Abstract
Src-family tyrosine kinases (SFKs) regulate cell proliferation, and increased SFK activity is common in human carcinomas, including cutaneous squamous cell carcinomas and its precursors. The elevated SFK activity in cutaneous SCCs was modeled using keratin 14-Fyn Y528F transgenic mice, which spontaneously form punctate keratotic lesions, scaly plaques, and large tumors resembling actinic keratoses (AKs), carcinoma in situ (SCIS), and SCCs, respectively.
Lesional tissue demonstrated increased levels of activated SFKs, PDK-1, STAT-3, and Erk1/2 while Notch 1/NICD protein and transcript levels were decreased. p53 levels also were decreased in SCIS and SCCs.
Raising Srcasm levels using a K14-Fyn Y528F/K14-Srcasm double transgenic model markedly inhibited cutaneous neoplasia. In contrast, increased expression of a non-phosphorylatable Srcasm mutant maintained the neoplastic phenotype. Raising Srcasm levels decreased levels of Fyn, activated SFKs, Erk 1/2, PDK-1, and phospho-STAT3, and raised Notch 1/NICD and p53 levels.
Analysis of human specimens revealed that levels of Fyn and activated SFKs were elevated in SCCs compared with adjacent non-lesional epidermis. In addition, Notch 1 and Srcasm protein and transcript levels were decreased in human SCCs compared to non-lesional epidermis. Therefore, the SCCs produced by the Fyn Y528F mice resemble their human counterparts at the molecular level.
K14-Fyn Y528F mice represent a robust model of cutaneous carcinogenesis that manifests precancerous lesions and SCCs resembling human disease. The Fyn/Srcasm signaling nexus modulates activity of STAT-3, PDK-1, Erk 1/2, Notch 1 and p53. Further study of Fyn and Srcasm should provide insights into the mechanisms regulating keratinocyte proliferation and skin carcinogenesis.
Introduction
Cutaneous squamous cell carcinoma (SCC) is the second most common form of cancer with over 250,000 cases annually in the US leading to approximately 2,500 deaths (1-3). Many cutaneous SCCs arise from a precursor lesion termed an actinic keratosis (AK), and approximately 60% of all individuals over the age of 40 will develop an AK requiring treatment during their lifetime, making AKs the most common precancerous lesion in the US (4). Such data show that AKs and cutaneous SCCs are a significant health problem, and demonstrate a need for an in vivo model resembling this human disease.
Src-family tyrosine kinases (SFKs) are known oncogenes and promote neoplasia in many tissues (5, 6). The majority of human carcinomas including colonic, breast, pancreatic, and cutaneous squamous cell demonstrate elevated SFK activity compared to corresponding non-neoplastic epithelium (7-11). However, the cellular mechanisms promoting increased SFK activity in human carcinomas remain unclear. Mutation of a C-terminal regulatory tyrosine can lead to increased SFK activity in tumors (5). However, such activating mutations in SFKs are rare in human carcinomas (12-15). These observations raise the hypothesis that additional cellular mechanisms could account for the elevated SFK activity in carcinomas, such as impaired negative regulation of SFKs.
Keratinocytes express three SFKs: Src, Fyn, and Yes (16). Fyn is a dually-acylated kinase containing covalently-linked myristoyl and palmitoyl moieties on its N-terminal SH4 domain (17). Palmitoylation targets SFKs to lipid rafts (18, 19), and Fyn localizes to lipid rafts and caveolae, subcellular domains associated with EGFR signaling and caveolae/raft-dependent endocytosis (20-22). These characteristics suggest that Fyn may play a significant role in regulating keratinocyte proliferation.
Srcasm (Src activating and signaling molecule) is an SFK substrate, that when phosphorylated, engages SFKs and downregulates them through a lysosomal-dependent mechanism (20, 23, 24). Srcasm localizes to the multi-vesicular body which is important for targeting endosomal proteins for lysosomal degradation (25, 26). Srcasm also contains two conserved Tsg101 tetrapeptide binding motifs (27); Tsg101 is a component of the ESCRT-1 complex and a regulator of the multi-vesicular body (25, 28). Srcasm lies at a signaling nexus between SFKs, Tsg101, and lysosomal protein degradation.
Human cutaneous squamous cell carcinoma in situs (SCISs) and SCCs show decreased Srcasm levels compared to unremarkable epidermis (20). SFK activity is elevated in AKs, SCIS, and SCC in human biopsies compared to adjacent non-lesional epidermis (8). Together, these data suggest an inverse relationship between SFK activity and Srcasm levels in human skin neoplasia.
Therefore, we hypothesized that increasing Fyn activity in the epidermal keratinocytes would promote cutaneous neoplasia and raising Srcasm levels would inhibit Fyn-induced neoplasia. These hypotheses were tested using transgenic mice expressing Fyn Y528F, Srcasm, and SrcasmDN, a non phosphorylatable Srcasm mutant. Increased Fyn Y528F expression induced the spontaneous formation of precancerous lesions and SCCs within 5 weeks. Increased expression of Srcasm, but not SrcasmDN, inhibited the formation of precancerous and cancerous lesions. Elevated Fyn levels promoted Erk 1/2, PDK-1, and STAT-3, activation and downregulated Notch 1 and p53. Increasing Srcasm lowered Fyn levels in vivo and normalized the activity and levels of these molecules.
Together, these data identify a mechanism of carcinogenesis in which the level of Fyn activity is inversely related to Srcasm levels; the data also demonstrate an important regulatory relationship between Fyn and Srcasm that involves Notch 1 and p53. The relationship between SFKs, Srcasm, Notch 1, and p53 may play an important role in regulating carcinogenesis.
