression of pRb and p53 pathway effectors was assessed by real-time PCR using a LightCycler RNA amplification kit (Roche Diagnostics, Nutley, NJ). Double-stranded urothelial cDNA was used for PCR at 95°C for 15’ for the first cycle; 95°C for 15”, 55–58°C for 20”, and 72°C for 30” for 50 cycles. The products were detected by direct incorporation of SYBR Green I into newly synthesized DNA. The relative abundance was expressed as a ratio to β-actin.
TUNEL Assay
De-paraffinized sections of mouse urinary bladders were incubated in a DNA labeling solution (APO-BrdU TUNEL Assay Kit; Invitrogen) for 1 hr at 37°C. The sections were then incubated with an anti-BrdU solution for 30 min.
Western Blot Analyses
Total urothelial proteins were dissolved in a lysis buffer containing 10% SDS, 20 mM Tris/HCl (pH7.5), 50 mM NaCl, 5 mM β-mercaptoethanol and a cocktail of protease inhibitors. Sixty micrograms of the proteins were resolved by SDS-PAGE, electrophoretically transferred onto PVDF membrane, incubated with primary antibodies, then with peroxidase-coupled secondary antibodies and developed using an enhanced chemiluminescent method (Amersham Biosciences, Piscataway, NJ). The primary antibodies were: anti-E2F1, anti-p107, anti-130, anti-E2F4, anti-caspase 3 (Cell Signaling Technology, Inc., Danvers, MA); anti-MAD2 (Proteintech Group, Inc., Chicago, IL); anti-p19, anti-p53, anti-p21 (Abcam, Inc., Cambridge, MA); anti-MDM2, anti-Bak, anti-Bax (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Antibody against MAPK (Cell Signaling Technology, Inc., Danvers, MA) served as a loading control.
Carcinogen Treatment of Knockout Mice
Five groups of mice (4–5 months old) were treated with N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN; TCI America, Portland, OR): (1) UPII-Cre (n = 23); (2) RB1−/− (n = 16); (3) p53−/− (n = 20); (4) RB1+/−/p53+/− (n = 8); (5) RB1−/−/p53−/− (n = 22). BBN was supplied ad libitum in the drinking water (final concentration = 0.01%) for 10 weeks. No difference in water consumption was observed among different groups.
Immunohistochemistry
De-paraffinized bladder sections were microwaved in citrate buffer (pH 6.0) for 20 min to unmask the antigens and were then incubated with primary and secondary antibodies conjugated with horseradish peroxidase and developed in a solution containing H2O2 and 3,3'-diaminobenzidine tetrahydrochloride. The primary antibodies were those for Western blotting and also included those against Ki67 (Abcam, Inc., Cambridge, MA), 34βE12 (Dako, Carpinteria, CA); E-cadherin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); and MMP9 (R&D Systems, Inc., Minneapolis, MN).
RESULTS
Loss of pRb Function in Mouse Urothelium Is Not Tumorigenic, Owing to a Compensatory Rescue by Multiple Secondary Tumor Defenses
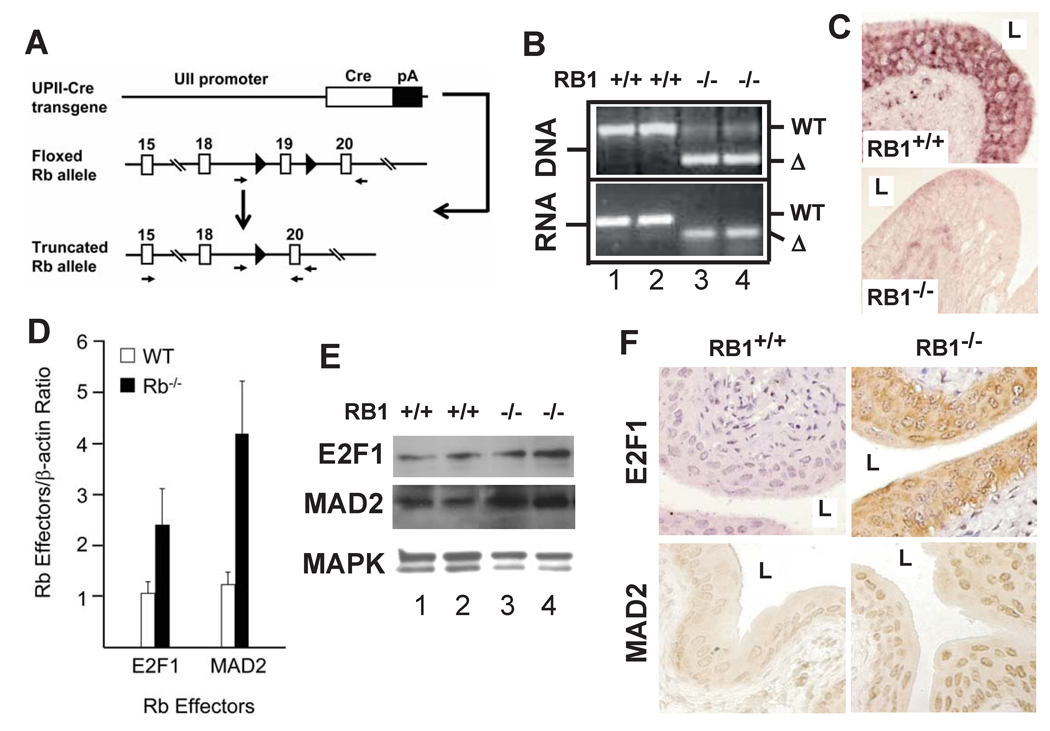
Despite a close association of pRb deficiency with advanced human urothelial carcinomas, it was unknown whether pRb deficiency alone can transform the urothelial cells. By expressing Cre recombinase under the control of a mouse uroplakin II promoter (UPII-Cre) in mice where exon 19 of the RB1 gene was flanked with two loxP sequences (floxed RB1; Fig. 1A), we achieved pRb inactivation exclusively in the urothelium. This circumvented the problem of embryonic lethality caused by global RB1 ablation. PCR using DNA and RNA from UPIICre/Cre/RB1flox/flox transgenic mice (or RB1−/− mice from here on) established the urothelium-specific truncation of RB1 (Fig. 1B). In situ hybridization using a deletion-site-specific ribo-probe (corresponding to the exon 19) detected no mRNA of the wild-type RB1 in any urothelial layer of the RB1−/− mice (Fig. 1C; lower panel), as opposed to the RB1+/+ mice where RB1 was detected in all layers (Fig. 1C; upper panel). Consistent with the previous finding that deletion of exon 19 is functionally equivalent to inactivation of the entire RB1 gene (29), urothelial truncation of RB1 led to a marked induction of pRb downstream effectors E2F1 and MAD2 (32), as evidenced by real-time quantitative PCR (Fig. 1D), Western blotting (Fig. 1E) and immunohistochemistry (Fig. 1F). These data firmly established the conditional knockout of RB1 in the urothelium.
