Abstract
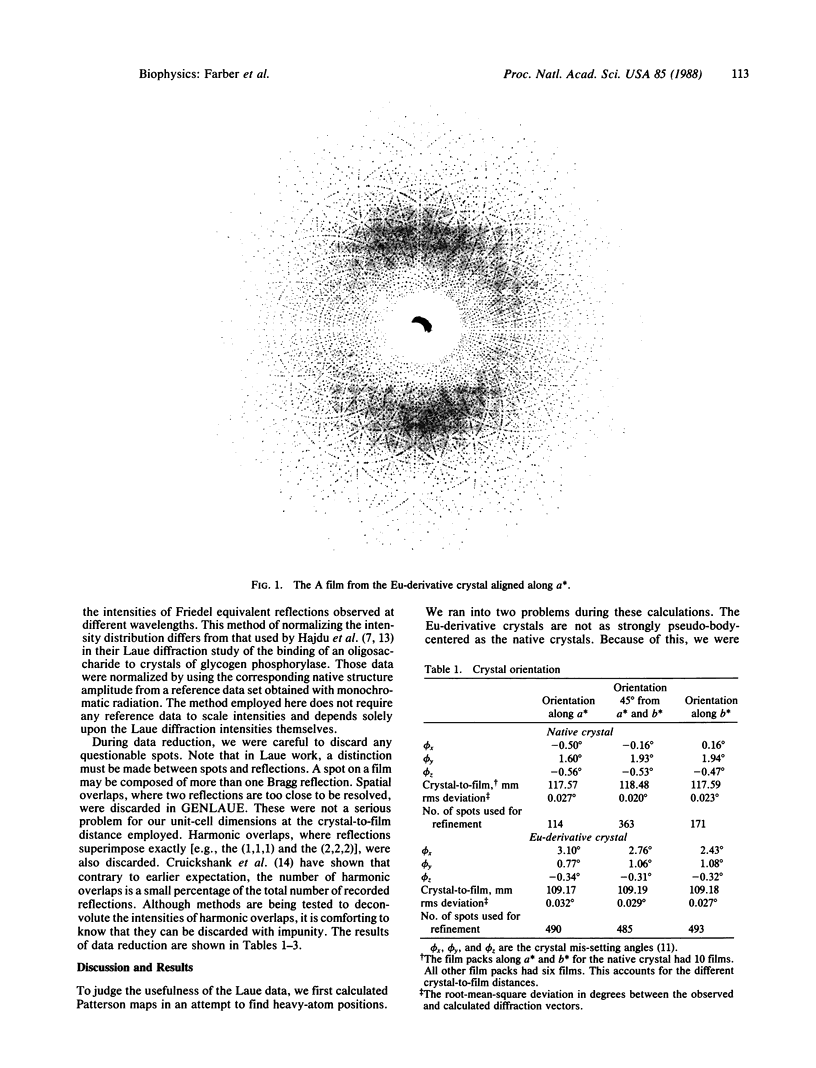
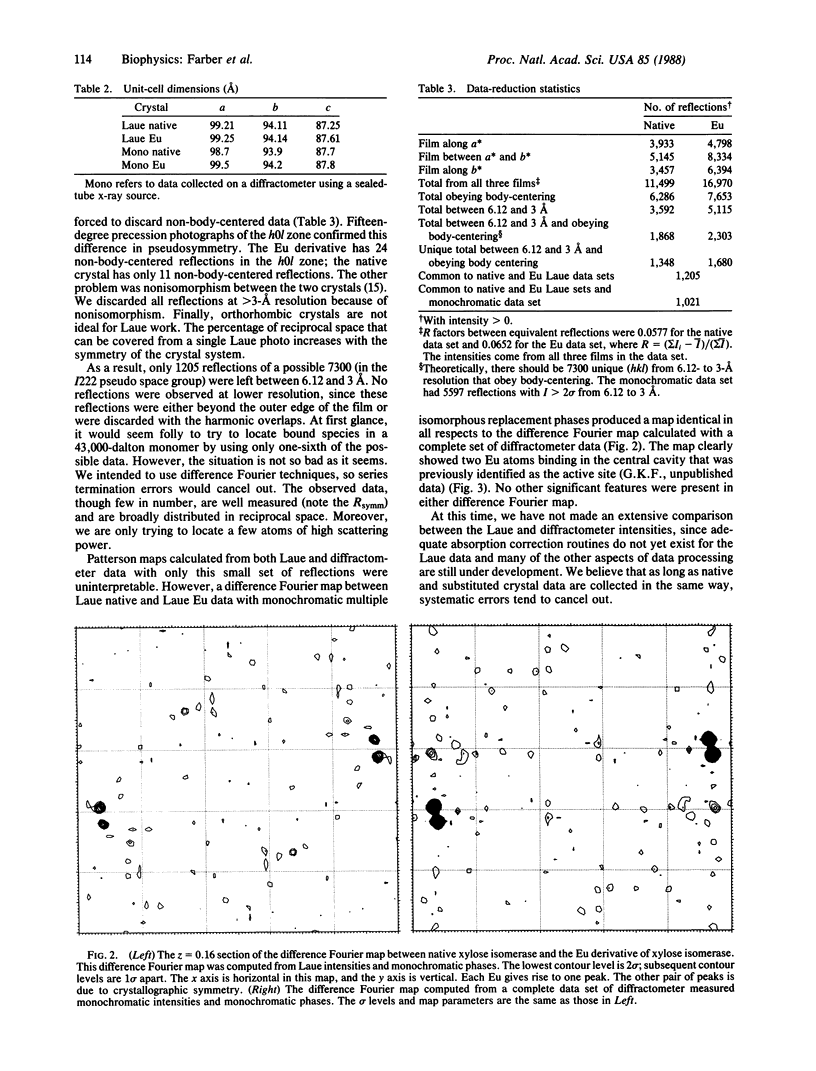
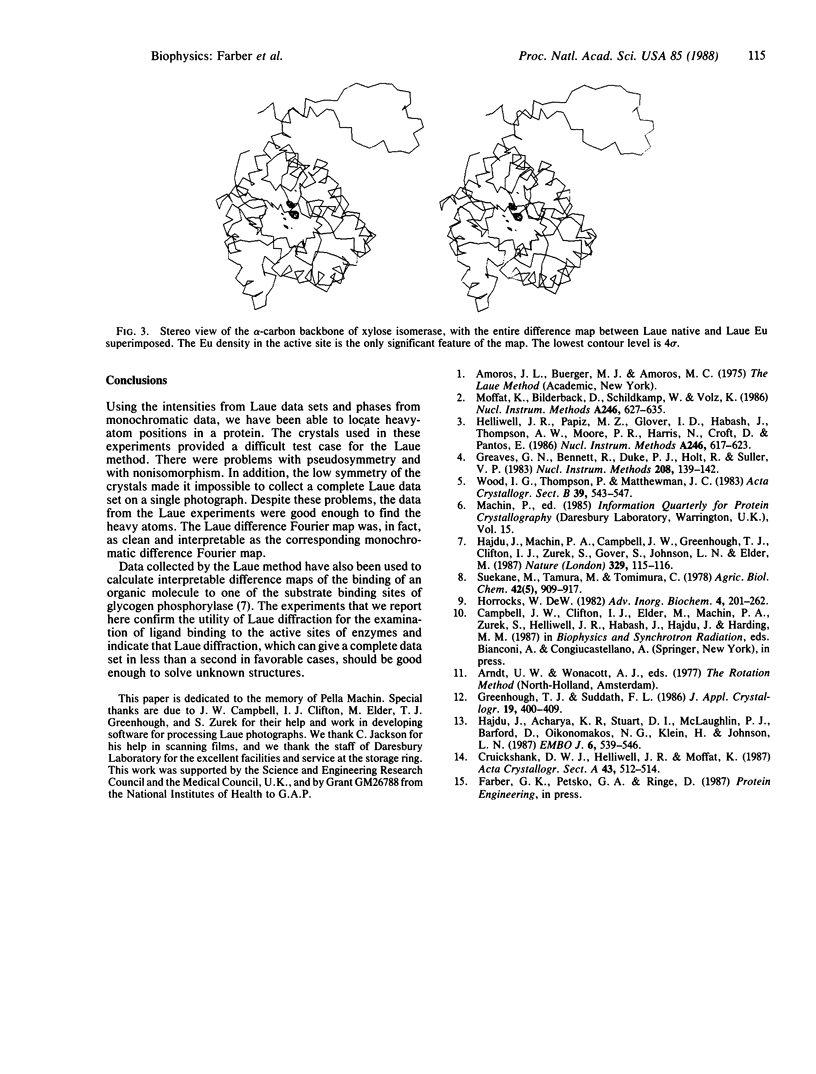
The Laue method (stationary crystal, polychromatic x-rays) was used to collect native and heavy-atom-derivative data on crystals of xylose isomerase (EC 5.3.1.5). These data were used to find the heavy-atom positions. The positions found by use of Laue data are the same as those found by use of monochromatic data collected on a diffractometer. These results confirm that Laue diffraction data sets, which can be obtained on a millisecond time scale, can be used to locate small molecules bound to protein active sites. The successful determination of heavy-atom positions also indicates that x-ray crystallographic data collected by the Laue method can be used to solve protein structures.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hajdu J., Acharya K. R., Stuart D. I., McLaughlin P. J., Barford D., Oikonomakos N. G., Klein H., Johnson L. N. Catalysis in the crystal: synchrotron radiation studies with glycogen phosphorylase b. EMBO J. 1987 Feb;6(2):539–546. doi: 10.1002/j.1460-2075.1987.tb04786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salibian A. H., Tesoro V. R., Wood D. L. Staged transfer of a free microvascular latissimus dorsi myocutaneous flap using saphenous vein grafts. Plast Reconstr Surg. 1983 Apr;71(4):543–547. doi: 10.1097/00006534-198304000-00018. [DOI] [PubMed] [Google Scholar]