Materials and Methods
Generation and characterization of transgenic mice
Murine HA-tagged Srcasm and HA-tagged Srcasm DN transgenic mice were generated previously (23). FynY528F cDNA was cloned into a vector driven by the human keratin 14 promoter (23). Transgene cassettes were excised and purified via Tris acetate-EDTA-agarose electrophoresis. C57BL/6 × CBA-fertilized oocytes were microinjected with the transgene cassettes using standard protocols at the University of Pennsylvania Transgenic Core Facility in accord with IACUC proposal 801519. Two independent K14-Fyn Y528F lines with hyperkeratotic plaques and tumor phenotype were derived. Founders were crossed with C57BL/6 mice to generate the C57BL/6 lines. The C57BL/6 lines were crossed with FVB/N and the F1 hybrids were characterized in this study. Up to three additional backcrosses of the Fyn Y528F transgene onto the FVB/N background yielded an identical phenotype. A two-sided Fisher's exact test was used to determine the statistical significance of differences in phenotype incidence.
Antibodies
Activated Src-family kinase, phospho-Erk 1/2, Fyn, keratin 6, phospho-STAT-3, phospho-PDK1, β-Actin, and α-Srcasm antibodies were used as previously described (25). For western blotting: α-p21 mouse mAb(F-5), sc-6246, 1:500 (Santa Cruz Biotechnology), α-MDM2 mouse mAb (2A10) #OP115, 2ug/mL, Calbiochem, α-p53 mouse mAb (1C12), 2524, Cell Signaling 1:1000, α-Notch1/NICD (C44H11), 3268, Cell Signaling 1:1000.
Histologic and Immunohistochemical Analysis
Skin samples were fixed in 10% NBF and subjected to standard processing and staining with hematoxylin and eosin. Tissue sections were subjected to immunohistochemistry as reported (8). Photomicrographs were obtained using a Leica DC300 digital camera coupled to a Zeiss Axiophot microscope; photos were obtained under identical conditions at the indicated magnifications as high resolution JPEG files.
Immunoblotting
Cell and tissue lysates were prepared and analyzed as described (24). Quantitative RT-PCR: Total RNA was isolated from epidermis and tumor samples using an OMNI homogenizer and the RNAeasy Fibrous Tissue Kit (Qiagen #74704) according to the manufacturer's instructions. RNA quantity was assessed using a NanoDrop spectrophotometer (ThermoScientific). Total RNA was reverse-transcribed with random hexamers using High Capacity RNA-to-cDNA Kit (Applied Biosystems,Foster City, CA) following the manufacturer's protocols. Equivalent amounts cDNA were subjected to quantitative PCR using the Power SYBR Green PCR master mix (Applied Biosystems) on an ABI 7000 instrument. Samples were run in triplicate on 96-well reaction plates with appropriate species specific primer pairs. Primers sets for each target gene were designed and purchased from IDT (Integrated DNA Technology, Coralville, Iowa). The comparative Ct method was used to determine the level of the target gene mRNA in tissue samples (Applied Biosystems User Bulletin #2, October 2001). Human samples were standardized to β-actin and murine samples were standardized to GADPH. Statistical analysis was performed using the independent groups T-test for means.
Dissection of human tissue
Unremarkable skin and SCC with non-lesional epidermis were obtained from the Moh's Surgical Unit, Dept. of Dermatology, University of Pennylvania Medical Center in accord with IRB protocol 808224. Histologic confirmation of specimen type was made using frozen sections stained with H+E. Portions of epidermis and SCC were isolated using a dissection scope and scalpels. The tissue was homogenized on ice in RIPA lysis buffer or RNA isolation buffer.
Results
C57BL/6 K14-Fyn Y528F transgenic mice exhibit hyperkeratotic plaques and spontaneous tumor formation
The effect of the K14-Fyn Y528F transgene was evaluated in the C57BL/6 genetic background, a tumor resistant genetic background (29). Increased epidermal expression of Fyn Y528F induces the development of multiple hyperkeratotic plaques in the epidermis, while control mice did not exhibit this phenotype (p< 1.2 × 10−19) (Fig. 1A and Table 1-Supplemental Data). The hyperkeratotic plaques are detectable at 3-4 days of age and persist until 3-4 weeks of age. Approximately 21% of the K14-Fyn Y528F C57BL/6 mice that exhibited hyperkeratotic plaques developed spontaneous keratotic tumors resembling cutaneous squamous cell carcinomas (SCCs) between three-to-seven months of age while littermate controls mice do not (p=0.001) (Fig. 1B). Spontaneous SCCs arising in the C57BL/6 background is unusual and suggests that Fyn Y528F functions as an oncogene in epidermal keratinocytes (31). To test the effects of increasing Srcasm levels on the K14-Fyn Y528F phenotype, K14-Fyn Y528F/K14-Srcasm double transgenic mice were generated; these double transgenic mice exhibited a significantly lower incidence of hyperkeratotic plaques compared to the parental line (p=0.003) (Table 1-Supplemental Data). In addition, no keratotic tumors formed in the K14-Fyn Y528F/K14Srcasm double transgenics; these data demonstrate that the Srcasm transgene inhibits tumor formation in C57BL/6 K14-Fyn Y528F mice (p=0.04).
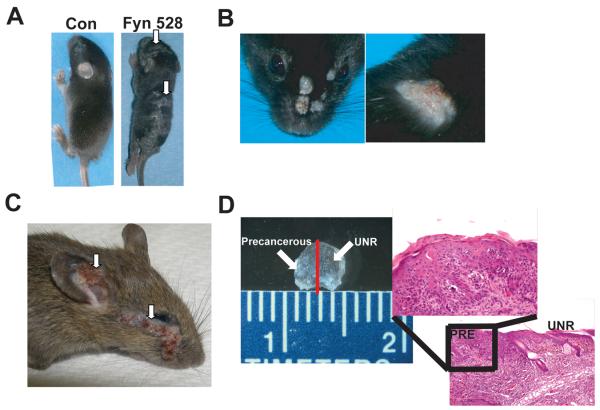
Figure 1. Phenotypes of K14-Fyn Y528F transgenic mice.
A) K14-Fyn Y528F transgenic mice exhibit hyperkeratotic plaques (arrows) on the head, ears, and back by 7 days. C57BL/6 mice shown. B) 21% of the C57BL/6 mice develop hyperkeratotic tumors on the head and mid-back. C) A 6 week-old C57BL/6-FVB/N F1 hybrid with large facial and auricular tumors (arrows). D) Magnified view of ear skin from a 5 week old C57BL/6-FVB/N F1 hybrid exhibiting punctate hyperkeratotic lesions. The red line denotes the plane of histologic section demonstrating the transition from unremarkable skin (UNR) to a precancerous lesion (PRE).