Figure 1. Conditional inactivation of RB1 in mouse urothelium.
(A) Targeting strategy. Top panel: UPII-Cre transgene in which a mouse uroplakin II promoter restricts Cre recombinase expression to urothelium; Middle panel: RB1 allele in which its exon 19 was flanked by two loxP sites (filled triangles); and Bottom panel: recombined RB1 allele with exon 19 deleted upon urothelial Cre expression. Arrows: primers for detecting RB1 truncation. (B) PCR detection of truncated RB1 in urothelium. Note a 260-bp truncated (Δ) RB1 DNA and a 50-bp truncated RB1 mRNA in UPIICre/Cre/RB1flox/flox transgenic mice (or RB1−/− mice) (lanes 3 and 4) and the absence of such truncated species in UPIICre/Cre only mice (or RB1+/+; lanes 1 and 2). (C) In situ hybridization. Anti-sense cRNA probe corresponding to exon 19 of RB1 reacted with urothelia of an RB1+/+ mouse (top) but not that of an RB1−/− mouse. Magnification: 200 ×. (D–F) Real-time RT-PCR (D), Western blotting (E) and immunohistochemistry (F) of pRb effectors, showing induction of E2F1 and MAD2 in the RB1−/− mice. Values in (D): means and standard deviation from 8 mice for each genotype. “L” in (C & F) denotes bladder lumen.
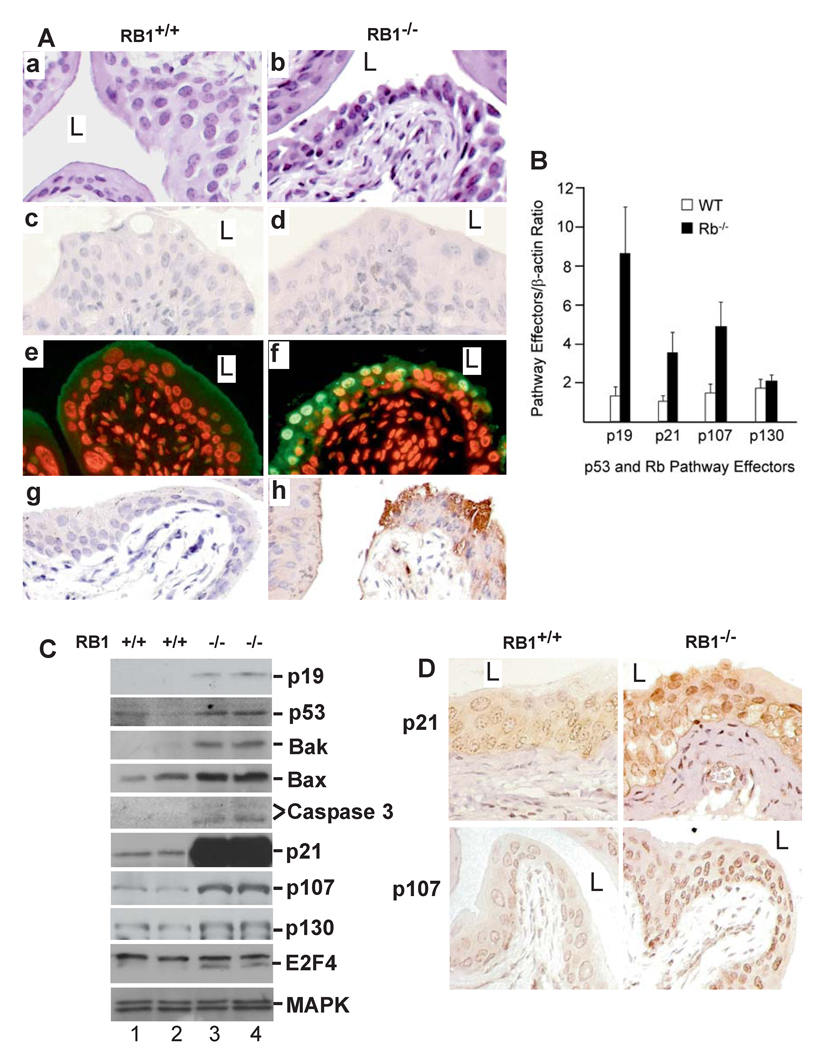
Unexpectedly, urothelial pRb inactivation failed to elicit hyper-proliferation or tumorigenesis after 28-months of follow-up of a cohort of 80 RB1−/− mice. The urothelia of the null mice were not thickened as compared to the wild-type control (Fig. 2A; panels (a) and (b)), and lacked expression of proliferative marker Ki67 (Fig. 2A; panels (c) and (d)). Instead, the pRb-deficient urothelial cells, particularly those in the superficial layer, exhibited condensed nuclei and increased intercellular space (Fig. 2A; panel b). TUNEL assay and anti-caspase 3 staining revealed many apoptotic cells (Fig. 2; panels f and h). These data provide compelling evidence that not only did RB1 deficiency fail to induce urothelial proliferation or tumorigenesis, it triggered apoptosis.
Figure 2. Urothelial response to pRb deficiency.
(A) H&E-stained urothelia of (a) an RB1+/+ mouse (15-month old) showing normal morphology and (b) an age-matched, RB1−/− mouse showing condensed nuclei and widened intercellular space. (c & d) anti-Ki67 staining showing lack of urothelial proliferation in the RB1−/− mouse. (e & f) TUNEL assay revealing apoptosis in the RB1−/− mouse (f) but not in the RB1+/+ control (e). (g & h) anti-caspase 3 antibody strongly labeling urothelial cells in RB1−/− mouse (h) but not in the RB1+/+ mouse (g). Magnification: 200 ×. (B–D) Real-time RT-PCR (B), Western blotting (C) and immunohistochemistry (D) showing strong induction of p53 pathway components p19, p53, Bak, Bax, activated caspase 3 fragments, p21 and pRb family member p107 and its binding partner E2F4, but not p130, in RB1−/− mice. n=8 in (B).