F1 hybrids between C57BL/6 K14-Fyn Y528F and FVB/N develop multiple spontaneous squamous cell carcinomas and precancerous lesions
To characterize the effect of the Fyn Y528F transgene in a standard tumor-permissive genetic background, the C57BL/6 K14-Fyn Y528F line was crossed with FVB/N to generate F1 progeny. These F1 hybrid progeny demonstrated hyperkeratotic plaques within four days postnatal. The C57BL/6-FVB/N F1 K14-Fyn Y528F hybrids developed tumors sooner than the C57BL/6 parental line, typically manifesting multiple tumors between 5-8 weeks. The formation of hyperkeratotic plaques in the K14-Fyn Y528F F1 hybrids was statistically significant compared with littermate controls (p < 4.5 × 10−56, Table 2-supplemental data). Spontaneous SCC formation was observed in 36% of K14-Fyn Y528F mice with none arising in littermate controls (Fig. 1C, p < 4.6 × 10−21). K14-Fyn Y528F transgenic mice derived from backcrossing the F1 hybrids one (N=20) or two (N=10) additional times onto the FVB/N background yielded a phenotype indistinguishable from the F1 hybrid.
The incidence of hyperkeratotic plaque formation in K14-Fyn Y528F/K14-Srcasm double transgenic mice (41%) was decreased compared to the parental line (81%) (p < 1.4 × 10−3, Table 2-supplemental data). Tumor formation was almost completely inhibited in the K14-Fyn Y528F/K14-Srcasm double transgenic mice (p < 1.5 × 10−3), suggesting that Srcasm can function as an anti-oncogene. The incidence of tumor formation was similar between K14-Fyn Y528F mice and K14-Fyn Y528F/K14-SrcasmDN double transgenic mice, that express a non-phosphorylatable form of Srcasm (p= 1). Therefore, the SrcasmDN transgene does not inhibit SCC formation and does not function as an anti-oncogene. The incidence of hyperkeratotic plaque and SCC formation was lower in the K14-Fyn Y528F/K14-Srcasm line compared with the K14-Fyn Y528F/K14-SrcasmDN lines with p < 5.3 × 10−3 and 0.01 respectively.
In addition to keratotic tumors, the C57BL/6 K14-Fyn Y528F-FVB/N F1 hybrids develop punctate (1-3 mm) hyperkeratotic lesions at 4-5 weeks that clinically resemble human precancerous lesions termed actinic keratoses (Fig. 1D). Histologic analysis demonstrates a precancerous lesion exhibiting hyperplasia, parakeratosis (retained nuclei in the stratum corneum), and keratinocyte atypia (Fig. 1D).
The K14-Fyn Y528F FVB/N mice with tumors were followed for at least 6 months and did not demonstrate evidence of metastasis, which mirrors the low metastatic potential seen with human cutaneous SCCs.
Histologic analysis of the hyperkeratotic plaques and tumors demonstrates carcinoma in situ and squamous cell carcinoma
Histologic analysis of the hyperkeratotic plaques that develop during the first week post-natal in the K14-Fyn Y528F C57BL/6 and C57BL/6-FVB/N F1 mice demonstrate a markedly thickened epidermis with increased mitotic activity, cytologic atypia, and architectural disorganization compared to adjacent non-lesional epidermis (Fig. 2A). These histologic features are consistent with squamous cell carcinoma in situ (SCIS) and resemble human SCIS (Fig. 2A).
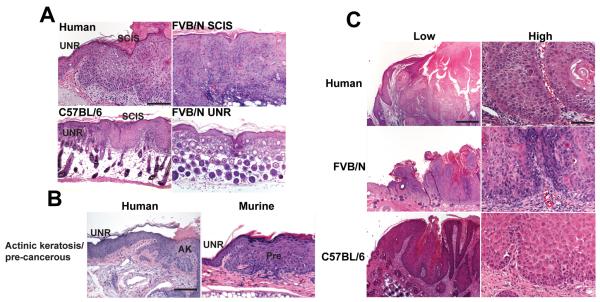
Figure 2. K14-Fyn Y528F lesions histologically mimic human lesions.
A) Human squamous cell carcinoma in situ (SCIS) demonstrates full-thickness keratinocyte atypia, epidermal hyperplasia, and hyperkeratosis without evidence of dermal invasion. Similar findings are seen in hyperkeratotic plaques taken from the back skin of K14-Fyn Y528F one week old mice of the indicated genetic backgrounds. UNR-unremarkable skin. Scale bar 200 μM, 125X B) C57BL/6-FVB/N F1 hybrid K14 Fyn Y528F mice demonstrate precancerous lesions that resemble human actinic keratoses. UNR-unremarkable skin, AK-actinic keratosis, Pre-precancerous lesion from the ear of a 5 week-old mouse. Scale bar 200 μM, 125X C) Biopsies of the spontaneous cutaneous tumors from a 5 week old FVB/N F1 hybrid and an 8 week old C57BL/6 mouse demonstrate SCCs resembling human cutaneous SCC. Scale bar 800 μM, 31.25X-low, Scale bar 100 μM, 250X-high. Data representative of three independent mice.
The C57BL/6-FVB/N F1 hybrid K14-Fyn Y528F mice exhibited small hyperkeratotic lesions ranging in size from 1-3 mm (Fig 2B); these lesions demonstrate focal epidermal hyperplasia, keratinocyte dysplasia, and hyperkeratosis, resembling a human actinic keratosis (AK) (Fig. 2B).
The spontaneous skin tumors that developed in both genetic backgrounds carrying the K14-Fyn Y528F transgene manifested enlarged keratinocytes with nuclear atypia, mitotic activity, dermal invasion, and focal keratinization (Fig. 2C). The cytologic and histologic features exhibited by these murine lesions are consistent with well-differentiated SCCs and closely resemble corresponding human lesions (30, 31). These data demonstrate that the Fyn Y528F transgene induces cutaneous lesions that are histologically similar to human AKs, SCIS, and SCCs.
Immunohistochemical analysis of precancerous lesions and cutaneous tumors in C57BL/6-FVB/N F1 hybrid K14-Fyn Y528F mice
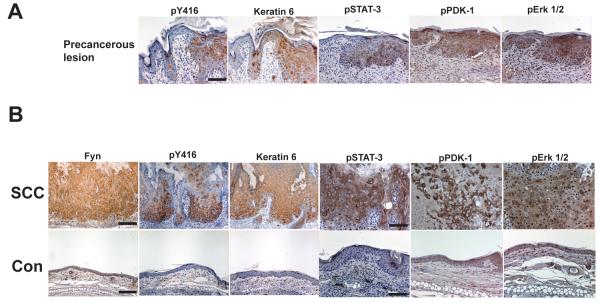
Immunohistochemical analysis of small precancerous lesions from a 5 week old C57BL/6-FVB/N F1 hybrid K14-Fyn Y528F mouse demonstrated increased levels of activated SFKs, keratin 6, phospho-STAT-3, phospho-Erk 1/2, and phospho-PDK-1 (Fig. 3A).
Figure 3. Immunohistochemical analysis of precancerous lesions and SCCs in C57BL/6-FVB/N F1 hybrid K14 Fyn Y528F mice.
A) Formalin-fixed serial sections of ear skin containing a precancerous lesion (right) and unremarkable epidermis (left) from a 5 week old transgenic mouse were subjected to immunohistochemical staining for the indicated markers; pY416-activated Src kinases. Scale bar 100 μM, 250X. B) Formalin-fixed sections of an ear SCC and adjacent non-lesional epidermis from a 5 week old transgenic mouse were subjected to immunohistochemical staining for the indicated markers; pY416-activated Src kinases. Scale bar 200 μM, 125X for Fyn, pY416, and keratin 6. Scale bar 100 μM, 250X for pSTAT-3, pPDK-1, and pErk1/2. Data representative of three independent mice.
Analysis of cutaneous SCCs from 5 week old C57BL/6-FVB/N F1 hybrid K14-Fyn Y528F mice demonstrated a pattern similar to that seen in the precancerous lesions with markedly increased levels of Fyn, activated SFKs, and keratin 6 compared with non-lesional epidermis (Fig. 3B). Increased staining for phospho-STAT-3, phospho-PDK-1, and phospho-p44/42 also was seen in the SCCs. Together, the data suggest that neoplastic lesions secondary to elevated Fyn activity also exhibit increased activation of STAT-3, PDK-1, and Erk 1/2. Similar staining was seen in SCIS lesions (data not shown) and in SCIS from K14-Fyn Y528F C57BL/6 mice (Sup. Fig. 1).
Increased Fyn activity decreases Notch1/NICD transcript levels
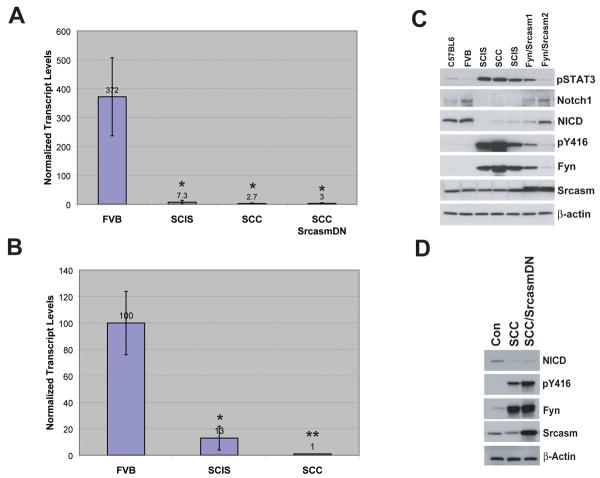
The formation of precancerous lesions and SCCs in C57BL/6-FVB/N F1 hybrid K14-Fyn Y528F mice phenocopies SM22α-DNMAML mice which have decreased epidermal Notch 1 signaling (32). Therefore, a link between increased Fyn activity and decreased Notch 1 signaling was assessed. qRT-PCR for Notch 1 transcript was performed on total RNA from unremarkable skin, SCIS lesions, and SCCs from K14-Fyn Y528F mice and littermate controls. Skin from FVB mice demonstrated relatively high levels of Notch 1 transcript (Fig. 4A). K14-Fyn Y528F SCIS lesions demonstrated approximately 2% of the Notch 1 transcript levels seen in control mice (p < 0.01). SCCs from K14-Fyn Y528F and double transgenic expressing SrcasmDN exhibited only 1% of the control Notch 1 transcript levels (p < 0.01). These data demonstrate that the cutaneous lesions associated with increased Fyn activity contain lower levels of Notch 1 transcript.
Figure 4. Regulation of Notch 1 by Fyn and Srcasm.
A) Fyn downregulates Notch 1 transcript levels. qRT-PCR was performed on cDNA from FVB/N F1 hybrid, K14-Fyn Y528F F1 hybrid SCIS, and SCCs from Fyn Y528F and Fyn Y528F/SrcasmDN mice to determine Notch 1 transcript levels. Three biopsies of each type were analyzed. Normalized mean levels and standard deviations shown. p-values compared with FVB: *- p < 0.01 B) Fyn downregulates levels of Srcasm transcript. qRT-PCR was performed on cDNA from FVB/N skin, K14-Fyn Y528F FVB/N F1 SCIS, and K14-Fyn Y528F FVB/N F1 SCC to determine Srcasm transcript levels as in A. p values compared with FVB: * p=0.041, **p=0.029 C) Srcasm regulates Fyn-dependent Notch inhibition and pSTAT-3 phosphorylation. Protein lysates from C57BL/6 and FVB/N F1 hybrid skin and SCIS or SCC lesions from FVB/N F1 hybrid lines were subjected to western blotting with the indicated antibodies. pY416-activated SFKs. Fyn/Srcasm1 exhibited a weak phenotype. Fyn/Srcasm2 exhibited no phenotype. D) SrcasmDN does not revert Fyn-dependent Notch inhibition. Protein lysates from unremarkable skin and SCCs were subjected to western blot analysis with the indicated antibodies as in C.