To determine the molecular mechanism(s) whereby RB1 deficiency led to the above consequences, we evaluated the key components of the p53 pathway and the pRb family. We found p19, p53 and p21 to be up-regulated in the RB1-deficient urothelial cells (Fig. 2B–D), with p21 protein increase being most dramatic (Fig. 2C). Pro-apoptotic molecules Bak and Bax and activated caspase fragments were also markedly upregulated (Fig. 2C), thus explaining the TUNEL results (Fig. 2A). Additionally, we observed a significant induction of p107, but not p130, albeit both members of the pRb family (Fig. 2B–D). Coinciding with the p107 induction was the increased expression of E2F4, which binds p107 and acts as a transcriptional repressor/growth inhibitor (33). These findings unravel several failsafe mechanisms with which the urothelial cells escape tumorigenesis during pRb deficiency.
Additional Loss of p53 in RB1-deficient Urothelial Cells Blunts p53-dependent Responses and Elicits Late-onset Urothelial Hyperproliferation
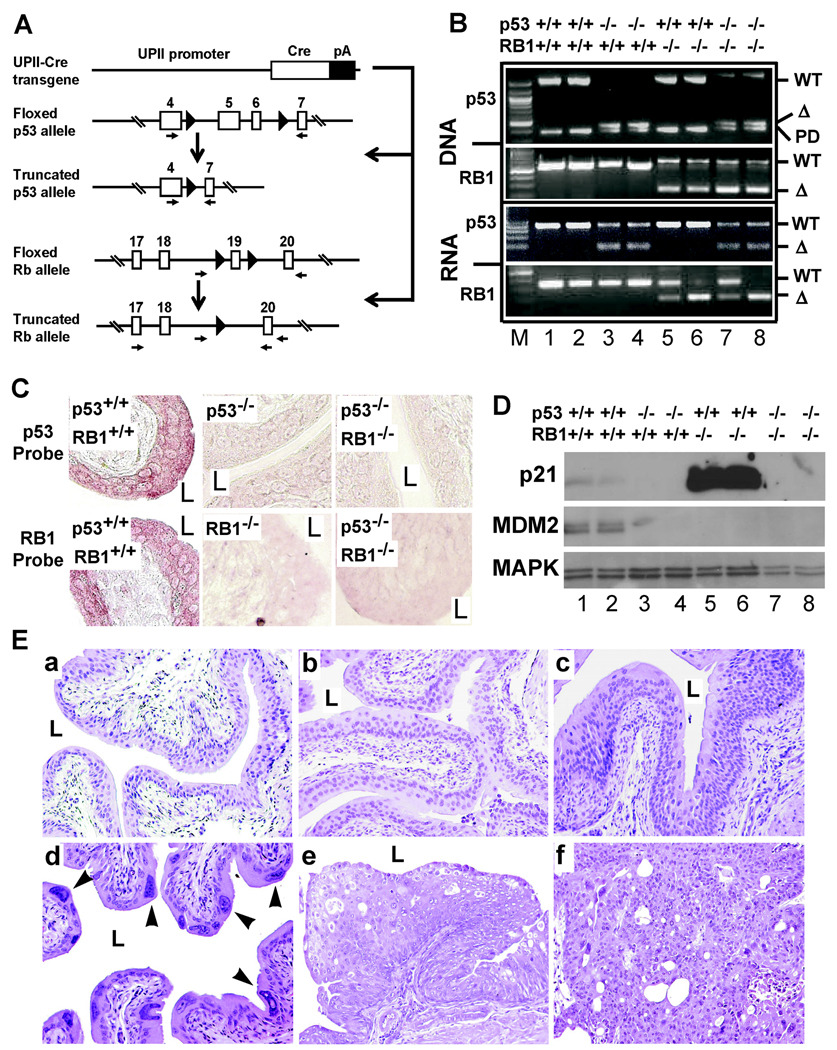
Because pRb deficiency activated the p53 pathway and urothelial apoptosis, we sought to dampen such “rescuing” effects, so as to promote urothelial proliferation. We crossed the UPIICre/Cre/RB1flox/flox with another mouse strain where exons 5 and 6 (encoding the DNA-binding domain) of the p53 gene were flanked with two loxP sites (Fig. 3A; (30)). Additional intercrosses among the littermates yielded mice deficient for RB1 (RB1−/−), p53 (p53−/−), or both (RB1−/−/p53−/−) in the urothelia. PCR and in situ hybridization proved urothelial truncation of p53 and/or RB1 DNA and RNA (Fig. 3B and 3C; data not shown). The functional effect of p53 deficiency was evident based on significant reduction of p53 downstream effectors p21 and MDM2 (Fig. 3D). Most notably, the pRb-deficiency-triggered induction of p21 (Fig. 3D; lanes 5 and 6) was completely abrogated in the p53−/−/RB1−/− mice (Fig. 3D; lanes 7 and 8). The reduced MDM2 expression in pRb−/− urothelium (Fig. 3D; lanes 5 and 6) was probably due to the increased expression of p19 (Fig. 2C), which binds MDM2 and targets it for ubiquitination (34). Like pRb deficiency, p53 deficiency alone did not enhance urothelial proliferation despite a long-term (up to 30-month) follow-up (Fig. 3E). Approximately 10% of the mice deficient for both p53 and pRb developed urothelial hyperplasia (Fig. 3E-c), as compared with wild-type mice (Fig. 3E-a) and p53−/−-only mice (Fig. 3E-b). Nearly 20% of the p53−/−/RB1−/− mice also exhibited nuclear abnormalities, particularly in the superficial urothelial layer, with large, irregularly shaped nuclei and dense chromatin (Fig. 3E-d). Finally, 2% of p53−/−/RB1−/− mice developed low-grade, superficial papillary bladder tumors (Fig. 3E-e & -f). No invasive urothelial tumor was observed throughout the 28-month observation period. These results indicate that, although deficiency of both p53 and pRb increases urothelial proliferation in aging animals, it is inadequate to trigger a high frequency of full-fledge urothelial tumors, let alone the invasive ones.
Figure 3. Spontaneous urothelial lesions in conditional p53/RB1 null mice.
(A) Co-inactivation of pRb and p53 in mouse urothelium. (1st line) UPII-Cre transgene; (2nd line) p53 allele whose exons 5 and 6 were flanked by loxP sites; (3rd line) recombined p53 allele upon urothelial Cre expression; (4th line) floxed RB1 allele; and (5th line) recombined RB1 allele upon urothelial Cre-expression. (B) PCR analyses of truncation of p53 and/or RB1 on DNA and RNA levels. Four major genotypes generated from multiple intercrosses were chosen for all studies: UPIICre/Cre (or p53+/+/RB1+/+; lanes 1 and 2), UPIICre/Cre/p53flox/flox (or p53−/−/RB1+/+; lanes 3 and 4), UPIICre/Cre/RB1flox/flox (or p53+/+/RB1−/−; lanes 5 and 6), and UPIICre/Cre/p53flox/flox//RB1flox/flox (or p53−/−/RB1−/−; lanes 7 and 8). WT, wild-type; Δ, truncated version; PD, p53 pseudogene. Note a 500-bp, truncated p53 DNA and an 85-bp truncated p53 mRNA in p53−/−/RB1+/+ mice (lanes 3 and 4) and p53−/−/RB1−/− mice (lanes 7 and 8). Also note a 260-bp truncated RB1 DNA and a 50-bp truncated RB1 mRNA in p53+/+/RB1−/− mice (lanes 5 and 6) and p53−/−/RB1−/− mice (lanes 7 and 8). (C) In situ hybridization. Anti-sense cRNA probe corresponding to exons 5 and 6 of p53 gene (p53 Probe) hybridized to the urothelium of wild-type mice, but not to those of p53-null mice or p53/RB1 null mice. Anti-sense cRNA probe corresponding to exon 19 of RB1 gene (Rb Probe) hybridized to the urothelium of the wild-type, but not to those of RB1-null mice or p53/RB1 null mice. Magnification: 200 ×. (D) Western blotting of p21 and MDM2 demonstrating that pRb-deficiency greatly induced p21 (lanes 5 and 6; also see Fig. 1), but this induction was abrogated by p53 inactivation (lanes 7 and 8). (E) H&E images of urinary bladders from a 15-month old wild-type mouse (a) showing normal urothelial morphology; an age-matched p53−/− mouse also showing normal morphology (b); a 12-month old p53−/−/RB1−/− mouse showing urothelial hyperplasia (c); and a 28-month old p53−/−/RB1−/− mouse showing nuclear atypia in the superficial layer (d; arrowheads). (e–f) two 28-month old p53−/−/RB1−/− mice exhibiting low-grade, superficial papillary tumors. Magnification: 200 ×.
Deficiency of Both pRb and p53, But Not Either Protein Alone, Predisposes Urothelium to Sub-threshold Chemical Carcinogenesis
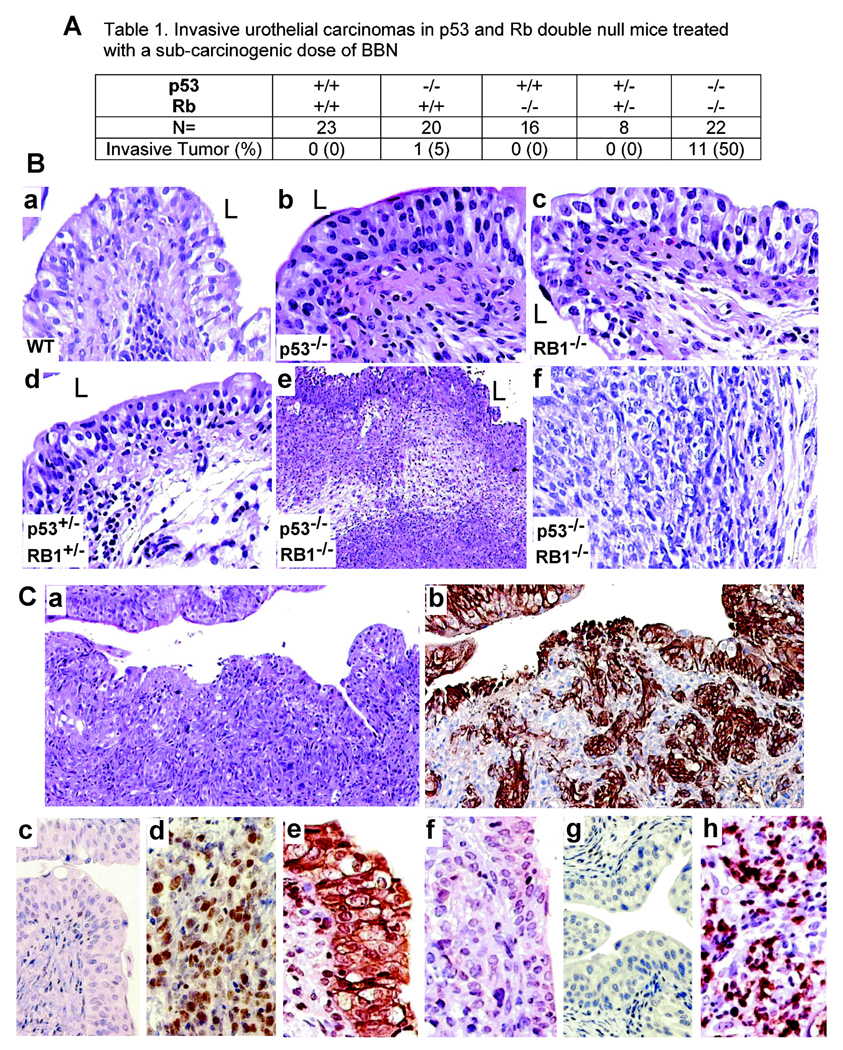
We next examined whether mice deficient for pRb and p53 were more susceptible to N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN), a bladder-specific carcinogen, than the wild-type counterparts or mice deficient for either protein (Fig. 4A). We treated groups of animals with BBN in the drinking water at a dose (0.01%) and time-frame (10 weeks) that were incapable of eliciting urothelial tumors in the wild-type mice (35). BBN is a human-relevant carcinogen and a metabolite of N-nitrosodibutylamine found in tobacco, food and industrial products (36). Inflammation and edema were evident in all groups (Fig. 4B). Urothelia of wild-type mice were non-neoplastic (Fig. 4B-a), as were those from mice deficient for either p53 (Fig. 4B-b) or RB1 (Fig. 4B-c). Heterozygous mice for both floxed p53 and floxed RB1 (p53+/−/RB1+/−) also exhibited non-neoplastic urothelia (Fig. 4B-d). In striking contrast, 50% of the homozygous mice deficient for both p53 and pRb (p53−/−/RB1−/−) developed early-onset, muscle-invasive urothelial tumors (Fig. 4A; Fig. 4B-e & -f; Fig. 4C-a & -b). The invasive tumor cells were positive for basal cell-specific keratins (Fig. 4C-b), establishing their urothelial origin. While normal urothelial cells lacked Ki67 (Fig. 4C-c), this proliferation marker was markedly induced in the invasive tumor cells (Fig. 4C-d). The invasive lesions had a profound reduction of E-cadherin (Fig. 4C-f), as compared with normal urothelium (Fig. 4C-e). Finally, whereas normal urothelium was negative for metalloprotease-9 (Fig. 4C-g), invasive cells expressed large amounts of this protease (Fig. 4C-h). These results reveal many features of mouse invasive urothelial tumors that mirror highly aggressive muscle-invasive urothelial carcinomas in humans (2), and indicate that the combined deficiency of p53 and pRb is cooperative and is necessary for promoting invasive urothelial tumorigenesis.