Increased Fyn activity decreases Srcasm transcript levels
Srcasm is a negative regulator of activated SFKs, including Fyn; therefore, the levels of Srcasm transcript were assessed in control skin, SCIS-like lesions, and SCCs (23). Control skin contained relatively high levels of Srcasm transcript while SCIS lesions contained only 13% (p=0.041) of control levels (Fig. 4B). SCCs from K14-Fyn Y528F mice contained only 1% of control levels of Srcasm transcript (p=0.029). As expected, SCCs from the K14-Fyn Y528F/SrcasmDN mice contained approximately 20 times the control level of Srcasm transcript because of the SrcasmDN transgene (data not shown).
Increased Fyn activity decreases Notch1/NICD levels and promotes STAT-3 phosphorylation
To determine if the Fyn-induced decrease in Notch 1 transcript levels correlated with a decrease in Notch 1/NICD protein levels, western blot analysis was performed on lysates of control skin, SCIS, and SCCs. Increased Fyn levels and activated SFK levels in SCIS and SCCs correlated with a dramatic drop in Notch 1 and NICD protein levels (Fig. 4C). K14-Fyn Y528F/Srcasm double transgenic mice demonstrating a weak phenotype, exhibited increased Srcasm levels associated with partially normalized Fyn and Notch 1/NICD levels (Fig. 4C). K14-Fyn Y528F/Srcasm double transgenic mice exhibiting a corrected phenotype demonstrated increased Srcasm levels associated with normalized levels of Fyn, activated SFKs, and Notch 1/NICD.
In oral SCCs, the level of phospho-STAT-3 is tightly correlated with the levels of SFK activity (33). Likewise, in the K14-Fyn Y528F skin lesions, the level of phospho-STAT-3 is proportional to the levels of Fyn and activated SFKs (Fig. 4C). Together, these data show that increased levels of activated Fyn enhance phospho-STAT-3 levels and downregulate Notch 1 transcript, Notch 1 protein, and Srcasm transcript levels. In K14-Fyn Y528F/K14-Srcasm mice, increasing Srcasm levels decreases Fyn and phospho-STAT-3 levels while restoring Notch 1/NICD levels. Together, Fyn and Srcasm can alter Notch 1 levels while promoting or inhibiting neoplasia, respectively.
Increased SrcasmDN expression does not downregulate levels of Fyn Y528F in transgenic skin
SrcasmDN has its known Fyn phosphorylation sites mutated to phenylalanines, and this mutant cannot be phosphorylated by Fyn nor downregulate native or activated Fyn in vitro (23). To better understand why SrcasmDN does not inhibit SCC formation, protein lysates from littermate control skin, K14-Fyn Y528F SCCs, and K14-Fyn Y528F/SrcasmDN SCCs were subjected to western blotting. Western blot analysis demonstrates that the SrcasmDN molecule does not downregulate Fyn Y528F like native Srcasm (Fig. 4D), and therefore, levels of activated SFKs remain elevated and Notch 1/NICD levels remain low. These data confirm that SrcasmDN cannot downregulate Fyn Y528F in vivo.
SFK activity is increased and Notch 1 and Srcasm levels are decreased in human SCC
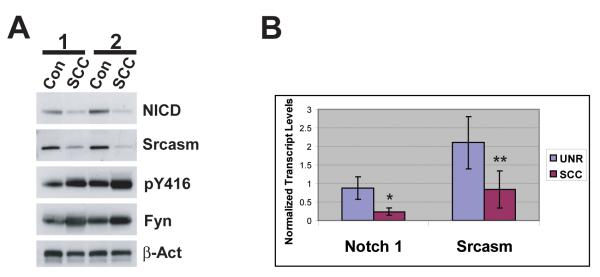
The relationship between Fyn, SFK activity, Srcasm, and Notch 1 has not been evaluated in genetically matched non-lesional human skin and SCCs (8, 20, 32, 34). To address this question, protein lysates of human SCCs and adjacent non-tumorigenic epidermis from two independent patients were subjected to western blot analysis for activated SFKs, Fyn, NICD, and Srcasm (Fig. 5A). Human SCCs demonstrate increased levels of activated SFKs and Fyn compared with unremarkable skin. Human SCCs also demonstrate decreased levels of NICD and Srcasm compared with unremarkable skin. These data show that the increased Fyn levels and SFK activity in human SCCs are associated with decreased NICD and Srcasm levels.
Figure 5. Elevated SFK levels correlate with decreased Notch 1 and Srcasm levels in human SCC.
A) Levels of activated SFKs are inversely related to NICD and Srcasm levels. Western blot analysis of lysates from paired non-lesional human skin and adjacent SCC using the indicated antibodies. Corresponding samples from two patients. B) Notch 1 and Srcasm transcripts are downregulated in human SCC. qRT-PCR was performed on mRNA derived from independent unremarkable human skin samples and SCCs to determine Notch 1 and Srcasm transcript levels. Normalized mean levels and standard deviations shown. Three independent unremarkable skin and SCC samples were analyzed. * p=0.025, **p=0.064
To determine if the decreased NICD and Srcasm levels were associated with lower mRNA levels, quantitative RT-PCR was performed on mRNA isolated from three randomly selected human SCCs and three independent unremarkable skin samples. Both NICD and Srcasm transcript levels were decreased in the SCCs compared to unremarkable skin (Fig. 5B). These data suggest that human cutaneous SCCs manifest decreased Notch 1 and Srcasm transcript and protein levels. Together, these data demonstrate that human SCCs and the K14-Fyn Y528F SCCs exhibit similarities regarding the levels of Fyn, SFK activity, Srcasm, and Notch 1.