Figure 4. Susceptibility of the p53/RB1 null mice to a sub-threshold treatment of a bladder-specific carcinogen, BBN.
(A) Frequency of invasive urothelial carcinomas. (B) H&E-stained cross-sections of the urinary bladders from age-matched (3-month) wild-type (a), p53−/− (b), RB1−/− (c) and p53+/−/RB1+/− (d) mice all exhibiting slight urothelial dysplasia with inflammation and edema in the lamina propria. (e & f) a p53−/−/RB1−/− double null mouse exhibiting an invasive tumor. Magnification: a–d, f, 200 ×; e, 50 ×. (C) Characteristics of BBN-triggered muscle-invasive urothelial carcinomas. Invasive lesions in mice null for p53 and pRb (a; H&E) showed strong staining for basal cell keratins (b; consecutive section), over-expression of Ki67 (d), decreased expression E-cadherin (f) and over-expression of MMP9 (h), as compared with their paired wild-type controls (c, e & g). Magnifications: a and b, 50 ×; c–h, 200 ×.
Selective Down-regulation of p107 in BBN-induced Invasive Urothelial Carcinomas
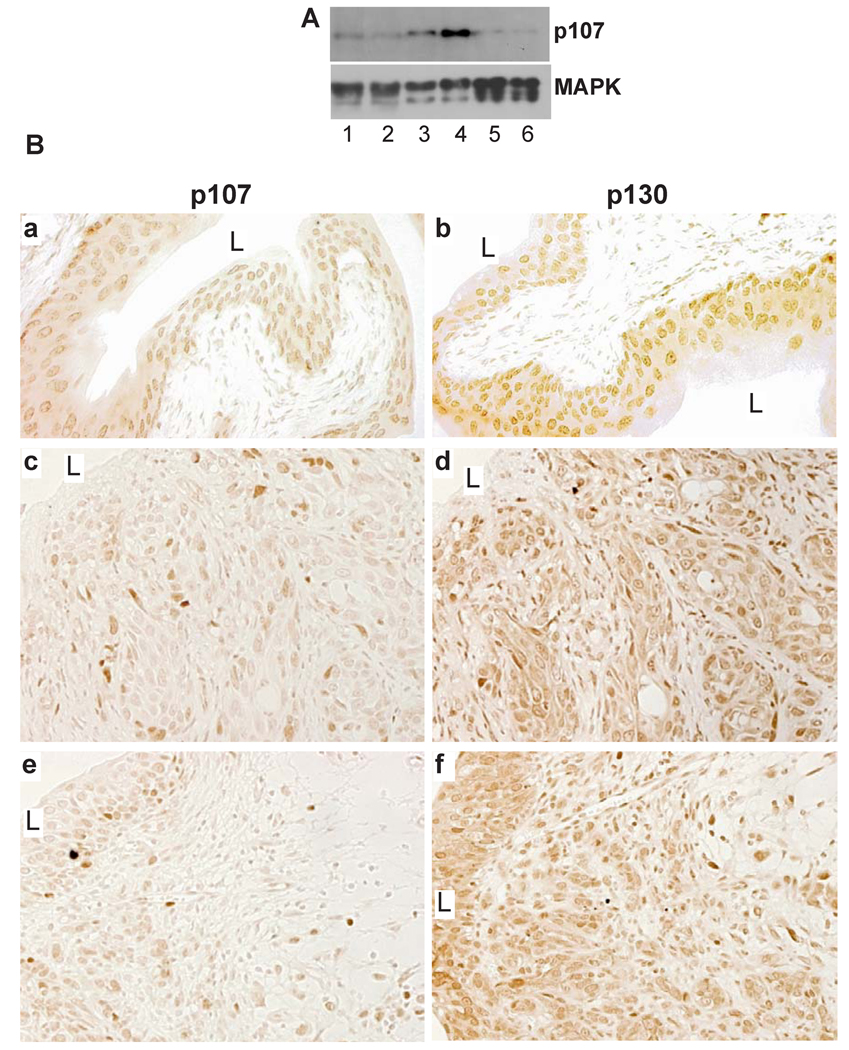
Our finding that inactivating pRb in urothelium induced p107 prompted us to examine whether these two tumor suppressors collaborate to inhibit urothelial tumorigenesis, and whether their co-inactivation could synergize with p53 deficiency to trigger invasive carcinomas. Western blotting and immunohistochemistry showed that, unlike normal urothelium where p107 was constitutively expressed (Fig. 5A, lanes 1 & 2; Fig. 5B-a) and unlike p53−/−/RB1−/− mice where p107 was strongly induced (Fig. 5A, lanes 3 & 4), p107 was significantly reduced in the BBN-treated, pRb/p53-deficient invasive carcinoma cells (Fig. 5A, lanes 5 & 6; Fig. 5B-c and 5B-e). However, p130 remained at high levels (Fig. 5B-d and 5B-f). These results suggest that the selective loss of p107 may play an important role in driving pRb/p53-deficient urothelial cells to form invasive carcinomas.
Figure 5. pRb family proteins in BBN-induced invasive urothelial carcinomas of p53−/−/RB1−/− double null mice.
(A) Western blotting showing the constitutive expression of p107 in wild-type mouse urothelia (lanes 1 & 2), elevated expression in p53−/−/RB1−/− mouse urothelia (3 & 4), and down-regulation in BBN-treated p53−/−/RB1−/− mouse urothelia that developed invasive urothelial carcinomas (5 & 6). (B) Immunohistochemistry. Wild-type mouse urothelia (a and b) or BBN-induced invasive urothelial carcinomas in the p53−/−/RB1−/− background (c–f) were stained with anti-p107 (left column) or anti-p130 (right column). (c and d) and (e and f) were consecutive sections. Note the significant down-regulation and heterogeneous staining of p107, but not p130, in the invasive lesions. Magnification: 200 ×.
DISCUSSION
The Pocket Family Proteins Work in Concert to Keep Normal Urothelium in a Quiescent State
Urothelium is one of the slowest cycling epithelia in the body, with a turnover rate of ~200 days and a tritium-thymidine labeling index of <0.01% (37). This remarkably low self-renewal rate is physiologically important because a stable urothelium is a necessity to maintain an effective permeability barrier (38). It is unclear how the urothelial cells are kept in a quiescent state despite constant exposure to carcinogens and mitogens (39). Base on our data, we conclude that the pocket family proteins play a key role in holding urothelial growth in check. All members of this family are significantly expressed in urothelial layers (Fig. 2 and Fig. 5). Conversely, E2F1, which drives cell cycle forward by transcribing multiple growth-promoting genes, is kept at a low level (Fig. 1). Given that pRb family proteins share significant structural and functional properties (40), these proteins may be redundant to ensure that the urothelial cells remain quiescent when challenged by growth stimuli. As we observed, pRb abrogation led to a marked upregulation of p107 and its transcriptional repressor E2F4 (Fig. 2 and Fig. 6). This may represent a compensatory urothelial response to pRb loss to restore the balance of growth inhibition. Because p107 is a transcriptional target of E2F1 (41), p107 induction may be a result of E2F1 overexpression associated with pRb-deficiency. Clearly, pRb family proteins work in a highly coordinated manner to restrict urothelial proliferation under normal and pRb-deficient conditions. We speculate that the loss of more than one pRb family member would be required to release the primary and the secondary blockade on urothelial growth, leading to proliferation. This notion is supported by our prior observation that all pRb family proteins are dramatically down-regulated in low-grade, superficial papillary urothelial carcinomas in transgenic mice that expressed an activated Ha-ras (42). Down-regulation of the entire pRb family proteins may be a prerequisite for urothelial tumorigenesis. It would be worthwhile to extend these observations through transgenic inactivation of all pRb family members in the urothelium.
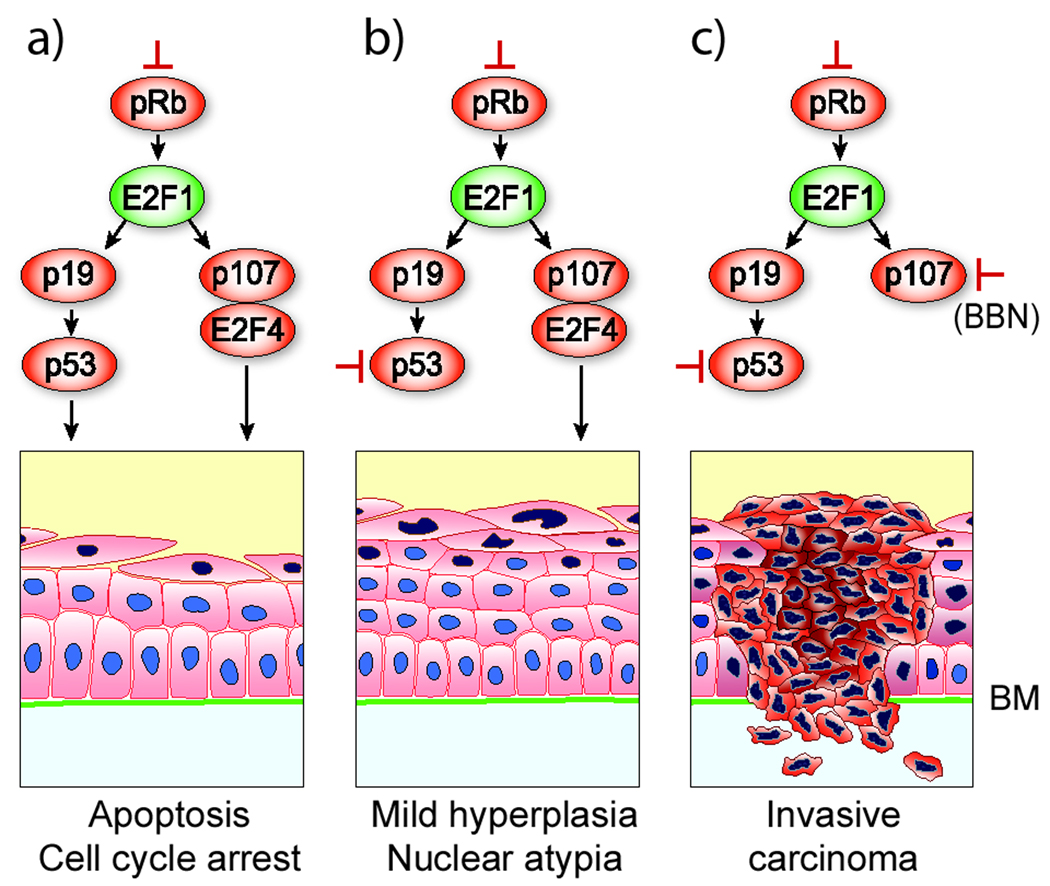
Figure 6. Urothelial responses to the loss of tumor suppressors.
(Column a) pRb deficiency in urothelium results in the overexpression of E2F1 which triggers two independent compensatory tumor defenses: one mediated by p19/p53 axis and another by pRb family member p107 and its transcriptional repressor partner E2F4. Together, these responses cause urothelial cell cycle arrest, apoptosis and aborted tumorigenesis. (b) loss of p53 in pRb-deficient cells mitigated urothelial apoptotic response, leading to late-onset hyperplasia and nuclear atypia. (c) additional loss of p107 in pRb/p53-deficient urothelial cells in carcinogen BBN-treated mice triggers invasive urothelial carcinomas. This model presents a new concept illustrating the collaborative relationship among pRb, p53 and p107 in invasive urothelial tumorigenesis.
Concurrent Defects of pRb and p53 Are Critical for Promoting, but not Initiating, Invasive Urothelial Carcinomas
We co-inactivated pRb and p53 in the urothelium, not only because the pRb deficiency strongly induced the p53 pathway (Fig. 2), but because their concurrent defects are closely correlated with the invasive urothelial carcinomas in humans (43). We were surprised to find, however, that defects in both genes failed to trigger invasive urothelial carcinomas. Our results challenge the prevailing theory based largely on the clinical correlative data that defects of pRb and p53 cause the invasive urothelial carcinomas. Rather, the combined deficiency of these two tumor suppressors is necessary to promote invasive urothelial tumors. When mice homozygously null for pRb and p53 were fed with 0.01% BBN for 10 weeks, 50% of the mice developed muscle-invasive urothelial carcinomas that strongly resembled the human counterparts morphologically and biochemically (Fig. 4). Few if any of such carcinomas occurred in identically treated mice that were homozygously null for pRb only or for p53 only, or in mice heterozygously null for both pRb and p53. These results indicate a pivotal collaborative role of the complete loss-of-function of both pRb and p53 in the promotion, but not in the initiation, of invasive urothelial carcinomas (Fig. 6).