Fyn and Srcasm modulate p53 levels
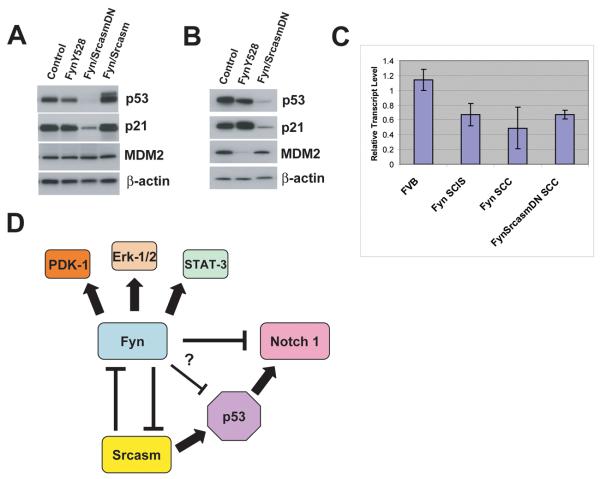
p53 is an important regulator of cutaneous carcinogenesis in humans, and impaired p53 function is associated with the formation of AKs and SCCs (34, 35). Therefore, we examined the levels of p53 protein and mRNA in skin samples from our transgenic lines. In C57BL/6-FVB/N F1 hybrid K14-Fyn Y528F SCIS lesions, increased Fyn levels were associated with decreased p53 levels compared to age-matched non-transgenic controls (Fig. 6A). Western blot analysis demonstrates that raising Srcasm levels elevates p53 levels in K14-Fyn Y528F/K14-Srcasm mice compared to non-transgenic controls and K14-Fyn Y528F mice (Fig. 6A). In contrast, increased expression of SrcasmDN lowers p53 levels in SCIS and SCCs in K14-Fyn Y528F/SrcasmDN double transgenic mice (Fig. 6A and B). In SCCs, as in SCIS lesions, increased Fyn expression was associated with lower p53 levels compared to controls (Fig. 6B).
Figure 6. Fyn and Srcasm modulate p53 levels.
A) Analysis of SCIS lesions and age-matched controls. Protein lysates from SCIS lesions of C57BL/6-FVB/N F1 hybrid transgenic lines or controls were subjected to western blot analysis for p53, p21, MDM2, β-actin. Data representative of two independent sets of mice. B) Analysis of SCC lesions and age-matched controls. Protein lysates from SCCs of the indicated lines or controls were subjected to western blot analysis as in A. Data representative of two independent sets of mice. C) qRT-PCR analysis for p53 transcript. mRNA from the indicated lesions and K14-Fyn Y528F FVB/N F1 hybrid transgenic lines or controls were subjected to qRT-PCR for p53. Data derived from two independent sets of samples. D) Fyn/Srcasm signaling nexus. Arrows denote positive regulatory relationships. T-bars denote negative regulatory relationships. Fyn increases levels of PDK-1, Erk 1/2, and STAT-3 activation. Fyn lowers Notch 1 transcript and protein levels, perhaps through p53-dependent and independent mechanisms. Fyn lowers Srcasm and p53 transcript levels. Srcasm lowers Fyn protein levels.
In all samples, p21 levels closely paralleled p53 levels implying that p53 is transcriptionally active (Figs. 6A and B) (36). MDM2 levels remained constant in all tissue samples except in SCCs from K14-Fyn Y528F mice; therefore, fluctuations in MDM2 levels do not correlate with the significant changes in p53 levels (Fig. 6A and B).
qRT-PCR analysis of RNA from these samples for p53 transcript demonstrates that p53 transcript levels were lower in mice harboring a Fyn Y528F transgene (Fig. 6C). However, the fluctuations in p53 protein levels amongst the various Fyn transgenic lines did not correlate with changes in p53 transcript levels (Fig. 6C). The p53 transcript level is significantly lower in the Fyn transgenic lines together compared with controls (p < 0.02). In the Fyn Y528F transgenic lines, raising Srcasm levels lowers Fyn levels and elevates p53 while increasing levels of SrcasmDN decreases p53 levels compared to controls. These in vivo data suggest that Fyn downregulates p53 transcript levels while Srcasm can modulate p53 protein levels independent of transcript levels. Therefore, changes in Fyn and Srcasm levels could alter cellular p53 levels and influence keratinocyte susceptibility to genotoxic stress.
Discussion
K14-Fyn Y528F transgenic mice represent a robust model of cutaneous carcinogenesis that spontaneously form neoplastic lesions resembling those seen in human cutaneous neoplasia. Within the first week post-natal, K14-Fyn Y528F transgenic mice exhibit hyperkeratotic plaques resembling human SCIS at the histologic and molecular levels. These SCIS lesions demonstrate increased activity of SFKs, the RAS-MAP kinase pathway, and the PI-3K/PDK-1/Akt kinase pathway, similar to human lesions and other murine models (8, 37-40).
At 4-5 weeks, the K14-Fyn Y528F FVB/N F1 hybrid transgenic mice spontaneously form punctate hyperkeratotic lesions resembling human AKs; both human AKs and these murine precancerous lesions are associated with increased levels of activated SFKs (8). Spontaneous generation of precancerous lesions within a short time makes the K14-Fyn Y528F model well suited for screening topical agents that may be efficacious in treating AKs.
At five weeks, K14-Fyn Y528F mice spontaneously develop cutaneous SCCs that have not metastasized within a 6 month follow up period, which is consistent with the low metastatic incidence of human cutaneous SCCs, approximately 4% in tumors 2-6 mm thick (41).
Transgenic mice expressing Src or activated mutant Src in the epidermis have been reported to develop SCCs spontaneously after 3 months, have an increased risk for developing papillomas secondary to two-stage chemical carcinogenesis, or develop SCCs in 25% of mice on the edges of healing wounds (42-44). However, these Src transgenic models take longer to manifest tumors and AK-like lesions have not been described. The K14-Fyn Y528F lines develop AK-like lesions and spontaneous SCCs in a 4-5 week time frame demonstrating that Fyn is a potent oncogene in keratinocytes.