Several mechanisms may underlie the collaborative effects between pRb and p53 deficiency. First, pRb loss in urothelium provokes a robust, p53-mediated apoptotic response (Fig. 2). This response was muted when pRb-deficient urothelial cells were also made p53-deficient (Fig. 3). Urothelial cells defective for both p53 and pRb are therefore much less capable than those defective for pRb only to respond to genotoxic agents such as BBN in mounting an apoptotic response. Instead, the pRb/p53-double deficient urothelial cells carrying carcinogen-damaged DNAs exit cell cycles uncontrollably via defective G1/S and G2/M checkpoints transpired by the pRb loss. Second, the collaborative effect could be due to a strong urothelial induction of MAD2 due to pRb loss (Fig. 1). MAD2 is a key kinetochore checkpoint protein and a downstream target of E2F1 (32). While normal level of MAD2 prevents premature cell cycle progression through the anaphase, excessive amounts can lead to abnormal chromosomal segregation and aneuploidy. Recent transgenic studies show that MAD2 overexpression leads to tumorigenesis, implicating MAD2 as an oncogene (44). Because p53 deficiency also leads to genome instability (45), these effects could be additive during BBN treatment.
It should be noted that, while pRb loss activates p53 pathway (Fig. 2), this compensatory response is not reciprocal. The loss of p53 in urothelium did not induce the expression of pRb family proteins, their E2F partners or downstream effectors (unpublished observation). Therefore, urothelial cells may be more resistant to pRb deficiency but more vulnerable to p53 deficiency. Such differential vulnerability to tumor suppressor loss may be applicable to other epithelia as well.
p107 Deficiency as a Potential Missing Link in Invasive Urothelial Tumorigenesis
The fact that even combined p53 and pRb inactivation failed to trigger muscle-invasive urothelial carcinomas suggests that additional genetic defects are required. Three lines of evidence suggest p107 deficiency as a potential missing link. First, p107, but not its family member p130, is highly up-regulated in pRb/p53-double deficient urothelial cells (Fig. 2), suggesting that p107 plays a critical tumor suppressive role during pRb/p53 deficiency (Fig. 6). Second, in BBN-treated pRb/p53-double knockout mice where 50% of the animals developed muscle-invasive carcinomas, p107 was significantly down-regulated (Fig. 5), whereas p130 remained at high levels. This selective loss of p107 suggests that this protein is an important target for inactivation by BBN. Third, as we previously showed, SV40T antigen, which functionally disables not only pRb and p53, but also pRb family proteins including p107 (28), was capable of inducing high-grade carcinoma in situ and invasive urothelial carcinomas (26, 46). A similar collaborative effect among pRb, p53 and p107 deficiencies was observed in the retina where deficiency of all three tumor suppressors, but not any combination of the two, induces retinal dysplasia or retinoblastoma (47). Together, the principle we demonstrated regarding the collaborative relationships among pRb, p53 and p107 may be applicable to the tumorigenic processes in many cell types (Fig. 6). Finally, our results suggest that pRb, p107 and p53 together could be a more reliable prognostic indicator than a combination of p53 and pRb for patients with invasive urothelial carcinomas.
ACKNOWLEDGEMENTS
This work was supported in part by grants from the United States National Institutes of Health (DK52206, DK69688) and from Veterans Administration (Merit Review Award), and by the Weinstein Foundation for Urological Research at New York University School of Medicine.
REFERENCES
- 1.Koss LG. Natural history and patterns of invasive cancer of the bladder. Eur Urol. 1998;33:2–4. doi: 10.1159/000052249. [DOI] [PubMed] [Google Scholar]
- 2.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 3.Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol. 2006;24:5552–5564. doi: 10.1200/JCO.2006.08.2073. [DOI] [PubMed] [Google Scholar]
- 4.Dinney CP, McConkey DJ, Millikan RE, et al. Focus on bladder cancer. Cancer Cell. 2004;6:111–116. doi: 10.1016/j.ccr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Dalbagni G, Presti J, Reuter V, Fair WR, Cordon-Cardo C. Genetic alterations in bladder cancer [see comments] Lancet. 1993;342:469–471. doi: 10.1016/0140-6736(93)91595-d. [DOI] [PubMed] [Google Scholar]
- 6.Wolff EM, Liang G, Jones PA. Mechanisms of Disease: genetic and epigenetic alterations that drive bladder cancer. Nat Clin Pract Urol. 2005;2:502–510. doi: 10.1038/ncpuro0318. [DOI] [PubMed] [Google Scholar]
- 7.Grossman HB. Superficial bladder cancer: decreasing the risk of recurrence. Oncology (Huntingt) 1996;10:1617–1624. discussion 24, 27–8. [PubMed] [Google Scholar]
- 8.Dalbagni G. The management of superficial bladder cancer. Nat Clin Pract Urol. 2007;4:254–260. doi: 10.1038/ncpuro0784. [DOI] [PubMed] [Google Scholar]
- 9.Grossman HB, Dinney CP. If cystectomy is insufficient, what is an urologist to do? Urol Oncol. 2003;21:475–478. doi: 10.1016/s1078-1439(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 10.Cordon-Cardo C, Cote RJ, Sauter G. Genetic and molecular markers of urothelial premalignancy and malignancy. Scand J Urol Nephrol Suppl. 2000:82–93. doi: 10.1080/003655900750169338. [DOI] [PubMed] [Google Scholar]
- 11.Knowles MA. Molecular pathogenesis of bladder cancer. Int J Clin Oncol. 2008;13:287–297. doi: 10.1007/s10147-008-0812-0. [DOI] [PubMed] [Google Scholar]
- 12.Rieger-Christ KM, Mourtzinos A, Lee PJ, et al. Identification of fibroblast growth factor receptor 3 mutations in urine sediment DNA samples complements cytology in bladder tumor detection. Cancer. 2003;98:737–744. doi: 10.1002/cncr.11536. [DOI] [PubMed] [Google Scholar]
- 13.Jebar AH, Hurst CD, Tomlinson DC, Johnston C, Taylor CF, Knowles MA. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene. 2005;24:5218–5225. doi: 10.1038/sj.onc.1208705. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZT, Pak J, Huang HY, et al. Role of Ha-ras activation in superficial papillary pathway of urothelial tumor formation. Oncogene. 2001;20:1973–1980. doi: 10.1038/sj.onc.1204315. [DOI] [PubMed] [Google Scholar]