The K14-Fyn Y528F transgenic lines phenocopy the SM22α-DNMAML mouse, though the SM22α-DNMAML line typically takes 6 months to manifest lesions (32). Western blot and qRT-PCR analysis of skin, SCIS, and SCCs from the various K14-Fyn Y528F lines demonstrated that increased levels of Fyn and activated SFKs are associated with decreased levels of Notch 1 mRNA, Notch 1 protein, and NICD. These data link increased SFK activity in keratinocytes with Notch 1 downregulation in vivo. This observation correlates with prior observations demonstrating that SFKs activate MEK1/ERK1 downstream of EGFR and that increased EGFR signaling downregulates Notch 1 transcription through a MEK1/ERK1-dependent pathway (20, 34). Notch 1 downregulation by Fyn may be important for promoting neoplasia; it will be interesting to determine if increased NICD expression can inhibit Fyn-dependent SCC induction.
Canonically, the EGFR-dependent downregulation of Notch 1 requires p53 function (34). Since Fyn downregulates Notch 1 in K14-Fyn Y528F/K14-Srcasm DN SCCs, which have very little p53 (Fig. 6B), Fyn may downregulate Notch 1 through a p53-independent mechanism (Fig. 6D). In vivo studies using p53 null mice will help determine if Fyn requires p53 for Notch 1 downregulation. Decreased p53 transcript levels in the K14-Fyn Y528F SCIS lesions and SCCs are consistent with prior observations that Fyn activates Erk1 and that EGFR downregulates p53 transcription through an Erk1/c-Jun dependent mechanism (34).
Increased SrcasmDN expression lowers p53 levels independent of transcript levels. Although SrcasmDN does not bind to Grb2, p85 PI-3 kinase, or SFKs, this molecule can still bind to Tsg101, Tollip, and mono-ubiquinated proteins. These altered intermolecular associations may enhance the ability of SrcasmDN to downregulate p53 at the proteomic level.
Supraphysiologic SFK levels and subphysiologic Srcasm levels are common in human AKs, SCIS, and cutaneous SCCs (8). These findings suggest that the inverse relationship between SFK activity and Srcasm levels may represent a general mechanism of carcinogenesis. Supporting this hypothesis, Srcasm levels are decreased in human basal cell carcinomas and in esophageal SCC tumor cell lines compared with non-neoplastic keratinocyte lines (data not shown) (45).
The data presented show that epidermal neoplasia develops when keratinocytic SFK activity exceeds the negative regulatory capacity of endogenous Srcasm (Fig 6D). However, the pro-oncogenic effect of Fyn can be inhibited by raising the Srcasm level such that its SFK-downregulatory capacity restores Fyn levels to near physiologic. The data presented suggest that one mechanism of SFK-dependent neoplasia would be to disrupt the balance between SFK activity and Srcasm-dependent SFK-downregulation so that it elevates SFK activity. Such an event could result by decreasing intracellular Srcasm levels. Further characterization of the mechanisms that regulate Srcasm levels may provide insights into SFK-dependent carcinogenesis.
The ability of Srcasm to inhibit Fyn-induced carcinogenesis suggests that Srcasm can function as an anti-oncogene. As such, Srcasm appears to represent a novel class of anti-oncogene that targets activated SFKs for degradation in a lysosomal-dependent manner (23). Such a pathway may be important for limiting SFK signaling and maintaining cell homeostasis. Manipulation of Srcasm levels may provide a tool for modulating the level of SFK signaling and a means of promoting or inhibiting epithelial cell growth.
Supplementary Material
Acknowledgements
This work was supported by NIAMS grant RO1-AR051380 to JTS and The Department of Dermatology, University of Pennsylvania Medical School. The authors thank Drs. John Stanley and Gary Koretzky for valuable advice and support.
References
- 1.American Academy of Dermatology Squamous Cell Carcinoma. 2008 April 15; 2008 [cited; Available from: [Google Scholar]
- 2.Kwa RE, Campana K, Moy RL. Biology of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992;26:1–26. doi: 10.1016/0190-9622(92)70001-v. [DOI] [PubMed] [Google Scholar]
- 3.MayoClinic.com Squamous Cell Carcinoma. 2007 March 7; 2008 [cited; Available from: [Google Scholar]
- 4.Czarnecki D, Meehan CJ, Bruce F, Culjak G. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207–9. doi: 10.1007/s10227-001-0041-x. [DOI] [PubMed] [Google Scholar]
- 5.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 6.Rous P. A SARCOMA OF THE FOWL TRANSMISSIBLE BY AN AGENT SEPARABLE FROM THE TUMOR CELLS. J Exp Med. 1911;13:397–411. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94:344–51. doi: 10.1002/cncr.10221. [DOI] [PubMed] [Google Scholar]
- 8.Ayli EE, Li W, Brown TT, Witkiewicz A, Elenitsas R, Seykora JT. Activation of Src-family tyrosine kinases in hyperproliferative epidermal disorders. J Cutan Pathol. 2008;35:273–7. doi: 10.1111/j.1600-0560.2007.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cam WR, Masaki T, Shiratori Y, et al. Reduced C-terminal Src kinase activity is correlated inversely with pp60(c-src) activity in colorectal carcinoma. Cancer. 2001;92:61–70. doi: 10.1002/1097-0142(20010701)92:1<61::aid-cncr1292>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Lutz MP, Esser IB, Flossmann-Kast BB, et al. Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem Biophys Res Commun. 1998;243:503–8. doi: 10.1006/bbrc.1997.8043. [DOI] [PubMed] [Google Scholar]
- 11.Yezhelyev MV, Koehl G, Guba M, et al. Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin Cancer Res. 2004;10:8028–36. doi: 10.1158/1078-0432.CCR-04-0621. [DOI] [PubMed] [Google Scholar]
- 12.Daigo Y, Furukawa Y, Kawasoe T, et al. Absence of genetic alteration at codon 531 of the human c-src gene in 479 advanced colorectal cancers from Japanese and Caucasian patients. Cancer Research. 1999;59:4222–4. [PubMed] [Google Scholar]
- 13.Irby RB, Mao W, Coppola D, et al. Activating SRC mutation in a subset of advanced human colon cancers. Nature Genetics. 1999;21:187–90. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- 14.Talamonti MS, Roh MS, Curley SA, Gallick GE. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest. 1993;91:53–60. doi: 10.1172/JCI116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang NM, Yeh KT, Tsai CH, Chen SJ, Chang JG. No evidence of correlation between mutation at codon 531 of src and the risk of colon cancer in Chinese. Cancer Letters. 2000;150:201–4. doi: 10.1016/s0304-3835(99)00398-5. [DOI] [PubMed] [Google Scholar]
- 16.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annual Review of Cell & Developmental Biology. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 17.Wolven A, Okamura H, Rosenblatt Y, Resh MD. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Molecular Biology of the Cell. 1997;8:1159–73. doi: 10.1091/mbc.8.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins SM, Quintrell NA, Bishop JM. Myristoylation and differential palmitoylation of the HCK protein-tyrosine kinases govern their attachment to membranes and association with caveolae. Mol Cell Biol. 1995;15:3507–15. doi: 10.1128/mcb.15.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–63. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Marshall C, Mei L, et al. Srcasm Modulates EGF and Src-kinase Signaling in Keratinocytes. J Biol Chem. 2005;280:6036–46. doi: 10.1074/jbc.M406546200. [DOI] [PubMed] [Google Scholar]
- 21.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–9. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 22.Wang XQ, Paller AS. Lipid rafts: membrane triage centers. J Invest Dermatol. 2006;126:951–3. doi: 10.1038/sj.jid.5700282. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Marshall C, Mei L, Gelfand J, Seykora JT. Srcasm Corrects Fyn-induced Epidermal Hyperplasia by Kinase Down-regulation. J Biol Chem. 2007;282:1161–9. doi: 10.1074/jbc.M606583200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seykora JT, Mei L, Dotto GP, Stein PL. ‘Srcasm: a novel Src activating and signaling molecule. J Biol Chem. 2002;277:2812–22. doi: 10.1074/jbc.M106813200. [DOI] [PubMed] [Google Scholar]
- 25.Nickerson DP, Russell MR, Odorizzi G. A concentric circle model of multivesicular body cargo sorting. EMBO Rep. 2007;8:644–50. doi: 10.1038/sj.embor.7401004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puertollano R. Interactions of TOM1L1 with the multivesicular body sorting machinery. J Biol Chem. 2005;280:9258–64. doi: 10.1074/jbc.M412481200. [DOI] [PubMed] [Google Scholar]
- 27.Yanagida-Ishizaki Y, Takei T, Ishizaki R, et al. Recruitment of Tom1L1/Srcasm to endosomes and the midbody by Tsg101. Cell Struct Funct. 2008;33:91–100. doi: 10.1247/csf.07037. [DOI] [PubMed] [Google Scholar]
- 28.Razi M, Futter CE. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell. 2006;17:3469–83. doi: 10.1091/mbc.E05-11-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakabayashi Y, Mao JH, Brown K, Girardi M, Balmain A. Promotion of Hras-induced squamous carcinomas by a polymorphic variant of the Patched gene in FVB mice. Nature. 2007;445:761–5. doi: 10.1038/nature05489. [DOI] [PubMed] [Google Scholar]
- 30.Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification--part two. J Cutan Pathol. 2006;33:261–79. doi: 10.1111/j.0303-6987.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 31.Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. J Cutan Pathol. 2006;33(Part one):191–206. doi: 10.1111/j.0303-6987.2006.00516_1.x. [DOI] [PubMed] [Google Scholar]
- 32.Proweller A, Tu L, Lepore JJ, et al. Impaired notch signaling promotes de novo squamous cell carcinoma formation. Cancer Res. 2006;66:7438–44. doi: 10.1158/0008-5472.CAN-06-0793. [DOI] [PubMed] [Google Scholar]
- 33.Xi S, Zhang Q, Dyer KF, et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278:31574–83. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 34.Kolev V, Mandinova A, Guinea-Viniegra J, et al. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat Cell Biol. 2008;10:902–11. doi: 10.1038/ncb1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziegler A, Jonason AS, Leffell DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–6. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 36.el-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 37.Khavari TA, Rinn J. Ras/Erk MAPK signaling in epidermal homeostasis and neoplasia. Cell Cycle. 2007;6:2928–31. doi: 10.4161/cc.6.23.4998. [DOI] [PubMed] [Google Scholar]
- 38.Qiao W, Li AG, Owens P, Xu X, Wang XJ, Deng CX. Hair follicle defects and squamous cell carcinoma formation in Smad4 conditional knockout mouse skin. Oncogene. 2006;25:207–17. doi: 10.1038/sj.onc.1209029. [DOI] [PubMed] [Google Scholar]
- 39.Scholl FA, Dumesic PA, Barragan DI, Charron J, Khavari PA. Mek1/2 gene dosage determines tissue response to oncogenic Ras signaling in the skin. Oncogene. 2009;28:1485–95. doi: 10.1038/onc.2008.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waterman EA, Sakai N, Nguyen NT, et al. A laminin-collagen complex drives human epidermal carcinogenesis through phosphoinositol-3-kinase activation. Cancer Res. 2007;67:4264–70. doi: 10.1158/0008-5472.CAN-06-4141. [DOI] [PubMed] [Google Scholar]
- 41.Brantsch KD, Meisner C, Schonfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9:713–20. doi: 10.1016/S1470-2045(08)70178-5. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto T, Jiang J, Kiguchi K, et al. Overexpression of a constitutively active form of c-src in skin epidermis increases sensitivity to tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Molecular Carcinogenesis. 2002;33:146–55. doi: 10.1002/mc.10030. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto T, Jiang J, Kiguchi K, et al. Targeted expression of c-Src in epidermal basal cells leads to enhanced skin tumor promotion, malignant progression, and metastasis. Cancer Res. 2003;63:4819–28. [PubMed] [Google Scholar]
- 44.Matsumoto T, Kiguchi K, Jiang J, et al. Development of transgenic mice that inducibly express an active form of c-Src in the epidermis. Mol Carcinog. 2004;40:189–200. doi: 10.1002/mc.20027. [DOI] [PubMed] [Google Scholar]
- 45.Meulener MC, Ayli EE, Elenitsas R, Seykora JT. Decreased Srcasm expression in hyperproliferative cutaneous lesions. J Cutan Pathol. 2009;36:291–5. doi: 10.1111/j.1600-0560.2008.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.