- 15.Mo L, Zheng X, Huang HY, et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J Clin Invest. 2007;117:314–325. doi: 10.1172/JCI30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoki Y, Niihori T, Kawame H, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 17.Cordon-Cardo C. p53 and Rb: simple interesting correlates or tumor markers of critical predictive nature? J Clin Oncol. 2004;22:975–977. doi: 10.1200/JCO.2004.12.994. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher O, Easton D, Anderson K, Gilham C, Jay M, Peto J. Lifetime risks of common cancers among retinoblastoma survivors. J Natl Cancer Inst. 2004;96:357–363. doi: 10.1093/jnci/djh058. [DOI] [PubMed] [Google Scholar]
- 19.Cordon-Cardo C, Reuter VE. Alterations of tumor suppressor genes in bladder cancer. Semin Diagn Pathol. 1997;14:123–132. [PubMed] [Google Scholar]
- 20.Shariat SF, Weizer AZ, Green A, et al. Prognostic value of P53 nuclear accumulation and histopathologic features in T1 transitional cell carcinoma of the urinary bladder. Urology. 2000;56:735–740. doi: 10.1016/s0090-4295(00)00756-1. [DOI] [PubMed] [Google Scholar]
- 21.Grossman HB, Liebert M, Antelo M, et al. p53 and Rb expression predict progression in T1 bladder cancer. Clin Cancer Res. 1998;4:829–834. [PubMed] [Google Scholar]
- 22.Cote RJ, Dunn MD, Chatterjee SJ, et al. Elevated and absent pRb expression is associated with bladder cancer progression and has cooperative effects with p53. Cancer Res. 1998;58:1090–1094. [PubMed] [Google Scholar]
- 23.Cordon-Cardo C, Zhang ZF, Dalbagni G, et al. Cooperative effects of p53 and pRB alterations in primary superficial bladder tumors. Cancer Res. 1997;57:1217–1221. [PubMed] [Google Scholar]
- 24.Donehower LA, Harvey M, Slagle BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Huang HY, Pak J, et al. p53 deficiency provokes urothelial proliferation and synergizes with activated Ha-ras in promoting urothelial tumorigenesis. Oncogene. 2004;23:687–696. doi: 10.1038/sj.onc.1207169. [DOI] [PubMed] [Google Scholar]
- 26.Grippo PJ, Sandgren EP. Highly invasive transitional cell carcinoma of the bladder in a simian virus 40 T-antigen transgenic mouse model. Am J Pathol. 2000;157:805–813. doi: 10.1016/S0002-9440(10)64594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang ZT, Pak J, Shapiro E, Sun TT, Wu XR. Urothelium-specific expression of an oncogene in transgenic mice induced the formation of carcinoma in situ and invasive transitional cell carcinoma. Cancer Res. 1999;59:3512–3517. [PubMed] [Google Scholar]
- 28.Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24:7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 29.Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- 30.Lin SC, Lee KF, Nikitin AY, et al. Somatic mutation of p53 leads to estrogen receptor alpha-positive and -negative mouse mammary tumors with high frequency of metastasis. Cancer Res. 2004;64:3525–3532. doi: 10.1158/0008-5472.CAN-03-3524. [DOI] [PubMed] [Google Scholar]
- 31.Mo L, Cheng J, Lee EY, Sun TT, Wu XR. Gene deletion in urothelium by specific expression of Cre recombinase. Am J Physiol Renal Physiol. 2005;289:F562–F568. doi: 10.1152/ajprenal.00368.2004. [DOI] [PubMed] [Google Scholar]
- 32.Hernando E, Nahle Z, Juan G, et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- 33.Crosby ME, Almasan A. Opposing roles of E2Fs in cell proliferation and death. Cancer Biol Ther. 2004;3:1208–1211. doi: 10.4161/cbt.3.12.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa K, Uzvolgyi E, St John MK, de Oliveira ML, Arnold L, Cohen SM. Frequent p53 mutations and occasional loss of chromosome 4 in invasive bladder carcinoma induced by N-butyl-N-(4-hydroxybutyl)nitrosamine in B6D2F1 mice. Mol Carcinog. 1998;21:70–79. doi: 10.1002/(sici)1098-2744(199801)21:1<70::aid-mc9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 36.Wu X-R, Sun T-T, McConkey DJ, Shrader M, Papageorgiou A. Animal Models of Bladder Cancer. In: Lerner SP, Schoenberg MP, Sternberg CN, editors. Textbook of Bladder Cancer. Boca Raton: Taylor & Francis; 2006. pp. 157–169. [Google Scholar]
- 37.Walker RE. Renewal of cell populations in the female mouse. Am. J. Anat. 1960;102:95–100. doi: 10.1002/aja.1001070202. [DOI] [PubMed] [Google Scholar]
- 38.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 39.Messing EM. Growth factors and bladder cancer: clinical implications of the interactions between growth factors and their urothelial receptors. Semin Surg Oncol. 1992;8:285–292. doi: 10.1002/ssu.2980080507. [DOI] [PubMed] [Google Scholar]
- 40.Sun A, Bagella L, Tutton S, Romano G, Giordano A. From G0 to S phase: a view of the roles played by the retinoblastoma (Rb) family members in the Rb-E2F pathway. J Cell Biochem. 2007;102:1400–1404. doi: 10.1002/jcb.21609. [DOI] [PubMed] [Google Scholar]
- 41.Dirks PB, Rutka JT, Hubbard SL, Mondal S, Hamel PA. The E2F-family proteins induce distinct cell cycle regulatory factors in p16-arrested, U343 astrocytoma cells. Oncogene. 1998;17:867–876. doi: 10.1038/sj.onc.1202008. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Espana A, Salazar E, Sun TT, Wu XR, Pellicer A. Differential expression of cell cycle regulators in phenotypic variants of transgenically induced bladder tumors: implications for tumor behavior. Cancer Res. 2005;65:1150–1157. doi: 10.1158/0008-5472.CAN-04-2074. [DOI] [PubMed] [Google Scholar]
- 43.Reznikoff CA, Belair CD, Yeager TR, et al. A molecular genetic model of human bladder cancer pathogenesis. Semin Oncol. 1996;23:571–584. [PubMed] [Google Scholar]
- 44.Sotillo R, Hernando E, Diaz-Rodriguez E, et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Efeyan A, Serrano M. p53: guardian of the genome and policeman of the oncogenes. Cell Cycle. 2007;6:1006–1010. doi: 10.4161/cc.6.9.4211. [DOI] [PubMed] [Google Scholar]
- 46.Cheng J, Huang H, Pak J, et al. Allelic loss of p53 gene is associated with genesis and maintenance, but not invasion, of mouse carcinoma in situ of the bladder. Cancer Res. 2003;63:179–185. [PubMed] [Google Scholar]
- 47.MacPherson D, Sage J, Kim T, Ho D, McLaughlin ME, Jacks T. Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 2004;18:1681–1694. doi: 10.1101/gad.1203304. [DOI] [PMC free article] [PubMed] [Google Scholar]